-
PDF
- Split View
-
Views
-
Cite
Cite
Susan Richter, Barbara Klink, Brit Nacke, Aguirre A. de Cubas, Anastasios Mangelis, Elena Rapizzi, Matthias Meinhardt, Christina Skondra, Massimo Mannelli, Mercedes Robledo, Mario Menschikowski, Graeme Eisenhofer, Epigenetic Mutation of the Succinate Dehydrogenase C Promoter in a Patient With Two Paragangliomas, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 2, 1 February 2016, Pages 359–363, https://doi.org/10.1210/jc.2015-3856
Close - Share Icon Share
Abstract
Mutational inactivation of the succinate dehydrogenase (SDH) complex is a well-described cause of tumor development in pheochromocytomas/paragangliomas (PPGLs) and gastrointestinal stromal tumors (GISTs). Epigenetic inactivation of the SDHC gene is a more recently discovered phenomenon, which so far has only been described in GISTs and PPGLs from patients with Carney triad syndrome.
A 33-year-old patient presented with two abdominal paragangliomas (PGLs) and an adrenocortical adenoma. Both PGLs showed high succinate:fumarate ratios indicative of SDHx mutations; however, no mutations in any of the known PPGL susceptibility genes were found in leucocyte or tumor DNA. We identified methylation of the SDHC promoter region in both PGLs, which coincided with decreased SDHC expression at mRNA and protein levels and a hypermethylated epigenomic signature (CpG island methylator phenotype). Low-level SDHC promoter methylation was also observed in the adenoma but not in normal adrenal tissue or blood, suggesting postzygotic somatic mosaicism for SDHC promoter methylation in the patient.
This report provides evidence that SDHC promoter methylation can cause PGLs due to SDHC inactivation, emphasizing the importance of considering epigenetic changes and functional readouts in the genetic evaluation of patients not only with GISTs and Carney triad but also with PPGL.
Pheochromocytomas/paragangliomas (PPGLs) are chromaffin cell tumors, with about 35% of cases due to germline mutations in one of many currently identified tumor susceptibility genes, including those encoding all succinate dehydrogenase (SDH) subunit genes. The latter mutations lead to profound changes in cellular metabolism, resulting in elevated succinate levels that mediate inhibition of α-ketoglutarate-dependent DNA and histone demethylases, thereby causing a CpG island methylator phenotype (CIMP) (1). Succinate:fumarate ratios can be used to reliably identify SDHx-mutated PPGLs by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (2). In that study, we also observed high succinate:fumarate ratios in six patients without SDHx mutations, among them the current case, suggesting alternative mechanisms causing loss of SDH function.
Recently, promoter methylation of SDHC has been described as a novel cause of SDH complex inactivation in gastrointestinal stromal tumor (GIST) patients with and without Carney triad syndrome, prompting us to evaluate the present case for SDHC promoter methylation (3–5).
Case Presentation
Clinical history
A 33-year-old female patient with a 2-year history of hypertension was referred to the University Hospital Dresden because of incidental findings of three abdominal masses by magnetic resonance imaging carried out for evaluation of back pain. Biochemical testing indicated elevated plasma normetanephrine of 272 pg/mL (upper cutoff, 112 pg/mL) and a normal metanephrine level. Urinary normetanephrine was also elevated to 843 μg/24 h (upper cutoff, 379 μg/24 h). After clonidine, plasma normetanephrine could only be suppressed to 216 pg/mL from 281 pg/mL. Computed tomography confirmed a right adrenal tumor of 2.2 cm, a retroperitoneal polycystic tumor of 3.0 × 4.6 cm (paraganglioma [PGL] 1), and a para-aortic mass of 2.6 cm (PGL2). Computed tomography of the thorax and abdomen excluded pulmonary or additional abdominal tumors. Meta-iodobenzylguanidine scintigraphy revealed both retroperitoneal masses, but not the adrenal mass. Histologically, the retroperitoneal tumors were both diagnosed as PGLs, and the adrenal tumor as an adrenocortical adenoma. On yearly follow-up, the patient has remained disease-free. The patient was investigated under an approved protocol with signed informed consent.
Genetic testing
Due to the presentation of two independent PGLs, mutation analyses of PPGL susceptibility genes was performed on DNA from the patient's blood. No germline mutations or deletions were identified in SDHAF2, SDHA, SDHB, SDHC, SDHD, VHL, MAX, TMEM127, MDH2, FH, HRAS, EPAS1, or RET using Sanger-sequencing and multiplex ligation-dependent probe amplification. Conventional cytogenetic analysis using GTG banding revealed a normal female karyotype, excluding large-scale chromosomal rearrangements. Additionally, tumor DNA was analyzed using panel next-generation sequencing (Illumina TrueSightCancer, and custom panel). No somatic mutations were identified in any of the known PPGL susceptibility genes indicated above, as well as in NF1, KIF1B, PDH1, PDH2, or other cancer-related genes included in the panel.
PGLs, but not the adenoma, display SDHx-metabolite phenotype
To assess functionality of the SDH complex, we performed an absorbance-based enzymatic assay as well as LC-MS/MS-based measurements of succinate and fumarate (2). SDH activity was reduced by 71 and 94% for PGL1 and PGL2, respectively, compared to the patient's adenoma as well as control PPGLs with functional SDH (Figure 1A). Succinate:fumarate ratios were strongly elevated in both PGLs, comparable to tumors with SDHx mutations, with PGL2 showing a greater degree of SDH complex inhibition (Figure 1A). A minor increase was also detected in the patient's adenoma compared to other adrenal adenomas.
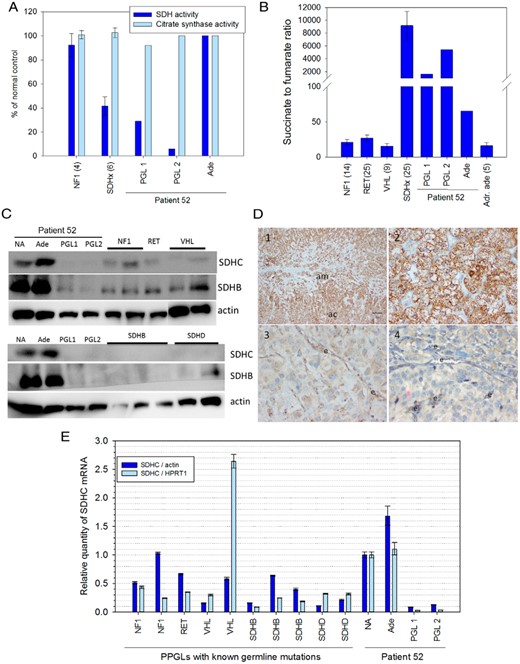
Patient PGLs (PGL1 and 2), but not the adenoma (Ade), show disruptions of SDH function with decreased SDHC expression. A, Enzymatic activity of SDH and citrate synthase were measured and compared to PPGLs from patients with known germline mutations (number of cases in parentheses). B, Succinate and fumarate were quantified by LC-MS/MS and compared to adrenal adenomas (Adr. ade), and PPGLs from patients with known germline mutations (number of cases in parentheses). C, Western blot analysis of SDHC and SDHB normalized to x-actin for tissue extracts from the patient's tumors and normal adrenal (NA), and PPGLs with known germline mutations (as indicated in the top row). D, Immunohistochemistry for SDHB: 1-normal adrenal cortex (ac) and medulla (am), as well as the 2-adenoma, show strong cytoplasmatic granular staining of SDHC, whereas 3-PGL1, and 4-PGL2 only show weak staining. e, endothelial cells. E, Relative expression of SDHC normalized to β-actin or HPRT1 in the same tissues analyzed in C. Primers and antibodies used in this study are listed in Supplemental Figure 1.
SDHB protein expression by Western blot was reduced in both PGLs, compared to the patient's normal adrenal tissue and adenoma, as well as PPGLs from other patients with functional SDH (Figure 1C). This was confirmed by SDHB immunohistochemistry, which was strongly positive for the adenoma and normal adrenal cortex and medulla (Figure 1D). Weak staining was observed in both PGLs, with stronger expression in endothelial cells within the tumors. Expression of SDHC was almost completely abolished at protein (Figure 1C) and RNA levels (Figure 1E) in both PGLs, but not in the adenoma or normal adrenal tissue of the patient.
Identification of somatic SDHC promotor methylation
Bisulfite-treated DNA from the patient's PGLs, adenoma, blood, and normal adrenal tissue was analyzed using methylation-sensitive high-resolution melting (MS-HRM) analysis and Sanger-sequencing of the SDHC promotor region (6). Primers are listed in Supplemental Figure 1. MS-HRM demonstrated SDHC promoter methylation at levels of 22–27% and 69–76% for PGL1 and PGL2, respectively (Figure 2, A and B). The patient's adenoma showed lower SDHC promotor methylation of 14–18%, whereas no methylation was detected in DNA from the patient's blood and normal adrenal tissue. MS-HRM analysis also did not detect SDHC promotor methylation in PPGLs from other patients with mutations in NF1, RET, VHL, SDHB, and SDHD (Figure 2B).
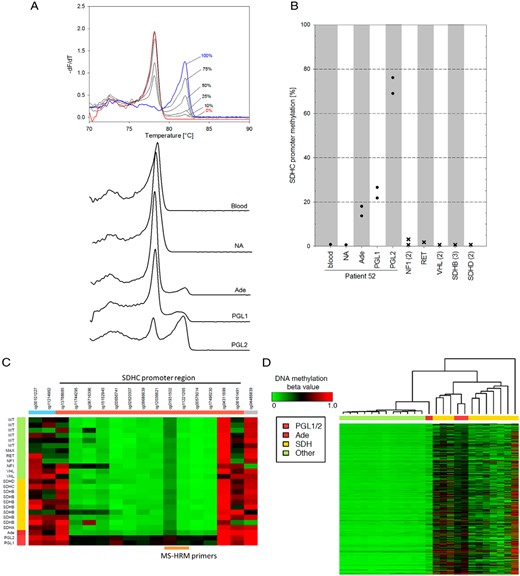
SDHC promoter methylation analysis. A, MS-HRM analysis of 16 CpGs in the proximal region of the SDHC gene covering the sequence from −97 to +147. Representative melting curves, plotted as negative derivative of fluorescence over temperature vs temperature, of standard DNA with various degrees of methylation and DNA extracted from samples of interest. B, SDHC promoter methylation compared to PPGLs from patients with known germline mutations. Circles indicate averages of two bisulfite modifications from two different DNA extractions of the same tumor; “x” indicates different tumor samples with the indicated mutations (number of samples in parentheses). C, Heatmap showing the methylation pattern (Illumina 450K array) of probes spanning the SDHC promoter (orange bar) and adjacent areas (blue and gray). Patient DNA from both PGLs and the adenoma is compared to PPGLs from SDHx-related cases (yellow bar) and SDHx-wild-type PPGLs (green bar). Probes (cg01931502/ cg11221265) covering the same region as MS-HRM primers indicate methylation of 55/40%, 57/44%, and 29/14% for PGL1, PGL2, and the adenoma, respectively. D, Heatmap of whole genome methylation profile with tumors ordered by similarity assessed by hierarchical cluster analysis. Abbreviations: NA, normal adrenal; Ade, patient's adenoma; PGL1/2, patient's paragangliomas.
Sanger sequencing of the SDHC promoter region confirmed the results of the MS-HRM analysis, but showed a higher percentage of methylation for PGL1 than indicated by MS-HRM (Supplemental Figure 2). For PGL1 and PGL2, methylation was estimated at 40–50% and 70–80%, respectively, whereas the adenoma showed minimal methylation of 5–10%.
In keeping with the MS-HRM results, Illumina 450K methylation array data showed consistently higher SDHC promoter methylation for both PGLs from our patient, compared to PPGLs with and without SDHx mutations (Figure 2C). In the adenoma, methylation of the downstream half of the SDHC promoter was also increased compared to other PPGLs. Hierarchical clustering of all 450K array probes showed that both PGLs associate with the CIMP cluster of SDHx tumors (Figure 2D), whereas the adenoma sits between the CIMP and the nonmethylated cluster. For PGL1, this was confirmed by expression analysis of genes known to be hypermethylated in SDHx tumors (Supplemental Figure 3) (7).
Discussion
Promoter methylation of the SDHC gene is a rare event, as expected for a housekeeping gene involved in central carbon metabolism and cellular energy production. Killian et al (4) demonstrated the lack of methylation in the SDHC promoter region by analyzing data from 854 normal and tumor reference tissues of various origins from the GEO database. Another study focusing on breast cancer also failed to identify tissues with methylated SDHC promoter (8). Previously, SDHC promoter epimutation has been described in 20 patients with GISTs, 50% of whom presented with Carney triad syndrome (3–5).
This report demonstrates that SDHC promoter methylation can provide a mechanism for inactivation of the SDH complex in sporadic PGLs. Although imaging studies indicated no evidence of GISTs, the future development of Carney triad-related neoplasms cannot be excluded. Results furthermore show a dose-dependent effect of the level of methylation of the SDHC promoter on SDH complex activity, with one PGL having the highest methylation status and the lowest SDH activity, which is also reflected in the higher succinate:fumarate ratio compared to the second PGL. The inactivation of SDHC by promoter methylation leads to the CIMP-like phenotype, similar to SDHx-mutated tumors (1).
Killian et al (4) identified low-level SDHC hypermethylation in blood and saliva of SDHx-wild-type/SDHC-epimutant GIST patients and interpreted this as a mosaic constitutional epimutation clonally expanding in tumor cells. For the present patient, blood and normal adrenal tissue were unmethylated at the SDHC promoter site, whereas the adenoma displayed low-level methylation. A form of postzygotic somatic mosaicism similar to EPAS1 mutations in patients with polycythemia-paraganglioma syndrome could provide an explanation for the observed phenotype (9). Mosaicism may occur due to unknown deregulatory events in a cell during an early developmental stage, eg, as morula or blastula, leading to a gain of CpG methylation at the SDHC promoter site in this cell and its descendants.
Somatic SDHC promoter methylation may account for PGL cases displaying a SDHx-tumor phenotype but without mutations in any of the SDHx genes. Evaluation of succinate:fumarate ratios can be an important diagnostic tool to identify such cases. This report further clarifies the results from Richter et al (2), in that one of the false positives was actually a true positive, suggesting a number of other undiscovered mechanisms for increased succinate:fumarate ratio in SDHx-wild-type tumors.
Acknowledgments
We thank Catleen Conrad and Romy Adler for technical assistance with DNA extractions and preparation of MS-HRM assays.
The project was funded by the Deutsche Forschungsgemeinschaft (EI855/1–2) and the Fondo de Investigaciones Sanitarias (PI14/00240).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- CIMP
CpG island methylator phenotype
- GIST
gastrointestinal stromal tumor
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- MS-HRM
methylation-sensitive high-resolution melting
- PGL
paraganglioma
- PPGL
pheochromocytoma/paraganglioma
- SDH
succinate dehydrogenase.



