-
PDF
- Split View
-
Views
-
Cite
Cite
Alice Pierre, Chrystèle Racine, Rodolfo A. Rey, Renato Fanchin, Joëlle Taieb, Joëlle Cohen-Tannoudji, Paul Carmillo, R. Blake Pepinsky, Richard L. Cate, Nathalie di Clemente, Most Cleaved Anti-Müllerian Hormone Binds Its Receptor in Human Follicular Fluid but Little Is Competent in Serum, The Journal of Clinical Endocrinology & Metabolism, Volume 101, Issue 12, 1 December 2016, Pages 4618–4627, https://doi.org/10.1210/jc.2016-1742
Close - Share Icon Share
Anti-Müllerian hormone (AMH) is an important clinical marker for diagnosing and assessing the reproductive status and/or disorders in men and women. Most studies have not distinguished between levels of inactive AMH precursor and the cleaved noncovalent complex that binds the AMH type II receptor (AMHRII) and initiates signaling.
The objective of the study was to measure the levels of AMH cleavage and bioactivity in human body fluids.
AMH cleavage levels and bioactivity were measured in the serum of six boys and in the follicular fluid and serum of nine control women and 13 women with the polycystic ovary syndrome (PCOS).
AMH cleavage levels were measured by capturing AMH with an anti-AMH antibody, followed by Western blotting. The bioactivity of cleaved AMH was assessed with an ELISA that measures the levels of AMH capable of binding AMHRII.
PCOS women have an elevated level of AMH cleavage in their follicular fluid (24% vs 8% in control women), and most of the cleaved AMH can bind AMHRII. Higher levels of cleavage are observed in female (60%) and male (79%) serum, but very little of the cleaved AMH can bind AMHRII.
These results support an autocrine role for AMH in the pathophysiology of PCOS in the follicle. In addition, they indicate that AMH undergoes interactions or structural changes after cleavage that prevent receptor binding, meaning, unexpectedly, that the level of cleaved AMH in biological fluids does not always reflect the level of bioactive AMH.
Anti-Müllerian Hormone (AMH), a member of the TGF-β family, is expressed in Sertoli cells of the fetal and postnatal testis and granulosa cells of the postnatal ovary (1). In the male vertebrate embryo, AMH is responsible for the regression of Müllerian ducts, the anlagen of the uterus, Fallopian tubes, and the upper part of the vagina, whereas in the adult male, AMH plays a role in Sertoli and Leydig cell differentiation and function (2, 3). In females, AMH has an inhibitory effect on primordial follicle recruitment and on the responsiveness of growing follicles to FSH (4, 5). In addition to its role in normal reproductive physiology, AMH is now recognized as an important clinical marker for diagnosing and assessing reproductive disorders in both men and women. In boys, serum AMH can be used to assess Sertoli cell function in children with intersex states (6) and distinguish between defects in testicular development (7–10). In women, serum AMH concentration is a reliable marker for the size of the ovarian follicle pool and a predictor of the ovarian response to controlled ovarian hyperstimulation (11). Furthermore, AMH levels are 2- to 3-fold higher in women with polycystic ovary syndrome (PCOS) (12), the most common cause of female infertility, and there is a correlation between the severity of the disease and AMH levels (13).
Like other members of the TGF-β family, AMH signals by assembling a transmembrane serine/threonine kinase receptor complex of type I and type II components and activating cytoplasmic small mothers against decapentaplegic (Smad) proteins (Figure 1A) (14, 15). AMH type II receptor (AMHRII) and AMH are mutually specific, whereas AMH shares its type I receptors and small mothers against decapentaplegic proteins with the bone morphogenetic proteins (16–18). AMH is translated as an inactive homodimeric precursor, containing an N-terminal proregion and a smaller C-terminal mature domain, which undergoes cleavage at monobasic sites between the two domains. After cleavage, the proregion and C-terminal homodimers remain associated in a noncovalent complex (cleaved AMH) (19) that can bind to AMHRII and initiate signaling (20).
![A, Model showing processing of AMH and assembly of the AMH receptor signaling complex. The AMH precursor must be cleaved to bind AMHRII and after binding the proregion domain dissociates from the mature domain. B, Biochemical analysis of AMH in control samples containing different ratios of uncleaved and cleaved AMH. Samples were prepared with various ratios of recombinant s-AMH (secreted AMH containing ∼95% uncleaved AMH precursor and ∼5% cleaved AMH) and c-AMH (100% cleaved AMH) in either FF or female serum with a total AMH level below 1 ng/mL; all samples contained the same level of total AMH (50 ng/mL). AMH was captured with an anti-N-terminal AMH mAb (10.6) conjugated to Sepharose, analyzed by SDS-PAGE under reducing conditions, followed by Western blotting using an anti-AMH polyclonal Ab. The level of AMH cleavage in each sample was calculated from a densitometry analysis (Image J) of the bands corresponding to the AMH precursor (70 kDa) and the N-terminal AMH proregion obtained after cleavage (58 kDa). The percentage of cleavage (proregion/[proregion + precursor] × 100) is shown at the bottom of each lane.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jcem/101/12/10.1210_jc.2016-1742/2/m_zeg0111628620001.jpeg?Expires=1750309372&Signature=wj43ksBhIH0MWUIymcpmfxEc2KMwTxuyjbGtdUdlmz5Rpt2aNpDcay4IYagYESUHQaItObpqn1Zz8rRvyYyO2pXjRZJUmNFkUqgdWsMDlEVqEqPbV0EhSes9CyblU0tmokQcE68fQ1s8xmOYB4IIZUUL92d-H3ZeBo7KLZQey--mMd6ZyVX-1yDWNgj3-mLR90Bm2NjW4DnQ9azM0z-JuM3QGE9qU8FgDWLhKiojkLkNe1Z1E0xDE-Lzk-tTlt2C~cTibu5CaLAkwSDxBsLbtHt-c8bJNZGLJlQPJ3P9SywZS7jBPy0jv8FjCkyeCY9yutE2uQ2oGZsmaO~hW7Ed9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
A, Model showing processing of AMH and assembly of the AMH receptor signaling complex. The AMH precursor must be cleaved to bind AMHRII and after binding the proregion domain dissociates from the mature domain. B, Biochemical analysis of AMH in control samples containing different ratios of uncleaved and cleaved AMH. Samples were prepared with various ratios of recombinant s-AMH (secreted AMH containing ∼95% uncleaved AMH precursor and ∼5% cleaved AMH) and c-AMH (100% cleaved AMH) in either FF or female serum with a total AMH level below 1 ng/mL; all samples contained the same level of total AMH (50 ng/mL). AMH was captured with an anti-N-terminal AMH mAb (10.6) conjugated to Sepharose, analyzed by SDS-PAGE under reducing conditions, followed by Western blotting using an anti-AMH polyclonal Ab. The level of AMH cleavage in each sample was calculated from a densitometry analysis (Image J) of the bands corresponding to the AMH precursor (70 kDa) and the N-terminal AMH proregion obtained after cleavage (58 kDa). The percentage of cleavage (proregion/[proregion + precursor] × 100) is shown at the bottom of each lane.
Because it is possible that certain aspects of endocrine status or disease may correlate with the level of AMH cleavage or activation, the ability to measure the level of cleaved and/or active AMH in body fluids may have important endocrine implications. In the current study, we have investigated the level of AMH cleavage in body fluids of males and females using a biochemical approach and an ELISA that measures the competency of the cleaved AMH for binding AMHRII. We find that AMH cleavage levels in body fluids vary significantly, with low levels observed in follicular fluid (FF) from control women, intermediate levels in FF from women with PCOS, and fairly high levels in female and male serum. Although most cleaved AMH in FF is competent for binding receptor, very little or none of the cleaved AMH in serum is competent.
Materials and Methods
Subjects
Male serum samples were obtained from an anonymized archive of the Hospital de Niños R Gutiérrez (Buenos Aires, Argentina), and provided boys attending the outpatient clinic met the following criteria: 1) written informed consent given by the participant’s mother/father; 2) anamnesis carried out by the physician ruled out genital or urological malformations, endocrine diseases, and chronic or acute general pathologies that could affect endocrine function; and 3) a clinical examination was performed to assess pubertal stage. Women undergoing in vitro fertilization (IVF) at the Antoine Béclère Hospital (Clamart) were included in this prospective study, in the control group, or in the PCOS group. All women of the control group met the following inclusion criteria: 1) age between 20 and 40 years; 2) both ovaries present, with no morphological abnormalities, adequately visualized in transvaginal ultrasound scans; 3) menstrual cycle length range between 26 and 30 days; 4) no current or past diseases affecting ovaries or gonadotropin or sex steroid secretion, clearance, or excretion; 5) no clinical signs of hyperandrogenism; and 6) no polycystic ovary morphology at ultrasonography. Infertility was due either to tubal or sperm abnormalities. Women with PCOS were selected according to the Rotterdam criteria (21): ovulatory disturbance, mainly oligomenorrhea and amenorrhea; and hyperandrogenism (clinical or biological) or polycystic ovary morphology at ultrasonography. An informed consent was obtained from all women, and this investigation received the approval of our internal institutional review board.
Collection of human body fluids
In our study, we included nine controls and 13 women with PCOS. FFs from each patient were pooled and centrifuged to separate granulosa and blood cells from the follicular fluid. A protease inhibitor cocktail (Sigma) was added to the FF samples, which were then aliquoted and stored at −80°C to avoid freezing and thawing cycles. All blood samples were obtained by venipuncture (on d 3 of the IVF cycle for women). The serum was then separated and frozen in aliquots at −20°C until ready for analysis.
Reagents
AMH proteins and anti-AMH antibodies (Abs) were previously described (20, 22). The AMHRII-Fc and the AMHRII-Fc/Fc fusion proteins have been previously described (20, 23).
Biochemical analysis of AMH in patient samples
Anti-N-terminal AMH monoclonal Ab (mAb) 10.6 (12.8 mg) was coupled to 5 g of rehydrated and washed cyanogen bromide activated Sepharose 4B (Sigma) as previously described (20). Prior to use, the resin was washed with PBS and then blocked with PBS containing 5% goat serum (Invitrogen) and 1% BSA (Sigma). For analysis of AMH, 50–400 μL of FF or serum was diluted to 1 mL with PBS and incubated with 25 μL of protein A Sepharose CL-4B (GE Healthcare) for 30 minutes. The resin was removed by centrifugation, and 25 μL of mAb 10.6 conjugated resin (washed and blocked as described above) was incubated with the supernatant with constant agitation for 120 minutes. The resin was washed five times with PBS/0.05% Tween 20. Forty microliters of nonreducing SDS-PAGE loading buffer was added and heated at 65°C for 5 minutes. β-Mercaptoethanol was added to various amounts of supernatant and analyzed by SDS-PAGE (7.5% gels; Bio-Rad Laboratories) and Western blotting. A rabbit anti-AMH polyclonal Ab was used to detect AMH and an antirabbit Ab conjugated to streptavidin-horseradish peroxidase (HRP) (Jackson ImmunoResearch) was used as the secondary Ab. The bands were quantified using the Image J software (National Institutes of Health, Bethesda, Maryland).
Enzyme-linked immunosorbent assays
Conditions for the receptor ELISA for measuring receptor binding competent (rbc)-AMH levels were as described previously (23) except that AMHRII-Fc/Fc and biotinylated mouse anti-C-terminal AMH mAb 22A2 were added at 0.75 and 0.5 μg/mL, respectively, and the biotinylated mAb was detected with a streptavidin-HRP conjugate (Jackson ImmunoResearch Laboratories). The rbc-AMH levels in patient samples were measured at four dilutions in duplicate. Standard curves were prepared using recombinant cleaved AMH diluted into human serum or FF with endogenous AMH levels less than 1 ng/mL and in a similar concentration range as the samples being tested. The serum and FF samples with very low AMH levels were also used to determine background values.
Statistics
All data were analyzed using the Prism 6 Software (GraphPad Software Inc) and the tests referred to in the figure and table legends.
Results
Biochemical analysis of AMH in human female and male body fluids
We characterized the forms of AMH in the body fluids of males and females using a biochemical approach. An anti-AMH mAb (10.6) that binds the N-terminal proregion, conjugated to Sepharose was used to capture AMH in body fluids. Captured AMH was then analyzed by SDS-PAGE under reducing conditions and Western blotting using an anti-AMH polyclonal Ab. Prior to analyzing AMH in body fluids, a control experiment was performed to validate this biochemical approach. Samples containing various ratios of recombinant cleaved AMH (c-AMH) and secreted AMH (s-AMH; 95% AMH precursor and ∼5% cleaved AMH) were diluted into either human female serum or FF, which had previously been shown to have very low AMH levels (<1 ng/mL) (Figure 1B, top panel). The AMH in each of these samples was then analyzed by precipitation and Western blotting under reducing conditions (Figure 1B, bottom panel). The major bands observed on the Western blot are the uncleaved AMH precursor (molecular mass 70 kDa) and the N-terminal proregion produced after cleavage (molecular mass 58 kDa); the C-terminal mature-region band is not detected by the anti-AMH polyclonal Ab. Densitometry analysis allowed an assessment of the relative levels of these bands and a calculation of the level of AMH cleavage, which is shown at the bottom of each lane in the Western blot. The level of AMH cleavage in each of these samples is consistent with the percentage of cleaved AMH in the s- and c-AMH that was added to each sample. These results indicate that AMH can be efficiently recovered from both serum and FF and that AMH is not undergoing additional cleavage during the biochemical analysis.
Next, we analyzed AMH in male and female body fluids, which had been frozen in aliquots and not subjected to multiple freeze/thaw cycles (Figure 2). The clinical parameters of the patients are shown in Table 1. The male serum samples analyzed in Figure 2A are from six boys ranging in age from 1 to 14 years and ranging in AMH levels from 200 to 30 ng/mL, respectively. A fairly uniform level of cleavage was observed in the six samples, ranging from 66% to 92%, indicating that a majority of the AMH in the serum of boys has been converted to the cleaved form of AMH. The mean level of AMH cleavage observed in the male serum samples was 79% (Figure 2D). Next, we compared the level of AMH cleavage in FF from control women (n = 9) and in FF and serum samples from PCOS women (n = 13). In our study, most of the PCOS women were only mildly hyperandronenic, but all were anovulatory, thus eligible for IVF according to our assisted reproduction technology center standards (24). In almost all cases, the PCOS FF samples showed higher levels of AMH cleavage than FF control samples, with mean levels of 24% and 8%, respectively (Figure 2D). Higher levels of AMH cleavage were observed in the serum from PCOS patients 399, 549, and 324 (compare lower panel of Figure 2B with Figure 2C), with a mean level of 60%.
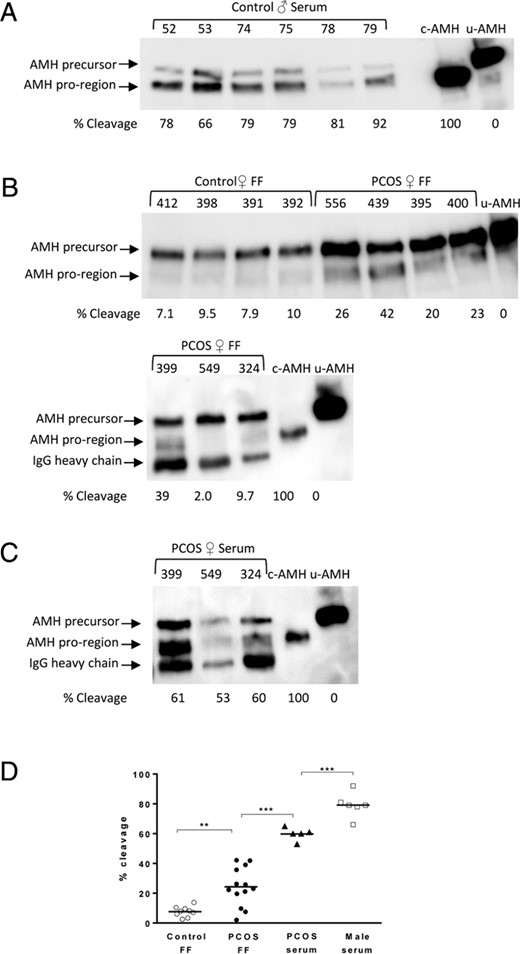
Characterization of AMH in male and female body fluids. Biochemical analysis of AMH in male serum samples (A), control and PCOS FF samples (B), and PCOS serum samples (C) was performed as described in Figure 1B. Representative Western blots are shown. c-AMH or u-AMH (100% uncleaved AMH with a mutated cleavage site) were run as controls to allow identification of the N-terminal proregion and AMH precursor bands. The band that runs just below the N-terminal proregion band in some of the Western blots with a molecular mass of approximately 50 kDa is the human IgG heavy chain, which is detected by the secondary antirabbit Fc Ab. Patient numbers are indicated in the top of the panels. Fifty microliters of male serum samples 52 and 53, 100 μL of samples 74 and 75, and 200 μL of samples 78 and 79 were analyzed in panel A and 400 μL of FF and female serum samples were analyzed in panels B and C. The following volumes of protein eluted from the Ab-Sepharose resin were analyzed by SDS-PAGE: 10 μL of male serum or control FF; 8 μL of PCOS serum; and 5 μL of PCOS FF. D, Comparison of the AMH cleavage levels in male and female body fluids. Mean values are shown for each group. Comparisons were made with proregion/(proregion + precursor) ratios using an ANOVA followed by Tukey’s multiple comparisons test. **, P < .001.
| . | Serum AMH, ng/mL . | FF AMH, ng/mL . | Age, y . | BMI, kg/m2 . | AFC . | T, ng/mL . | E2, pg/mL . | FSH, IU/mL . | LH, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Control women | |||||||||
| 367 | 3.1 | 0.82 | 37 | 19.1 | 17 | 0.62 | 37 | 8.8 | 6.6 |
| 377 | 3.5 | 1.03 | 26 | 21.2 | 18 | 0.53 | 38 | 6.4 | 4.2 |
| 378 | 2.5 | 1.25 | 35 | 19.6 | 14 | 0.51 | 37 | 9.7 | 3.4 |
| 391 | 2.3 | 1.32 | 30 | 23.6 | 13 | 0.49 | 49 | 8.1 | 4.4 |
| 392 | 1.6 | 1.15 | 36 | 24.8 | 13 | 0.32 | 18 | 7.3 | 4 |
| 398 | 1.7 | 1.49 | 29 | 18.8 | 20 | 0.8 | 46 | 6.9 | 3.8 |
| 412 | 2.7 | 1.3 | 37 | 18.5 | 10 | 0.57 | 43 | 8.7 | 8 |
| 414 | 2.6 | 1.17 | 34 | 21.2 | 21 | 0.81 | 32 | 8.6 | 4.4 |
| 416 | 3.7 | 0.61 | 28 | 22.3 | 16 | 0.29 | 12 | 8.2 | 7.6 |
| Median | 2.6 | 1.17 | 34 | 21.19 | 16 | 0.53 | 37 | 8.2 | 4.4 |
| PCOS women | |||||||||
| 3 | 19.4 | 37.6 | 31 | 19.5 | 28 | 0.77 | 41.9 | 5.7 | 7.9 |
| 208 | 7.4 | 10.6 | 33 | 23.1 | 50 | 0.65 | 42 | 5.2 | 5 |
| 324 | 13.4 | 9.7 | 22 | 27 | 34 | 0.6 | 60 | 5.2 | 2.4 |
| 376 | 9.8 | 2.3 | 38 | 28.5 | 34 | 0.87 | 41 | 7 | 5 |
| 387 | 6.4 | 3.7 | 28 | 23.2 | 33 | 0.78 | 26 | 7.2 | 9.2 |
| 395 | 7.7 | 6.2 | 37 | 21.5 | 35 | 0.46 | 26 | 5.3 | 7.1 |
| 399 | 15.4 | 8.5 | 31 | 22.5 | 67 | 0.4 | 49 | 6.2 | 10 |
| 400 | 8.3 | 20.8 | 26 | 20.4 | 31 | 0.41 | 40 | 7.9 | 7.6 |
| 428 | 10.7 | 5.6 | 36 | 24.5 | 47 | 0.78 | 30 | 4.1 | 3.1 |
| 439 | 9.9 | 5.4 | 32 | 23.7 | 60 | 0.43 | 152 | 2.2 | 2.2 |
| 549 | 2 | 8.1 | 35 | 18 | 40 | 0.96 | 42 | 5.6 | 5.3 |
| 553 | 23.3 | 3.9 | 28 | 27.1 | 75 | 1.02 | 66 | 4.7 | 18.7 |
| 556 | 6.4 | 4.8 | 29 | 23.7 | 39 | 1.86 | 18 | 6.8 | 9.1 |
| Median | 9.8a | 6.2a | 31, NS | 23.2, NS | 39a | 0.78, NS | 41, NS | 5.6a | 7.1, NS |
| Males | |||||||||
| 52 | 243.6 | 1 | |||||||
| 53 | 289.9 | 1 | |||||||
| 74 | 103 | 8 | |||||||
| 75 | 137.1 | 8 | |||||||
| 78 | 30 | 13 | |||||||
| 79 | 39 | 14 |
| . | Serum AMH, ng/mL . | FF AMH, ng/mL . | Age, y . | BMI, kg/m2 . | AFC . | T, ng/mL . | E2, pg/mL . | FSH, IU/mL . | LH, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Control women | |||||||||
| 367 | 3.1 | 0.82 | 37 | 19.1 | 17 | 0.62 | 37 | 8.8 | 6.6 |
| 377 | 3.5 | 1.03 | 26 | 21.2 | 18 | 0.53 | 38 | 6.4 | 4.2 |
| 378 | 2.5 | 1.25 | 35 | 19.6 | 14 | 0.51 | 37 | 9.7 | 3.4 |
| 391 | 2.3 | 1.32 | 30 | 23.6 | 13 | 0.49 | 49 | 8.1 | 4.4 |
| 392 | 1.6 | 1.15 | 36 | 24.8 | 13 | 0.32 | 18 | 7.3 | 4 |
| 398 | 1.7 | 1.49 | 29 | 18.8 | 20 | 0.8 | 46 | 6.9 | 3.8 |
| 412 | 2.7 | 1.3 | 37 | 18.5 | 10 | 0.57 | 43 | 8.7 | 8 |
| 414 | 2.6 | 1.17 | 34 | 21.2 | 21 | 0.81 | 32 | 8.6 | 4.4 |
| 416 | 3.7 | 0.61 | 28 | 22.3 | 16 | 0.29 | 12 | 8.2 | 7.6 |
| Median | 2.6 | 1.17 | 34 | 21.19 | 16 | 0.53 | 37 | 8.2 | 4.4 |
| PCOS women | |||||||||
| 3 | 19.4 | 37.6 | 31 | 19.5 | 28 | 0.77 | 41.9 | 5.7 | 7.9 |
| 208 | 7.4 | 10.6 | 33 | 23.1 | 50 | 0.65 | 42 | 5.2 | 5 |
| 324 | 13.4 | 9.7 | 22 | 27 | 34 | 0.6 | 60 | 5.2 | 2.4 |
| 376 | 9.8 | 2.3 | 38 | 28.5 | 34 | 0.87 | 41 | 7 | 5 |
| 387 | 6.4 | 3.7 | 28 | 23.2 | 33 | 0.78 | 26 | 7.2 | 9.2 |
| 395 | 7.7 | 6.2 | 37 | 21.5 | 35 | 0.46 | 26 | 5.3 | 7.1 |
| 399 | 15.4 | 8.5 | 31 | 22.5 | 67 | 0.4 | 49 | 6.2 | 10 |
| 400 | 8.3 | 20.8 | 26 | 20.4 | 31 | 0.41 | 40 | 7.9 | 7.6 |
| 428 | 10.7 | 5.6 | 36 | 24.5 | 47 | 0.78 | 30 | 4.1 | 3.1 |
| 439 | 9.9 | 5.4 | 32 | 23.7 | 60 | 0.43 | 152 | 2.2 | 2.2 |
| 549 | 2 | 8.1 | 35 | 18 | 40 | 0.96 | 42 | 5.6 | 5.3 |
| 553 | 23.3 | 3.9 | 28 | 27.1 | 75 | 1.02 | 66 | 4.7 | 18.7 |
| 556 | 6.4 | 4.8 | 29 | 23.7 | 39 | 1.86 | 18 | 6.8 | 9.1 |
| Median | 9.8a | 6.2a | 31, NS | 23.2, NS | 39a | 0.78, NS | 41, NS | 5.6a | 7.1, NS |
| Males | |||||||||
| 52 | 243.6 | 1 | |||||||
| 53 | 289.9 | 1 | |||||||
| 74 | 103 | 8 | |||||||
| 75 | 137.1 | 8 | |||||||
| 78 | 30 | 13 | |||||||
| 79 | 39 | 14 |
Abbreviations: AFC, antral follicular count; BMI, body mass index; E2, estradiol; NS, nonsignificant. Comparisons between control and PCOS women were performed with the Mann Whitney test.
P < .001 vs control women.
| . | Serum AMH, ng/mL . | FF AMH, ng/mL . | Age, y . | BMI, kg/m2 . | AFC . | T, ng/mL . | E2, pg/mL . | FSH, IU/mL . | LH, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Control women | |||||||||
| 367 | 3.1 | 0.82 | 37 | 19.1 | 17 | 0.62 | 37 | 8.8 | 6.6 |
| 377 | 3.5 | 1.03 | 26 | 21.2 | 18 | 0.53 | 38 | 6.4 | 4.2 |
| 378 | 2.5 | 1.25 | 35 | 19.6 | 14 | 0.51 | 37 | 9.7 | 3.4 |
| 391 | 2.3 | 1.32 | 30 | 23.6 | 13 | 0.49 | 49 | 8.1 | 4.4 |
| 392 | 1.6 | 1.15 | 36 | 24.8 | 13 | 0.32 | 18 | 7.3 | 4 |
| 398 | 1.7 | 1.49 | 29 | 18.8 | 20 | 0.8 | 46 | 6.9 | 3.8 |
| 412 | 2.7 | 1.3 | 37 | 18.5 | 10 | 0.57 | 43 | 8.7 | 8 |
| 414 | 2.6 | 1.17 | 34 | 21.2 | 21 | 0.81 | 32 | 8.6 | 4.4 |
| 416 | 3.7 | 0.61 | 28 | 22.3 | 16 | 0.29 | 12 | 8.2 | 7.6 |
| Median | 2.6 | 1.17 | 34 | 21.19 | 16 | 0.53 | 37 | 8.2 | 4.4 |
| PCOS women | |||||||||
| 3 | 19.4 | 37.6 | 31 | 19.5 | 28 | 0.77 | 41.9 | 5.7 | 7.9 |
| 208 | 7.4 | 10.6 | 33 | 23.1 | 50 | 0.65 | 42 | 5.2 | 5 |
| 324 | 13.4 | 9.7 | 22 | 27 | 34 | 0.6 | 60 | 5.2 | 2.4 |
| 376 | 9.8 | 2.3 | 38 | 28.5 | 34 | 0.87 | 41 | 7 | 5 |
| 387 | 6.4 | 3.7 | 28 | 23.2 | 33 | 0.78 | 26 | 7.2 | 9.2 |
| 395 | 7.7 | 6.2 | 37 | 21.5 | 35 | 0.46 | 26 | 5.3 | 7.1 |
| 399 | 15.4 | 8.5 | 31 | 22.5 | 67 | 0.4 | 49 | 6.2 | 10 |
| 400 | 8.3 | 20.8 | 26 | 20.4 | 31 | 0.41 | 40 | 7.9 | 7.6 |
| 428 | 10.7 | 5.6 | 36 | 24.5 | 47 | 0.78 | 30 | 4.1 | 3.1 |
| 439 | 9.9 | 5.4 | 32 | 23.7 | 60 | 0.43 | 152 | 2.2 | 2.2 |
| 549 | 2 | 8.1 | 35 | 18 | 40 | 0.96 | 42 | 5.6 | 5.3 |
| 553 | 23.3 | 3.9 | 28 | 27.1 | 75 | 1.02 | 66 | 4.7 | 18.7 |
| 556 | 6.4 | 4.8 | 29 | 23.7 | 39 | 1.86 | 18 | 6.8 | 9.1 |
| Median | 9.8a | 6.2a | 31, NS | 23.2, NS | 39a | 0.78, NS | 41, NS | 5.6a | 7.1, NS |
| Males | |||||||||
| 52 | 243.6 | 1 | |||||||
| 53 | 289.9 | 1 | |||||||
| 74 | 103 | 8 | |||||||
| 75 | 137.1 | 8 | |||||||
| 78 | 30 | 13 | |||||||
| 79 | 39 | 14 |
| . | Serum AMH, ng/mL . | FF AMH, ng/mL . | Age, y . | BMI, kg/m2 . | AFC . | T, ng/mL . | E2, pg/mL . | FSH, IU/mL . | LH, IU/mL . |
|---|---|---|---|---|---|---|---|---|---|
| Control women | |||||||||
| 367 | 3.1 | 0.82 | 37 | 19.1 | 17 | 0.62 | 37 | 8.8 | 6.6 |
| 377 | 3.5 | 1.03 | 26 | 21.2 | 18 | 0.53 | 38 | 6.4 | 4.2 |
| 378 | 2.5 | 1.25 | 35 | 19.6 | 14 | 0.51 | 37 | 9.7 | 3.4 |
| 391 | 2.3 | 1.32 | 30 | 23.6 | 13 | 0.49 | 49 | 8.1 | 4.4 |
| 392 | 1.6 | 1.15 | 36 | 24.8 | 13 | 0.32 | 18 | 7.3 | 4 |
| 398 | 1.7 | 1.49 | 29 | 18.8 | 20 | 0.8 | 46 | 6.9 | 3.8 |
| 412 | 2.7 | 1.3 | 37 | 18.5 | 10 | 0.57 | 43 | 8.7 | 8 |
| 414 | 2.6 | 1.17 | 34 | 21.2 | 21 | 0.81 | 32 | 8.6 | 4.4 |
| 416 | 3.7 | 0.61 | 28 | 22.3 | 16 | 0.29 | 12 | 8.2 | 7.6 |
| Median | 2.6 | 1.17 | 34 | 21.19 | 16 | 0.53 | 37 | 8.2 | 4.4 |
| PCOS women | |||||||||
| 3 | 19.4 | 37.6 | 31 | 19.5 | 28 | 0.77 | 41.9 | 5.7 | 7.9 |
| 208 | 7.4 | 10.6 | 33 | 23.1 | 50 | 0.65 | 42 | 5.2 | 5 |
| 324 | 13.4 | 9.7 | 22 | 27 | 34 | 0.6 | 60 | 5.2 | 2.4 |
| 376 | 9.8 | 2.3 | 38 | 28.5 | 34 | 0.87 | 41 | 7 | 5 |
| 387 | 6.4 | 3.7 | 28 | 23.2 | 33 | 0.78 | 26 | 7.2 | 9.2 |
| 395 | 7.7 | 6.2 | 37 | 21.5 | 35 | 0.46 | 26 | 5.3 | 7.1 |
| 399 | 15.4 | 8.5 | 31 | 22.5 | 67 | 0.4 | 49 | 6.2 | 10 |
| 400 | 8.3 | 20.8 | 26 | 20.4 | 31 | 0.41 | 40 | 7.9 | 7.6 |
| 428 | 10.7 | 5.6 | 36 | 24.5 | 47 | 0.78 | 30 | 4.1 | 3.1 |
| 439 | 9.9 | 5.4 | 32 | 23.7 | 60 | 0.43 | 152 | 2.2 | 2.2 |
| 549 | 2 | 8.1 | 35 | 18 | 40 | 0.96 | 42 | 5.6 | 5.3 |
| 553 | 23.3 | 3.9 | 28 | 27.1 | 75 | 1.02 | 66 | 4.7 | 18.7 |
| 556 | 6.4 | 4.8 | 29 | 23.7 | 39 | 1.86 | 18 | 6.8 | 9.1 |
| Median | 9.8a | 6.2a | 31, NS | 23.2, NS | 39a | 0.78, NS | 41, NS | 5.6a | 7.1, NS |
| Males | |||||||||
| 52 | 243.6 | 1 | |||||||
| 53 | 289.9 | 1 | |||||||
| 74 | 103 | 8 | |||||||
| 75 | 137.1 | 8 | |||||||
| 78 | 30 | 13 | |||||||
| 79 | 39 | 14 |
Abbreviations: AFC, antral follicular count; BMI, body mass index; E2, estradiol; NS, nonsignificant. Comparisons between control and PCOS women were performed with the Mann Whitney test.
P < .001 vs control women.
Validation of an assay for assessing AMHRII binding competency of AMH
To assess whether the cleaved AMH in body fluids is competent for binding AMHRII, we used a soluble receptor referred to as AMHRII-Fc/Fc, a heterodimer consisting of one chain of the extracellular domain of human AMHRII fused to the Fc portion of human IgG and a second Fc chain (23). AMHRII-Fc/Fc was captured on an antihuman Fc Ab and incubated with c- and 100% uncleaved AMH with a mutated cleavage site (u-AMH) at various concentrations; AMH bound to the soluble receptor was detected with biotinylated anti-C-terminal AMH mAb 22A2 and a streptavidin-HRP conjugate. The receptor ELISA detected c-AMH but did not detect u-AMH (Figure 3A). In contrast, an ELISA for total AMH detected both forms of AMH with similar sensitivity (Figure 3B). We refer to the AMH detected with the receptor ELISA as receptor binding competent-AMH (rbc-AMH).
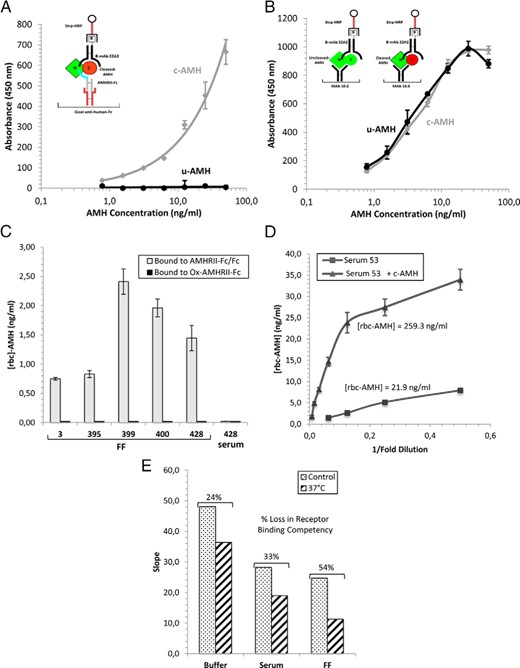
Analysis of AMH in body fluids with the receptor ELISA. A, The receptor ELISA detects only cleaved AMH. The schematic diagram in the inset shows the receptor ELISA format used to detect AMH competent for binding AMHRII. AMHRII-Fc/Fc was captured on a goat antihuman Fc Ab coated on the ELISA plate and incubated with c- and u-AMH at various concentrations. Bound AMH was detected with a biotinylated anti-C-terminal mAb (22A2) and streptavidin conjugated to HRP. B, The ELISA for total AMH detects c- and u-AMH with similar sensitivity. The schematic diagram shows the ELISA format for detecting both cleaved and uncleaved AMH precursor (total AMH). AMH was captured on an anti-N-terminal AMH mAb (10.6) and detected as described above. C, The receptor ELISA was used to measure rbc-AMH levels in PCOS FF and serum samples at four dilutions in duplicate. No signals were obtained when the inactive oxidized receptor (Ox-AMHRII-Fc) was used in place of AMHRII-Fc/Fc, indicating the signals obtained with AMHRII-Fc/Fc are due to specific binding. D, c-AMH added to male serum sample 53 can be detected with the receptor ELISA. Sample 53 (with or without 200 ng/ml c-AMH added) was assayed at various dilutions in the receptor ELISA in duplicate. When sample 53 was assayed by itself, the level of rbc-AMH detected with the receptor ELISA was 21.9 ng/mL (determined from the three lowest dilutions), despite containing 192 ng/mL cleaved AMH as assessed by biochemical analysis. When sample 53 containing 200 ng/mL c-AMH was assayed, the level of rbc-AMH detected was 259 ng/mL (determined from the four lowest dilutions), showing that the added c-AMH can be detected. E, c-AMH loses receptor binding competency when incubated at 37°C for 5 days. c-AMH was incubated for 5 days at 37°C in either ELISA buffer (1% BSA, 1% goat serum in PBS) or serum or FF at 200 ng/mL and then assayed in the receptor ELISA at various dilutions in duplicate. Control samples were prepared immediately before the ELISA assay and kept on ice prior to the assay. Slopes were determined from the lowest dilutions in which the slopes were linear. c-AMH shows a decrease in receptor binding competency of 24%, 33%, and 54% after 5 days at 37°C in the ELISA buffer, serum, or FF, respectively. The differences in the slopes of the control curves are due to the AMHRII binding component in serum and FF, which reduces the amount of AMH that can bind to the plate. Data points in panels A, B, and D are averages of two replicates and error bars represent SE; data in panel C are also shown in Table 3 and represent mean values ± SE.
When c-AMH was spiked into serum or FF (with endogenous AMH levels <1 ng/mL) and assayed in the receptor ELISA, a decrease of approximately 30%–50% was observed in the maximal signal at high c-AMH concentrations, compared with when the c-AMH was diluted into ELISA buffer (Supplemental Figure 1). This effect is consistent with a prevalent component in both FF and serum that can bind to the AMHRII-Fc/Fc protein on the ELISA plate with low affinity and reduce the amount of AMH that can bind. This phenomenon does not affect the accuracy of the receptor ELISA results, provided that standard curves are generated in the same body fluid and a similar concentration range as the AMH sample being tested.
We next tested whether the receptor ELISA could measure the level of AMH that is competent for binding AMHRII in the same samples that were analyzed for cleavage by Western blot analysis in Figure 1B. In Table 2, the levels of rbc-AMH measured by the AMHRII-Fc/Fc ELISA in the eight samples are shown along with the calculated percentages of AMH that are receptor binding competent, which are very similar to the cleavage levels determined by biochemical analysis. These results validate the usefulness of the receptor ELISA for assessing AMHRII binding competency of AMH and also indicate that in this experiment, most of the cleaved AMH is competent for binding AMHRII.
Levels of rbc-AMH Determined by the Receptor ELISA in Control Samples With Various Ratios of s- and c-AMH and a Comparison With Cleavage Levels Determined by Western Blot Analysis
| . | Samples . | rbc-AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |||
|---|---|---|---|---|---|---|---|---|
| s-AMH, ng/mL . | c-AMH, ng/mL . | c-AMH, % . | Individual Samples . | Average of Samples . | ||||
| AMH diluted into female serum | 50.0 | 0.0 | 0.0 | 2.5 ± 0.3 (6)a | 5.1 ± 0.7 | 9.3 | 54.5 | 84.2 |
| 37.5 | 12.5 | 25.0 | 9.0 ± 0.6 (8) | 18.1 ± 1.2 | 20.7 | 87.3 | ||
| 25.0 | 25.0 | 50.0 | 22.0 ± 2.4 (6) | 44.0 ± 4.8 | 35.9 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 36.3 ± 2.6 (6) | 72.6 ± 5.2 | 78.3 | 92.7 | ||
| AMH diluted into FF | 50.0 | 0.0 | 0.0 | 2.1 ± 0.2 (8)a | 4.2 ± 0.3 | 9.6 | 43.4 | |
| 37.5 | 12.5 | 25.0 | 14.7 ± 1.3 (8) | 29.5 ± 2.6 | 21.1 | 100.0b | ||
| 25.0 | 25.0 | 50.0 | 26.1 ± 1.7 (7) | 52.2 ± 3.4 | 51.4 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 39.5 ± 1.4 (8) | 79.0 ± 2.9 | 82.7 | 95.5 | ||
| . | Samples . | rbc-AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |||
|---|---|---|---|---|---|---|---|---|
| s-AMH, ng/mL . | c-AMH, ng/mL . | c-AMH, % . | Individual Samples . | Average of Samples . | ||||
| AMH diluted into female serum | 50.0 | 0.0 | 0.0 | 2.5 ± 0.3 (6)a | 5.1 ± 0.7 | 9.3 | 54.5 | 84.2 |
| 37.5 | 12.5 | 25.0 | 9.0 ± 0.6 (8) | 18.1 ± 1.2 | 20.7 | 87.3 | ||
| 25.0 | 25.0 | 50.0 | 22.0 ± 2.4 (6) | 44.0 ± 4.8 | 35.9 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 36.3 ± 2.6 (6) | 72.6 ± 5.2 | 78.3 | 92.7 | ||
| AMH diluted into FF | 50.0 | 0.0 | 0.0 | 2.1 ± 0.2 (8)a | 4.2 ± 0.3 | 9.6 | 43.4 | |
| 37.5 | 12.5 | 25.0 | 14.7 ± 1.3 (8) | 29.5 ± 2.6 | 21.1 | 100.0b | ||
| 25.0 | 25.0 | 50.0 | 26.1 ± 1.7 (7) | 52.2 ± 3.4 | 51.4 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 39.5 ± 1.4 (8) | 79.0 ± 2.9 | 82.7 | 95.5 | ||
s-AMH and c-AMH were mixed at the indicated concentrations into serum or FF at a total concentration of 50 ng/mL and assayed in the receptor ELISA at four dilutions in duplicate. Results are expressed as mean ± SE, and n indicates the number of measurements included in the reported average value; high signals out of the linear range of the standard curve were omitted as were very low signals. The cleavage levels determined by Western blot analysis are from Figure 1B.
Levels of cleaved AMH in these samples were determined by adding s-AMH to the serum or FF at 200 ng/mL and assaying at four dilutions; the values are the concentrations of cleaved AMH detected in 50 ng/mL s-AMH. The serum and FF used for this experiment have total AMH levels below 1 ng/mL.
Values were capped at 100%.
Levels of rbc-AMH Determined by the Receptor ELISA in Control Samples With Various Ratios of s- and c-AMH and a Comparison With Cleavage Levels Determined by Western Blot Analysis
| . | Samples . | rbc-AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |||
|---|---|---|---|---|---|---|---|---|
| s-AMH, ng/mL . | c-AMH, ng/mL . | c-AMH, % . | Individual Samples . | Average of Samples . | ||||
| AMH diluted into female serum | 50.0 | 0.0 | 0.0 | 2.5 ± 0.3 (6)a | 5.1 ± 0.7 | 9.3 | 54.5 | 84.2 |
| 37.5 | 12.5 | 25.0 | 9.0 ± 0.6 (8) | 18.1 ± 1.2 | 20.7 | 87.3 | ||
| 25.0 | 25.0 | 50.0 | 22.0 ± 2.4 (6) | 44.0 ± 4.8 | 35.9 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 36.3 ± 2.6 (6) | 72.6 ± 5.2 | 78.3 | 92.7 | ||
| AMH diluted into FF | 50.0 | 0.0 | 0.0 | 2.1 ± 0.2 (8)a | 4.2 ± 0.3 | 9.6 | 43.4 | |
| 37.5 | 12.5 | 25.0 | 14.7 ± 1.3 (8) | 29.5 ± 2.6 | 21.1 | 100.0b | ||
| 25.0 | 25.0 | 50.0 | 26.1 ± 1.7 (7) | 52.2 ± 3.4 | 51.4 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 39.5 ± 1.4 (8) | 79.0 ± 2.9 | 82.7 | 95.5 | ||
| . | Samples . | rbc-AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |||
|---|---|---|---|---|---|---|---|---|
| s-AMH, ng/mL . | c-AMH, ng/mL . | c-AMH, % . | Individual Samples . | Average of Samples . | ||||
| AMH diluted into female serum | 50.0 | 0.0 | 0.0 | 2.5 ± 0.3 (6)a | 5.1 ± 0.7 | 9.3 | 54.5 | 84.2 |
| 37.5 | 12.5 | 25.0 | 9.0 ± 0.6 (8) | 18.1 ± 1.2 | 20.7 | 87.3 | ||
| 25.0 | 25.0 | 50.0 | 22.0 ± 2.4 (6) | 44.0 ± 4.8 | 35.9 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 36.3 ± 2.6 (6) | 72.6 ± 5.2 | 78.3 | 92.7 | ||
| AMH diluted into FF | 50.0 | 0.0 | 0.0 | 2.1 ± 0.2 (8)a | 4.2 ± 0.3 | 9.6 | 43.4 | |
| 37.5 | 12.5 | 25.0 | 14.7 ± 1.3 (8) | 29.5 ± 2.6 | 21.1 | 100.0b | ||
| 25.0 | 25.0 | 50.0 | 26.1 ± 1.7 (7) | 52.2 ± 3.4 | 51.4 | 100.0b | ||
| 12.5 | 37.5 | 75.0 | 39.5 ± 1.4 (8) | 79.0 ± 2.9 | 82.7 | 95.5 | ||
s-AMH and c-AMH were mixed at the indicated concentrations into serum or FF at a total concentration of 50 ng/mL and assayed in the receptor ELISA at four dilutions in duplicate. Results are expressed as mean ± SE, and n indicates the number of measurements included in the reported average value; high signals out of the linear range of the standard curve were omitted as were very low signals. The cleavage levels determined by Western blot analysis are from Figure 1B.
Levels of cleaved AMH in these samples were determined by adding s-AMH to the serum or FF at 200 ng/mL and assaying at four dilutions; the values are the concentrations of cleaved AMH detected in 50 ng/mL s-AMH. The serum and FF used for this experiment have total AMH levels below 1 ng/mL.
Values were capped at 100%.
Most cleaved AMH in FF can bind AMHRII, but very little or none is competent in serum
Receptor ELISA results obtained from the analysis of five FF samples from women with PCOS are shown in Figure 3C and Table 3. A negative control was also performed: AMHRII-Fc oxidized with sodium metaperiodate (Ox-AMHRII-Fc), which almost completely inactivates the receptor for binding AMH (data not shown). When this fusion protein was used instead of AMHRII-Fc/Fc, no rbc-AMH was detected in the FF samples, indicating that the rbc-AMH level observed with AMHRII-Fc/Fc results from specific binding to AMH (Figure 3C). The levels of AMH cleavage determined from the Western blot in Figure 2B correlate fairly well with the levels that are receptor binding competent (Table 3); on average 61.6% of the cleaved AMH in the FF samples was detected by the receptor ELISA, indicating that most of the cleaved AMH in FF is competent for binding AMHRII. In contrast, we were unable to detect any rbc-AMH in female serum samples 428, 324, 399, or 549 (Figure 3C and Table 3) indicating that most or all of the cleaved AMH in female serum is not competent.
Levels of rbc-AMH in Female and Male Samples Determined by the Receptor ELISA and a Comparison to Cleavage Levels Determined by Western Blot Analysis
| . | Patient Sample Number . | rbc-AMH Determined by ELISA, ng/mL, n . | Total AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |
|---|---|---|---|---|---|---|---|
| Individual Samples . | Average of Samples . | ||||||
| ♀ FF | 3 | 0.75 ± 0.02 (4) | 37.6 ± 3.1 (4) | 2.0 ± 0.2 | 7.5 | 26.6 | 61.6 |
| 395 | 0.83 ± 0.06 (6) | 6.2 ± 0.5 (8) | 13.4 ± 1.5 | 19.6 | 68.5 | ||
| 399 | 2.41 ± 0.22 (8) | 8.5 ± 0.3 (8) | 28.3 ± 2.7 | 38.5 | 73.6 | ||
| 400 | 1.96 ± 0.15 (6) | 20.8 ± 1.6 (6) | 9.4 ± 1.0 | 23.0 | 41.0 | ||
| 428 | 1.44 ± 0.21 (5) | 5.6 ± 0.3 (8) | 25.8 ± 4.1 | 26.2 | 98.5 | ||
| ♀ Serum | 324 | 0.0 (4) | 13.4 | 0.0 | 59.6 | 0.0 | 0.0 |
| 399 | 0.0 (4) | 15.4 | 0.0 | 61.4 | 0.0 | ||
| 428 | 0.0 (4) | 10.7 | 0.0 | ND | ND | ||
| 549 | 0.0 (4) | 2.0 | 0.0 | 52.6 | 0.0 | ||
| ♂ Serum | 52 | 23.1 ± 2.6 (6) | 244 ± 9.7 (6) | 9.5 ± 1.1 | 77.9 | 12.1 | 10.9 |
| 53 | 21.9 ± 1.9 (6) | 290 ± 19.5 (6) | 7.6 ± 0.8 | 66.1 | 11.3 | ||
| 74 | 10.4 ± 1.7 (6) | 103 ± 4.0 (6) | 10.1 ± 1.7 | 79.0 | 12.8 | ||
| 75 | 3.6 ± 0.7 (6) | 137 ± 6.5 (6) | 2.6 ± 0.5 | 79.0 | 3.3 | ||
| 78 | 3.1 ± 0.5 (4) | 30.0 ± 1.2 (6) | 10.3 ± 1.7 | 81.3 | 12.7 | ||
| 79 | 4.7 ± 0.7 (4) | 39.0 ± 2.5 (6) | 12.2 ± 2.0 | 92.4 | 13.2 | ||
| . | Patient Sample Number . | rbc-AMH Determined by ELISA, ng/mL, n . | Total AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |
|---|---|---|---|---|---|---|---|
| Individual Samples . | Average of Samples . | ||||||
| ♀ FF | 3 | 0.75 ± 0.02 (4) | 37.6 ± 3.1 (4) | 2.0 ± 0.2 | 7.5 | 26.6 | 61.6 |
| 395 | 0.83 ± 0.06 (6) | 6.2 ± 0.5 (8) | 13.4 ± 1.5 | 19.6 | 68.5 | ||
| 399 | 2.41 ± 0.22 (8) | 8.5 ± 0.3 (8) | 28.3 ± 2.7 | 38.5 | 73.6 | ||
| 400 | 1.96 ± 0.15 (6) | 20.8 ± 1.6 (6) | 9.4 ± 1.0 | 23.0 | 41.0 | ||
| 428 | 1.44 ± 0.21 (5) | 5.6 ± 0.3 (8) | 25.8 ± 4.1 | 26.2 | 98.5 | ||
| ♀ Serum | 324 | 0.0 (4) | 13.4 | 0.0 | 59.6 | 0.0 | 0.0 |
| 399 | 0.0 (4) | 15.4 | 0.0 | 61.4 | 0.0 | ||
| 428 | 0.0 (4) | 10.7 | 0.0 | ND | ND | ||
| 549 | 0.0 (4) | 2.0 | 0.0 | 52.6 | 0.0 | ||
| ♂ Serum | 52 | 23.1 ± 2.6 (6) | 244 ± 9.7 (6) | 9.5 ± 1.1 | 77.9 | 12.1 | 10.9 |
| 53 | 21.9 ± 1.9 (6) | 290 ± 19.5 (6) | 7.6 ± 0.8 | 66.1 | 11.3 | ||
| 74 | 10.4 ± 1.7 (6) | 103 ± 4.0 (6) | 10.1 ± 1.7 | 79.0 | 12.8 | ||
| 75 | 3.6 ± 0.7 (6) | 137 ± 6.5 (6) | 2.6 ± 0.5 | 79.0 | 3.3 | ||
| 78 | 3.1 ± 0.5 (4) | 30.0 ± 1.2 (6) | 10.3 ± 1.7 | 81.3 | 12.7 | ||
| 79 | 4.7 ± 0.7 (4) | 39.0 ± 2.5 (6) | 12.2 ± 2.0 | 92.4 | 13.2 | ||
Female FF and male serum samples were assayed in the receptor ELISA at four dilutions in duplicate. Results are expressed as mean ± SE, and n indicates the number of measurements included in the reported average value; high signals out of the linear range of the standard curve were omitted as were very low signals. The cleavage levels determined by Western blot analysis are from Figure 2, A–C.
Levels of rbc-AMH in Female and Male Samples Determined by the Receptor ELISA and a Comparison to Cleavage Levels Determined by Western Blot Analysis
| . | Patient Sample Number . | rbc-AMH Determined by ELISA, ng/mL, n . | Total AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |
|---|---|---|---|---|---|---|---|
| Individual Samples . | Average of Samples . | ||||||
| ♀ FF | 3 | 0.75 ± 0.02 (4) | 37.6 ± 3.1 (4) | 2.0 ± 0.2 | 7.5 | 26.6 | 61.6 |
| 395 | 0.83 ± 0.06 (6) | 6.2 ± 0.5 (8) | 13.4 ± 1.5 | 19.6 | 68.5 | ||
| 399 | 2.41 ± 0.22 (8) | 8.5 ± 0.3 (8) | 28.3 ± 2.7 | 38.5 | 73.6 | ||
| 400 | 1.96 ± 0.15 (6) | 20.8 ± 1.6 (6) | 9.4 ± 1.0 | 23.0 | 41.0 | ||
| 428 | 1.44 ± 0.21 (5) | 5.6 ± 0.3 (8) | 25.8 ± 4.1 | 26.2 | 98.5 | ||
| ♀ Serum | 324 | 0.0 (4) | 13.4 | 0.0 | 59.6 | 0.0 | 0.0 |
| 399 | 0.0 (4) | 15.4 | 0.0 | 61.4 | 0.0 | ||
| 428 | 0.0 (4) | 10.7 | 0.0 | ND | ND | ||
| 549 | 0.0 (4) | 2.0 | 0.0 | 52.6 | 0.0 | ||
| ♂ Serum | 52 | 23.1 ± 2.6 (6) | 244 ± 9.7 (6) | 9.5 ± 1.1 | 77.9 | 12.1 | 10.9 |
| 53 | 21.9 ± 1.9 (6) | 290 ± 19.5 (6) | 7.6 ± 0.8 | 66.1 | 11.3 | ||
| 74 | 10.4 ± 1.7 (6) | 103 ± 4.0 (6) | 10.1 ± 1.7 | 79.0 | 12.8 | ||
| 75 | 3.6 ± 0.7 (6) | 137 ± 6.5 (6) | 2.6 ± 0.5 | 79.0 | 3.3 | ||
| 78 | 3.1 ± 0.5 (4) | 30.0 ± 1.2 (6) | 10.3 ± 1.7 | 81.3 | 12.7 | ||
| 79 | 4.7 ± 0.7 (4) | 39.0 ± 2.5 (6) | 12.2 ± 2.0 | 92.4 | 13.2 | ||
| . | Patient Sample Number . | rbc-AMH Determined by ELISA, ng/mL, n . | Total AMH Determined by ELISA, ng/mL, n . | AMH That Is Receptor Binding Competent, % . | Cleavage Determined From Western Blot, % . | Cleaved AMH That Is Receptor Binding Competent, % . | |
|---|---|---|---|---|---|---|---|
| Individual Samples . | Average of Samples . | ||||||
| ♀ FF | 3 | 0.75 ± 0.02 (4) | 37.6 ± 3.1 (4) | 2.0 ± 0.2 | 7.5 | 26.6 | 61.6 |
| 395 | 0.83 ± 0.06 (6) | 6.2 ± 0.5 (8) | 13.4 ± 1.5 | 19.6 | 68.5 | ||
| 399 | 2.41 ± 0.22 (8) | 8.5 ± 0.3 (8) | 28.3 ± 2.7 | 38.5 | 73.6 | ||
| 400 | 1.96 ± 0.15 (6) | 20.8 ± 1.6 (6) | 9.4 ± 1.0 | 23.0 | 41.0 | ||
| 428 | 1.44 ± 0.21 (5) | 5.6 ± 0.3 (8) | 25.8 ± 4.1 | 26.2 | 98.5 | ||
| ♀ Serum | 324 | 0.0 (4) | 13.4 | 0.0 | 59.6 | 0.0 | 0.0 |
| 399 | 0.0 (4) | 15.4 | 0.0 | 61.4 | 0.0 | ||
| 428 | 0.0 (4) | 10.7 | 0.0 | ND | ND | ||
| 549 | 0.0 (4) | 2.0 | 0.0 | 52.6 | 0.0 | ||
| ♂ Serum | 52 | 23.1 ± 2.6 (6) | 244 ± 9.7 (6) | 9.5 ± 1.1 | 77.9 | 12.1 | 10.9 |
| 53 | 21.9 ± 1.9 (6) | 290 ± 19.5 (6) | 7.6 ± 0.8 | 66.1 | 11.3 | ||
| 74 | 10.4 ± 1.7 (6) | 103 ± 4.0 (6) | 10.1 ± 1.7 | 79.0 | 12.8 | ||
| 75 | 3.6 ± 0.7 (6) | 137 ± 6.5 (6) | 2.6 ± 0.5 | 79.0 | 3.3 | ||
| 78 | 3.1 ± 0.5 (4) | 30.0 ± 1.2 (6) | 10.3 ± 1.7 | 81.3 | 12.7 | ||
| 79 | 4.7 ± 0.7 (4) | 39.0 ± 2.5 (6) | 12.2 ± 2.0 | 92.4 | 13.2 | ||
Female FF and male serum samples were assayed in the receptor ELISA at four dilutions in duplicate. Results are expressed as mean ± SE, and n indicates the number of measurements included in the reported average value; high signals out of the linear range of the standard curve were omitted as were very low signals. The cleavage levels determined by Western blot analysis are from Figure 2, A–C.
Receptor ELISA results obtained from the analysis of six male serum samples are shown in Table 3. In these samples, the percentages of AMH that are receptor binding competent are much lower than the cleavage levels obtained from the biochemical analysis. On average, only 10.9% of the cleaved AMH in the six male serum samples was competent for binding AMHRII. To demonstrate that there is not a problem with the detection of cleaved AMH in male serum, 200 ng/mL c-AMH was added to serum sample 53 (containing an rbc-AMH level of 21.9 ng/mL) and reassayed (Figure 3D). The rbc-AMH level in the sample with the added c-AMH was 259 ng/mL, which indicates that the added c-AMH can be detected in male serum with the receptor ELISA.
Because in the female, the serum pool of AMH is derived from the FF pool of AMH secreted by granulosa cells, the observation that cleaved AMH is less active in female serum than in FF indicates that either a mechanism exists that prevents cleaved AMH from binding to AMHRII or that one exists for inactivating it. Therefore, we investigated whether cleaved AMH might be losing activity in the serum over time while at 37°C. As shown in Figure 3E, we found that when c-AMH is incubated for 5 days at 37°C in ELISA buffer (1% BSA; 1% goat serum in PBS), human male serum, or human FF, the c-AMH lost 24%, 33%, or 54% of its receptor binding competency, respectively. The fact that c-AMH also loses activity in FF and ELISA buffer suggests that this may be a nonspecific phenomenon and that the higher fraction of cleaved AMH in FF that is competent for binding AMHRII is due to the continued production of AMH by granulosa cells.
Discussion
The goal of this study was to measure levels of AMH cleavage and activation in body fluids and investigate whether these features of AMH processing correlate with aspects of endocrine status. We have previously shown that the AMH precursor undergoes an obligatory cleavage, which allows it to assume a conformation that can bind AMHRII and initiate signaling (20). To measure AMH cleavage levels in body fluids, we used a biochemical approach involving the capture of endogenous AMH with an anti-AMH mAb, followed by Western blotting. We found a range of AMH cleavage levels: 8% in FF from control women, 24% in FF from women with PCOS, 60% in serum samples from women with PCOS, and 79% in male serum samples from 1- to 14-year-old boys. To assess whether the cleaved AMH was bioactive, we developed an ELISA that measures the levels of bioactive AMH capable of binding AMHRII. We found that recombinant AMH is almost completely competent for binding receptor after cleavage but that competency decreases after incubation at 37°C in FF or serum. Furthermore, only a fraction of the cleaved endogenous AMH measured in body fluids was found to be competent for binding AMHRII: 62% in FF, 11% in male serum, and 0% in female serum. Thus, using cleaved AMH as a surrogate marker for bioactive AMH could obscure an underlying relationship with endocrine status.
In previous studies, AMH cleavage levels have been assessed in human plasma samples using a biochemical approach similar to one that we have used (25) and in male and female serum samples using ELISA approaches that detect the AMH precursor (26–28). A high level of AMH cleavage was observed in all of these studies, in agreement with the high level of cleavage observed in our study in female (60%) and male (79%) serum. In contrast, we found only 8% AMH cleavage in FF from control women and 24% in FF from women with PCOS. Thus, the predominant form of AMH in FF is the AMH precursor, similar to what has been found in bovine testis (29) and ovine granulosa cells (30). Altogether these results indicate that a small amount of locally cleaved AMH is sufficient for the observed biological effects within the gonads as well as for Müllerian duct regression. A mechanism clearly exists in the ovary for cleaving AMH, however, which appears to be accentuated in PCOS. Based on the high AMH cleavage levels observed in serum, it can be inferred that the AMH precursor is also subjected to cleavage during or after transfer to the blood.
It is well known that PCOS women have higher serum and FF AMH levels. This is due to both more small antral follicles and an overexpression of AMH by granulosa cells (31, 32). We have reported recently that in dysovulatory PCOS women, this could be partly explained by an up-regulation of AMH expression by LH (24). We show here that the high serum and follicular AMH levels observed in PCOS women are also associated with elevated levels of AMH cleavage and an increased ability of FF AMH to bind AMHRII. These results and the overexpression of AMHRII by PCOS granulosa cells (32) strongly support the hypothesis that AMH effects, in particular its inhibitory action on granulosa cell sensitivity to FSH, are more pronounced in PCOS women and that AMH is likely involved in the follicular arrest and the ovulation defects observed in these women.
Despite the high level of cleaved AMH within the blood, most of this cleaved AMH does not remain active because as our results show, it is not competent for binding AMHRII. This suggests that either a mechanism exists that prevents cleaved AMH from binding to AMHRII or that one exists for inactivating it. Two observations favor the latter possibility: recombinant cleaved AMH remains active when diluted in FF or serum and maintained at 4°C and loses partial activity only when incubated at 37°C for 5 days. A number of proteins have been identified that bind other members of the TGF-β family and block access to their receptors (33), but the apparent slow rate at which receptor binding competency of cleaved AMH is lost is inconsistent with a serum or FF protein that can bind cleaved AMH and prevent it from binding AMHRII. Rather, our data are more consistent with the cleaved AMH undergoing a structural change. One possibility is that the cleaved AMH undergoes some dissociation of the N-terminal pro- and C-terminal mature domains. We have shown previously that the C-terminal mature domain is not very stable in the absence of the N-terminal prodomain (34). However, this would not explain our current results, because the ELISA we used for measuring total AMH concentrations does not detect dissociated complex. Another possibility is that the cleaved AMH is undergoing further cleavage in the blood as suggested by Mamsen et al (27); cleavage within the mature domain or a change in its conformation could render it incapable of binding AMHRII. We are currently investigating these possibilities.
In addition to the well-documented paracrine and autocrine roles of AMH in the regression of the Müllerian duct and the male and female gonads, a number of endocrine roles have been proposed in the nervous and cardiovascular systems (reviewed in reference 35). For AMH to have an effect on cells within these target organs, the AMH ligand must be able to bind to AMHRII. In addition to the requirement for being cleaved, which allows AMH to assume the right conformation for binding to AMHRII, the activated ligand must reach a concentration close to the dissociation constant of the AMH/AMHRII interaction so that a sufficient amount can bind. A consequence of the loss of receptor binding competency, which we have demonstrated occurs in the blood, is that the active AMH ligand concentration is also reduced. If the ligand concentration is decreased substantially below the dissociation constant, then fewer receptors per cell will be bound and activated, leading to a decreased cellular response. Thus, loss of receptor binding competency should be considered when evaluating potential endocrine roles of AMH.
Acknowledgments
We thank the IVF laboratory of Antoine Béclère Hospital for technical assistance.
This work was supported by Idex Sorbonne Paris Cité and Agence Nationale pour la Recherche (Grant ANR-12-BSV1-0034-01) to N.d.C.
Disclosure Summary: A patent application (EP14153715.9) has been filed on the AMHRII-Fc/Fc soluble receptor by R.L.C. and N.d.C. The other authors have nothing to disclose.
Abbreviations
- Ab
antibody
- AMH
anti-Müllerian hormone
- AMHRII
AMH type II receptor
- c-AMH
cleaved AMH
- FF
follicular fluid
- HRP
horseradish peroxidase
- IVF
in vitro fertilization
- mAb
monoclonal antibody
- PCOS
polycystic ovary syndrome
- rbc-AMH
receptor binding competent-AMH
- s-AMH
secreted AMH
- u-AMH
uncleaved AMH with a mutated cleavage site.



