-
PDF
- Split View
-
Views
-
Cite
Cite
Laurie A. Wing, John V. Conaglen, Goswin Y. Meyer-Rochow, Marianne S. Elston, Paraganglioma in Pregnancy: A Case Series and Review of the Literature, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 8, 1 August 2015, Pages 3202–3209, https://doi.org/10.1210/jc.2015-2122
Close - Share Icon Share
Pregnancies complicated by a pheochromocytoma or paraganglioma are very rare, being estimated to occur in 0.007% of all pregnancies. Both the well-being of the mother and fetus need to be considered, and management can be challenging. The optimal management of women with a pheochromocytoma or paraganglioma in pregnancy is not well established.
The objective of the study was to assess whether there is a difference in fetal or maternal mortality between pheochromocytomas and paragangliomas in pregnancy.
We present an experience of eight pregnancies in four SDHB germline mutation-positive women with sympathetic paragangliomas, followed by a systematic review of the literature to compare the outcome of paragangliomas with that of pheochromocytomas occurring in pregnancy.
In our case series, favorable fetal and maternal outcomes were seen in all eight pregnancies. From the systematic review, maternal and fetal mortality were lower in women with paragangliomas, at 3.6% and 12% respectively, compared with 9.8% and 16% in women with pheochromocytomas.
Pregnant women with paragangliomas may be at a lower risk of adverse outcome than those with pheochromocytomas, but both maternal and fetal mortality rates are still higher than that of the general obstetric population.
Pregnancies complicated by a pheochromocytoma or paraganglioma (PGL) are very rare, estimated to occur in 0.007% of all pregnancies (1). Due to the rarity of pheochromocytomas and PGLs in pregnancy, the recommended management for the mother and baby is based on case reports, small case series, and expert opinion.
Improving maternal and fetal mortality in patients with a pheochromocytoma/PGL in pregnancy have been published since the initial case reports of the 1950s. In the 1960s, maternal and fetal mortality in pheochromocytoma/PGL were reported as 48% and 55%, respectively (2). In the period between 1980 and 1987, maternal and fetal mortality in these conditions were cited as being 17% and 26%, respectively (3). These respective mortalities in pregnancy-associated pheochromocytoma/PGL decreased further to 12% and 17% in cases reported from 1998 through 2008 (4). The diagnosis of pheochromocytoma/PGL is increasingly being recognized in the antenatal period, resulting in 12% fetal mortality (and 0% maternal mortality), compared with 29% fetal and maternal mortality when the diagnosis is made during labor or immediately postpartum (5).
Current recommendations for the management of a pheochromocytoma/PGL discovered in pregnancy are that if the condition is diagnosed in the first 24 weeks of gestation, the tumor should be removed, preferably by a laparascopic approach in the second trimester (6), whereas when a pheochromocytoma/PGL is discovered in the third trimester, surgical excision should be delayed until the fetus is viable and able to be delivered, with the tumor being removed either immediately after delivery or at a later date (6). Due to historical data, in which fetal mortality from vaginal delivery was much higher, cesarean section is the recommended mode of delivery of the baby (7). For imaging, MRI is recommended for pheochromocytoma/PGL in pregnancy due to the associated risk of fetal exposure to ionizing radiation with computed tomography imaging. Preoperative α-receptor blockade should be given as for nonpregnant patients (although a target blood pressure (BP) has not been established) along with increased salt and fluid intake (6). Phenoxybenazmine does cross the placenta and may cause neonatal hypotension and respiratory depression (8).
PGLs are extraadrenal chromaffin cell tumors, which, in a recent review of their incidence in pregnancy, were reported to comprise only 19% of chromaffin cell tumors, whereas the majority were pheochromocytomas (5). The clinical and biochemical patterns of pheochromocytomas may differ when compared with PGLs. Differences in tumor size, catecholamine content, and secretion may result in differential hemodynamic effects. As a result, the maternal and fetal risk may vary according to tumor site (adrenal vs extraadrenal).
We report our experience of eight pregnancies in four women with SDHB-related PGLs. In addition, we conducted a systematic review of the literature to analyze maternal and fetal outcomes in pregnant women with pheochromocytomas compared with PGLs. The aim of the review was to assess whether there was a difference in fetal or maternal mortality between pheochromocytomas and PGLs in pregnancy.
Subjects and Methods
We conducted a systematic literature review of articles published over the 15-year period between 1998 and 2013. OVID MEDLINE and PubMed search engines were used to identify relevant articles. The key words, pheochromocytoma, paraganglioma, and extraadrenal pheochromocytoma, were used. A separate search was performed to identify articles specifically related to PGL and pheochromocytoma in pregnancy, using the key words pregnancy or pregnant. All articles published in the period between 1998 and 2013 were included. Non-English articles were identified and translated. Cases were also included if the diagnosis of pheochromocytoma/PGL was made within 6 months of delivery.
A total of 189 articles were identified. Sixty-four articles did not adequately describe a case of pheochromocytoma or PGL and were excluded. Four additional papers were excluded because the cases had previously been described by another author. A total of 117 articles were included in the review comprising 143 cases of chromaffin cell tumors in pregnancy (112 adrenal pheochromocytomas, 28 extraadrenal PGLs, and three patients who had synchronous pheochromocytomas and PGLs). Data were collected on maternal and fetal mortality, symptoms, fold elevation in urinary or plasma catecholamines or metanephrines, genetic testing, and stage of pregnancy at which the diagnosis was made. Confidence intervals were calculated using the modified Wald method. The process whereby articles were selected for the systematic review is shown in Figure 1.
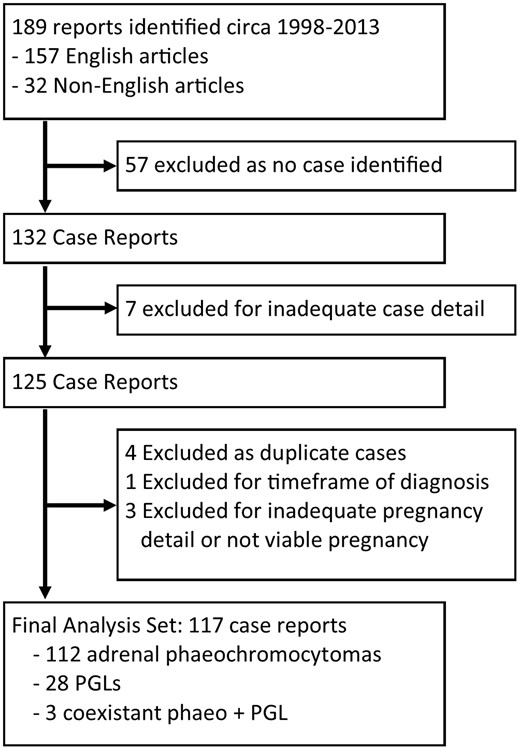
Schematic of articles included in the systematic review.
PGL = paraganglioma.
Case series (summarized in Table 1)
| Case . | Pregnancy . | Lesion . | CA Excess . | Normet . | Other . | CgA . | α-Blocker . | Delivery . | Fetal Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | PGL | Yes | ND | Plasma NA 2-fold increase | ND | Nil | NVD | Healthy |
| 2008 | PGL | Yes | 1.3-fold increase | Doxazosin | Elec LSCS | Healthy | |||
| 2010 | PGL | Yes | 2-fold increase | Doxazosin | Emerg LSCS | NICU, respiratory depression | |||
| 2010 | PGL | Yes | ND | Nil | Termination | ||||
| 2013 | Met PGL | Yes | 3-fold increase | Doxazosin | Emerg LSCS | Healthy | |||
| 2 | 2010 | HNPGL PGL | Yesa | Normal | 65 | Doxazosin | NVD | Healthy | |
| 3 | 2013 | PGL | No | Normal | Plasma 3-MT 92-fold increase | 138 | Nil | NVD | Healthy |
| 4 | 2010 | PGL | Yes | 8.6-fold increase | 31 | PBZ | Elec LSCS | Healthy | |
| 2013 | PGL | Yes | 4.5-fold increase | 9 | PBZ | Elec LSCS | NICU admission |
| Case . | Pregnancy . | Lesion . | CA Excess . | Normet . | Other . | CgA . | α-Blocker . | Delivery . | Fetal Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | PGL | Yes | ND | Plasma NA 2-fold increase | ND | Nil | NVD | Healthy |
| 2008 | PGL | Yes | 1.3-fold increase | Doxazosin | Elec LSCS | Healthy | |||
| 2010 | PGL | Yes | 2-fold increase | Doxazosin | Emerg LSCS | NICU, respiratory depression | |||
| 2010 | PGL | Yes | ND | Nil | Termination | ||||
| 2013 | Met PGL | Yes | 3-fold increase | Doxazosin | Emerg LSCS | Healthy | |||
| 2 | 2010 | HNPGL PGL | Yesa | Normal | 65 | Doxazosin | NVD | Healthy | |
| 3 | 2013 | PGL | No | Normal | Plasma 3-MT 92-fold increase | 138 | Nil | NVD | Healthy |
| 4 | 2010 | PGL | Yes | 8.6-fold increase | 31 | PBZ | Elec LSCS | Healthy | |
| 2013 | PGL | Yes | 4.5-fold increase | 9 | PBZ | Elec LSCS | NICU admission |
Abbreviations: CA, catecholamine; CgA, chromogranin A; Elec LSCS, elective lower segment cesarean section; Emerg LSCS, emergency lower segment cesarean section; HNPGL, head and neck paraganglioma; MT, methoxytyramine; NA, noradrenaline; ND, not done; NICU, newborn intensive care unit; Normet, normetanephrine; NVD, normal vaginal delivery; PBZ, phenoxybenzamine.
This case was likely secretory based on hemodynamic changes during tumor handling at the time of later tumor resection, but plasma free metanephrine levels were not elevated on serial samples.
| Case . | Pregnancy . | Lesion . | CA Excess . | Normet . | Other . | CgA . | α-Blocker . | Delivery . | Fetal Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | PGL | Yes | ND | Plasma NA 2-fold increase | ND | Nil | NVD | Healthy |
| 2008 | PGL | Yes | 1.3-fold increase | Doxazosin | Elec LSCS | Healthy | |||
| 2010 | PGL | Yes | 2-fold increase | Doxazosin | Emerg LSCS | NICU, respiratory depression | |||
| 2010 | PGL | Yes | ND | Nil | Termination | ||||
| 2013 | Met PGL | Yes | 3-fold increase | Doxazosin | Emerg LSCS | Healthy | |||
| 2 | 2010 | HNPGL PGL | Yesa | Normal | 65 | Doxazosin | NVD | Healthy | |
| 3 | 2013 | PGL | No | Normal | Plasma 3-MT 92-fold increase | 138 | Nil | NVD | Healthy |
| 4 | 2010 | PGL | Yes | 8.6-fold increase | 31 | PBZ | Elec LSCS | Healthy | |
| 2013 | PGL | Yes | 4.5-fold increase | 9 | PBZ | Elec LSCS | NICU admission |
| Case . | Pregnancy . | Lesion . | CA Excess . | Normet . | Other . | CgA . | α-Blocker . | Delivery . | Fetal Outcome . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2006 | PGL | Yes | ND | Plasma NA 2-fold increase | ND | Nil | NVD | Healthy |
| 2008 | PGL | Yes | 1.3-fold increase | Doxazosin | Elec LSCS | Healthy | |||
| 2010 | PGL | Yes | 2-fold increase | Doxazosin | Emerg LSCS | NICU, respiratory depression | |||
| 2010 | PGL | Yes | ND | Nil | Termination | ||||
| 2013 | Met PGL | Yes | 3-fold increase | Doxazosin | Emerg LSCS | Healthy | |||
| 2 | 2010 | HNPGL PGL | Yesa | Normal | 65 | Doxazosin | NVD | Healthy | |
| 3 | 2013 | PGL | No | Normal | Plasma 3-MT 92-fold increase | 138 | Nil | NVD | Healthy |
| 4 | 2010 | PGL | Yes | 8.6-fold increase | 31 | PBZ | Elec LSCS | Healthy | |
| 2013 | PGL | Yes | 4.5-fold increase | 9 | PBZ | Elec LSCS | NICU admission |
Abbreviations: CA, catecholamine; CgA, chromogranin A; Elec LSCS, elective lower segment cesarean section; Emerg LSCS, emergency lower segment cesarean section; HNPGL, head and neck paraganglioma; MT, methoxytyramine; NA, noradrenaline; ND, not done; NICU, newborn intensive care unit; Normet, normetanephrine; NVD, normal vaginal delivery; PBZ, phenoxybenzamine.
This case was likely secretory based on hemodynamic changes during tumor handling at the time of later tumor resection, but plasma free metanephrine levels were not elevated on serial samples.
Case 1
A 26-year-old Cook Island Māori woman presented in her third pregnancy to another center with an incidental finding of a right perirenal mass on obstetric ultrasound scan. An MRI scan performed at 30 weeks' gestation demonstrated a complex 81- × 77- × 76-mm retroperitoneal mass (with T2 enhancement and central necrosis) near the right kidney (Figure 3). This mass was initially reported as consistent with a retroperitoneal sarcoma. Labor was induced at 38 weeks and she had an uncomplicated normal vaginal delivery. A subsequent biopsy of the tumor was suggestive of a PGL. Twenty-four-hour urine catecholamines demonstrated elevated dopamine and noradrenaline levels of 3482 nmol per 24 hours [reference range (RR) 400–2600] and 979 nmol per 24 hours (RR 70–500), respectively. Repeat MRI imaging postpartum demonstrated a second mass in the right presacral region. After α-blockade the masses were resected without complication. Histology confirmed the diagnosis of paragangliomas. Genetic testing demonstrated a germline missense mutation (R46Q) in exon 2 of the SDHB gene.
The patient re-presented 2 years later during her fifth pregnancy after a first-trimester miscarriage 1 year previously. Her plasma normetanephrine level was mildly elevated at 1130 pmol/L (RR < 900 pmol/L) with a normal plasma metanephrine level. She was treated with α-blockade (doxazosin) and remained normotensive with no hyperadrenergic symptoms during the pregnancy. An uncomplicated elective cesarean section was performed at 38 weeks' gestation, delivering a healthy male infant weighing 3450 g. A sixth pregnancy in 2010 was complicated by placenta previa and recurrent vaginal bleeding. Further MRI imaging showed two lesions, both 2.2 cm in maximum diameter lesions, one to the left of the aorta and one between the superior mesenteric artery and the aorta. Plasma normetanephrine levels were elevated at 1860 pmol/L (RR 0–900). She was monitored as an inpatient from 31 weeks and required an emergency cesarean section at 32 weeks for bleeding. Her baby required a prolonged admission to the newborn unit for respiratory distress. A seventh pregnancy was electively terminated and she did not present again until 20 weeks into her eighth pregnancy. She had been offered multiple endocrinology clinic appointments but did not attend for follow-up.
During the eighth pregnancy, she remained asymptomatic and normotensive. Plasma normetanephrines were 3-fold elevated. Widespread metastatic PGL was evident on imaging. Doxazosin was commenced. She developed obstetric cholestasis, and due to concerns about fetal well-being, she underwent elective cesarean section at 38 weeks' gestation, giving birth to a live healthy female infant weighing 3050 g.
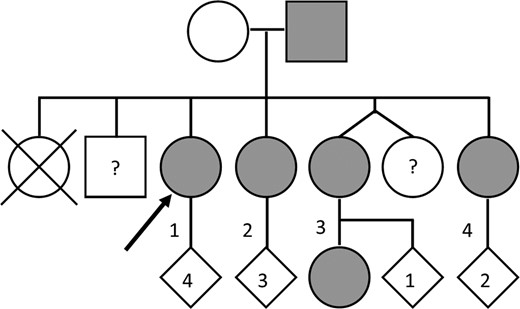
The family of the index patient underwent predictive testing for the R46Q mutation (Figure 2). Her father and three of her four sisters were identified as carriers. Two siblings declined testing.
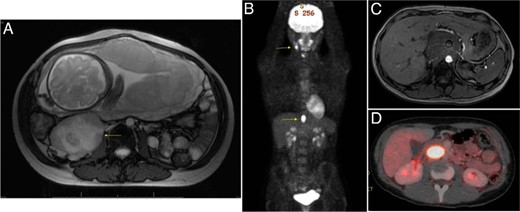
Selected imaging of our case series patients.
A, Patient 1: 30/40 MRI scan (81 × 77 × 76 mm) retroperitoneal mass with central necrosis and presacral lesion. B, Patient 2: FDG-PET demonstrating right carotid body and abdominal PGLs (postpartum). C, Patient 3: MRI scan showing large retroperitoneal mass encasing the mesenteric vessels. D, Patient 4: postpartum FDG-PET demonstrating the abdominal PGL.
Case 2
Patient 2, a sister of the index case, presented in the third trimester of her third pregnancy at age 23 years. She had a history of two previous normal vaginal deliveries. The most recent delivery had occurred after her genetic testing confirmed she was an SDHB mutation carrier but prior to any biochemical testing because she had failed to attend her scheduled specialist appointments. She delivered the second baby without complications by vaginal delivery. During the third pregnancy, she was normotensive and treated with doxazosin as an inpatient from 33 weeks' gestation and remained normotensive with blood pressures between 100/50 and 116/50 mm Hg. MRI imaging demonstrated a 13-mm lesion with high signal on T2-weighted imaging in the paraaortic region near the left renal hilum. Plasma metanephrines were normal with a normetanephrine of 382 pmol/L (RR 0–900) and a metanephrine of less than 100 pmol/L (RR 0–500). The chromogranin A was mildly elevated at 65 U/L (RR 0–20U/L). On the basis of the normal plasma metanephrines and the nonspecific nature of the lesion seen on MRI, the decision was made to allow a trial of normal vaginal delivery under close monitoring. This occurred without complication.
After delivery of the baby, she underwent an 18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) scan. This confirmed two lesions: one adjacent to the right carotid body and a second lesion adjacent to the inferior vena cava posterior to the caudate lobe of the liver. She was recommenced on doxazosin and had the two lesions resected without complication, although it was noted during resection of the abdominal lesion that there was hemodynamic changes with tumor handling, suggesting that indeed the paraaortic tumor was secretory. Follow-up plasma metanephrines remained normal. Histology was consistent, with both of these lesions being paragangliomas.
Case 3
Patient 3, another sister of the index case, was identified as carrying the R46Q SDHB germline mutation in 2008 when aged 17 years. She had an extensive history of nonattendance at the clinic and was first assessed by the endocrine service at age 21 years when she was at 14 weeks' gestation into her third pregnancy. Two previous vaginal deliveries had been uncomplicated. At assessment, plasma-free metanephrines were normal [normetanephrine, 342 pmol/L (RR 0–900); metanephrine, 104 pmol/L (RR 0–500)], but a plasma 3-methoxytyramine level was markedly elevated at 10 115 pmol/L (RR < 110 pmol/L). Chromogranin A was elevated at 135 U/L (RR 0–20 U/L). An MRI of the abdomen showed a large retroperitoneal mass surrounding the mesenteric vessels and encasing the superior mesenteric artery. Due to the isolated dopamine hypersecretion, she was considered at low risk of a hypertensive crisis, and a trial of normal vaginal delivery was offered under close monitoring. She had an uncomplicated normal vaginal delivery of a healthy male infant weighing 3100 g. She was normotensive throughout the labor and delivery. Resection of the tumor was discussed with multiple endocrine and gastrointestinal surgeons; however, the patient was not considered a suitable surgical candidate. Further treatment options are being explored.
Case 4
Patient 4, the youngest sister of the index patient, presented at age 17 years in the second trimester of her first pregnancy. Plasma normetanephrine was significantly elevated at 7770 pmol/L (RR < 900 pmol/L), with normal metanephrine of 102 pmol/L and a chromogranin A of 31 U/L (RR 0–20 U/L). She was commenced on phenoxybenzamine and remained normotensive throughout the pregnancy, maintaining an average BP of 100/60 mm Hg. An MRI scan revealed a 3.5- × 2.2-cm lesion behind the uncinate process of the pancreas, compressing the inferior vena cava. She had an elective cesarean section under an epidural anesthetic at 38 weeks and gave birth to a healthy baby boy. She failed to attend her scheduled follow-up appointments to discuss surgical resection.
She represented 2 years later during her second pregnancy. The plasma normetanephrine level again was significantly elevated at 7680 pmol/L (RR < 900). Plasma metanephrine and 3-methoxytyramine levels were normal. The patient was commenced on phenoxybenzamine at 37 weeks' gestation. She was monitored as an inpatient for 3 days prior to surgery and remained normotensive. Her phenoxybenzamine dose was uptitrated to 20 mg twice daily with no significant postural hypotension. She was commenced on a high-salt diet and fluid loaded for 24 hours prior to surgery. She underwent an uncomplicated elective lower segment cesarean section followed by resection of the paracaval lesion under general anesthesia at 39 weeks' gestation. Her baby required intubation for 2 hours after delivery and newborn unit admission due to sedation at delivery, thought to be due to the combination of phenoxybenzamine and fentanyl, but was well on discharge after a 3-day admission. On direct inspection a placental mass was noted. Histologically the paracaval tumor was subsequently confirmed to be a PGL and the placental lesion to be a chorangioma (a benign tumor found in ∼1% of placentas) (9). Chorangioma has not previously been reported in association with pheochromocytoma or PGL. The patient's postoperative plasma metanephrines were normal.
Results
Results of the systematic review are shown in Table 2. A total of 117 articles were included in the review, comprising 143 cases of chromaffin cell tumors in pregnancy (112 adrenal pheochromocytomas, 28 extraadrenal paragangliomas, and three patients who had synchronous pheochromocytomas and paragangliomas).
| . | Pheochromocytoma (n = 115) . | Paraganglioma (n = 31) . |
|---|---|---|
| Hypertension | ||
| Unilateral/isolated | 83 (0.85, CI 0.76–0.91) | 23 (0.82, CI 0.64–0.93) |
| Bilateral | 11 (0.73, CI 0.48–0.90) | |
| Concurrent PGL | 3 (1, CI 0.38–1) | |
| Hypertensive crisis | 29 (0.27, CI 0.19–0.36) | 4 (0.14, CI 0.051–0.32) |
| Mode of delivery | ||
| Cesarean | 62 (0.70, CI 0.60–0.79) | 22 (0.92, CI 0.73–0.99) |
| Vaginal delivery | 26 (0.3, CI 0.21–0.40) | 2 (0.083, CI 0.12–0.27) |
| Maternal mortality | 11 (0.098, CI 0.054–0.17) | 1 (0.036, CI <0.0001–0.19) |
| Fetal mortality | ||
| Unilateral/isolated | 17 (0.16, CI 0.10–0.24) | 3 (0.12, CI 0.032–0.30) |
| Bilateral | 3 (0.2, CI 0.063–0.46) | |
| Concurrent PGL | 1 (0.5, CI 0.095–0.91) | |
| . | Pheochromocytoma (n = 115) . | Paraganglioma (n = 31) . |
|---|---|---|
| Hypertension | ||
| Unilateral/isolated | 83 (0.85, CI 0.76–0.91) | 23 (0.82, CI 0.64–0.93) |
| Bilateral | 11 (0.73, CI 0.48–0.90) | |
| Concurrent PGL | 3 (1, CI 0.38–1) | |
| Hypertensive crisis | 29 (0.27, CI 0.19–0.36) | 4 (0.14, CI 0.051–0.32) |
| Mode of delivery | ||
| Cesarean | 62 (0.70, CI 0.60–0.79) | 22 (0.92, CI 0.73–0.99) |
| Vaginal delivery | 26 (0.3, CI 0.21–0.40) | 2 (0.083, CI 0.12–0.27) |
| Maternal mortality | 11 (0.098, CI 0.054–0.17) | 1 (0.036, CI <0.0001–0.19) |
| Fetal mortality | ||
| Unilateral/isolated | 17 (0.16, CI 0.10–0.24) | 3 (0.12, CI 0.032–0.30) |
| Bilateral | 3 (0.2, CI 0.063–0.46) | |
| Concurrent PGL | 1 (0.5, CI 0.095–0.91) | |
Confidence intervals calculated using the modified Wald method.
| . | Pheochromocytoma (n = 115) . | Paraganglioma (n = 31) . |
|---|---|---|
| Hypertension | ||
| Unilateral/isolated | 83 (0.85, CI 0.76–0.91) | 23 (0.82, CI 0.64–0.93) |
| Bilateral | 11 (0.73, CI 0.48–0.90) | |
| Concurrent PGL | 3 (1, CI 0.38–1) | |
| Hypertensive crisis | 29 (0.27, CI 0.19–0.36) | 4 (0.14, CI 0.051–0.32) |
| Mode of delivery | ||
| Cesarean | 62 (0.70, CI 0.60–0.79) | 22 (0.92, CI 0.73–0.99) |
| Vaginal delivery | 26 (0.3, CI 0.21–0.40) | 2 (0.083, CI 0.12–0.27) |
| Maternal mortality | 11 (0.098, CI 0.054–0.17) | 1 (0.036, CI <0.0001–0.19) |
| Fetal mortality | ||
| Unilateral/isolated | 17 (0.16, CI 0.10–0.24) | 3 (0.12, CI 0.032–0.30) |
| Bilateral | 3 (0.2, CI 0.063–0.46) | |
| Concurrent PGL | 1 (0.5, CI 0.095–0.91) | |
| . | Pheochromocytoma (n = 115) . | Paraganglioma (n = 31) . |
|---|---|---|
| Hypertension | ||
| Unilateral/isolated | 83 (0.85, CI 0.76–0.91) | 23 (0.82, CI 0.64–0.93) |
| Bilateral | 11 (0.73, CI 0.48–0.90) | |
| Concurrent PGL | 3 (1, CI 0.38–1) | |
| Hypertensive crisis | 29 (0.27, CI 0.19–0.36) | 4 (0.14, CI 0.051–0.32) |
| Mode of delivery | ||
| Cesarean | 62 (0.70, CI 0.60–0.79) | 22 (0.92, CI 0.73–0.99) |
| Vaginal delivery | 26 (0.3, CI 0.21–0.40) | 2 (0.083, CI 0.12–0.27) |
| Maternal mortality | 11 (0.098, CI 0.054–0.17) | 1 (0.036, CI <0.0001–0.19) |
| Fetal mortality | ||
| Unilateral/isolated | 17 (0.16, CI 0.10–0.24) | 3 (0.12, CI 0.032–0.30) |
| Bilateral | 3 (0.2, CI 0.063–0.46) | |
| Concurrent PGL | 1 (0.5, CI 0.095–0.91) | |
Confidence intervals calculated using the modified Wald method.
The overall maternal mortality rate was 9.8% [95% confidence interval (CI) 0.054–0.17] in pheochromocytomas and 3.6% in paragangliomas (95% CI < 0.0001–0.19). Fetal mortality was calculated after excluding those pregnancies, which resulted in elective termination. Fetal mortality in women with pheochromocytomas was 16% (95% CI 0.1–0.24), compared with 12% (95% CI 0.032–0.3) for those with paragangliomas. The diagnosis was made antenatally in 84% of patients with paragangliomas and 80.3% of those with pheochromocytomas. When we add our case series of eight pregnancies, of which seven were secretory paragangliomas, overall maternal mortality for PGLs decreased to 2.8% (95% CI < 0.001–0.15) and fetal mortality to 8.8% (95% CI 0.023–0.24). Of those women reported to have bilateral pheochromocytomas, there was no maternal mortality reported; however, fetal mortality occurred in 3 of 15 of these cases (0.2, CI 0.063–0.46) (10–12). There were three cases of pheochromocytoma with Cushing's syndrome (13–15). There was no fetal or maternal mortality in these cases.
The degree of elevation in the urinary/plasma catecholamines or metanephrines was documented in 54.8% of cases in the literature. In those cases in which catecholamine levels were documented, there did not appear to be a correlation between the degree of elevation in the catecholamines and fetal or maternal mortality. There was also no clear correlation between the size of the lesions and the probability of fetal or maternal mortality, but again only incomplete data were available for tumor size.
Cardiovascular collapse, pulmonary edema, or fatal arrhythmia was the cause of death in 6 of the 11 maternal deaths (1, 16–20). One patient died of multiorgan failure (21), and another suffered from a subarachnoid hemorrhage (22). The cause of death was not reported in three cases (14, 23, 24). There was one maternal death in a woman with a paraganglioma. The cause of death was reported as cardiovascular collapse resulting in a ventricular fibrillation arrest (25).
Hypertension was the most common presenting feature in pregnancy, reported in 87% of pheochromocytomas and 86% of PGLs. Hypertensive crises were more commonly seen in patients with pheochromocytomas, occurring in 27 women (0.24, CI 0.17–0.33) compared with four women with PGLs (0.14, 95% CI 0.051–0.32). Patients with PGLs were more likely to be asymptomatic than those with pheochromocytoma (10 of 36 vs 5 of 112). Of particular note, in our case series, all four women were asymptomatic and normotensive throughout the eight pregnancies.
Discussion
Pregnancies complicated by a PGL or pheochromocytoma are extremely rare, estimated to occur in 1 in 15 000 pregnancies (1). PGLs are less common than pheochromocytomas in pregnancy. In a recent review of pheochromocytoma in pregnancy, extraadrenal lesions accounted for only 14 of the cases described (19%) (5).
Based on our experience from our case series, we hypothesized that the risk of hypertensive crisis during delivery may be less in patients with functioning PGLs compared with pheochromocytomas. Our review of the literature indicates that maternal mortality may be lower in patients with PGLs compared with those with pheochromocytomas. Fetal mortality also may be lower in patients with PGLs compared with those with pheochromocytomas.
In addition, there is a lower rate of hypertensive crises reported in patients with PGLs, four patients (0.14, 95% CI 0.051–0.32) compared with 27 patients (0.27, 95% CI 0.19–0.36). This may relate to less effective catecholamine secretion compared with pheochromocytomas. Pheochromocytomas and PGLs can have diverse biochemical phenotypes. It is recognized that PGLs may exhibit different biochemical behavior from pheochromocytomas. PGLs secrete noradrenaline and/or dopamine, whereas pheochromocytomas may secrete either noradrenaline or adrenaline. The total tissue concentrations of catecholamines are highest in adrenaline-secreting tumors (26). Tumors related to an underlying genetic abnormality (VHL, NF1, MEN2, SDHB, and SDHD) show different catecholamine secretory profiles (26). In patients with SDHB mutations, the tumor catecholamine concentration is lower than in tumors caused by other genetic mutations and noradrenaline-secreting tumors in which a mutation has not been identified (27). Catecholamine secretion rates into plasma were higher in the nonhereditary noradrenaline-secreting tumors and in those with von Hippel Lindau syndrome compared with other hereditary syndromes (multiple endocrine neoplasia-2, neurofibromatosis type 1, SDHB, and SDHD) (26). In our review of the literature, the degree of epinephrine or metanephrine level elevation was reported in 19 of 29 patients with pheochromocytomas who developed a hypertensive crisis (67%). There were six maternal deaths in which no catecholamine level was reported, and the type or degree of catecholamine excess was not reported in four cases. Mixed noradrenaline and adrenaline elevation was reported in 13 patients. Isolated noradrenaline elevation was reported in two cases, isolated adrenaline elevation in two cases, and vanillylmandelic acid alone in two cases. There were four cases of hypertensive crisis reported in patients with paragangliomas. In these four patients, there was one maternal death (25) and one fetal death in a different patient (28).
The management of BP in pregnant woman with pheochromocytoma is a careful balance between providing adequate catecholamine blockade to reduce the risk of catecholamine excess while not compromising placental blood flow by lowering the BP excessively. In nonpregnant patients, a preoperative target BP of less than 130/80 mm Hg sitting but greater than 80/45 mm Hg standing has been recommended (29). It has been suggested that excessive BP lowering in pregnancy may result in uteroplacental insufficiency and intrauterine growth restriction, with a 176-g decrease in fetal weight with a 10-mm Hg decrease in mean arterial pressure in pregnant mothers without pheochromocytoma/PGLs (30). In the context of pregnant women without pheochromocytomas, the threshold for treatment of hypertension in pregnancy varies, depending on which guideline is used, but treatment is suggested only if the BP is greater than 140–159 mm Hg systolic or 90–109 mm Hg diastolic (31). However, the issue with pheochromocytoma/PGL patients is the potentially labile BP with the risk of severe peaks of BP if no α-blockade is given. Women with pheochromocytoma or PGLs are at risk of placental abruption and fetal loss (7). In our series of patients, the women were normotensive in all of the pregnancies described. This is unusual, given that 82% (23 women) were hypertensive in 31 cases of PGL reported in the literature. Despite the normotension we did use low-dose α-blockade in combination with a high-salt diet to try to prevent marked BP fluctuations. As demonstrated by patient 2, normal plasma-free metanephrine levels do not necessarily mean that the lesion is nonsecretory, particularly with manipulation at the time of surgery, and low-dose α-blockade should be considered in these cases.
The choice of specific α-blockade needs to be carefully considered in the management of pheochromocytoma/PGL in pregnancy. Phenoxybenzamine is an irreversible α-adrenergic antagonist and has previously been suggested as the treatment of choice in women with pheochromocytoma or PGL (32). Phenoxybenzamine has a half-life of 24 hours, and the clinical effects may continue for up to a week after discontinuation (33). Phenoxybenzamine has been shown to cross the placenta and accumulate in the fetus (8), potentially leading to neonatal respiratory depression and hypotension. As such, it has been recommended that neonates of women who have taken phenoxybenzamine in pregnancy are monitored for hypotension and respiratory depression in the first few days of life (34). It is theorized that the respiratory depression observed in these babies may result from an element of cardiac failure due to the large proportion of α-adrenergic receptors in the fetal heart (34). Despite no teratogenic effects of phenoxybenzamine having been reported in animal testing, there are no well-controlled studies in pregnant woman. In the United States and the United Kingdom, phenoxybenzamine is a pregnancy category C, indicating that the pharmacological effects of the drug may cause harm to the fetus or neonate without causing malformation.
Doxazosin is a competitive α-1 selective α-adrenergic blocker with a plasma half-life of 20 hours (33). There have been no reports of adverse effects on the neonate with the use of doxazosin in pregnant women with pheochromocytoma or paragangliomas. In our series of patients, doxazosin was used in four of eight pregnancies. For patient 1, doxazosin was used in the sixth pregnancy. This baby was born prematurely at 32 weeks and experienced respiratory depression requiring neonatal unit admission. Given the premature delivery, it is difficult to determine what extent the doxazosin may have contributed to the respiratory depression. Doxazosin was used without complication in three of the remaining eight pregnancies. Phenoxybenzamine was used in the two pregnancies of patient 4. Despite low BP, she delivered appropriate-for-gestational-age babies in both cases.
In gestational hypertension, prazosin, another selective α-1 blocker, has been suggested as a possible second-line antihypertensive during pregnancy (35), and its use is reported in cases of pheochromocytoma and PGL in pregnancy without adverse effects. However, prazosin has a much shorter half-life of only 2–3 hours, meaning that a multiple daily dosing regimen is required (33). In our systematic review of the literature, phenoxybenzamine was the α-blocker most commonly used. In the 62 cases of pheochromocytoma in which the type of α-blockade was specified, there were 36 cases of phenoxybenzamine use (58%), 18 cases of prazosin use (26%), and eight cases in which doxazosin was used (12.9%). There were 17 cases of PGL in which the type of α-blockade was specified. Phenoxybenzamine was the most commonly reported (15 cases, 88%), and two cases reported the use of doxazosin (12%). Based on the literature review, doxazosin appears to be the least commonly used of these three α-blockers. However, if no additional safety concerns are identified, due to its more favorable duration of action, doxazosin may become the α-blocker of choice for these women during pregnancy.
Traditionally it has been recommended that vaginal delivery is best avoided in pregnant women with pheochromocytomas or paragangliomas (36). There is a potential risk of precipitating a hypertensive crisis from active labor. There have been cases reported in the literature of successful normal vaginal deliveries without maternal or fetal mortality (37–43). However, in a case reported by Lyman (41), the postpartum period was complicated by pulmonary edema in the mother. In our series, three of the eight neonates were delivered by vaginal delivery. In one of these cases, the delivery was performed elsewhere prior to the diagnosis of PGL. In the other two, one was purely dopamine secreting (patient 3) so deemed to be at low risk of hypertensive crisis. The third case (patient 2) had normal plasma-free metanephrine levels but in retrospect likely did have a secretory lesion because the later intraoperative behavior was consistent with a functioning tumor at the time of surgical manipulation. Despite her normal plasma-free metanephrines, she was pretreated with α-blockade and salt and fluid loading, and an uncomplicated vaginal delivery was undertaken with close monitoring.
After the diagnosis of PGL or pheochromocytoma in pregnancy, a multidisciplinary approach is essential. It is important that the mother with pheochromocytoma/PGL deliver in a tertiary hospital with an experienced obstetric, anesthetic, and endocrine service as well as a neonatal intensive unit. The delivery can be performed under a spinal anesthetic. Spinal anesthetic may have a theoretical advantage of reducing neural stimulation to the adrenal glands and sympathetic chain if the block is high enough. It is also important to remember to avoid medications in these women that may precipitate a crisis, in particular agents such as metoclopramide (33). Ondansetron was successfully used in our case series without hemodynamic instability and has previously been reported to be used without adverse effects in metastatic pheochromocytoma (44).
One limitation of this review is that it is vulnerable to publication bias, given that centers with more favorable pregnancy outcomes may be more likely to publish their case reports; however, it would be expected that this should pertain to both pregnancies with pheochromocytoma and those with PGL. It is also possible that additional pregnancies had unrecognized synchronous tumors; however, due to the varied locations, it would be expected that PGLs are more likely to have been missed than pheochromocytomas.
In conclusion, pregnant women with PGLs may be at a lower risk of adverse outcome than those with pheochromocytomas, but both maternal and fetal mortality rates are still higher than that of the general obstetric population, and a multidisciplinary approach with careful monitoring is recommended.
Acknowledgments
We acknowledge Professor Ken Huddle and Dr B. D. Djurhuus for providing additional information regarding their published cases.
Author contributions include the following: L.A.W. helped with the study design, data collection and analysis, drafting the article, and approval of the final version; J.V.C. helped with the study design, revising the article for important intellectual content, and approval of the final version; G.Y.M.-R. helped with the conception of the study, revising the article for important intellectual content, and approval of the final version; M.S.E. helped with the study design, interpretation of the data, revising the article for important intellectual content, and approval of the final version.
This study was funded by Waikato District Health Board (Hamilton, New Zealand).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations
- BP
blood pressure
- CI
confidence interval
- FDG-PET
18F-fluorodeoxyglucose-positron emission tomography
- MRI
magnetic resonance imaging
- PGL
paraganglioma
- RR
reference range



