-
PDF
- Split View
-
Views
-
Cite
Cite
Rodis D Paparodis, Dimitra Bantouna, Evangelos Karvounis, Shahnawaz Imam, Juan Carlos Jaume, Higher TSH Is Not Associated With Thyroid Cancer Risk in the Presence of Thyroid Autoimmunity, The Journal of Clinical Endocrinology & Metabolism, Volume 105, Issue 7, July 2020, Pages e2389–e2397, https://doi.org/10.1210/clinem/dgaa237
Close - Share Icon Share
Abstract
Higher-but-within-normal thyrotropin (thyroid-stimulating hormone, TSH) is associated with higher risk for differentiated thyroid cancer (DTC) in surgical series. Our recent clinical observations suggest that this is not the case in the presence of autoimmune thyroid disease (AITD). We designed the present study to clarify this controversy.
We analyzed our prospectively collected database of patients referred for thyroid surgery at 2 tertiary care referral centers in Greece and the United States. We collected data for preoperative TSH, postoperative pathology, and thyroid peroxidase (TPO) antibodies titers. Subjects were subdivided into 2 groups, those with AITD (i.e., lymphocytic thyroiditis) and non-AITD. We excluded subjects with Graves disease, abnormal TSH (< 0.40 or > 4.50 mIU/mL), or recent use of levothyroxine. We compared the serum TSH among different groups using the Mann-Whitney test.
A total of 3973 subjects were screened; 1357 met exclusion criteria. After all exclusions, data from 1731 non-AITD subjects and 329 AITD subjects were included in the analysis. AITD subjects had higher TSH than non-AITD subjects (2.09 vs 1.48; P < 0.0001). TSH values were higher in DTC compared with benign histology only in non-AITD subjects (1.65 vs 1.40; P < 0.0001). Progressively higher TSH was associated with higher incidence of DTC only in non-AITD subjects (P < 0.0001). In AITD subjects, TSH was similar between groups with or without DTC (2.02 vs 2.14; P = 0.21).
TSH concentrations are not associated with the risk of developing DTC in the presence of thyroid autoimmunity, even though this seems to be the case for all other patients.
Differentiated thyroid cancer (DTC) consists of papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC). The overall incidence of DTC has been rising over the past many years, at a rate of approximately 3% per year, mostly due to a rise in the incidence of PTC (1). This has been attributed in part to the concurrent rise in the use of point-of-care ultrasound imaging of the thyroid gland (2). In order to understand better this rising incidence, researchers evaluated the role of potential risk factors. In multiple studies, a positive role was attributed to serum thyrotropin (thyroid-stimulating hormone, TSH) concentration, which was found to be associated with the risk for DTC when it is elevated, or even within the higher end of the reference range (3-13). On the other hand, patients with autoimmune thyroid disease (AITD, chronic lymphocytic thyroiditis) are frequently found to present with elevated TSH concentrations due to partial or complete destruction of the thyroid gland from the autoimmune response (14). These patients are found to harbor DTC in the thyroidectomy surgical specimen more frequently than expected (15, 16), even though development of DTC has rarely been described in prospective observational studies of patients with full-blown Hashimoto’s disease (17).
Despite the presence of abundant data on the role of increased serum TSH and thyroid cancer, recent conflicting clinical and research data argue against this hypothesis in patients with Hashimoto thyroiditis. In order to evaluate whether higher-but-within-normal serum TSH concentrations affect the risk for DTC in the presence or absence of AITD, we conducted the present study.
Materials and Methods
Our Thyroid Multidisciplinary Clinics are located in 2 tertiary referral centers: 1 in Greece and the other one in the United States. In these clinics, patients from throughout the regions are referred for evaluation and management of nodular thyroid disease. All patients are registered in our prospectively collected databases, which contain clinical, laboratory, imaging, cytological, and pathological data. For the present study, we collected data from patients cared for at our clinics for 10 consecutive years, who had undergone total thyroidectomy for clinical indications (Table 1). This analysis includes the subject age, gender, preoperative serum TSH, thyroid peroxidase (TPO) antibodies titers (when available), and surgical pathology report from the thyroidectomy specimen. Blood samples were collected in the morning, after an overnight fast. The hormone assays were performed with chemiluminescent immunoassays, at the clinical laboratories of both institutions. All surgical pathology reports were generated by academic pathologists and reviewed for this study. The collection of patient data and subsequent analysis was approved by Human Subjects Institutional Review Boards. Consent was obtained from each subject after full explanation of the purpose and nature of all procedures used.
| Indication . | Overall . | AITD . | Non-AITD . |
|---|---|---|---|
| MNG | 867 | 59 | 808 |
| Thyroid cancer | 447 | 84 | 363 |
| Compression | 182 | 62 | 120 |
| Radiation history | 6 | 3 | 3 |
| Large/growing | 142 | 46 | 96 |
| Genetic | 11 | 2 | 9 |
| Atypia | 71 | 23 | 48 |
| FN/HCN | 286 | 60 | 226 |
| Thyroglossal duct | 2 | 0 | 2 |
| Lymphoma | 4 | 2 | 2 |
| Patient selection | 5 | 2 | 3 |
| Other | 78 | 2 | 76 |
| Unclear | 7 | 2 | 5 |
| Indication . | Overall . | AITD . | Non-AITD . |
|---|---|---|---|
| MNG | 867 | 59 | 808 |
| Thyroid cancer | 447 | 84 | 363 |
| Compression | 182 | 62 | 120 |
| Radiation history | 6 | 3 | 3 |
| Large/growing | 142 | 46 | 96 |
| Genetic | 11 | 2 | 9 |
| Atypia | 71 | 23 | 48 |
| FN/HCN | 286 | 60 | 226 |
| Thyroglossal duct | 2 | 0 | 2 |
| Lymphoma | 4 | 2 | 2 |
| Patient selection | 5 | 2 | 3 |
| Other | 78 | 2 | 76 |
| Unclear | 7 | 2 | 5 |
Note: The sum of all patients entered in this table is larger than the overall number of patients included in our study, since each patient could fulfill multiple surgical criteria simultaneously.
Abbreviations/definitions: AITD, autoimmune thyroid disease; Atypia, patients with a history of FNA cytology consistent with atypia of undetermined significance or other atypical features; Compression, the presence of long-standing, significant compressive symptoms of the neck, such as dysphagia, vocal cord paralysis, pressure, or neck pain; FN/HCN, patients with FNA cytology consistent with follicular neoplasm/Hurthle cell neoplasm or suspicion for any of the above; Genetic: patients carrying mutations known to cause specific histological types of thyroid cancer, or patients operated for thyroid nodules, in the presence of strong family history of thyroid cancer; Large/growing, patients with thyroid nodules > 4 cm in largest diameter or nodules growing over time; Lymphoma, patients operated with a total thyroidectomy for primary thyroid lymphoma; MNG, multinodular goiter; Other, patients operated in the thyroid for various reasons (e.g., abscess, intrathyroidal parathyroids, cancers metastatic to the thyroid, operation for tracheostomy); Patient selection, patients operated with a total thyroidectomy for nodular disease, not fulfilling other established criteria, who were operated due to their choice; Radiation history, patients with thyroid nodules and history of exposure to ionizing radiation who opted directly for surgery; Thyroglossal duct, patients undergoing total thyroidectomy, for the main reason of the presence of a large thyroglossal duct cyst; Thyroid cancer, any fine needle aspiration cytological report consistent with a specimen positive or suspicious for any type of thyroid cancer.
| Indication . | Overall . | AITD . | Non-AITD . |
|---|---|---|---|
| MNG | 867 | 59 | 808 |
| Thyroid cancer | 447 | 84 | 363 |
| Compression | 182 | 62 | 120 |
| Radiation history | 6 | 3 | 3 |
| Large/growing | 142 | 46 | 96 |
| Genetic | 11 | 2 | 9 |
| Atypia | 71 | 23 | 48 |
| FN/HCN | 286 | 60 | 226 |
| Thyroglossal duct | 2 | 0 | 2 |
| Lymphoma | 4 | 2 | 2 |
| Patient selection | 5 | 2 | 3 |
| Other | 78 | 2 | 76 |
| Unclear | 7 | 2 | 5 |
| Indication . | Overall . | AITD . | Non-AITD . |
|---|---|---|---|
| MNG | 867 | 59 | 808 |
| Thyroid cancer | 447 | 84 | 363 |
| Compression | 182 | 62 | 120 |
| Radiation history | 6 | 3 | 3 |
| Large/growing | 142 | 46 | 96 |
| Genetic | 11 | 2 | 9 |
| Atypia | 71 | 23 | 48 |
| FN/HCN | 286 | 60 | 226 |
| Thyroglossal duct | 2 | 0 | 2 |
| Lymphoma | 4 | 2 | 2 |
| Patient selection | 5 | 2 | 3 |
| Other | 78 | 2 | 76 |
| Unclear | 7 | 2 | 5 |
Note: The sum of all patients entered in this table is larger than the overall number of patients included in our study, since each patient could fulfill multiple surgical criteria simultaneously.
Abbreviations/definitions: AITD, autoimmune thyroid disease; Atypia, patients with a history of FNA cytology consistent with atypia of undetermined significance or other atypical features; Compression, the presence of long-standing, significant compressive symptoms of the neck, such as dysphagia, vocal cord paralysis, pressure, or neck pain; FN/HCN, patients with FNA cytology consistent with follicular neoplasm/Hurthle cell neoplasm or suspicion for any of the above; Genetic: patients carrying mutations known to cause specific histological types of thyroid cancer, or patients operated for thyroid nodules, in the presence of strong family history of thyroid cancer; Large/growing, patients with thyroid nodules > 4 cm in largest diameter or nodules growing over time; Lymphoma, patients operated with a total thyroidectomy for primary thyroid lymphoma; MNG, multinodular goiter; Other, patients operated in the thyroid for various reasons (e.g., abscess, intrathyroidal parathyroids, cancers metastatic to the thyroid, operation for tracheostomy); Patient selection, patients operated with a total thyroidectomy for nodular disease, not fulfilling other established criteria, who were operated due to their choice; Radiation history, patients with thyroid nodules and history of exposure to ionizing radiation who opted directly for surgery; Thyroglossal duct, patients undergoing total thyroidectomy, for the main reason of the presence of a large thyroglossal duct cyst; Thyroid cancer, any fine needle aspiration cytological report consistent with a specimen positive or suspicious for any type of thyroid cancer.
Subjects
Based on surgical pathology, our subjects were subdivided into 2 groups: those found to harbor bilateral lymphocytic infiltrates in the surgical specimen consistent with chronic lymphocytic thyroiditis, who were designated the autoimmune thyroid disease group (AITD); and those without evidence of autoimmunity, who were designated the non-AITD group and served as our control group. Subjects with postoperative diagnosis of AITD, not on levothyroxine (see exclusion criteria below), were further subdivided in those with TPO antibody titers > 100 IU/mL (TPO+) and those with TPO antibody titers from 0 to < 100 IU/mL (TPO−). In addition, we subdivided our subjects based on TSH concentration quintiles (Q1-Q5). These values were different in each subgroup and in the entire population. The quintiles in each subgroup were of equal number. A secondary analysis was performed dividing the subjects in a unified scale of quintiles (all subjects combined, subdivided in 5 groups based on serum TSH).
Exclusion criteria
We excluded subjects with Graves disease (by history or surgical pathology), those with other forms of thyroid disease not pertaining to the current analysis (e.g., De Quervain’s thyroiditis), those with a preoperative abnormal TSH concentration (< 0.40 or > 4.50 IU/L), those who received any medications, which could affect the concentrations of thyroid hormones during the last 3 months prior to the study enrollment and those with incomplete data.
Statistical analysis
The statistical analysis was performed using the GraphPad Prism v.6.1 software (GraphPad Software, San Diego, CA). We compared noncategorical variables using the Mann-Whitney test and calculated the means and standard deviations. We compared categorical variables using the Fisher’s exact test and we calculated the odds ratios (OR) and 95% confidence intervals (95% CI). P values < 0.05 were deemed significant.
Results
A total of 3973 subjects were screened for study eligibility. Out of these, 1357 subjects met exclusion criteria, consisting of 563 subjects with known hypothyroidism on levothyroxine replacement therapy, 205 subjects with Graves disease, and 384 subjects with abnormal TSH concentration at baseline (< 0.40 or > 4.50 IU/L). An additional 78 subjects were excluded due to incomplete pathology data.
A total of 2060 subjects were included in our analysis, consisting of 1731 non-AITD subjects and 329 AITD subjects. Of the latter, TPO antibody titers were available in 118 subjects: 73 subjects were TPO− and 45 subjects were TPO+. The age, gender, and serum TSH concentrations of the subject groups and group comparisons are presented in Table 2. Subjects with pathologically identified AITD have higher serum TSH concentrations; the same holds true for those subjects with higher serum TPO antibody titers. The incidence of DTC in the different subject groups and their comparisons are also presented in Table 2. Subjects with AITD had higher risk for DTC compared with their non-AITD counterparts. Serum TSH concentrations were compared among subjects from different groups; the results are presented in Table 3, revealing a statistically significantly higher TSH in subjects with DTC in the non-AITD, but not in those comprising the AITD group. The quintiles of serum TSH concentration and the incidence of DTC in each subgroup, as well as their comparisons are presented in Table 4a (Table 4b, excluding patients with thyroid microcarcinomas) and depicted in Fig. 1a (Fig. 1b, excluding patients with thyroid microcarcinomas), revealing a strong, statistically significant linear association between the risk of DTC and TSH in subjects without AITD, compared with an insignificant inverse association in the AITD subjects.
| . | n . | Gender Female: n (%) Male: n (%) . | Age (years) Mean ± SD . | TSH (IU/mL) Mean ± SD . | Benign n (%) . | DTC n (%) . |
|---|---|---|---|---|---|---|
| Non-AITD | 1731 | 1300 (75.1%) 431 (24.9%) | 49.8 ± 16.0 | 1.48 ± 0.81 | 1205 (69.6%) | 576 (30.4%) |
| AITD | 329 | 280 (85.1%) 49 (14.9%) | 45.6 ± 15.9 | 2.09 ± 0.98 | 176 (53.5%) | 153 (46.5%) |
| OR (95% CI) P value | n/a | 1.89 (1.37-2.62) 0.0001 | P < 0.0001 | P < 0.0001 | 0.55 (0.43-0.70) < 0.0001 | |
| TPO+ | 103 | 52/2 | 42.5 ± 14.6 | 2.44 ± 0.99 | 30 (55.6%) | 24 (44.4%) |
| TPO− | 54 | 88/15 | 45.1 ± 15.3 | 1.87 ± 0.91 | 48 (46.6%) | 55 (53.4%) |
| OR (95% CI) P value | n/a | 4.43 (0.97-20.16) 0.056 | P = 0.29 | P = 0.0007 | 0.70 (0.36-1.35) 0.32 |
| . | n . | Gender Female: n (%) Male: n (%) . | Age (years) Mean ± SD . | TSH (IU/mL) Mean ± SD . | Benign n (%) . | DTC n (%) . |
|---|---|---|---|---|---|---|
| Non-AITD | 1731 | 1300 (75.1%) 431 (24.9%) | 49.8 ± 16.0 | 1.48 ± 0.81 | 1205 (69.6%) | 576 (30.4%) |
| AITD | 329 | 280 (85.1%) 49 (14.9%) | 45.6 ± 15.9 | 2.09 ± 0.98 | 176 (53.5%) | 153 (46.5%) |
| OR (95% CI) P value | n/a | 1.89 (1.37-2.62) 0.0001 | P < 0.0001 | P < 0.0001 | 0.55 (0.43-0.70) < 0.0001 | |
| TPO+ | 103 | 52/2 | 42.5 ± 14.6 | 2.44 ± 0.99 | 30 (55.6%) | 24 (44.4%) |
| TPO− | 54 | 88/15 | 45.1 ± 15.3 | 1.87 ± 0.91 | 48 (46.6%) | 55 (53.4%) |
| OR (95% CI) P value | n/a | 4.43 (0.97-20.16) 0.056 | P = 0.29 | P = 0.0007 | 0.70 (0.36-1.35) 0.32 |
Abbreviations: AITD, autoimmune thyroid disease (see text); DTC, differentiated thyroid cancer; OR, odds ratio; TPO+, thyroid peroxidase antibodies titers > 100 IU/ml; TPO−, thyroid peroxidase antibodies titers < 100 IU/ml; SD, standard deviation.
| . | n . | Gender Female: n (%) Male: n (%) . | Age (years) Mean ± SD . | TSH (IU/mL) Mean ± SD . | Benign n (%) . | DTC n (%) . |
|---|---|---|---|---|---|---|
| Non-AITD | 1731 | 1300 (75.1%) 431 (24.9%) | 49.8 ± 16.0 | 1.48 ± 0.81 | 1205 (69.6%) | 576 (30.4%) |
| AITD | 329 | 280 (85.1%) 49 (14.9%) | 45.6 ± 15.9 | 2.09 ± 0.98 | 176 (53.5%) | 153 (46.5%) |
| OR (95% CI) P value | n/a | 1.89 (1.37-2.62) 0.0001 | P < 0.0001 | P < 0.0001 | 0.55 (0.43-0.70) < 0.0001 | |
| TPO+ | 103 | 52/2 | 42.5 ± 14.6 | 2.44 ± 0.99 | 30 (55.6%) | 24 (44.4%) |
| TPO− | 54 | 88/15 | 45.1 ± 15.3 | 1.87 ± 0.91 | 48 (46.6%) | 55 (53.4%) |
| OR (95% CI) P value | n/a | 4.43 (0.97-20.16) 0.056 | P = 0.29 | P = 0.0007 | 0.70 (0.36-1.35) 0.32 |
| . | n . | Gender Female: n (%) Male: n (%) . | Age (years) Mean ± SD . | TSH (IU/mL) Mean ± SD . | Benign n (%) . | DTC n (%) . |
|---|---|---|---|---|---|---|
| Non-AITD | 1731 | 1300 (75.1%) 431 (24.9%) | 49.8 ± 16.0 | 1.48 ± 0.81 | 1205 (69.6%) | 576 (30.4%) |
| AITD | 329 | 280 (85.1%) 49 (14.9%) | 45.6 ± 15.9 | 2.09 ± 0.98 | 176 (53.5%) | 153 (46.5%) |
| OR (95% CI) P value | n/a | 1.89 (1.37-2.62) 0.0001 | P < 0.0001 | P < 0.0001 | 0.55 (0.43-0.70) < 0.0001 | |
| TPO+ | 103 | 52/2 | 42.5 ± 14.6 | 2.44 ± 0.99 | 30 (55.6%) | 24 (44.4%) |
| TPO− | 54 | 88/15 | 45.1 ± 15.3 | 1.87 ± 0.91 | 48 (46.6%) | 55 (53.4%) |
| OR (95% CI) P value | n/a | 4.43 (0.97-20.16) 0.056 | P = 0.29 | P = 0.0007 | 0.70 (0.36-1.35) 0.32 |
Abbreviations: AITD, autoimmune thyroid disease (see text); DTC, differentiated thyroid cancer; OR, odds ratio; TPO+, thyroid peroxidase antibodies titers > 100 IU/ml; TPO−, thyroid peroxidase antibodies titers < 100 IU/ml; SD, standard deviation.
TSH Concentrations Comparisons Between Subjects With Benign Histology and Those With DTC
| . | Benign . | DTC . | P value . | DTMc . | P value . |
|---|---|---|---|---|---|
| Non-AITD | 1.40 ± 0.79 | 1.65 ± 0.84 | < 0.0001 | 1.72 ± 0.85 | < 0.0001 |
| AITD | 2.14 ± 1.00 | 2.02 ± 0.98 | 0.21 | 1.94 ± 0.97 | 0.09 |
| P value | < 0.0001 | < 0.0001 | . | < 0.0001 | . |
| TPO+ | 0.92 ± 0.18 | 0.98 ± 0.14 | 0.28 | . | . |
| TPO− | 0.93 ± 0.13 | 0.98 ± 0.15 | 0.12 | . | . |
| P value | 0.86 | 0.91 | . | . | . |
| . | Benign . | DTC . | P value . | DTMc . | P value . |
|---|---|---|---|---|---|
| Non-AITD | 1.40 ± 0.79 | 1.65 ± 0.84 | < 0.0001 | 1.72 ± 0.85 | < 0.0001 |
| AITD | 2.14 ± 1.00 | 2.02 ± 0.98 | 0.21 | 1.94 ± 0.97 | 0.09 |
| P value | < 0.0001 | < 0.0001 | . | < 0.0001 | . |
| TPO+ | 0.92 ± 0.18 | 0.98 ± 0.14 | 0.28 | . | . |
| TPO− | 0.93 ± 0.13 | 0.98 ± 0.15 | 0.12 | . | . |
| P value | 0.86 | 0.91 | . | . | . |
Abbreviations: AITD, autoimmune thyroid disease (see text); DTC, differentiated thyroid cancer; DTMc, differentiated thyroid cancer > 1 cm in largest diameter (macrocarcinoma);TPO+, thyroid peroxidase antibodies titers > 100 IU/ml; TPO−, thyroid peroxidase antibodies titers < 100 IU/ml.
TSH Concentrations Comparisons Between Subjects With Benign Histology and Those With DTC
| . | Benign . | DTC . | P value . | DTMc . | P value . |
|---|---|---|---|---|---|
| Non-AITD | 1.40 ± 0.79 | 1.65 ± 0.84 | < 0.0001 | 1.72 ± 0.85 | < 0.0001 |
| AITD | 2.14 ± 1.00 | 2.02 ± 0.98 | 0.21 | 1.94 ± 0.97 | 0.09 |
| P value | < 0.0001 | < 0.0001 | . | < 0.0001 | . |
| TPO+ | 0.92 ± 0.18 | 0.98 ± 0.14 | 0.28 | . | . |
| TPO− | 0.93 ± 0.13 | 0.98 ± 0.15 | 0.12 | . | . |
| P value | 0.86 | 0.91 | . | . | . |
| . | Benign . | DTC . | P value . | DTMc . | P value . |
|---|---|---|---|---|---|
| Non-AITD | 1.40 ± 0.79 | 1.65 ± 0.84 | < 0.0001 | 1.72 ± 0.85 | < 0.0001 |
| AITD | 2.14 ± 1.00 | 2.02 ± 0.98 | 0.21 | 1.94 ± 0.97 | 0.09 |
| P value | < 0.0001 | < 0.0001 | . | < 0.0001 | . |
| TPO+ | 0.92 ± 0.18 | 0.98 ± 0.14 | 0.28 | . | . |
| TPO− | 0.93 ± 0.13 | 0.98 ± 0.15 | 0.12 | . | . |
| P value | 0.86 | 0.91 | . | . | . |
Abbreviations: AITD, autoimmune thyroid disease (see text); DTC, differentiated thyroid cancer; DTMc, differentiated thyroid cancer > 1 cm in largest diameter (macrocarcinoma);TPO+, thyroid peroxidase antibodies titers > 100 IU/ml; TPO−, thyroid peroxidase antibodies titers < 100 IU/ml.
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.91 (0.41-1.24) | 1.46 (1.25-1.68) | 1.98 (1.68-2.31) | 2.68 (2.34-3.03) | 3.65 (3.07-4.45) | 0.57 (0.40-0.73) | 0.98 (0.73-1.11) | 1.29 (1.11-1.48) | 1.73 (1.48-2.02) | 2.73 (2.03-4.48) |
| n | 66 | 66 | 65 | 66 | 66 | 356 | 356 | 357 | 356 | 356 |
| DTC, n (%) | 31 (47.0) | 30 (45.5) | 29 (44.6) | 30 (45.5) | 27 (40.9) | 71 (19.9) | 98 (27.5) | 100 (28.0) | 125 (35.1) | 143 (40.2) |
| BEN, n (%) | 35 (53.0) | 36 (54.5) | 36 (55.4) | 36 (54.5) | 39 (59.1) | 284 (80.1) | 258 (72.5) | 257 (72.0) | 231 (64.9) | 213 (59.8) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c |
| χ 2P value | 0.97 | < 0.0001 | ||||||||
| FTC, n (%DTC) | 0 (0.0) | 1 (3.3) | 3 (10.3) | 1 (3.3) | 1 (3.7) | 3 (4.2) | 3 (3.1) | 6 (6.0) | 10 (8.0) | 9 (6.3) |
| PTC, n (%DTC) | 31 (100) | 29 (96.7) | 26 (89.7) | 29 (96.7) | 26 (96.3) | 68 (95.8) | 96 (96.9) | 94 (94.0) | 115 (92.0) | 134 (93.7) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.36 | 0.57 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.91 (0.41-1.24) | 1.46 (1.25-1.68) | 1.98 (1.68-2.31) | 2.68 (2.34-3.03) | 3.65 (3.07-4.45) | 0.57 (0.40-0.73) | 0.98 (0.73-1.11) | 1.29 (1.11-1.48) | 1.73 (1.48-2.02) | 2.73 (2.03-4.48) |
| n | 66 | 66 | 65 | 66 | 66 | 356 | 356 | 357 | 356 | 356 |
| DTC, n (%) | 31 (47.0) | 30 (45.5) | 29 (44.6) | 30 (45.5) | 27 (40.9) | 71 (19.9) | 98 (27.5) | 100 (28.0) | 125 (35.1) | 143 (40.2) |
| BEN, n (%) | 35 (53.0) | 36 (54.5) | 36 (55.4) | 36 (54.5) | 39 (59.1) | 284 (80.1) | 258 (72.5) | 257 (72.0) | 231 (64.9) | 213 (59.8) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c |
| χ 2P value | 0.97 | < 0.0001 | ||||||||
| FTC, n (%DTC) | 0 (0.0) | 1 (3.3) | 3 (10.3) | 1 (3.3) | 1 (3.7) | 3 (4.2) | 3 (3.1) | 6 (6.0) | 10 (8.0) | 9 (6.3) |
| PTC, n (%DTC) | 31 (100) | 29 (96.7) | 26 (89.7) | 29 (96.7) | 26 (96.3) | 68 (95.8) | 96 (96.9) | 94 (94.0) | 115 (92.0) | 134 (93.7) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.36 | 0.57 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; %DTC, percentage of the total number of DTCs; FTC, Follicular thyroid cancer; PTC, papillary thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3; d Statistically significantly higher risk for DTC compared with Non-AITD Q4.
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.91 (0.41-1.24) | 1.46 (1.25-1.68) | 1.98 (1.68-2.31) | 2.68 (2.34-3.03) | 3.65 (3.07-4.45) | 0.57 (0.40-0.73) | 0.98 (0.73-1.11) | 1.29 (1.11-1.48) | 1.73 (1.48-2.02) | 2.73 (2.03-4.48) |
| n | 66 | 66 | 65 | 66 | 66 | 356 | 356 | 357 | 356 | 356 |
| DTC, n (%) | 31 (47.0) | 30 (45.5) | 29 (44.6) | 30 (45.5) | 27 (40.9) | 71 (19.9) | 98 (27.5) | 100 (28.0) | 125 (35.1) | 143 (40.2) |
| BEN, n (%) | 35 (53.0) | 36 (54.5) | 36 (55.4) | 36 (54.5) | 39 (59.1) | 284 (80.1) | 258 (72.5) | 257 (72.0) | 231 (64.9) | 213 (59.8) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c |
| χ 2P value | 0.97 | < 0.0001 | ||||||||
| FTC, n (%DTC) | 0 (0.0) | 1 (3.3) | 3 (10.3) | 1 (3.3) | 1 (3.7) | 3 (4.2) | 3 (3.1) | 6 (6.0) | 10 (8.0) | 9 (6.3) |
| PTC, n (%DTC) | 31 (100) | 29 (96.7) | 26 (89.7) | 29 (96.7) | 26 (96.3) | 68 (95.8) | 96 (96.9) | 94 (94.0) | 115 (92.0) | 134 (93.7) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.36 | 0.57 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.91 (0.41-1.24) | 1.46 (1.25-1.68) | 1.98 (1.68-2.31) | 2.68 (2.34-3.03) | 3.65 (3.07-4.45) | 0.57 (0.40-0.73) | 0.98 (0.73-1.11) | 1.29 (1.11-1.48) | 1.73 (1.48-2.02) | 2.73 (2.03-4.48) |
| n | 66 | 66 | 65 | 66 | 66 | 356 | 356 | 357 | 356 | 356 |
| DTC, n (%) | 31 (47.0) | 30 (45.5) | 29 (44.6) | 30 (45.5) | 27 (40.9) | 71 (19.9) | 98 (27.5) | 100 (28.0) | 125 (35.1) | 143 (40.2) |
| BEN, n (%) | 35 (53.0) | 36 (54.5) | 36 (55.4) | 36 (54.5) | 39 (59.1) | 284 (80.1) | 258 (72.5) | 257 (72.0) | 231 (64.9) | 213 (59.8) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c |
| χ 2P value | 0.97 | < 0.0001 | ||||||||
| FTC, n (%DTC) | 0 (0.0) | 1 (3.3) | 3 (10.3) | 1 (3.3) | 1 (3.7) | 3 (4.2) | 3 (3.1) | 6 (6.0) | 10 (8.0) | 9 (6.3) |
| PTC, n (%DTC) | 31 (100) | 29 (96.7) | 26 (89.7) | 29 (96.7) | 26 (96.3) | 68 (95.8) | 96 (96.9) | 94 (94.0) | 115 (92.0) | 134 (93.7) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.36 | 0.57 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; %DTC, percentage of the total number of DTCs; FTC, Follicular thyroid cancer; PTC, papillary thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3; d Statistically significantly higher risk for DTC compared with Non-AITD Q4.
TSH Quintiles by Subgroup and Incidence of Differentiated Thyroid Macrocacrinomas (all patients with differentiated thyroid cancer < 1 cm in largest diameter are excluded)
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.88 (0.40-1.21) | 1.44 (1.21-1.67) | 1.94 (1.68-2.30) | 2.63 (2.30-3.02) | 3.59 (3.03-4.45) | 0.58 (0.40-0.74) | 0.93 (0.75-1.13) | 1.32 (1.13-1.53) | 1.80 (1.53-2.13) | 2.79 (2.15-4.48) |
| n | 65 | 64 | 65 | 64 | 65 | 335 | 335 | 335 | 335 | 336 |
| DTC n (%) | 30 (46.2) | 34 (53.1) | 29 (44.6) | 28 (43.8) | 26 (40.0) | 62 (18.5) | 89 (26.6) | 101 (30.1) | 128 (38.2) | 134 (39.9) |
| BEN n (%) | 35 (53.8) | 30 (46.9) | 36 (55.4) | 36 (56.2) | 39 (60.0) | 273 (81.5) | 249 (73.4) | 234 (69.9) | 207 (61.8) | 202 (60.1) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c, d |
| χ 2P value | 0.66 | < 0.0001 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.88 (0.40-1.21) | 1.44 (1.21-1.67) | 1.94 (1.68-2.30) | 2.63 (2.30-3.02) | 3.59 (3.03-4.45) | 0.58 (0.40-0.74) | 0.93 (0.75-1.13) | 1.32 (1.13-1.53) | 1.80 (1.53-2.13) | 2.79 (2.15-4.48) |
| n | 65 | 64 | 65 | 64 | 65 | 335 | 335 | 335 | 335 | 336 |
| DTC n (%) | 30 (46.2) | 34 (53.1) | 29 (44.6) | 28 (43.8) | 26 (40.0) | 62 (18.5) | 89 (26.6) | 101 (30.1) | 128 (38.2) | 134 (39.9) |
| BEN n (%) | 35 (53.8) | 30 (46.9) | 36 (55.4) | 36 (56.2) | 39 (60.0) | 273 (81.5) | 249 (73.4) | 234 (69.9) | 207 (61.8) | 202 (60.1) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c, d |
| χ 2P value | 0.66 | < 0.0001 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; %DTC, percentage of the total number of DTCs; FTC, Follicular thyroid cancer; PTC, papillary thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant.; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3; d Statistically significantly higher risk for DTC compared with Non-AITD Q4.
TSH Quintiles by Subgroup and Incidence of Differentiated Thyroid Macrocacrinomas (all patients with differentiated thyroid cancer < 1 cm in largest diameter are excluded)
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.88 (0.40-1.21) | 1.44 (1.21-1.67) | 1.94 (1.68-2.30) | 2.63 (2.30-3.02) | 3.59 (3.03-4.45) | 0.58 (0.40-0.74) | 0.93 (0.75-1.13) | 1.32 (1.13-1.53) | 1.80 (1.53-2.13) | 2.79 (2.15-4.48) |
| n | 65 | 64 | 65 | 64 | 65 | 335 | 335 | 335 | 335 | 336 |
| DTC n (%) | 30 (46.2) | 34 (53.1) | 29 (44.6) | 28 (43.8) | 26 (40.0) | 62 (18.5) | 89 (26.6) | 101 (30.1) | 128 (38.2) | 134 (39.9) |
| BEN n (%) | 35 (53.8) | 30 (46.9) | 36 (55.4) | 36 (56.2) | 39 (60.0) | 273 (81.5) | 249 (73.4) | 234 (69.9) | 207 (61.8) | 202 (60.1) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c, d |
| χ 2P value | 0.66 | < 0.0001 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, mean (range) | 0.88 (0.40-1.21) | 1.44 (1.21-1.67) | 1.94 (1.68-2.30) | 2.63 (2.30-3.02) | 3.59 (3.03-4.45) | 0.58 (0.40-0.74) | 0.93 (0.75-1.13) | 1.32 (1.13-1.53) | 1.80 (1.53-2.13) | 2.79 (2.15-4.48) |
| n | 65 | 64 | 65 | 64 | 65 | 335 | 335 | 335 | 335 | 336 |
| DTC n (%) | 30 (46.2) | 34 (53.1) | 29 (44.6) | 28 (43.8) | 26 (40.0) | 62 (18.5) | 89 (26.6) | 101 (30.1) | 128 (38.2) | 134 (39.9) |
| BEN n (%) | 35 (53.8) | 30 (46.9) | 36 (55.4) | 36 (56.2) | 39 (60.0) | 273 (81.5) | 249 (73.4) | 234 (69.9) | 207 (61.8) | 202 (60.1) |
| SS | a | b | c | - | - | - | a | a | a, b, c | a, b, c, d |
| χ 2P value | 0.66 | < 0.0001 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; %DTC, percentage of the total number of DTCs; FTC, Follicular thyroid cancer; PTC, papillary thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant.; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3; d Statistically significantly higher risk for DTC compared with Non-AITD Q4.
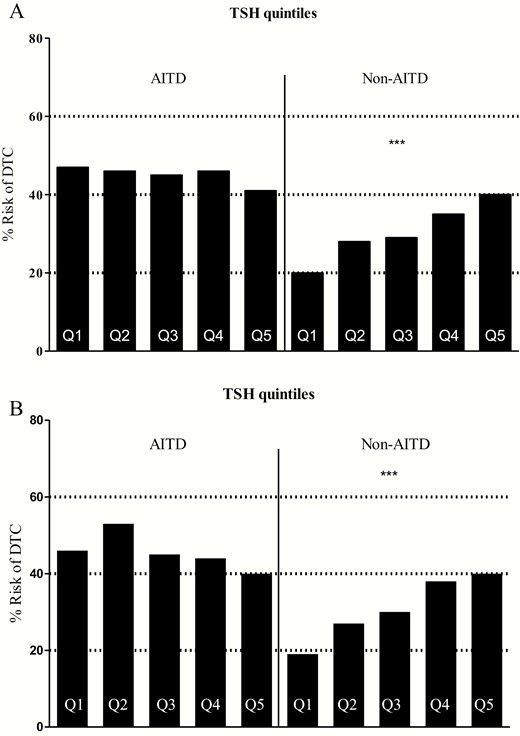
(a) Incidence of DTC by serum TSH quintiles in subjects with and without autoimmune thyroid disease by pathology; (b) Incidence of DTC by serum TSH quintiles in subjects with and without autoimmune thyroid disease by pathology, excluding patients with thyroid microcarcinomas. Abbreviations: AITD: autoimmune thyroid disease; DTC: differentiated thyroid cancer; Q1-Q5: serum TSH concentration quintiles 1-5.
Serum TSH is usually higher in patients with AITD relative to the general population, and that could lead to a potential bias in our analysis. In order to prevent that bias, we performed a secondary analysis of the data, after pooling all our data together and recalculating the serum TSH concentration quintiles for the entire population. The results of that analysis are presented in Table 5, and are almost identical to the outcomes of our primary analysis.
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, range | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 |
| n | 24 | 36 | 68 | 70 | 131 | 397 | 379 | 354 | 358 | 290 |
| DTC, n (%) | 12 (50.0) | 14 (36.4) | 39 (57.3) | 32 (45.7) | 56 (42.7) | 86 (21.7) | 106 (28.0) | 101 (28.5) | 125 (34.9) | 118 (40.7) |
| BEN, n (%) | 12 (50.0) | 22 (63.6) | 29 (42.7) | 38 (54.3) | 75 (57.3) | 311 (78.3) | 273 (72.0) | 253 (71.5) | 233 (65.1) | 172 (59.3) |
| SS | a | - | c | - | - | - | a | a | a, b | a, b, c |
| χ 2P value | 0.29 | < 0.0001 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, range | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 |
| n | 24 | 36 | 68 | 70 | 131 | 397 | 379 | 354 | 358 | 290 |
| DTC, n (%) | 12 (50.0) | 14 (36.4) | 39 (57.3) | 32 (45.7) | 56 (42.7) | 86 (21.7) | 106 (28.0) | 101 (28.5) | 125 (34.9) | 118 (40.7) |
| BEN, n (%) | 12 (50.0) | 22 (63.6) | 29 (42.7) | 38 (54.3) | 75 (57.3) | 311 (78.3) | 273 (72.0) | 253 (71.5) | 233 (65.1) | 172 (59.3) |
| SS | a | - | c | - | - | - | a | a | a, b | a, b, c |
| χ 2P value | 0.29 | < 0.0001 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3.
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, range | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 |
| n | 24 | 36 | 68 | 70 | 131 | 397 | 379 | 354 | 358 | 290 |
| DTC, n (%) | 12 (50.0) | 14 (36.4) | 39 (57.3) | 32 (45.7) | 56 (42.7) | 86 (21.7) | 106 (28.0) | 101 (28.5) | 125 (34.9) | 118 (40.7) |
| BEN, n (%) | 12 (50.0) | 22 (63.6) | 29 (42.7) | 38 (54.3) | 75 (57.3) | 311 (78.3) | 273 (72.0) | 253 (71.5) | 233 (65.1) | 172 (59.3) |
| SS | a | - | c | - | - | - | a | a | a, b | a, b, c |
| χ 2P value | 0.29 | < 0.0001 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH, range | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 | 0.40-0.79 | 0.79-1.20 | 1.20-1.63 | 1.63-2.27 | 2.28-4.48 |
| n | 24 | 36 | 68 | 70 | 131 | 397 | 379 | 354 | 358 | 290 |
| DTC, n (%) | 12 (50.0) | 14 (36.4) | 39 (57.3) | 32 (45.7) | 56 (42.7) | 86 (21.7) | 106 (28.0) | 101 (28.5) | 125 (34.9) | 118 (40.7) |
| BEN, n (%) | 12 (50.0) | 22 (63.6) | 29 (42.7) | 38 (54.3) | 75 (57.3) | 311 (78.3) | 273 (72.0) | 253 (71.5) | 233 (65.1) | 172 (59.3) |
| SS | a | - | c | - | - | - | a | a | a, b | a, b, c |
| χ 2P value | 0.29 | < 0.0001 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); BEN, benign histology; DTC, differentiated thyroid cancer; Q1-Q5, TSH concentration quintiles 1-5; SS, statistically significant; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher risk for DTC compared with Non-AITD Q1; b Statistically significantly higher risk for DTC compared with Non-AITD Q2; c Statistically significantly higher risk for DTC compared with Non-AITD Q3.
The features of tumor aggressiveness of DTC in our subject groups and the between-group comparisons are presented in Table 6. In brief, serum TSH concentration does not seem to play any significant role in the presence of micro vs macrocarcinoma, distant metastasis, lymphatic involvement, extrathyroidal extension (ETE), or main tumor size. The only feature of tumor aggressiveness which was found to correlate with rising serum TSH is the cancer maximal diameter, which was higher in subjects in the highest quintile relative to almost all the rest, only in non-AITD subjects.
Comparison of the Incidence of Features of Tumor Aggressiveness Between Subgroups
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH range | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 |
| LNs n (%) | 1/25 (4.0) | 6/29 (20.7) | 6/27 (22.2) | 5/25 (20.0) | 4/25 (19.0) | 9/53 (17.0) | 15/76 (19.7) | 25/83 (30.1) | 17/110 (15.5) | 29/114 (25.4) |
| SS | - | - | - | - | - | - | - | d | - | - |
| χ 2P value | 0.40 | 0.10 | ||||||||
| MF n (%) | 1/25 (4.0) | 2/29 (6.9%) | 4/36 (11.1) | 5/32 (15.7) | 6/51 (11.7) | 17/53 (32.1) | 29/76 (38.2) | 34/78 (43.6) | 32/100 (32.0) | 41/107 (38.3) |
| SS | - | - | - | - | - | f | g | h | - | j |
| χ 2P value | 0.54 | 0.52 | ||||||||
| ETE | 2/26 (7.7) | 6/29 (20.7) | 5/27 (18.5) | 5/26 (19.2) | 3/21 (14.3) | 6/51 (11.8) | 8/70 (11.4) | 16/79 (20.2) | 11/101 (10.9) | 25/107 (23.4) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.71 | 0.06 | ||||||||
| DM | 1/26 (3.8) | 0/29 (0.0) | 0/27 (0.0) | 0/26 (0.0) | 0/21 (0.0) | 1/51 (1.9) | 1/71 (1.4) | 2/79 (2.5) | 2/102 (2.0) | 7/107 (6.5) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.41 | 0.24 | ||||||||
| Size in cm (SD) | 1.3 (0.9) | 1.4 (1.1) | 1.5 (1.1) | 1.2 (1.0) | 1.3 (1.2) | 1.7 (1.7) | 1.6 (1.6) | 1.7 (1.7) | 1.6 (1.6) | 2.2 (1.9) |
| SS | - | - | - | - | - | - | - | - | - | a,b,d,j |
| KW P value | 0.84 | 0.035 | ||||||||
| MC | 10/26 | 14/30 | 12/27 | 14/26 | 11/22 | 19/52 | 28/73 | 28/91 | 35/106 | 30/110 |
| SS | - | - | - | - | e | - | - | - | - | - |
| χ 2P value | 0.85 | 0.57 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH range | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 |
| LNs n (%) | 1/25 (4.0) | 6/29 (20.7) | 6/27 (22.2) | 5/25 (20.0) | 4/25 (19.0) | 9/53 (17.0) | 15/76 (19.7) | 25/83 (30.1) | 17/110 (15.5) | 29/114 (25.4) |
| SS | - | - | - | - | - | - | - | d | - | - |
| χ 2P value | 0.40 | 0.10 | ||||||||
| MF n (%) | 1/25 (4.0) | 2/29 (6.9%) | 4/36 (11.1) | 5/32 (15.7) | 6/51 (11.7) | 17/53 (32.1) | 29/76 (38.2) | 34/78 (43.6) | 32/100 (32.0) | 41/107 (38.3) |
| SS | - | - | - | - | - | f | g | h | - | j |
| χ 2P value | 0.54 | 0.52 | ||||||||
| ETE | 2/26 (7.7) | 6/29 (20.7) | 5/27 (18.5) | 5/26 (19.2) | 3/21 (14.3) | 6/51 (11.8) | 8/70 (11.4) | 16/79 (20.2) | 11/101 (10.9) | 25/107 (23.4) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.71 | 0.06 | ||||||||
| DM | 1/26 (3.8) | 0/29 (0.0) | 0/27 (0.0) | 0/26 (0.0) | 0/21 (0.0) | 1/51 (1.9) | 1/71 (1.4) | 2/79 (2.5) | 2/102 (2.0) | 7/107 (6.5) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.41 | 0.24 | ||||||||
| Size in cm (SD) | 1.3 (0.9) | 1.4 (1.1) | 1.5 (1.1) | 1.2 (1.0) | 1.3 (1.2) | 1.7 (1.7) | 1.6 (1.6) | 1.7 (1.7) | 1.6 (1.6) | 2.2 (1.9) |
| SS | - | - | - | - | - | - | - | - | - | a,b,d,j |
| KW P value | 0.84 | 0.035 | ||||||||
| MC | 10/26 | 14/30 | 12/27 | 14/26 | 11/22 | 19/52 | 28/73 | 28/91 | 35/106 | 30/110 |
| SS | - | - | - | - | e | - | - | - | - | - |
| χ 2P value | 0.85 | 0.57 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); DM, distant metastasis; ETE, extrathyroidal extension; KW, Kruskal Wallis statistical test; LNs, presence of lymph node involvement by surgical pathology; MC, microcarcinomas; MF, presence of a multifocal tumor; Q1-Q5, TSH concentration quintiles 1-5; SD, standard deviation; SS, statistical significance; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher compared with Non-AITD Q1; b Statistically significantly higher compared to Non-AITD Q2; c Statistically significantly higher compared to Non-AITD Q3; d Statistically significantly higher compared to Non-AITD Q4; e Statistically significantly higher compared to Non-AITD Q5; f Statistically significantly higher compared to AITD Q1; g Statistically significantly higher compared to AITD Q2; h Statistically significantly higher compared to AITD Q3; i Statistically significantly higher compared to AITD Q4; j Statistically significantly higher compared to AITD Q5.
Comparison of the Incidence of Features of Tumor Aggressiveness Between Subgroups
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH range | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 |
| LNs n (%) | 1/25 (4.0) | 6/29 (20.7) | 6/27 (22.2) | 5/25 (20.0) | 4/25 (19.0) | 9/53 (17.0) | 15/76 (19.7) | 25/83 (30.1) | 17/110 (15.5) | 29/114 (25.4) |
| SS | - | - | - | - | - | - | - | d | - | - |
| χ 2P value | 0.40 | 0.10 | ||||||||
| MF n (%) | 1/25 (4.0) | 2/29 (6.9%) | 4/36 (11.1) | 5/32 (15.7) | 6/51 (11.7) | 17/53 (32.1) | 29/76 (38.2) | 34/78 (43.6) | 32/100 (32.0) | 41/107 (38.3) |
| SS | - | - | - | - | - | f | g | h | - | j |
| χ 2P value | 0.54 | 0.52 | ||||||||
| ETE | 2/26 (7.7) | 6/29 (20.7) | 5/27 (18.5) | 5/26 (19.2) | 3/21 (14.3) | 6/51 (11.8) | 8/70 (11.4) | 16/79 (20.2) | 11/101 (10.9) | 25/107 (23.4) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.71 | 0.06 | ||||||||
| DM | 1/26 (3.8) | 0/29 (0.0) | 0/27 (0.0) | 0/26 (0.0) | 0/21 (0.0) | 1/51 (1.9) | 1/71 (1.4) | 2/79 (2.5) | 2/102 (2.0) | 7/107 (6.5) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.41 | 0.24 | ||||||||
| Size in cm (SD) | 1.3 (0.9) | 1.4 (1.1) | 1.5 (1.1) | 1.2 (1.0) | 1.3 (1.2) | 1.7 (1.7) | 1.6 (1.6) | 1.7 (1.7) | 1.6 (1.6) | 2.2 (1.9) |
| SS | - | - | - | - | - | - | - | - | - | a,b,d,j |
| KW P value | 0.84 | 0.035 | ||||||||
| MC | 10/26 | 14/30 | 12/27 | 14/26 | 11/22 | 19/52 | 28/73 | 28/91 | 35/106 | 30/110 |
| SS | - | - | - | - | e | - | - | - | - | - |
| χ 2P value | 0.85 | 0.57 | ||||||||
| . | AITD . | Non-AITD . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . | Q1 . | Q2 . | Q3 . | Q4 . | Q5 . |
| TSH range | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 | 0.40-0.78 | 0.78-1.19 | 1.19-1.60 | 1.60-2.28 | 2.28-4.48 |
| LNs n (%) | 1/25 (4.0) | 6/29 (20.7) | 6/27 (22.2) | 5/25 (20.0) | 4/25 (19.0) | 9/53 (17.0) | 15/76 (19.7) | 25/83 (30.1) | 17/110 (15.5) | 29/114 (25.4) |
| SS | - | - | - | - | - | - | - | d | - | - |
| χ 2P value | 0.40 | 0.10 | ||||||||
| MF n (%) | 1/25 (4.0) | 2/29 (6.9%) | 4/36 (11.1) | 5/32 (15.7) | 6/51 (11.7) | 17/53 (32.1) | 29/76 (38.2) | 34/78 (43.6) | 32/100 (32.0) | 41/107 (38.3) |
| SS | - | - | - | - | - | f | g | h | - | j |
| χ 2P value | 0.54 | 0.52 | ||||||||
| ETE | 2/26 (7.7) | 6/29 (20.7) | 5/27 (18.5) | 5/26 (19.2) | 3/21 (14.3) | 6/51 (11.8) | 8/70 (11.4) | 16/79 (20.2) | 11/101 (10.9) | 25/107 (23.4) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.71 | 0.06 | ||||||||
| DM | 1/26 (3.8) | 0/29 (0.0) | 0/27 (0.0) | 0/26 (0.0) | 0/21 (0.0) | 1/51 (1.9) | 1/71 (1.4) | 2/79 (2.5) | 2/102 (2.0) | 7/107 (6.5) |
| SS | - | - | - | - | - | - | - | - | - | - |
| χ 2P value | 0.41 | 0.24 | ||||||||
| Size in cm (SD) | 1.3 (0.9) | 1.4 (1.1) | 1.5 (1.1) | 1.2 (1.0) | 1.3 (1.2) | 1.7 (1.7) | 1.6 (1.6) | 1.7 (1.7) | 1.6 (1.6) | 2.2 (1.9) |
| SS | - | - | - | - | - | - | - | - | - | a,b,d,j |
| KW P value | 0.84 | 0.035 | ||||||||
| MC | 10/26 | 14/30 | 12/27 | 14/26 | 11/22 | 19/52 | 28/73 | 28/91 | 35/106 | 30/110 |
| SS | - | - | - | - | e | - | - | - | - | - |
| χ 2P value | 0.85 | 0.57 | ||||||||
Abbreviations: AITD, autoimmune thyroid disease (see text); DM, distant metastasis; ETE, extrathyroidal extension; KW, Kruskal Wallis statistical test; LNs, presence of lymph node involvement by surgical pathology; MC, microcarcinomas; MF, presence of a multifocal tumor; Q1-Q5, TSH concentration quintiles 1-5; SD, standard deviation; SS, statistical significance; TSH, thyrotropin (thyroid-stimulating hormone).
a Statistically significantly higher compared with Non-AITD Q1; b Statistically significantly higher compared to Non-AITD Q2; c Statistically significantly higher compared to Non-AITD Q3; d Statistically significantly higher compared to Non-AITD Q4; e Statistically significantly higher compared to Non-AITD Q5; f Statistically significantly higher compared to AITD Q1; g Statistically significantly higher compared to AITD Q2; h Statistically significantly higher compared to AITD Q3; i Statistically significantly higher compared to AITD Q4; j Statistically significantly higher compared to AITD Q5.
Discussion
The overall thyroid cancer incidence has been rising at a high rate during the last 4 decades (1). This rise is thought to occur mainly due to a higher detection rate, mostly mediated by the common use of point-of-care ultrasound examination at endocrinology and primary care practices alike (2, 18). However, some studies have implied that this iatrogenic rise in the detection rate could not completely explain the worldwide epidemic of thyroid cancer in its entirety (2). Since autoimmune thyroid disorders are on the rise as well (19), an association between these two conditions has been proposed, mostly based on surgical series outcomes (4, 16).
One of the most prominent findings in patients with AITD in general is a rise in TSH concentration above the reference range, which marks the passage from a euthyroid to a hypothyroid state (14, 20). When TSH is within the reference range, higher TSH has been clearly shown to be associated with a higher likelihood of developing thyroid nodules and thyroid cancer (3-13).
This higher likelihood of developing thyroid neoplasms is thought to be due to the pro-proliferative capacity of TSH that, when bound to its receptor on the follicular thyroid cells, induces growth (7, 21), even though this hypothesis is not accepted by all scholars (22). Reciprocally, low TSH, similar to what is usually seen in toxic adenomas, is known to lower the risk of DTC in most but not all studies. In subgroups of patients with DTC, a lower likelihood of tumor recurrence and a higher disease-free survival has also been found with adequate TSH suppression. Similar findings have been observed in patients with iatrogenically suppressed serum TSH concentrations, when this was employed to treat thyroid nodules, even though these practices are discouraged today due to the questionable effectiveness (23) and the common occurrence of adverse consequences on bone metabolism and cardiac rhythm (24).
If the hypothesis of TSH pro-proliferative capacity were to be true, thyroid cancers would have been observed, in patients with overt/untreated hypothyroidism, where serum TSH concentrations could reach high titers. This theory, however, has been disproven, since patients with significant thyroid failure, requiring high doses of thyroid hormone supplementation are found to have a much lower incidence of DTC on surgical specimens (16). Moreover, a pivotal prospective study of 829 patients with hypothyroidism due to full-blown Hashimoto disease, who were followed for 22 years, did not find increased risk for DTC development as well (17).
Of note, in multiple postsurgical series, patients with bilateral lymphocytic infiltrates in their thyroid glands, with normal TSH and thyroid hormone concentrations (also known as euthyroid Hashimoto) harbor DTCs much more commonly than those without such histological changes (16, 25). Although these patients have higher serum TSH concentrations compared with those without AITD, TSH remains within the normal range. If that subtle elevation of TSH were to be causative of the higher incidence of DTC, a linearly correlated degree of elevation in serum TSH, as shown for the patients without AITD (3-13), should have been expected.
In the present study, patients with AITD not on levothyroxine therapy, had serum TSH concentrations within the reference range not linearly correlated with high incidence of DTC. Regardless of the TSH quintile, the incidence was not statistically different among the AITD subgroups. On the other hand, the non-AITD population exhibited the well-known linear relationship between serum TSH and DTC incidence (3-13)
The population with non-AITD had a similar risk with their AITD counterparts, only when TSH rose to the fifth quintile (> 2.73 IU/L) always within the reference range. Therefore, at least a differential role of TSH in these 2 distinct patient populations need to be postulated. TSH could still be a mediator of tumorigenesis in the absence of thyroid autoimmunity, but not in the presence of chronic lymphocytic thyroiditis. In addition, the presence of very high rates of DTCs in patients with chronic lymphocytic thyroiditis (euthyroid Hashimoto) suggests that this “clinically silent autoimmune condition” could produce an inflammatory milieu within the gland, capable of promoting cancer generation, similar to what is known to occur in other organs (26).
This potential carcinogenic role of thyroid auto-inflammation is supported by the findings of previously published studies, assessing the number and the activity of immune cells found in the thyroid glands of patients with chronic lymphocytic thyroiditis (27). Specifically, thyroid cancer-associated double-negative (DN) T cells might downregulate proliferation and effector functions of cytotoxic T cells and thereby contribute to tumor tolerance and active avoidance of tumor immunity (27). Even the subtype of macrophages (M1 vs M2) and quality of natural killer (NK) cells in the immune microenvironment of a tumor have been implicated in thyroid cancer development regardless of TSH level (28).
Finally, in our population, TSH elevations were not that strongly associated with worse disease stage, and the only association was that of larger tumors in patients with TSH > 2.73 IU/L (fifth quintile) in the absence of AITD.
Our study has several limitations, given its cross-sectional nature. A main limitation is the presence of potential selection bias, since all cases had been originally selected for thyroid surgery by the treating endocrinologist. That type of bias though, does not affect serum TSH or its role on thyroid cancer development. The large number of patients enrolled in the present study further limits this effect, if any exists. Our study results are limited by the presence of small numbers of patients evaluated in each quintile for AITD patients, even though the overall number of patients with euthyroid autoimmune thyroiditis in total is quite large. Also, the small numbers of patients with available TPO antibody titers does not allow for conclusions generalizable to the entire population of patients with thyroid nodules. Studies with larger sample size should follow to further characterize the effects of high TPO antibody titers with regard to the development of DTC. Finally, we know that serum TSH has a significant diurnal and day-to-day variation, rendering a single measurement less indicative of the overall status of thyroid epithelial cell stimulation in any patient at any given time, allowing for introduction of additional bias to our study results, even though, this has been the case for many prior studies published on this subject.
In conclusion, in this large database study from 2 tertiary institutions, in 2 different continents, we showed that even though TSH concentrations might play a role in thyroid cancer development and severity in patients with thyroid nodular disease in the absence of chronic thyroid autoimmunity, the latter seems to be a more potent mediator of thyroid carcinogenesis, independent of serum TSH elevations.
Abbreviations
- AITD
autoimmune thyroid disease
- DTC
differentiated thyroid cancer
- FTC
follicular thyroid cancer
- PTC
papillary thyroid cancer
- TPO
thyroid peroxidase
- TSH
thyrotropin (thyroid-stimulating hormone)
Additional Information
Disclosure Summary: No competing financial interests exist.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References



