-
PDF
- Split View
-
Views
-
Cite
Cite
Manna Zhang, Guoyu Tong, Yanling Liu, Yiming Mu, Jianping Weng, Yaoming Xue, Zuojie Luo, Yuanming Xue, Lixin Shi, Xueyan Wu, Shouyue Sun, Yanhua Zhu, Ying Cao, Jie Zhang, Hong Huang, Ben Niu, Hong Li, Qinghua Guo, Yan Gao, Zhibin Li, Guang Ning, Dalong Zhu, Xiaoying Li, on Behalf of the HHIS Study Group, Sequential Versus Continual Purified Urinary FSH/hCG in Men With Idiopathic Hypogonadotropic Hypogonadism, The Journal of Clinical Endocrinology & Metabolism, Volume 100, Issue 6, 1 June 2015, Pages 2449–2455, https://doi.org/10.1210/jc.2014-3802
Close - Share Icon Share
Gonadotropin therapy using a human chorionic gonadotropin (hCG) and FSH preparation is an effective regimen in inducing masculinization and spermatogenesis in men with idiopathic hypogonadotropic hypogonadism (IHH). However, the high cost of medication and frequent injections affect compliance.
The aim of this study was to determine the efficacy of sequential use of highly purified urinary FSH (uFSH)/hCG in men with IHH.
A randomized, open-label, prospective, controlled noninferiority trial with an 18-month follow-up was conducted in 9 tertiary hospitals.
A total of 67 Chinese men with IHH were randomly allocated into group A receiving continual uFSH (75 U, 3 times a week) and hCG (2000 U, twice a week) injection and group B receiving sequential uFSH (75 U, 3 times a week every other 3 months) and hCG (2000 U, twice a week) injection.
The primary outcome was the proportion of subjects with a sperm concentration of ≥1.0 ×106/mL during the 18 months. The efficacy between groups A and B was compared for noninferiority.
Of the patients, 17/33 (51.5%) receiving continual uFSH/hCG and 19/34 (55.9%) receiving sequential uFSH/hCG achieved sperm concentrations of ≥1.0 × 106/mL. The efficacy in the sequential uFSH/hCG group was not inferior to that in the continual uFSH/hCG group (noninferiority, P = .008) by intention-to-treat analysis.
The efficacy of the sequential uFSH/hCG regimen is not inferior to that of the continual uFSH/hCG regimen in inducing spermatogenesis and masculinization of patients with IHH.
Congenital idiopathic hypogonadotropic hypogonadism (IHH) is characterized by absent or incomplete sexual maturation and infertility caused by an isolated defect of GnRH release or action (1–3). IHH is classified as Kallmann syndrome in the presence of anosmia or hyposmia and normosmic IHH with an intact sense of smell (2, 4). The goal of therapy for male patients with IHH consists of achievement of physical and psychological sexual maturation and fertility. Pulsatile GnRH or exogenous gonadotropins are usually used to induce spermatogenesis and promote testicular enlargement (5–11). The regimen for gonadotropin replacement includes an initial use of human chorionic gonadotropin (hCG) for 6 to 12 months and then addition of FSH or human menopausal gonadotropin (hMG) until pregnancy (12).
However, the current regimen is costly and poorly complied with by patients. It was documented that spermatogenesis could be well maintained using hCG alone, provided it was once induced by pulsatile GnRH or continual hCG/FSH treatment in some patients with IHH (13, 14). It was also reported that hCG alone could induce spermatogenesis in patients with IHH (7). Thus, the objective of this study was to explore the efficacy of intermittent or sequential use of FSH preparations in combination with hCG in inducing spermatogenesis in patients with IHH. This modified regimen might greatly reduce the medication cost.
Subjects and Methods
Study design
This randomized, open-label, multicenter, noninferiority trial was conducted between October 2009 and December 2012 in accordance with the ethical principles of the Declaration of Helsinki and the principles of current good clinical practice.
The study protocol was reviewed and approved by the Institutional Review Board of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from each participant. This study was conducted in 9 tertiary hospitals in China. A complete medical history was recorded, and a physical examination was performed by a senior endocrinologist.
The GnRH- and hCG-stimulating tests were performed at the initial screening. A total of 96 male patients with IHH were initially screened, and 67 patients met the inclusion criteria and were randomly allocated into 2 groups; continual uFSH (group A, n = 33) and sequential uFSH (group B, n = 34) as shown in Figure 1. The patients were followed up every 3 months at 3, 6, 9, 12, 15, and 18 months after treatment initiation, including physical examination, semen analysis, and hormone measurements.
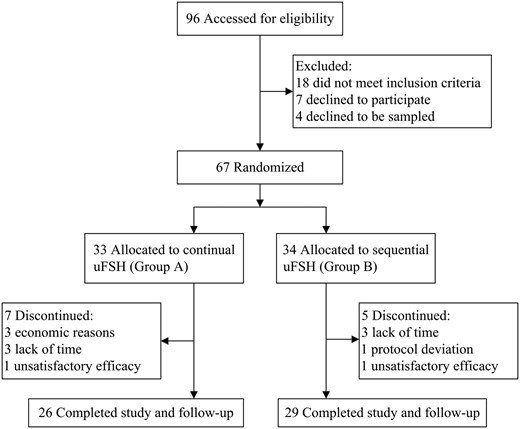
Study subjects
Eligibility criteria included (1) men aged 18 to 45 years (2) without spontaneous puberty, (3) who had serum testosterone levels of <100 ng/dL (3.5 nmol/L) in the presence of low or normal gonadotropins and (4) otherwise had normal testing of the anterior pituitary gland. Exclusion criteria included (1) acquired hypogonadotropic hypogonadism (HH), (2) previous exposure to pulsatile GnRH- or FSH-containing preparations, (3) ever having received testosterone replacement for more than 6 months duration, (4) a sperm concentration of ≥1.0 × 106/mL, (5) the presence or history of unilateral or bilateral cryptorchidism, (6) a serum testosterone level of <150 ng/dL (5.2 nmol/L) by an hCG-stimulating test, or (7) moderate or severe liver and renal dysfunction, including serum alanine aminotransferase of > 120 IU/L, aspartate aminotransferase of > 80 IU/L, and serum creatinine of > 115 μmol/L.
A total of 96 patients were initially screened. Of those, 18 patients did not meet the inclusion criteria, 7 declined to participate, and 4 declined to undergo blood sampling. Twelve patients did not complete the study, including 7 from group A and 5 from group B. The reasons for the dropouts are indicated in Figure 1.
Randomization and masking
Participants were randomly assigned to group A and B (1:1). The randomization was conducted independently at a central office using a computer-generated random allocation sequence table with permuted blocks of 6 and with stratification by centers. Allocation concealment was performed by enclosing assignments in sequentially numbered, opaque, closed envelopes. Patients, investigators, trial staff, and statisticians were masked to treatment allocation.
Treatment
The patients in both groups A and B received injections of 2000 U of hCG twice a week from the start to the 18th month. In group A, patients received injections of 75 U of uFSH 3 times a week from the 6th to 18th month. In group B, patients received injections of 75 U of uFSH 3 times a week from the 6th to the 18th month every other 3 months. Both hCG and uFSH were injected intramuscularly by nurses or by the patients themselves.
Outcomes
The primary outcome was defined as a sperm concentration of ≥1.0 × 106/mL during the 18-month course of treatment. A value of 1.0 × 106/mL is commonly used in clinical trials because it is representative of the sperm concentration that is compatible with achieving pregnancy (15–17). The secondary outcomes included single testicular volume, serum testosterone concentration, sperm activity, sperm count per ejaculate, and time of spermatogenesis. Self-reported pregnancy was also recorded.
Clinical measurements
Body weight, height, body mass index, Tanner stage, and penis length were evaluated at each visit. Body weight, expressed in 0.1-kg intervals, was measured at fasting states in the morning. Harpenden stadiometers were used for the measurement of body height. Each subject was measured 3 times to the nearest 5 mm. Body mass index was calculated as body weight in kilograms divided by height in squared meters. Pubic and gonad Tanner stages were evaluated by a senior physician. Penis length was measured from the base all the way to the end of the tip. Testicular volumes were examined by ultrasound examinations of scrotal content (GE LOGIQ E9; GE Healthcare) and calculated by the formula of length × width × depth × 0.71 (18).
Biochemical measurements
Blood samples were collected in the morning within 24 to 72 hours after the last hCG and uFSH injections and immediately centrifuged. Serum was frozen at −80°C until assayed. Serum LH, FSH, estradiol, and testosterone were measured by chemiluminescence immunoassay (Abbott). The normal range for LH is 1.1 to 8.8 IU/L, for FSH is 1.6 to 6.4 IU/L, and testosterone is 160 to 800 ng/dL (5.6–27.8 nmol/L) in men.
Semen specimens were collected from the patients at each visit upon successful ejaculation and analyzed according to World Health Organization guidelines (19). All of the samples were collected after 4 to 7 days of sexual abstinence and obtained by masturbation. Azoospermia is defined as no sperm detected in semen. Sperm motility was classified as grade A, B, and C.
Statistical analysis
A total of 67 patients were randomly allocated at a ratio of 1:1 into 2 groups. Efficacy was compared between groups A and B for noninferiority. The expected efficacy rate in the reference group (continual uFSH, group A) was 70% (20). The margin of acceptable difference between group B and group A was set at 25%. The criterion for establishing noninferiority was that the lower bound of the two-sided 95% confidence interval (CI) for the differences in rates of group B vs group A must exceed the predefined noninferiority margin of −3%. Under these assumptions, a sample size of 33 patients in each of the 2 treatment groups was needed to yield 80% power to conclude that group B was not inferior to group A with α = .05.
All efficacy analyses were performed based on intention to treat (ITT). The primary outcome for noninferiority was also analyzed using the per-protocol (PP) principle, which included the subjects who completed the whole process of the 18-month treatment. The Student t test was used for normal distributed variables. A nonparametric Wilcoxon rank sum test was performed for skewed parameters. Kaplan-Meier plots were used to analyze the times for the primary outcome and initiation of spermatogenesis, which were compared between groups with the use of the log-rank test. The χ2 or Fisher exact test was used for comparison of binary outcomes. Statistical analysis was performed using SAS version 8.1 software (SAS Institute). The results are presented as means ± SD or medians (interquartile range).
Results
Baseline characteristics
The clinical characteristics and hormonal profiles of the participants at baseline are shown in Table 1. The mean ages were 23.1 ± 5.1 years in group A and 24.1 ± 4.9 years in group B; 14 (42.4%) individuals in group A and 16 (47.1%) individuals in group B presented with complete anosmia or hyposmia. All of the patients lacked signs of secondary sexual characteristics and had small testes and short penises. Serum testosterone, LH, and FSH concentrations were reduced. Sperm count was null in the ejaculates at baseline.
| . | Continual uFSH (group A, n = 33) . | Sequential uFSH (group B, n = 34) . | P value . |
|---|---|---|---|
| Age, y | 23.1 ± 5.1 | 24.1 ± 4.9 | .413 |
| Height, cm | 172.1 ± 7.1 | 172.6 ± 6.4 | .730 |
| Body mass index, kg/m2 | 21.3 ± 4.5 | 22.2 ± 6.6 | .537 |
| Penis length, cm | 3.9 ± 1.4 | 4.3 ± 1.0 | .193 |
| Testicular volume, ml | 1.7 (0.9–3.4) | 1.5 (1.0–3.0) | .783 |
| Pubic hair stage | 1.9 ± 0.9 | 2.1 ± 1.0 | .302 |
| Genital Tanner stage | 1.6 ± 0.9 | 1.7 ± 0.7 | .401 |
| Plasma LH, IU/L | 0.3 (0.1–0.7) | 0.3 (0.1–0.8) | .580 |
| Plasma FSH, IU/L | 0.8 (0.4–1.6) | 1.0 (0.5–1.4) | .624 |
| Plasma testosterone, ng/dL | 40 (30–50) | 40 (30–50) | .841 |
| . | Continual uFSH (group A, n = 33) . | Sequential uFSH (group B, n = 34) . | P value . |
|---|---|---|---|
| Age, y | 23.1 ± 5.1 | 24.1 ± 4.9 | .413 |
| Height, cm | 172.1 ± 7.1 | 172.6 ± 6.4 | .730 |
| Body mass index, kg/m2 | 21.3 ± 4.5 | 22.2 ± 6.6 | .537 |
| Penis length, cm | 3.9 ± 1.4 | 4.3 ± 1.0 | .193 |
| Testicular volume, ml | 1.7 (0.9–3.4) | 1.5 (1.0–3.0) | .783 |
| Pubic hair stage | 1.9 ± 0.9 | 2.1 ± 1.0 | .302 |
| Genital Tanner stage | 1.6 ± 0.9 | 1.7 ± 0.7 | .401 |
| Plasma LH, IU/L | 0.3 (0.1–0.7) | 0.3 (0.1–0.8) | .580 |
| Plasma FSH, IU/L | 0.8 (0.4–1.6) | 1.0 (0.5–1.4) | .624 |
| Plasma testosterone, ng/dL | 40 (30–50) | 40 (30–50) | .841 |
Data are presented as means ± SD or medians (interquartile range).
| . | Continual uFSH (group A, n = 33) . | Sequential uFSH (group B, n = 34) . | P value . |
|---|---|---|---|
| Age, y | 23.1 ± 5.1 | 24.1 ± 4.9 | .413 |
| Height, cm | 172.1 ± 7.1 | 172.6 ± 6.4 | .730 |
| Body mass index, kg/m2 | 21.3 ± 4.5 | 22.2 ± 6.6 | .537 |
| Penis length, cm | 3.9 ± 1.4 | 4.3 ± 1.0 | .193 |
| Testicular volume, ml | 1.7 (0.9–3.4) | 1.5 (1.0–3.0) | .783 |
| Pubic hair stage | 1.9 ± 0.9 | 2.1 ± 1.0 | .302 |
| Genital Tanner stage | 1.6 ± 0.9 | 1.7 ± 0.7 | .401 |
| Plasma LH, IU/L | 0.3 (0.1–0.7) | 0.3 (0.1–0.8) | .580 |
| Plasma FSH, IU/L | 0.8 (0.4–1.6) | 1.0 (0.5–1.4) | .624 |
| Plasma testosterone, ng/dL | 40 (30–50) | 40 (30–50) | .841 |
| . | Continual uFSH (group A, n = 33) . | Sequential uFSH (group B, n = 34) . | P value . |
|---|---|---|---|
| Age, y | 23.1 ± 5.1 | 24.1 ± 4.9 | .413 |
| Height, cm | 172.1 ± 7.1 | 172.6 ± 6.4 | .730 |
| Body mass index, kg/m2 | 21.3 ± 4.5 | 22.2 ± 6.6 | .537 |
| Penis length, cm | 3.9 ± 1.4 | 4.3 ± 1.0 | .193 |
| Testicular volume, ml | 1.7 (0.9–3.4) | 1.5 (1.0–3.0) | .783 |
| Pubic hair stage | 1.9 ± 0.9 | 2.1 ± 1.0 | .302 |
| Genital Tanner stage | 1.6 ± 0.9 | 1.7 ± 0.7 | .401 |
| Plasma LH, IU/L | 0.3 (0.1–0.7) | 0.3 (0.1–0.8) | .580 |
| Plasma FSH, IU/L | 0.8 (0.4–1.6) | 1.0 (0.5–1.4) | .624 |
| Plasma testosterone, ng/dL | 40 (30–50) | 40 (30–50) | .841 |
Data are presented as means ± SD or medians (interquartile range).
After 6 months of hCG monotherapy, the clinical characteristics and hormone concentrations are shown in Supplemental Table 1 and Figure 3. Serum testosterone concentrations were markedly increased in the first 3 months in both groups (Supplemental Table 1 and Figure 4B). The testis volumes and penis lengths were significantly increased at 6 months in both groups. However, there were no significant differences in those secondary characteristics between group A and group B at the sixth month (P > .05).
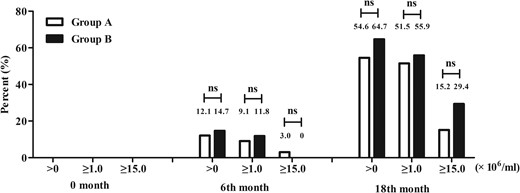
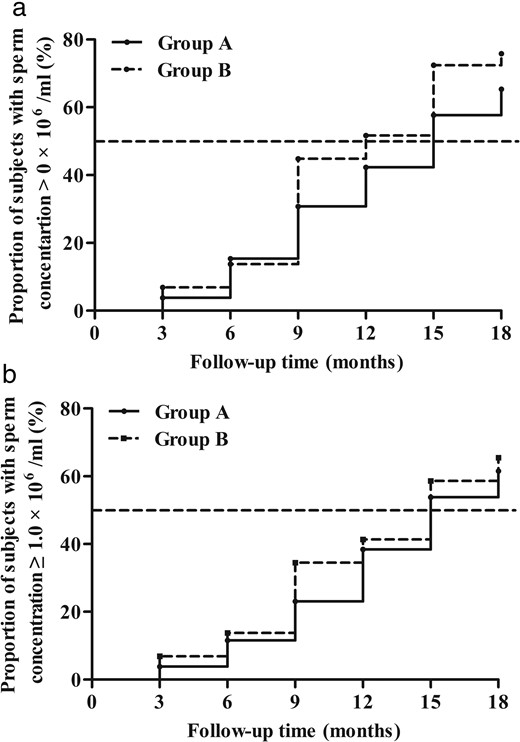
Proportion of subjects with sperm concentrations of >0, ≥1.0, and ≥15.0 × 106/mL by ITT analysis at 0 month, 6th month, and 18th month.
0 month, the patients had no treatment; 6th month, the patients had received 6 months of hCG monotherapy; 18th month, the patients had received 6 month of hCG monotherapy and an additional 12 month of hCG and uFSH combination therapy. ns, not significant
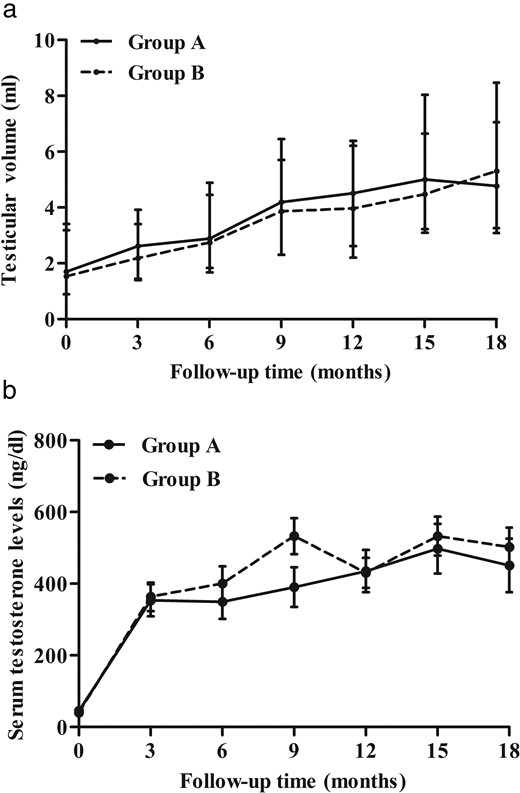
The changes in testicular volume and serum testosterone concentration throughout the period of the 18-month follow-up.
Single testicular volume (A) and serum testosterone concentration (B) in the continual uFSH group and the sequential uFSH group through the 18-month treatment period. The data are presented as means ± SD.
After 6 months of hCG monotherapy, 4 of 33 (12.1%) patients in group A and 5 of 34(14.7%) patients in group B had spermatogenesis (sperm density of >0 × 106/mL). Three of 33 (9.1%) patients in group A and 4 of 34 (11.8%) patients in group B achieved a sperm density of ≥1.0 × 106/mL. However, there was no significant difference in spermatogenesis between the 2 groups (see Figure 3).
Primary outcome
After an additional 12 months of combination treatment, a sperm density of ≥1.0 × 106/mL was achieved in 17 of 33 (51.5%; 95% CI, 33.5%–69.2%) patients in group A who received continual uFSH injection and in 19 of 34 (55.9%; 95% CI, 37.9%–72.8%) patients in group B who received sequential uFSH injection by ITT analysis (P = .008 for noninferiority) (Figures 2 and 3). A sperm density of ≥1.0 × 106/mL was achieved in 16 of 26 (61.5%; 95% CI, 40.6%–79.8%) patients in group A and 19 of 29 (65.5%; 95% CI, 45.7%–82.1%) patients in group B by PP principal analysis (P = .013 for noninferiority) (Figure 2).
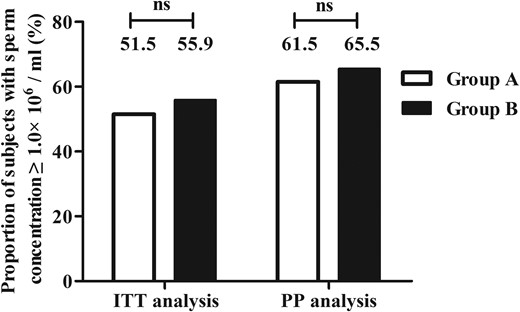
Proportion of subjects with a sperm concentration of ≥1.0 × 106/mL by ITT and PP analysis after the 18-month treatment.
n.s., not significant.
Testis volume and penis length
Testis volumes and penis lengths were not significantly different between group A and group B at the 18th month (Supplemental Table 1). The median single testicular volume was increased from 1.7 to 4.8 mL in group A and from 1.5 to 5.3 mL in group B at the 18th month (Supplemental Table 1 and Figure 4A). The median penis length increased from 3.9 to 6.7 cm in group A and from 4.3 to 6.9 cm in group B (P = .912; Supplemental Table 1).
Serum hormone concentrations
Serum testosterone concentrations were not significantly different between group A and group B at 18 months (P = .288; Supplemental Table 1). The Tanner stage for pubic hair was increased from 1.9 at baseline to 4.3 at 18 months in group A and from 2.1 at baseline to 4.6 at 18 months in group B (P = .963; Supplemental Table 1). The genital Tanner stage was increased from 1.6 to 4.3 in group A and from 1.7 to 4.3 in group B (P = .626; Supplemental Table 1). In addition, obvious beard growth and voice changes were observed in all subjects in both groups.
Sperm count and motility
Eighteen of 33 (54.6%; 95% CI, 36.4%–71.9%) patients in group A and 22 of 34 (64.7%; 95% CI, 46.5%–80.3%) patients in group B had spermatogenesis during the 18-month treatment by ITT analysis (P = .397) (Figure 3). Median sperm concentrations were 1.1 × 106/mL in group A and 1.5 × 106/mL in group B, which was not significantly different (P = .317, Supplemental Table 1). Five of 33 (15.2%) patients in group A and 10 of 34 (29.4%) patients in group B achieved sperm concentrations of >15 × 106/mL (P = .162, Figure 3). Median sperm activity was 32.8% in group A and 44.7% in group B (P = .745, Supplemental Table 1).
Timing of spermatogenesis
Kaplan-Meier survival analysis showed that the median time for initial spermatogenesis was 9 months in group A and 6 months in group B after hCG and uFSH combination treatment (Figure 5A). The median time to achieve the primary outcome was 9 months in both groups after hCG and uFSH combination treatment (Figure 5B). There were no significant differences between the 2 groups in the timing of initial spermatogenesis and achievement of the primary outcome.
Kaplan-Meier survival analysis for the median times of spermatogenesis (A) and achievement of a sperm concentration of ≥1.0 × 106 /mL (B).
The median times of spermatogenesis and achievement of the primary outcome were 15 months in the continual uFSH group. The median time of spermatogenesis was 12 months and of achievement of the primary outcome was 15 months in the sequential uFSH group. There were no significant differences between these 2 groups in the median times of spermatogenesis and achievement of the primary outcome (P > .05).
Economic effectiveness
The estimated medication cost was $4,800 ($175 for hCG and $4,625 for uFSH) for group A and $2,490 ($175 for hCG and $2,315 for uFSH) for group B for the 18-month treatment. Compared with the continual uFSH regimen in group A, the sequential uFSH regimen in group B reduced cost by $2,310 for each patient to achieve the noninferior efficacy in spermatogenesis, which could be a tremendous saving for ordinary families of patients with IHH in China.
Discussion
In the American Association of Clinical Endocrinology guidelines, hCG combined with FSH preparations is described as an effective regimen in inducing spermatogenesis in male patients with IHH (12). However, the frequent injections and costly medication result in poor compliance. In our present study, the intermittent use of uFSH every other 3 months produced noninferior efficacy as with the continual use of uFSH in inducing spermatogenesis. The modified regimen was meaningful in reducing medication cost.
The efficacy of continual use of uFSH in achieving a sperm concentration of ≥1.0 × 106/mL was 51.5%, which is lower than that in other studies (17, 21–24). This may be attributed to the shorter term (1 year), fewer courses, severe IHH, a fixed dose (75 U), and lack of pretreatment with an FSH preparation in our present study, whereas the uFSH dosage could be increased from 75 to 150 U according to the response (21–24) and 1 or more courses of pretreatment with hMG or FSH had been received in patients with IHH in other studies (20, 25). Furthermore, the extracted urine FSH was not as pure as the recombinant FSH used in other studies (22, 24, 26, 27). It was reported that biological efficacy of 150 U of recombinant human (rh) FSH might be comparable with 225 U of uFSH (50 U of rhFSH vs 75 U of uFSH) (28). Although hMG and uFSH preparations have been replaced by rhFSH in many countries, hMG and uFSH are still mostly used in patients with IHH in China because of its low cost.
Liu et al (25) reported that the median sperm concentrations for unassisted pregnancies in infertile Chinese men were >8.0 million/mL. In our study, we found that 8 of 33 (24.2%) patients in the continual uFSH group and 13 of 34 (38.2%) patients in the sequential uFSH group achieved this outcome by ITT analysis, which was not significantly different between those 2 groups (P = .217).
As reported by Ogawa and colleagues (29), the intermittent use of FSH-containing preparations was also assessed in patients with HH with GH deficiency. Their regimen included 3000 U of hCG 2 or 3 times a week for the first 6 months and then addition of 75 U of hMG for 3 months and was repeated until the patients were married. All 4 patients achieved spermatogenesis at a concentration of 24 to 100 × 106/mL in several years by intermittent use of HMG. Another study showed that spermatogenesis induced by treatment with GnRH or hCG/FSH could be maintained for 3 to 24 months by hCG treatment alone in patients with HH (7, 13). It was also reported that spermatogenesis could be maintained longer by using hCG alone in hypophysectomized patients (14). Those findings indicate that Sertoli cells in the testis could maintain function even while receiving intermittent stimulation of FSH. As expected, testicular volumes, penis length, and pubic hair were progressively increased during the period of treatment. The serum testosterone concentration was abruptly increased by use of hCG alone. The testicular volumes were measured by ultrasonography and calculated by the formula of length × width × depth × 0.71 in our present study. The baseline median single testicular volumes were 1.7 and 1.5 mL in groups A and B, which indicates that the subjects had severe IHH. Sakamoto et al (18) proposed that the measurement of testicular volume by ultrasonography was more accurate than that by a Prader orchidometer. However, we also measured testicular volume using a Prader orchidometer. The average single testicular volumes were 3.0 mL in both groups at baseline and 13.0 mL in group A and 12.0 mL in group B after 18 months of treatment by Prader orchidometer. The median motile sperms (grade A, B, and C) were 32.8% in group A and 44.7% in group B, which is similar with that in other studies (17, 22).
The median time to induce spermatogenesis was 15 months in group A and 12 months in group B since initiation of the treatment. The median time for achieving primary outcomes was 15 months, which is consistent with those in other studies (10, 24, 27, 29). The intermittent use of FSH has a trend for an earlier induction of spermatogenesis although there is no significant difference between the 2 groups.
The ability to impregnate condition was recorded in 3.0% (1 of 33) patients in the continual uFSH treatment group and in 14.7% (5 of 34) patients in the sequential uFSH treatment group 6 months after the end of the trial. Most patients (80%) in our study had no desire for an immediate pregnancy since they were not yet married during this study.
The cost of gonadotropins and frequent injections are the 2 major obstacles for patients with IHH to receive this medication. Our data showed that the sequential FSH regimen could reduce the cost by almost half and reduce injection times. However, improvement in compliance was not assessed in this randomized controlled trial. It should be noted that the rhFSH and hCG could be administered subcutaneously, which can definitely improve the compliance of the patients.
Our current study has several limitations: (1) an open-label rather than blind trial was performed because of the injection preparations; (2) the uFSH dosage could not be adjusted according to the spermatogenesis response; (3) those patients who had a history of cryptorchidism and a poor response to hCG (serum testosterone peak of <150 ng/dL by hCG stimulation) were excluded; and (4) a sperm concentration of 1.0 million/mL was used as the endpoint, which might be weakly compatible for unassisted pregnancies.
In conclusion, our results indicate that sequential use of FSH preparations every other 3 months is effective in inducing spermatogenesis in IHH patients. This approach would greatly reduce medication costs for patients with IHH.
Acknowledgments
We are indebted to all the patients who participated in this study.
This work was supported by the Shanghai Shenkang Hospital Development Center (Grant SHDC12012102) and the Shanghai Science and Technology Committee (Grant 09DJ1400402).
This trial is registered at clinicaltrials.gov (registration number NCT01403532).
Disclosure Summary: The authors have nothing to disclose.
M.Z., G.T., and Y.L. contributed equally to the study.
Abbreviations
- CI
confidence interval
- hCG
human chorionic gonadotropin
- HH
hypogonadotropic hypogonadism
- hMG
human menopausal gonadotropin
- IHH
idiopathic hypogonadotropic hypogonadism
- ITT
intention to treat
- PP
per-protocol
- rh
recombinant human
- uFSH
urinary FSH.



