-
PDF
- Split View
-
Views
-
Cite
Cite
Benita S Arakal, Richard S Rowlands, Michael McCarthy, David E Whitworth, Sarah E Maddocks, Philip E James, Paul G Livingstone, Corallococcus senghenyddensis sp. nov., a myxobacterium with potent antimicrobial activity, Journal of Applied Microbiology, Volume 135, Issue 5, May 2024, lxae102, https://doi.org/10.1093/jambio/lxae102
Close - Share Icon Share
Abstract
Corallococcus species are diverse in the natural environment with 10 new Corallococcus species having been characterized in just the last 5 years. As well as being an abundant myxobacterial genus, they produce several secondary metabolites, including Corallopyronin, Corramycin, Coralmycin, and Corallorazine. We isolated a novel strain Corallococcus spp RDP092CA from soil in South Wales, UK, using Candida albicans as prey bait and characterized its predatory activities against pathogenic bacteria and yeast.
The size of the RDP092CA genome was 8.5 Mb with a G + C content of 71.4%. Phylogenetically, RDP092CA is closely related to Corallococcus interemptor, C. coralloides, and C. exiguus. However, genome average nucleotide identity and digital DNA–DNA hybridization values are lower than 95% and 70% when compared to those type strains, implying that it belongs to a novel species. The RDP092CA genome harbours seven types of biosynthetic gene clusters (BGCs) and 152 predicted antimicrobial peptides. In predation assays, RDP092CA showed good predatory activity against Escherichia coli, Pseudomonas aeruginosa, Citrobacter freundii, and Staphylococcus aureus but not against Enterococcus faecalis. It also showed good antibiofilm activity against all five bacteria in biofilm assays. Antifungal activity against eight Candida spp. was variable, with particularly good activity against Meyerozyma guillermondii DSM 6381. Antimicrobial peptide RDP092CA_120 exhibited potent antibiofilm activity with >50% inhibition and >60% dispersion of biofilms at concentrations down to 1 μg/ml.
We propose that strain RDP092CA represents a novel species with promising antimicrobial activities, Corallococcus senghenyddensis sp. nov. (=NBRC 116490T =CCOS 2109T), based on morphological, biochemical, and genomic features.
In the era of antimicrobial resistance, the search for novel antimicrobial agents is of prime importance. With the traditional antibiotic pipeline running dry, predatory bacteria are explored as alternative therapeutic agents. This study has characterized a novel bacterium, Corallococcus senghenyddensis, with potential antimicrobial properties.
Introduction
The genus Corallococcus belongs to the phylum Myxococcota, class Myxococcia, order Myxococcales, and family Myxococcaceae (Waite et al. 2020). Being ubiquitous and highly diverse, Corallococcus spp. have been isolated from natural sources like soil, decayed leaves, the bark of trees, and dung of herbivorous mammals (Reichenbach 1999, Dawid 2000). They exhibit predatory social behaviour secreting lytic and toxic components that kill prey organisms and subsequently feed on the macromolecules of the dead carcasses (Thiery and Kaimer 2020, Phillips et al. 2022). Other interesting multicellular behaviours of Corallococcus spp. include swarming growth, social gliding motility, and fruiting body development with sporulation when depleted of nutrients (Zhao et al. 2021). Currently, there are 12 validly described species within the genus Corallococcus, of which nine have been described in the last 3 years (Livingstone et al. 2020, Babadi et al. 2022, Zhang et al. 2022). Genomic analysis has shown an unprecedented diversity in Corallococcus spp., and the further identification of novel species is inevitable (Livingstone et al. 2018).
Several bioactive compounds with strong antimicrobial activities have been reported in Corallococcus spp., including Corallopyronin (Krome et al. 2022), Corramycin (Couturier et al. 2022), Coralmycin (Kim et al. 2016), and Corallorazine (Schmitz et al. 2014). Arakal et al. (2022) reported promising antimicrobial peptides (AMPs) against pathogenic bacteria from Corallococcus coralloides. In addition, several enzymes with potential industrial applications, such as maltohexaose-forming α-amylase AmyM (Li et al. 2015), glucoamylase GlucaM (Li et al. 2017), β-(1,3)-glucanase (Zhou et al. 2019), glycogen-branching enzyme, CcGBE (Ye et al. 2022), and an aldo-keto reductase with antiprelog stereospecificity (Yajuan et al. 2020), have been reported in Corallococcus spp., thus they are both interesting and beneficial microbes. Here, we report a novel Corallococcus species (Corallococcus senghenyddensis), which has potent antimicrobial and anticandidal properties.
Materials and methods
Isolation of strain RDP092CA
RDP092CA was isolated from garden soil collected at Senghenydd, Caerphilly County Borough, Wales, UK (51°35′43″N, 003°15′54″W) in November 2021. Isolation of the strain involved a baiting technique using Candida albicans DSM 1386 as bait with the intention of selectively isolating predatory bacteria that can kill Candida spp. Briefly, C. albicans DSM1386 was grown on Saboraud’s dextrose agar (Thermo Scientific, Leicestershire, UK), colonies were suspended in phosphate buffered saline (PBS) (Thermo Scientific) to an OD600 ∼6 and spotted onto WCX agar (0.1% CaCl2.2H2O, 1.5% agar, 20 mM HEPES with 2.5% cycloheximide). A soil sample (∼5 g) was mixed with 1 ml of 95% cycloheximide and inoculated onto WCX agar close to spots of C. albicans DSM1386. After incubation, fruiting bodies and swarming growth were subcultured onto VY-2 agar (0.5% dried baker’s yeast, 0.1% CaCl2.2H2O, and 1.5% agar). Subculturing was repeated until pure cultures were obtained. The isolate was identified by 16S rRNA sequencing using F27 and R1389 primers as previously described (Livingstone et al. 2017). The closest sequences from the NCBI database (Sayers et al. 2021) were retrieved to construct a phylogenetic tree using MEGA 11 (Tamura et al. 2021).
Genome sequencing and sequence analysis
Strain RDP092CA was subjected to whole genome sequencing using the Illumina HiSeq 2500 platform at MicrobesNG (University of Birmingham, UK). Kraken (Wood and Salzberg 2014) was used to identify the closest reference genome and SPAdes (Prjibelski et al. 2020) for de_novo assembly of the reads. Genome annotations were generated using prokka (Seemann 2014) and RAST (Aziz et al. 2008). Average nucleotide identity (ANI) (Richter Rosselló-Móra 2009) and in silico DNA–DNA hybridization were estimated using the genome-to-genome distance calculator GGDC 2.1 (Meier-Kolthoff et al. 2013). Biosynthetic gene clusters (BGCs) for secondary metabolites were identified using antiSMASH v.5.0 (Blin et al. 2019) and BAGEL4 (van Heel et al. 2018). DBAASP (Pirtskhalava et al. 2021) was employed to predict AMPs. AMPs predicted in silico to have potent activity were further investigated for their physiochemical properties and antimicrobial properties via the APD3 tool (Wang et al. 2016). The sequenced genome is available in the NCBI database under the accession number JAYESI000000000.
Growth and biochemical characteristics
Growth parameters were tested on VY-2 agar at temperatures 4°C, 10°C, 20°C, 30°C, 37°C, and 40°C (pH 7.2), and pH 4, 5, 6, 7, 8, 9, and 10 (30°C). Growth in the presence of 0%, 0.5%, 1%, 1.5%, 2%, and 3% NaCl concentrations was tested on VY-2 agar (30°C and pH 7.2). Antibiotic susceptibility testing for cefotaxime, ceftazidime, gentamicin, and trimethoprim–sulfamethoxazole was undertaken by the Stokes method (Gosden et al. 1998) using Escherichia coli ATCC 25922 as control. Enzymatic activity of the isolate was characterized by API ZYM (BioMérieux) and API CORYNE (BioMérieux) kits according to the manufacturer’s instructions.
Fatty acid analysis
Fatty acid analysis was performed at the Leibniz Institute, DSMZ-German Collection of Microorganisms and Cell Cultures. Saponification, methylation, and extraction of the cellular fatty acids were carried out using standard protocols to convert fatty acids into their methyl esters (FAME) (Sasser 2001). FAME analysis and identification were performed by GC-MS on the Agilent 7000D system with a phenyl methyl silicone fused silica capillary column.
Predation and biofilm assays
Predation assays were based on a lawn culture method (Livingstone et al. 2017, Zwarycz and Whitworth 2023) using non-nutrient WAT agar (0.1% CaCl2.2H2O, 1.5% agar, pH 7.2, and 20 mM HEPES). A range of bacterial and fungal organisms were used to make prey lawns (Table 1). Briefly, prey organisms were grown in nutrient broth for 48 h, cells were washed and suspended in PBS (OD600 ∼6) to make lawns on WAT agar. Strain RDP092CA was grown on VY-2 agar and colonies were scraped, washed, and resuspended in PBS to OD600 ∼3. Prey suspensions were then inoculated onto WAT agar to make lawns and dried. 10 μl RDP092CA cells were spotted onto prey lawns, incubated for 4 days (30°C) and zone diameters of predation were measured.
| Prey strains . |
|---|
| Staphylococcus aureus EMRSA-15 |
| Pseudomonas aeruginosa ATCC 9027 |
| Citrobacter freundii NCTC 6272 |
| Escherichia coli ATCC 10418 |
| Enterococcus faecalis ATCC 19433 |
| Candida albicans DSM 1386 |
| Candida auris DSM 21092 |
| Candida dubliniensis DSM 13268 |
| Candida glabrata DSM 11226 |
| Candida parapsilosis DSM 4237 |
| Candida tropicalis DSM 1346 |
| Clavispora lusitaniae DSM 70102 |
| Meyerozyma guilliermondii DSM 6381 |
| Prey strains . |
|---|
| Staphylococcus aureus EMRSA-15 |
| Pseudomonas aeruginosa ATCC 9027 |
| Citrobacter freundii NCTC 6272 |
| Escherichia coli ATCC 10418 |
| Enterococcus faecalis ATCC 19433 |
| Candida albicans DSM 1386 |
| Candida auris DSM 21092 |
| Candida dubliniensis DSM 13268 |
| Candida glabrata DSM 11226 |
| Candida parapsilosis DSM 4237 |
| Candida tropicalis DSM 1346 |
| Clavispora lusitaniae DSM 70102 |
| Meyerozyma guilliermondii DSM 6381 |
| Prey strains . |
|---|
| Staphylococcus aureus EMRSA-15 |
| Pseudomonas aeruginosa ATCC 9027 |
| Citrobacter freundii NCTC 6272 |
| Escherichia coli ATCC 10418 |
| Enterococcus faecalis ATCC 19433 |
| Candida albicans DSM 1386 |
| Candida auris DSM 21092 |
| Candida dubliniensis DSM 13268 |
| Candida glabrata DSM 11226 |
| Candida parapsilosis DSM 4237 |
| Candida tropicalis DSM 1346 |
| Clavispora lusitaniae DSM 70102 |
| Meyerozyma guilliermondii DSM 6381 |
| Prey strains . |
|---|
| Staphylococcus aureus EMRSA-15 |
| Pseudomonas aeruginosa ATCC 9027 |
| Citrobacter freundii NCTC 6272 |
| Escherichia coli ATCC 10418 |
| Enterococcus faecalis ATCC 19433 |
| Candida albicans DSM 1386 |
| Candida auris DSM 21092 |
| Candida dubliniensis DSM 13268 |
| Candida glabrata DSM 11226 |
| Candida parapsilosis DSM 4237 |
| Candida tropicalis DSM 1346 |
| Clavispora lusitaniae DSM 70102 |
| Meyerozyma guilliermondii DSM 6381 |
To assess antibiofilm properties, biofilm inhibition and dispersal assays were carried out separately (Arakal et al. 2022). Biofilm inhibition was assessed by incubating prey organisms (100 µl, OD600 ∼1) and RDP092CA strain cultures (100 µl, OD600 ∼2) for 16 h in a 96-well plate. After incubation, unattached bacteria were removed by washing with PBS and adherent cells (biofilms) were scraped and enumerated by total viable count (TVC) (Miles et al. 1938). To assess biofilm dispersal, prey organisms were first grown for 16 h in 96-well plates to form biofilms. After washing with PBS, active cultures of RDP092CA (100 µl, OD600 ∼2) were added and incubated for a further 16 h, followed with washing and TVCs as previously described.
AMPs—MIC and antibiofilm properties of RDP092CA_120
Based on in silico analysis, RDP092CA_120, a 35-amino acid AMP predicted to have potent antimicrobial activity was synthesized at Peptide Protein Research Ltd, Fareham, UK. A 1% DMSO stock solution of the crude desalted AMP was diluted to obtain a final concentration of 1000 μg/ml. MIC and antibiofilm properties of RDP092CA_120 were determined using E. coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 19433, and C. albicans DSM1386. A micro broth dilution method was used for MIC assays according to Clinical and Laboratory Standards Institute (CLSI) methods (Watts 1999). Briefly, 2-fold dilutions of the AMP in Mueller–Hinton broth (MHB), ranging from 512 to 7.8 μg/ml were made, to which 1 × 105 CFU/ml bacterial suspensions were added and incubated for 18 h. Appropriate antibiotics (Amoxycillin, Ampicillin, Vancomycin, Piperacillin, and Fluconazole) and, MHB without AMP were used as positive and negative controls respectively. MICs were the lowest dilution at which there was no visible growth. To check antibiofilm efficacy of the AMP, biofilm prevention and biofilm disruption assays were carried out. The assays were performed as for MICs with the AMP. After incubation, unattached bacteria were removed by washing with PBS (Merck Life Science UK Limited, Dorset, UK) and biofilms were scraped and enumerated by TVCs (Miles et al. 1938). For biofilm disruption, bacteria were grown in 96-well plates without the AMP for 16 h at 37°C. Unattached bacteria were removed by washing with PBS and biofilms were treated with dilutions of AMP overnight, followed with TVCs to enumerate viable bacteria.
Wound flow infection model
A biofilm flow device was used to investigate the killing effects of RDP092CA against five pathogenic organisms in mixed culture, that are commonly isolated from chronic wound infections (Khalid et al. 2023). 1 ml of prey organism culture (OD600 ∼2) was added to 20 ml of 1.5% (w/v) molten agarose and allowed to set. 8 mm diameter plugs of agarose were cut using a sterile leather punch and placed in the biofilm flow device. The device was filled with simulated wound fluid (SWF; Bradford et al. 2009) and connected to a peristaltic pump to replenish SWF at a constant flow rate (2 ml/h). At 24, 48, 72, and 96 h, agarose plugs were removed and homogenized in PBS with a glass homogenizer and with enumeration by TVCs, using Chromogenic UTI Agar for E. coli, Cetrimide Agar Ps. aeruginosa, Mannitol Salt Agar Staph. aureus, Slanetz and Bartley Agar for Ent. faecalis, and Chrome Coliform Agar for Citrobacter freundii (Thermo Scientific).
Results
Morphological, cultural, and biochemical properties
The growth characteristics and biochemical activities of all 12 previously described Corallococcus species type strains and RDP092CA were thoroughly assessed (Table 2). Strain RDP092CA is a nonmotile, Gram-negative, and slender bacillus. On VY-2 the vegetative phase of growth was seen as a thin film of transparent swarming growth with rippling (Fig. 1A). Vegetative cells measure 1.2–1.4 μm in length and 3–6 μm in width (Fig. 1B). Fruiting bodies were friable, yellow, or orange pigmented, and coral shaped (Fig. 1C). Myxospores within the fruiting bodies were ∼1 μm in diameter (Fig. 1D) Growth was seen at temperatures ranging between 20oC and 37°C (optimum temperature 30°C) and no growth observed at 4oC and 40oC. The isolate grew at a pH between 5 and 9 with an optimum pH of 7, but no growth at pH 4 and 10. RDP092CA grew at a salinity between 0.5% and 1.5% with an optimum growth at 1% NaCl and no growth at 2% and 3%. The strain was susceptible to cefotaxime and gentamicin while resistant to ceftazidime and trimethoprim sulfamethoxazole (Table 2). RDP092CA cells exhibited high levels (+++) of catalase, β-glucosidase, N-acetyl-β-glucosaminidase, acid phosphatase, esterase lipase, lipase, and leucine arylamidase enzyme activities; and moderate (++) to weak (+) pyrazinamidase, gelatinase, ⍺-glucosidase, naphthol-AS-BI-phosphohydrolase, valine arylamidase, alkaline phosphatase, and esterase (C4) activities. Breakdown of sugars such as glucose, maltose, ribose, and xylose were seen but no fermentation of lactose, saccharose, glycogen, or mannitol. The major fatty acids (>5%) were iso-C15:0 (13-methyltetradecanoic) (33.58%), iso-C14:0 3-OH (15.21%), and iso-C17:0 (isomargaric) (10.12%) (Table 3). The abundance of iso-C17:0 (isomargaric) and iso-C15:0 (13-methyltetradecanoic) was in agreement with previous studies of fatty acid profiling of Corallococcus spp., however, iso-C14:0 3-OH (which was in trace amounts in other myxobacteria including other Corallococcus spp.) was abundant in strain RDP092CA, further indicating uniqueness of the isolate (Garcia et al. 2011). Apart from the presence of C10:0 (decanoic) and C11:0 (undecanoic), RDP092CA has a similar fatty acid profile to C. silvisoli. (Supplementary file).

Morphological features of RDP092CA. Growth on VY-2 agar (1A), vegetative cells (1B), stereoscopic micrograph of the fruiting bodies on VY-2 agar (1C), SEM image of fruiting body (1D).
Physiological and biochemical features of strain RDP092CA compared with 12 other type strains of Corallococcus spp. (1) RDP092CA, (2) Corallococcus exiguous, (3) Corallococcus interemptor, (4) Corallococcus praedator, (5) Corallococcus aberystwythensis, (6) Corallococcus llansteffanensis, (7) Corallococcus sicarius, (8) Corallococcus exercitus, (9) Corallococcus carmarthensis, (10) Corallococcus terminator, (11) Corallococcus silvisoli, (12) Corallococcus soli, and (13) C. coralloides.
| . | Species designation (see legend) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| Growth at | |||||||||||||
| pH5 | + | + | + | – | – | – | – | + | + | − | NA | − | − |
| pH6 | ++ | ++ | + | − | + | + | + | ++ | + | + | +++ | +++ | ++ |
| pH7 | +++ | +++ | ++ | + | + | ++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ |
| pH8 | ++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | ++ | +++ | +++ |
| pH9 | + | +++ | ++ | + | − | ++ | ++ | ++ | +++ | ++ | + | +++ | +++ |
| 30∘C | +++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | +++ | ++ | ++ |
| 35∘C | ++ | + | + | − | ++ | + | ++ | ++ | ++ | − | ++ | +++ | + |
| Biochemical reactions | |||||||||||||
| Esculin test | + | − | + | + | − | + | + | − | + | + | + | − | + |
| Gelatin test | + | + | + | − | − | − | + | + | + | + | + | + | + |
| Glucose assimilation | + | − | − | − | − | − | − | + | − | − | + | − | − |
| Maltose assimilation | + | + | − | − | − | − | − | − | − | + | + | − | − |
| Nitrate reduction | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Antibiotic resistance | |||||||||||||
| Cefotaxime | − | + | + | + | + | + | + | + | − | + | − | − | − |
| Ceftazidime | + | + | + | + | + | + | + | + | + | + | − | − | − |
| Gentamicin | − | + | + | + | + | + | + | + | − | + | − | + | + |
| Trimethoprim sulfamethoxazole | + | − | − | − | − | − | − | − | − | − | − | + | + |
| Genome comparison | |||||||||||||
| Contigs | 234 | 36 | 459 | 1491 | 625 | 1244 | 802 | 961 | 530 | 863 | 62 | 68 | 68 |
| Genome size (Mb) | 8.48 | 10.41 | 9.47 | 10.51 | 9.98 | 10.53 | 10.39 | 10.15 | 10.79 | 10.35 | 9.23 | 9.44 | 9.44 |
| Mol% GC | 71.4 | 69.6 | 70 | 69.7 | 70 | 70.3 | 70.2 | 70.2 | 69.9 | 69.5 | 69.8 | 69.8 | 69.8 |
| No. of CDS | 7166 | 8416 | 7892 | 9011 | 8353 | 8867 | 8442 | 8611 | 8959 | 8506 | 7412 | 7445 | 7445 |
| Pseudogene | 159 | 216 | 286 | 211 | 270 | 307 | 272 | 257 | 197 | 131 | 140 | 140 | |
| . | Species designation (see legend) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| Growth at | |||||||||||||
| pH5 | + | + | + | – | – | – | – | + | + | − | NA | − | − |
| pH6 | ++ | ++ | + | − | + | + | + | ++ | + | + | +++ | +++ | ++ |
| pH7 | +++ | +++ | ++ | + | + | ++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ |
| pH8 | ++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | ++ | +++ | +++ |
| pH9 | + | +++ | ++ | + | − | ++ | ++ | ++ | +++ | ++ | + | +++ | +++ |
| 30∘C | +++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | +++ | ++ | ++ |
| 35∘C | ++ | + | + | − | ++ | + | ++ | ++ | ++ | − | ++ | +++ | + |
| Biochemical reactions | |||||||||||||
| Esculin test | + | − | + | + | − | + | + | − | + | + | + | − | + |
| Gelatin test | + | + | + | − | − | − | + | + | + | + | + | + | + |
| Glucose assimilation | + | − | − | − | − | − | − | + | − | − | + | − | − |
| Maltose assimilation | + | + | − | − | − | − | − | − | − | + | + | − | − |
| Nitrate reduction | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Antibiotic resistance | |||||||||||||
| Cefotaxime | − | + | + | + | + | + | + | + | − | + | − | − | − |
| Ceftazidime | + | + | + | + | + | + | + | + | + | + | − | − | − |
| Gentamicin | − | + | + | + | + | + | + | + | − | + | − | + | + |
| Trimethoprim sulfamethoxazole | + | − | − | − | − | − | − | − | − | − | − | + | + |
| Genome comparison | |||||||||||||
| Contigs | 234 | 36 | 459 | 1491 | 625 | 1244 | 802 | 961 | 530 | 863 | 62 | 68 | 68 |
| Genome size (Mb) | 8.48 | 10.41 | 9.47 | 10.51 | 9.98 | 10.53 | 10.39 | 10.15 | 10.79 | 10.35 | 9.23 | 9.44 | 9.44 |
| Mol% GC | 71.4 | 69.6 | 70 | 69.7 | 70 | 70.3 | 70.2 | 70.2 | 69.9 | 69.5 | 69.8 | 69.8 | 69.8 |
| No. of CDS | 7166 | 8416 | 7892 | 9011 | 8353 | 8867 | 8442 | 8611 | 8959 | 8506 | 7412 | 7445 | 7445 |
| Pseudogene | 159 | 216 | 286 | 211 | 270 | 307 | 272 | 257 | 197 | 131 | 140 | 140 | |
Physiological and biochemical features of strain RDP092CA compared with 12 other type strains of Corallococcus spp. (1) RDP092CA, (2) Corallococcus exiguous, (3) Corallococcus interemptor, (4) Corallococcus praedator, (5) Corallococcus aberystwythensis, (6) Corallococcus llansteffanensis, (7) Corallococcus sicarius, (8) Corallococcus exercitus, (9) Corallococcus carmarthensis, (10) Corallococcus terminator, (11) Corallococcus silvisoli, (12) Corallococcus soli, and (13) C. coralloides.
| . | Species designation (see legend) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| Growth at | |||||||||||||
| pH5 | + | + | + | – | – | – | – | + | + | − | NA | − | − |
| pH6 | ++ | ++ | + | − | + | + | + | ++ | + | + | +++ | +++ | ++ |
| pH7 | +++ | +++ | ++ | + | + | ++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ |
| pH8 | ++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | ++ | +++ | +++ |
| pH9 | + | +++ | ++ | + | − | ++ | ++ | ++ | +++ | ++ | + | +++ | +++ |
| 30∘C | +++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | +++ | ++ | ++ |
| 35∘C | ++ | + | + | − | ++ | + | ++ | ++ | ++ | − | ++ | +++ | + |
| Biochemical reactions | |||||||||||||
| Esculin test | + | − | + | + | − | + | + | − | + | + | + | − | + |
| Gelatin test | + | + | + | − | − | − | + | + | + | + | + | + | + |
| Glucose assimilation | + | − | − | − | − | − | − | + | − | − | + | − | − |
| Maltose assimilation | + | + | − | − | − | − | − | − | − | + | + | − | − |
| Nitrate reduction | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Antibiotic resistance | |||||||||||||
| Cefotaxime | − | + | + | + | + | + | + | + | − | + | − | − | − |
| Ceftazidime | + | + | + | + | + | + | + | + | + | + | − | − | − |
| Gentamicin | − | + | + | + | + | + | + | + | − | + | − | + | + |
| Trimethoprim sulfamethoxazole | + | − | − | − | − | − | − | − | − | − | − | + | + |
| Genome comparison | |||||||||||||
| Contigs | 234 | 36 | 459 | 1491 | 625 | 1244 | 802 | 961 | 530 | 863 | 62 | 68 | 68 |
| Genome size (Mb) | 8.48 | 10.41 | 9.47 | 10.51 | 9.98 | 10.53 | 10.39 | 10.15 | 10.79 | 10.35 | 9.23 | 9.44 | 9.44 |
| Mol% GC | 71.4 | 69.6 | 70 | 69.7 | 70 | 70.3 | 70.2 | 70.2 | 69.9 | 69.5 | 69.8 | 69.8 | 69.8 |
| No. of CDS | 7166 | 8416 | 7892 | 9011 | 8353 | 8867 | 8442 | 8611 | 8959 | 8506 | 7412 | 7445 | 7445 |
| Pseudogene | 159 | 216 | 286 | 211 | 270 | 307 | 272 | 257 | 197 | 131 | 140 | 140 | |
| . | Species designation (see legend) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| Growth at | |||||||||||||
| pH5 | + | + | + | – | – | – | – | + | + | − | NA | − | − |
| pH6 | ++ | ++ | + | − | + | + | + | ++ | + | + | +++ | +++ | ++ |
| pH7 | +++ | +++ | ++ | + | + | ++ | ++ | +++ | +++ | ++ | +++ | +++ | ++ |
| pH8 | ++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | ++ | +++ | +++ |
| pH9 | + | +++ | ++ | + | − | ++ | ++ | ++ | +++ | ++ | + | +++ | +++ |
| 30∘C | +++ | +++ | ++ | + | + | ++ | ++ | ++ | +++ | ++ | +++ | ++ | ++ |
| 35∘C | ++ | + | + | − | ++ | + | ++ | ++ | ++ | − | ++ | +++ | + |
| Biochemical reactions | |||||||||||||
| Esculin test | + | − | + | + | − | + | + | − | + | + | + | − | + |
| Gelatin test | + | + | + | − | − | − | + | + | + | + | + | + | + |
| Glucose assimilation | + | − | − | − | − | − | − | + | − | − | + | − | − |
| Maltose assimilation | + | + | − | − | − | − | − | − | − | + | + | − | − |
| Nitrate reduction | − | − | + | − | − | − | − | − | − | − | − | − | − |
| Antibiotic resistance | |||||||||||||
| Cefotaxime | − | + | + | + | + | + | + | + | − | + | − | − | − |
| Ceftazidime | + | + | + | + | + | + | + | + | + | + | − | − | − |
| Gentamicin | − | + | + | + | + | + | + | + | − | + | − | + | + |
| Trimethoprim sulfamethoxazole | + | − | − | − | − | − | − | − | − | − | − | + | + |
| Genome comparison | |||||||||||||
| Contigs | 234 | 36 | 459 | 1491 | 625 | 1244 | 802 | 961 | 530 | 863 | 62 | 68 | 68 |
| Genome size (Mb) | 8.48 | 10.41 | 9.47 | 10.51 | 9.98 | 10.53 | 10.39 | 10.15 | 10.79 | 10.35 | 9.23 | 9.44 | 9.44 |
| Mol% GC | 71.4 | 69.6 | 70 | 69.7 | 70 | 70.3 | 70.2 | 70.2 | 69.9 | 69.5 | 69.8 | 69.8 | 69.8 |
| No. of CDS | 7166 | 8416 | 7892 | 9011 | 8353 | 8867 | 8442 | 8611 | 8959 | 8506 | 7412 | 7445 | 7445 |
| Pseudogene | 159 | 216 | 286 | 211 | 270 | 307 | 272 | 257 | 197 | 131 | 140 | 140 | |
Cellular fatty acid composition of strain RDP092CA. tr, trace amount (below 0.1%).
| Fatty acid . | Percentage . | Equivalent chain length (ECL) values . |
|---|---|---|
| C10:0 | 0.53 | 9.997 |
| C11:0 | 0.23 | 11.001 |
| C14:0 | 0.90 | 14.001 |
| C15:0 | 0.46 | 15.001 |
| C16:0 | 1.59 | 15.996 |
| C16:1 w11c | 1.01 | 15.761 |
| C16:1 w5c | 1.44 | 15.905 |
| Total SCFA | 6.16 | |
| iso-C11:0 | 0.16 | 10.606 |
| iso-C13:0 | 0.98 | 12.616 |
| iso-C14:0 | 0.25 | 13.621 |
| iso-C14:1 | tr | 13.394 |
| iso-C15:0 | 33.58 | 14.625 |
| iso-C16:0 | 2.44 | 15.625 |
| iso-C17:0 | 10.12 | 16.625 |
| iso-C18:0 | 0.36 | 17.638 |
| iso-C14:0 3-OH | 15.21 | 15.114 |
| iso-C15:0 3-OH | 0.57 | 16.127 |
| iso-C15:1 w9c | 0.33 | 14.393 |
| iso-C17:1 w10c | 2.06 | 16.382 |
| Total BCFA | 66.15 |
| Fatty acid . | Percentage . | Equivalent chain length (ECL) values . |
|---|---|---|
| C10:0 | 0.53 | 9.997 |
| C11:0 | 0.23 | 11.001 |
| C14:0 | 0.90 | 14.001 |
| C15:0 | 0.46 | 15.001 |
| C16:0 | 1.59 | 15.996 |
| C16:1 w11c | 1.01 | 15.761 |
| C16:1 w5c | 1.44 | 15.905 |
| Total SCFA | 6.16 | |
| iso-C11:0 | 0.16 | 10.606 |
| iso-C13:0 | 0.98 | 12.616 |
| iso-C14:0 | 0.25 | 13.621 |
| iso-C14:1 | tr | 13.394 |
| iso-C15:0 | 33.58 | 14.625 |
| iso-C16:0 | 2.44 | 15.625 |
| iso-C17:0 | 10.12 | 16.625 |
| iso-C18:0 | 0.36 | 17.638 |
| iso-C14:0 3-OH | 15.21 | 15.114 |
| iso-C15:0 3-OH | 0.57 | 16.127 |
| iso-C15:1 w9c | 0.33 | 14.393 |
| iso-C17:1 w10c | 2.06 | 16.382 |
| Total BCFA | 66.15 |
Cellular fatty acid composition of strain RDP092CA. tr, trace amount (below 0.1%).
| Fatty acid . | Percentage . | Equivalent chain length (ECL) values . |
|---|---|---|
| C10:0 | 0.53 | 9.997 |
| C11:0 | 0.23 | 11.001 |
| C14:0 | 0.90 | 14.001 |
| C15:0 | 0.46 | 15.001 |
| C16:0 | 1.59 | 15.996 |
| C16:1 w11c | 1.01 | 15.761 |
| C16:1 w5c | 1.44 | 15.905 |
| Total SCFA | 6.16 | |
| iso-C11:0 | 0.16 | 10.606 |
| iso-C13:0 | 0.98 | 12.616 |
| iso-C14:0 | 0.25 | 13.621 |
| iso-C14:1 | tr | 13.394 |
| iso-C15:0 | 33.58 | 14.625 |
| iso-C16:0 | 2.44 | 15.625 |
| iso-C17:0 | 10.12 | 16.625 |
| iso-C18:0 | 0.36 | 17.638 |
| iso-C14:0 3-OH | 15.21 | 15.114 |
| iso-C15:0 3-OH | 0.57 | 16.127 |
| iso-C15:1 w9c | 0.33 | 14.393 |
| iso-C17:1 w10c | 2.06 | 16.382 |
| Total BCFA | 66.15 |
| Fatty acid . | Percentage . | Equivalent chain length (ECL) values . |
|---|---|---|
| C10:0 | 0.53 | 9.997 |
| C11:0 | 0.23 | 11.001 |
| C14:0 | 0.90 | 14.001 |
| C15:0 | 0.46 | 15.001 |
| C16:0 | 1.59 | 15.996 |
| C16:1 w11c | 1.01 | 15.761 |
| C16:1 w5c | 1.44 | 15.905 |
| Total SCFA | 6.16 | |
| iso-C11:0 | 0.16 | 10.606 |
| iso-C13:0 | 0.98 | 12.616 |
| iso-C14:0 | 0.25 | 13.621 |
| iso-C14:1 | tr | 13.394 |
| iso-C15:0 | 33.58 | 14.625 |
| iso-C16:0 | 2.44 | 15.625 |
| iso-C17:0 | 10.12 | 16.625 |
| iso-C18:0 | 0.36 | 17.638 |
| iso-C14:0 3-OH | 15.21 | 15.114 |
| iso-C15:0 3-OH | 0.57 | 16.127 |
| iso-C15:1 w9c | 0.33 | 14.393 |
| iso-C17:1 w10c | 2.06 | 16.382 |
| Total BCFA | 66.15 |
Phylogenomics and genomic analysis
The complete 16S rRNA gene sequence of RDP092CA was 1536 bp. The maximum-likelihood phylogenetic tree based on16S rRNA sequences grouped RDP092CA with C. interemptor, C. coralloides, and C. exiguus (Fig. 2). ANI and dDDH values are concordant with relationships suggested by the 16S rRNA tree (Fig. 3 and Table 4). RDP092CA had an ANI value of 92% with C. coralloides and 91% with C. interemptor and C. exiguus, and dDDH values of 44.8, 43.8, and 43.2 with C. coralloides, C. exiguus, and C. interemptor, respectively. The ANI values were less than 95% and dDDH values less than 70% (the species threshold as defined by Yoon et al., (2017)), compared to the closest type strains implying RDP092CA represents a novel species within the Corallococcus genus.
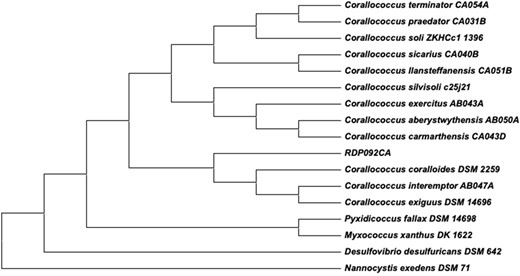
16S rRNA phylogenetic tree showing the position of strain RDP092CA among the 12 type strains of Corallococcus spp.
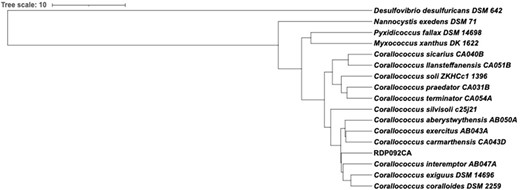
ANI-based phylogenetic tree showing the position of strain RDP092CA among the 12 type strains of Corallococcus spp.
ANI/dDDH. (1) RDP092/CA, (2) C. soli, (3) C. praedator, (4) C. terminator, (5) C. sicarius, (6) C . llansteffanensis, (7) C. exercitus, (8) C. aberystwythensis, (9) C. carmarthensis, (10) C. interemptor, (11) C. coralloides, (12) C. exiguous, and (13) C. silvisoli.
| ANI/dDDH value (%) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dDDH\ANI . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| 1 | 100 | 87 | 87 | 86 | 87 | 87 | 91 | 91 | 91 | 91 | 92 | 91 | 89 |
| 2 | 30.5 | 100 | 92 | 91 | 89 | 89 | 87 | 87 | 87 | 86 | 86 | 86 | 87 |
| 3 | 30.9 | 44 | 100 | 91 | 88 | 87 | 84 | 84 | 84 | 85 | 86 | 87 | 87 |
| 4 | 30.1 | 43 | 50 | 100 | 88 | 87 | 84 | 84 | 84 | 84 | 85 | 86 | 87 |
| 5 | 30.8 | 36 | 36 | 35 | 100 | 92 | 85 | 85 | 86 | 85 | 86 | 86 | 87 |
| 6 | 31.7 | 36 | 37 | 35 | 50 | 100 | 85 | 85 | 85 | 85 | 86 | 87 | 88 |
| 7 | 43 | 31 | 32 | 31 | 32 | 32 | 100 | 91 | 91 | 90 | 92 | 92 | 89 |
| 8 | 42.6 | 31 | 31 | 30 | 31 | 32 | 47 | 100 | 91 | 90 | 91 | 91 | 89 |
| 9 | 43.1 | 31 | 31 | 31 | 31 | 33 | 48 | 48 | 100 | 90 | 91 | 92 | 89 |
| 10 | 43.2 | 30 | 30 | 30 | 30 | 31 | 42 | 43 | 42 | 100 | 92 | 92 | 88 |
| 11 | 44.8 | 30 | 31 | 30 | 30 | 31 | 44 | 44 | 44 | 46 | 100 | 94 | 88 |
| 12 | 43.8 | 30 | 30 | 30 | 30 | 31 | 43 | 43 | 44 | 44 | 54 | 100 | 88 |
| 13 | 35.2 | 32 | 32 | 31 | 32 | 33 | 36 | 35 | 36 | 34 | 35 | 34 | 100 |
| ANI/dDDH value (%) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dDDH\ANI . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| 1 | 100 | 87 | 87 | 86 | 87 | 87 | 91 | 91 | 91 | 91 | 92 | 91 | 89 |
| 2 | 30.5 | 100 | 92 | 91 | 89 | 89 | 87 | 87 | 87 | 86 | 86 | 86 | 87 |
| 3 | 30.9 | 44 | 100 | 91 | 88 | 87 | 84 | 84 | 84 | 85 | 86 | 87 | 87 |
| 4 | 30.1 | 43 | 50 | 100 | 88 | 87 | 84 | 84 | 84 | 84 | 85 | 86 | 87 |
| 5 | 30.8 | 36 | 36 | 35 | 100 | 92 | 85 | 85 | 86 | 85 | 86 | 86 | 87 |
| 6 | 31.7 | 36 | 37 | 35 | 50 | 100 | 85 | 85 | 85 | 85 | 86 | 87 | 88 |
| 7 | 43 | 31 | 32 | 31 | 32 | 32 | 100 | 91 | 91 | 90 | 92 | 92 | 89 |
| 8 | 42.6 | 31 | 31 | 30 | 31 | 32 | 47 | 100 | 91 | 90 | 91 | 91 | 89 |
| 9 | 43.1 | 31 | 31 | 31 | 31 | 33 | 48 | 48 | 100 | 90 | 91 | 92 | 89 |
| 10 | 43.2 | 30 | 30 | 30 | 30 | 31 | 42 | 43 | 42 | 100 | 92 | 92 | 88 |
| 11 | 44.8 | 30 | 31 | 30 | 30 | 31 | 44 | 44 | 44 | 46 | 100 | 94 | 88 |
| 12 | 43.8 | 30 | 30 | 30 | 30 | 31 | 43 | 43 | 44 | 44 | 54 | 100 | 88 |
| 13 | 35.2 | 32 | 32 | 31 | 32 | 33 | 36 | 35 | 36 | 34 | 35 | 34 | 100 |
ANI/dDDH. (1) RDP092/CA, (2) C. soli, (3) C. praedator, (4) C. terminator, (5) C. sicarius, (6) C . llansteffanensis, (7) C. exercitus, (8) C. aberystwythensis, (9) C. carmarthensis, (10) C. interemptor, (11) C. coralloides, (12) C. exiguous, and (13) C. silvisoli.
| ANI/dDDH value (%) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dDDH\ANI . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| 1 | 100 | 87 | 87 | 86 | 87 | 87 | 91 | 91 | 91 | 91 | 92 | 91 | 89 |
| 2 | 30.5 | 100 | 92 | 91 | 89 | 89 | 87 | 87 | 87 | 86 | 86 | 86 | 87 |
| 3 | 30.9 | 44 | 100 | 91 | 88 | 87 | 84 | 84 | 84 | 85 | 86 | 87 | 87 |
| 4 | 30.1 | 43 | 50 | 100 | 88 | 87 | 84 | 84 | 84 | 84 | 85 | 86 | 87 |
| 5 | 30.8 | 36 | 36 | 35 | 100 | 92 | 85 | 85 | 86 | 85 | 86 | 86 | 87 |
| 6 | 31.7 | 36 | 37 | 35 | 50 | 100 | 85 | 85 | 85 | 85 | 86 | 87 | 88 |
| 7 | 43 | 31 | 32 | 31 | 32 | 32 | 100 | 91 | 91 | 90 | 92 | 92 | 89 |
| 8 | 42.6 | 31 | 31 | 30 | 31 | 32 | 47 | 100 | 91 | 90 | 91 | 91 | 89 |
| 9 | 43.1 | 31 | 31 | 31 | 31 | 33 | 48 | 48 | 100 | 90 | 91 | 92 | 89 |
| 10 | 43.2 | 30 | 30 | 30 | 30 | 31 | 42 | 43 | 42 | 100 | 92 | 92 | 88 |
| 11 | 44.8 | 30 | 31 | 30 | 30 | 31 | 44 | 44 | 44 | 46 | 100 | 94 | 88 |
| 12 | 43.8 | 30 | 30 | 30 | 30 | 31 | 43 | 43 | 44 | 44 | 54 | 100 | 88 |
| 13 | 35.2 | 32 | 32 | 31 | 32 | 33 | 36 | 35 | 36 | 34 | 35 | 34 | 100 |
| ANI/dDDH value (%) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| dDDH\ANI . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| 1 | 100 | 87 | 87 | 86 | 87 | 87 | 91 | 91 | 91 | 91 | 92 | 91 | 89 |
| 2 | 30.5 | 100 | 92 | 91 | 89 | 89 | 87 | 87 | 87 | 86 | 86 | 86 | 87 |
| 3 | 30.9 | 44 | 100 | 91 | 88 | 87 | 84 | 84 | 84 | 85 | 86 | 87 | 87 |
| 4 | 30.1 | 43 | 50 | 100 | 88 | 87 | 84 | 84 | 84 | 84 | 85 | 86 | 87 |
| 5 | 30.8 | 36 | 36 | 35 | 100 | 92 | 85 | 85 | 86 | 85 | 86 | 86 | 87 |
| 6 | 31.7 | 36 | 37 | 35 | 50 | 100 | 85 | 85 | 85 | 85 | 86 | 87 | 88 |
| 7 | 43 | 31 | 32 | 31 | 32 | 32 | 100 | 91 | 91 | 90 | 92 | 92 | 89 |
| 8 | 42.6 | 31 | 31 | 30 | 31 | 32 | 47 | 100 | 91 | 90 | 91 | 91 | 89 |
| 9 | 43.1 | 31 | 31 | 31 | 31 | 33 | 48 | 48 | 100 | 90 | 91 | 92 | 89 |
| 10 | 43.2 | 30 | 30 | 30 | 30 | 31 | 42 | 43 | 42 | 100 | 92 | 92 | 88 |
| 11 | 44.8 | 30 | 31 | 30 | 30 | 31 | 44 | 44 | 44 | 46 | 100 | 94 | 88 |
| 12 | 43.8 | 30 | 30 | 30 | 30 | 31 | 43 | 43 | 44 | 44 | 54 | 100 | 88 |
| 13 | 35.2 | 32 | 32 | 31 | 32 | 33 | 36 | 35 | 36 | 34 | 35 | 34 | 100 |
The draft genome size of the strain RDP092CA was 8 482 601 bp with a G + C content of 71.4% dispersed across 234 contigs, with a N50 of 198 581 bp and L50 of 14 (L50 referring to the longest 14 contigs making up more than half the size of the whole genome and the 14th largest contig having a size of 198 581 bp). The genome had 7371 coding genes and 55 RNA sequences, of which 4186 (57%) coded for putative functions, and the remaining 3185 (43%) were hypothetical proteins. Genome sequences of the 13 type strains of Corallococcus spp. harbour 13 BGCs of which, strain RDP092CA possesses 7 (Table 5). The RDP092CA genome was screened for AMPs using the DBAASP tool which retrieved a total of 52 predicted AMPs with possible antimicrobial properties. Based on their physiochemical and antimicrobial properties 21 AMPs were predicted to have ‘potent’ activity (Table 6), being predicted to have activity against fungi, viruses, and more than two bacteria.
BGCs. (1) Carotenoid, (2) VEPE/AEPE/TG-1, (3) Myxochelin-A/Myxochelin-B, (4) Geosmin, (5) Anabaenopeptin NZ857/Nostamide A, (6) Dawenol, (7) 1-nonadecene/(14Z)-1, 14-nonadecadiene, (8) 1-heptadecene, (9) RhizomideA/RhizomideB/RhizomideC, (10) IcosalideA/IcosalideB, (11) Xenotetrapeptide, (12) BicornutinA1/BicornutinA2, and (13) Bacillomycin.
| . | Percentage similarity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| RDP092CA | 100 | 100 | 83 | 100 | − | − | − | − | 100 | 100 | − | − | 20 |
| C. soli ZKHCc1 1396T | 100 | 100 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | − | − | − | 20 |
| C. praedator CA031BT | 100 | 80 | 33 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. terminator CA054AT | 100 | 80 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | 100 | − | − | − |
| C. sicarius CA040BT | 45 | 80 | 75 | 100 | − | 22 | − | − | 100 | − | − | − | 20 |
| C. llansteffanensis CA051BT | 27 | 80 | 83 | 100 | 100 | − | − | 100 | 100 | − | 100 | − | − |
| C. exercitus AB043AT | 90 | 80 | 33 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| C. aberystwythensis AB050AT | 63 | 100 | 75 | 100 | − | − | − | 100 | 100 | − | − | 100 | − |
| C. carmarthensis CA043DT | 100 | 100 | 83 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. interemptor AB047AT | 100 | 60 | 83 | 100 | − | − | − | 100 | 100 | − | − | − | 20 |
| C. coralloides DSM 2259T | 100 | 100 | 83 | 100 | − | − | − | − | − | − | − | − | − |
| C. exiguus DSM 14696T | 100 | 100 | 83 | 100 | − | − | 100 | − | − | − | − | − | 20 |
| C. silvisoli c25j21T | 100 | 100 | 83 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| . | Percentage similarity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| RDP092CA | 100 | 100 | 83 | 100 | − | − | − | − | 100 | 100 | − | − | 20 |
| C. soli ZKHCc1 1396T | 100 | 100 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | − | − | − | 20 |
| C. praedator CA031BT | 100 | 80 | 33 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. terminator CA054AT | 100 | 80 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | 100 | − | − | − |
| C. sicarius CA040BT | 45 | 80 | 75 | 100 | − | 22 | − | − | 100 | − | − | − | 20 |
| C. llansteffanensis CA051BT | 27 | 80 | 83 | 100 | 100 | − | − | 100 | 100 | − | 100 | − | − |
| C. exercitus AB043AT | 90 | 80 | 33 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| C. aberystwythensis AB050AT | 63 | 100 | 75 | 100 | − | − | − | 100 | 100 | − | − | 100 | − |
| C. carmarthensis CA043DT | 100 | 100 | 83 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. interemptor AB047AT | 100 | 60 | 83 | 100 | − | − | − | 100 | 100 | − | − | − | 20 |
| C. coralloides DSM 2259T | 100 | 100 | 83 | 100 | − | − | − | − | − | − | − | − | − |
| C. exiguus DSM 14696T | 100 | 100 | 83 | 100 | − | − | 100 | − | − | − | − | − | 20 |
| C. silvisoli c25j21T | 100 | 100 | 83 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
BGCs. (1) Carotenoid, (2) VEPE/AEPE/TG-1, (3) Myxochelin-A/Myxochelin-B, (4) Geosmin, (5) Anabaenopeptin NZ857/Nostamide A, (6) Dawenol, (7) 1-nonadecene/(14Z)-1, 14-nonadecadiene, (8) 1-heptadecene, (9) RhizomideA/RhizomideB/RhizomideC, (10) IcosalideA/IcosalideB, (11) Xenotetrapeptide, (12) BicornutinA1/BicornutinA2, and (13) Bacillomycin.
| . | Percentage similarity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| RDP092CA | 100 | 100 | 83 | 100 | − | − | − | − | 100 | 100 | − | − | 20 |
| C. soli ZKHCc1 1396T | 100 | 100 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | − | − | − | 20 |
| C. praedator CA031BT | 100 | 80 | 33 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. terminator CA054AT | 100 | 80 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | 100 | − | − | − |
| C. sicarius CA040BT | 45 | 80 | 75 | 100 | − | 22 | − | − | 100 | − | − | − | 20 |
| C. llansteffanensis CA051BT | 27 | 80 | 83 | 100 | 100 | − | − | 100 | 100 | − | 100 | − | − |
| C. exercitus AB043AT | 90 | 80 | 33 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| C. aberystwythensis AB050AT | 63 | 100 | 75 | 100 | − | − | − | 100 | 100 | − | − | 100 | − |
| C. carmarthensis CA043DT | 100 | 100 | 83 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. interemptor AB047AT | 100 | 60 | 83 | 100 | − | − | − | 100 | 100 | − | − | − | 20 |
| C. coralloides DSM 2259T | 100 | 100 | 83 | 100 | − | − | − | − | − | − | − | − | − |
| C. exiguus DSM 14696T | 100 | 100 | 83 | 100 | − | − | 100 | − | − | − | − | − | 20 |
| C. silvisoli c25j21T | 100 | 100 | 83 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| . | Percentage similarity . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . |
| RDP092CA | 100 | 100 | 83 | 100 | − | − | − | − | 100 | 100 | − | − | 20 |
| C. soli ZKHCc1 1396T | 100 | 100 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | − | − | − | 20 |
| C. praedator CA031BT | 100 | 80 | 33 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. terminator CA054AT | 100 | 80 | 83 | 100 | 100 | 88 | 100 | 100 | 100 | 100 | − | − | − |
| C. sicarius CA040BT | 45 | 80 | 75 | 100 | − | 22 | − | − | 100 | − | − | − | 20 |
| C. llansteffanensis CA051BT | 27 | 80 | 83 | 100 | 100 | − | − | 100 | 100 | − | 100 | − | − |
| C. exercitus AB043AT | 90 | 80 | 33 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| C. aberystwythensis AB050AT | 63 | 100 | 75 | 100 | − | − | − | 100 | 100 | − | − | 100 | − |
| C. carmarthensis CA043DT | 100 | 100 | 83 | 100 | 100 | − | − | − | 100 | − | − | − | − |
| C. interemptor AB047AT | 100 | 60 | 83 | 100 | − | − | − | 100 | 100 | − | − | − | 20 |
| C. coralloides DSM 2259T | 100 | 100 | 83 | 100 | − | − | − | − | − | − | − | − | − |
| C. exiguus DSM 14696T | 100 | 100 | 83 | 100 | − | − | 100 | − | − | − | − | − | 20 |
| C. silvisoli c25j21T | 100 | 100 | 83 | 100 | 100 | − | − | 100 | 100 | 100 | − | − | − |
| AMP . | Normalized hydrophobicity . | Net charge . | Amphiphilicity index . |
|---|---|---|---|
| RDP092CA_9 MASSPRRPRSYWRKLLGGCVVSIARVLAPTSVLADGPPVLDGRLRYGRVPVPVRGSQSLGIRGAPRLPG | −0.91 | 10 | 0.71 |
| RDP092 CA _10 MANSKSKHRRVQMKIRQHWKKRIKKQKEAAKAAAAEGKKK | 1.04 | 14 | 1.75 |
| RDP092 CA _21 MAWKCDLCGKRPLVGNNVSHANNKTKKRTLPNLQKLRANVNGSIERVLACTRCIKAGKVVKAA | 0 | 12 | 0.89 |
| RDP092 CA _36 MATKTQKQTIQRKARQMKAKTVKAVSRAGKQARRVQVTLGDLIAAAFDTVGGEARKVAKVVSSTDMTLATGRHIVFVG | −0.09 | 12 | 0.77 |
| RDP092 CA _48 MSDGLRMVSGMEAMMQTMLVFVPVALLVGAVVMIPRSRRRVRRWKVRAG | −0.95 | 7 | 0.67 |
| RDP092 CA _53 MSAAGRTRRTAHRMRGARRPESHDAPCIAFVMRRPAMGPPHAAPHTPDPTPLATQAPRKDRPACA | −0.47 | 8 | 0.6 |
| RDP092 CA _57 MFSTLWMGLMAGGWGRMKDALGELRARSHRLR | −0.55 | 4 | 1.02 |
| RDP092 CA _65 ARGFRQLLLRGLQKARGEWALICLTHNLLKLHRAQLAA | −0.55 | 6 | 0.91 |
| RDP092 CA _70 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092 CA _75 MAAKKKTAKKAATKKTATKKTARKAPAAKRGTAKKAAAKKPAAKKAAKKKTASRRKAAAPAALATPEA | 0.47 | 24 | 1.3 |
| RDP092 CA _81 MPPEFPARGVRRRDAARRGRAFLRVSVDGLWQ | −0.59 | 5 | 0.91 |
| RDP092 CA _92 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092 CA _104 MAKRTAGKAKGDTAVRRQGRRVMPALRRTAKRARVAAQKAQLTVGEVIAAAFDTAGGEMSGVLELVTSPQMTRALGRRIVVVG | −0.39 | 12 | 0.68 |
| RDP092 CA _115 MAGNERLGVARVGLGGAVATTAGAGMKLTRRGRVERLPERSRS | −0.33 | 6 | 0.63 |
| RDP092 CA _119 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_120 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092CA_138 MLPSMSTTRIRCHVKAEQAKEIISRDTLKRGRAHCNVLGAWN | −0.12 | 5 | 0.88 |
| RDP092CA_140 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_145 MATTKTRVPRTIDAYEVREAMRQRWSKLIAQVA | −0.39 | 4 | 1.11 |
| RDP092CA_146 MKVRASVKKICDKCKVVRRKGIVRVICASNPRHKQRQG | 0.38 | 12 | 1.17 |
| RDP092CA_147 MAKLSKIAQAKRKLKFPVRQYNRCGLCGRPRAFLRKFKMCRICLRHRALRGEITGVTKSSW | −0.18 | 17 | 1.17 |
| AMP . | Normalized hydrophobicity . | Net charge . | Amphiphilicity index . |
|---|---|---|---|
| RDP092CA_9 MASSPRRPRSYWRKLLGGCVVSIARVLAPTSVLADGPPVLDGRLRYGRVPVPVRGSQSLGIRGAPRLPG | −0.91 | 10 | 0.71 |
| RDP092 CA _10 MANSKSKHRRVQMKIRQHWKKRIKKQKEAAKAAAAEGKKK | 1.04 | 14 | 1.75 |
| RDP092 CA _21 MAWKCDLCGKRPLVGNNVSHANNKTKKRTLPNLQKLRANVNGSIERVLACTRCIKAGKVVKAA | 0 | 12 | 0.89 |
| RDP092 CA _36 MATKTQKQTIQRKARQMKAKTVKAVSRAGKQARRVQVTLGDLIAAAFDTVGGEARKVAKVVSSTDMTLATGRHIVFVG | −0.09 | 12 | 0.77 |
| RDP092 CA _48 MSDGLRMVSGMEAMMQTMLVFVPVALLVGAVVMIPRSRRRVRRWKVRAG | −0.95 | 7 | 0.67 |
| RDP092 CA _53 MSAAGRTRRTAHRMRGARRPESHDAPCIAFVMRRPAMGPPHAAPHTPDPTPLATQAPRKDRPACA | −0.47 | 8 | 0.6 |
| RDP092 CA _57 MFSTLWMGLMAGGWGRMKDALGELRARSHRLR | −0.55 | 4 | 1.02 |
| RDP092 CA _65 ARGFRQLLLRGLQKARGEWALICLTHNLLKLHRAQLAA | −0.55 | 6 | 0.91 |
| RDP092 CA _70 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092 CA _75 MAAKKKTAKKAATKKTATKKTARKAPAAKRGTAKKAAAKKPAAKKAAKKKTASRRKAAAPAALATPEA | 0.47 | 24 | 1.3 |
| RDP092 CA _81 MPPEFPARGVRRRDAARRGRAFLRVSVDGLWQ | −0.59 | 5 | 0.91 |
| RDP092 CA _92 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092 CA _104 MAKRTAGKAKGDTAVRRQGRRVMPALRRTAKRARVAAQKAQLTVGEVIAAAFDTAGGEMSGVLELVTSPQMTRALGRRIVVVG | −0.39 | 12 | 0.68 |
| RDP092 CA _115 MAGNERLGVARVGLGGAVATTAGAGMKLTRRGRVERLPERSRS | −0.33 | 6 | 0.63 |
| RDP092 CA _119 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_120 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092CA_138 MLPSMSTTRIRCHVKAEQAKEIISRDTLKRGRAHCNVLGAWN | −0.12 | 5 | 0.88 |
| RDP092CA_140 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_145 MATTKTRVPRTIDAYEVREAMRQRWSKLIAQVA | −0.39 | 4 | 1.11 |
| RDP092CA_146 MKVRASVKKICDKCKVVRRKGIVRVICASNPRHKQRQG | 0.38 | 12 | 1.17 |
| RDP092CA_147 MAKLSKIAQAKRKLKFPVRQYNRCGLCGRPRAFLRKFKMCRICLRHRALRGEITGVTKSSW | −0.18 | 17 | 1.17 |
| AMP . | Normalized hydrophobicity . | Net charge . | Amphiphilicity index . |
|---|---|---|---|
| RDP092CA_9 MASSPRRPRSYWRKLLGGCVVSIARVLAPTSVLADGPPVLDGRLRYGRVPVPVRGSQSLGIRGAPRLPG | −0.91 | 10 | 0.71 |
| RDP092 CA _10 MANSKSKHRRVQMKIRQHWKKRIKKQKEAAKAAAAEGKKK | 1.04 | 14 | 1.75 |
| RDP092 CA _21 MAWKCDLCGKRPLVGNNVSHANNKTKKRTLPNLQKLRANVNGSIERVLACTRCIKAGKVVKAA | 0 | 12 | 0.89 |
| RDP092 CA _36 MATKTQKQTIQRKARQMKAKTVKAVSRAGKQARRVQVTLGDLIAAAFDTVGGEARKVAKVVSSTDMTLATGRHIVFVG | −0.09 | 12 | 0.77 |
| RDP092 CA _48 MSDGLRMVSGMEAMMQTMLVFVPVALLVGAVVMIPRSRRRVRRWKVRAG | −0.95 | 7 | 0.67 |
| RDP092 CA _53 MSAAGRTRRTAHRMRGARRPESHDAPCIAFVMRRPAMGPPHAAPHTPDPTPLATQAPRKDRPACA | −0.47 | 8 | 0.6 |
| RDP092 CA _57 MFSTLWMGLMAGGWGRMKDALGELRARSHRLR | −0.55 | 4 | 1.02 |
| RDP092 CA _65 ARGFRQLLLRGLQKARGEWALICLTHNLLKLHRAQLAA | −0.55 | 6 | 0.91 |
| RDP092 CA _70 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092 CA _75 MAAKKKTAKKAATKKTATKKTARKAPAAKRGTAKKAAAKKPAAKKAAKKKTASRRKAAAPAALATPEA | 0.47 | 24 | 1.3 |
| RDP092 CA _81 MPPEFPARGVRRRDAARRGRAFLRVSVDGLWQ | −0.59 | 5 | 0.91 |
| RDP092 CA _92 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092 CA _104 MAKRTAGKAKGDTAVRRQGRRVMPALRRTAKRARVAAQKAQLTVGEVIAAAFDTAGGEMSGVLELVTSPQMTRALGRRIVVVG | −0.39 | 12 | 0.68 |
| RDP092 CA _115 MAGNERLGVARVGLGGAVATTAGAGMKLTRRGRVERLPERSRS | −0.33 | 6 | 0.63 |
| RDP092 CA _119 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_120 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092CA_138 MLPSMSTTRIRCHVKAEQAKEIISRDTLKRGRAHCNVLGAWN | −0.12 | 5 | 0.88 |
| RDP092CA_140 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_145 MATTKTRVPRTIDAYEVREAMRQRWSKLIAQVA | −0.39 | 4 | 1.11 |
| RDP092CA_146 MKVRASVKKICDKCKVVRRKGIVRVICASNPRHKQRQG | 0.38 | 12 | 1.17 |
| RDP092CA_147 MAKLSKIAQAKRKLKFPVRQYNRCGLCGRPRAFLRKFKMCRICLRHRALRGEITGVTKSSW | −0.18 | 17 | 1.17 |
| AMP . | Normalized hydrophobicity . | Net charge . | Amphiphilicity index . |
|---|---|---|---|
| RDP092CA_9 MASSPRRPRSYWRKLLGGCVVSIARVLAPTSVLADGPPVLDGRLRYGRVPVPVRGSQSLGIRGAPRLPG | −0.91 | 10 | 0.71 |
| RDP092 CA _10 MANSKSKHRRVQMKIRQHWKKRIKKQKEAAKAAAAEGKKK | 1.04 | 14 | 1.75 |
| RDP092 CA _21 MAWKCDLCGKRPLVGNNVSHANNKTKKRTLPNLQKLRANVNGSIERVLACTRCIKAGKVVKAA | 0 | 12 | 0.89 |
| RDP092 CA _36 MATKTQKQTIQRKARQMKAKTVKAVSRAGKQARRVQVTLGDLIAAAFDTVGGEARKVAKVVSSTDMTLATGRHIVFVG | −0.09 | 12 | 0.77 |
| RDP092 CA _48 MSDGLRMVSGMEAMMQTMLVFVPVALLVGAVVMIPRSRRRVRRWKVRAG | −0.95 | 7 | 0.67 |
| RDP092 CA _53 MSAAGRTRRTAHRMRGARRPESHDAPCIAFVMRRPAMGPPHAAPHTPDPTPLATQAPRKDRPACA | −0.47 | 8 | 0.6 |
| RDP092 CA _57 MFSTLWMGLMAGGWGRMKDALGELRARSHRLR | −0.55 | 4 | 1.02 |
| RDP092 CA _65 ARGFRQLLLRGLQKARGEWALICLTHNLLKLHRAQLAA | −0.55 | 6 | 0.91 |
| RDP092 CA _70 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092 CA _75 MAAKKKTAKKAATKKTATKKTARKAPAAKRGTAKKAAAKKPAAKKAAKKKTASRRKAAAPAALATPEA | 0.47 | 24 | 1.3 |
| RDP092 CA _81 MPPEFPARGVRRRDAARRGRAFLRVSVDGLWQ | −0.59 | 5 | 0.91 |
| RDP092 CA _92 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092 CA _104 MAKRTAGKAKGDTAVRRQGRRVMPALRRTAKRARVAAQKAQLTVGEVIAAAFDTAGGEMSGVLELVTSPQMTRALGRRIVVVG | −0.39 | 12 | 0.68 |
| RDP092 CA _115 MAGNERLGVARVGLGGAVATTAGAGMKLTRRGRVERLPERSRS | −0.33 | 6 | 0.63 |
| RDP092 CA _119 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_120 LFSRLKQNRRVATRYEKRAANYLAMVHIASIRLWL | −0.54 | 7 | 1.23 |
| RDP092CA_138 MLPSMSTTRIRCHVKAEQAKEIISRDTLKRGRAHCNVLGAWN | −0.12 | 5 | 0.88 |
| RDP092CA_140 LFNRLKQNRRVATRYEKRAANYLAMVQIASIRLWL | −0.55 | 7 | 1.22 |
| RDP092CA_145 MATTKTRVPRTIDAYEVREAMRQRWSKLIAQVA | −0.39 | 4 | 1.11 |
| RDP092CA_146 MKVRASVKKICDKCKVVRRKGIVRVICASNPRHKQRQG | 0.38 | 12 | 1.17 |
| RDP092CA_147 MAKLSKIAQAKRKLKFPVRQYNRCGLCGRPRAFLRKFKMCRICLRHRALRGEITGVTKSSW | −0.18 | 17 | 1.17 |
In vitro antimicrobial properties using live RDP092CA
Antimicrobial killing activities investigated by predation assays showed a good predatory activity against E. coli, Ps. aeruginosa, Cit. freundii, and Staph. aureus but not against Ent. faecalis. The antifungal activity via predation assays against eight the Candida spp. were varied, with good activity against Meyerozyma guillermondii DSM 6381, and low activity against C. auris DSM 21092 (Table 7). All five prey bacteria were prevented from forming biofilms and preformed biofilms were degraded to a similar extent (Fig. 4A). Similarly, there were significant antibiofilm activity against all eight Candida spp. tested (Fig. 4B). In the five-species wound-like biofilm model, there was some reduction in the TVCs but not significant (P > .05) (Fig. 4C).
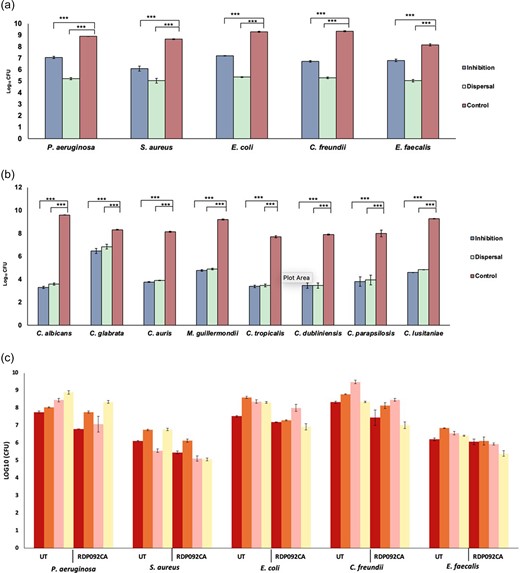
(A) Antibiofilm activities of live RDP092CA strain against bacteria. Statistical significance was determined by nonparametric one-way ANOVA (*** P < .05). (B) Antibiofilm activities against Candida spp. The significances were determined by nonparametric one-way ANOVA (*** P < .05). (C) Predatory activity of RDP092CA in the wound flow infection model (UT–untreated, RDPO92CA—treated with RDP092CA).
| Prey strains . | Predation zone sizes (mm) . |
|---|---|
| Staphylococcus aureus EMRSA-15 | 28.5 |
| Pseudomonas aeruginosa ATCC 9027 | 24 |
| Citrobacter freundii NCTC 6272 | 31 |
| Escherichia coli ATCC 10418 | 31 |
| Enterococcus faecalis ATCC 19433 | 10 |
| C. glabrata DSM 11226 | 22 |
| C. auris DSM 21092 | 18 |
| C. dubliniensis DSM 13268 | 20 |
| M. guillermondii DSM 6381 | 34 |
| C. parapsilosis DSM 4237 | 29 |
| C. lusitaniae DSM 70102 | 29 |
| C. albicans DSM 1386 | 19 |
| C. tropicalis DSM 1346 | 22 |
| Prey strains . | Predation zone sizes (mm) . |
|---|---|
| Staphylococcus aureus EMRSA-15 | 28.5 |
| Pseudomonas aeruginosa ATCC 9027 | 24 |
| Citrobacter freundii NCTC 6272 | 31 |
| Escherichia coli ATCC 10418 | 31 |
| Enterococcus faecalis ATCC 19433 | 10 |
| C. glabrata DSM 11226 | 22 |
| C. auris DSM 21092 | 18 |
| C. dubliniensis DSM 13268 | 20 |
| M. guillermondii DSM 6381 | 34 |
| C. parapsilosis DSM 4237 | 29 |
| C. lusitaniae DSM 70102 | 29 |
| C. albicans DSM 1386 | 19 |
| C. tropicalis DSM 1346 | 22 |
| Prey strains . | Predation zone sizes (mm) . |
|---|---|
| Staphylococcus aureus EMRSA-15 | 28.5 |
| Pseudomonas aeruginosa ATCC 9027 | 24 |
| Citrobacter freundii NCTC 6272 | 31 |
| Escherichia coli ATCC 10418 | 31 |
| Enterococcus faecalis ATCC 19433 | 10 |
| C. glabrata DSM 11226 | 22 |
| C. auris DSM 21092 | 18 |
| C. dubliniensis DSM 13268 | 20 |
| M. guillermondii DSM 6381 | 34 |
| C. parapsilosis DSM 4237 | 29 |
| C. lusitaniae DSM 70102 | 29 |
| C. albicans DSM 1386 | 19 |
| C. tropicalis DSM 1346 | 22 |
| Prey strains . | Predation zone sizes (mm) . |
|---|---|
| Staphylococcus aureus EMRSA-15 | 28.5 |
| Pseudomonas aeruginosa ATCC 9027 | 24 |
| Citrobacter freundii NCTC 6272 | 31 |
| Escherichia coli ATCC 10418 | 31 |
| Enterococcus faecalis ATCC 19433 | 10 |
| C. glabrata DSM 11226 | 22 |
| C. auris DSM 21092 | 18 |
| C. dubliniensis DSM 13268 | 20 |
| M. guillermondii DSM 6381 | 34 |
| C. parapsilosis DSM 4237 | 29 |
| C. lusitaniae DSM 70102 | 29 |
| C. albicans DSM 1386 | 19 |
| C. tropicalis DSM 1346 | 22 |
In vitro antimicrobial properties of the AMP RDP092CA_120
Predicted AMP RDP092CA_120 has a length of 35 amino acids, a molecular weight of 4246.062 Da, a net charge of +7 and is predicted (in silico by the DBAASP) to be potent against E. coli ATCC 25922, Ps. aeruginosa ATCC 27853, K. pneumoniae, Staph. aureus ATCC 25923, C. albicans, and S. cerevisiae. RDP092CA_120 was chosen for synthesis and tested against a panel of bacteria and yeast. AMP RDP092CA_120 exhibited high MICs (>512 μg/ml) against E. coli, Ps. aeruginosa, Cit. freundii, Staph. aureus, Ent. faecalis, and C. albicans in comparison to traditional antibiotics which had MICs ranging between 1 and 4 μg/ml. However, the AMP gave >50% inhibition of biofilm formation and >60% dispersion of biofilms at concentrations as low as 1 μg/ml, which is similar to traditional antibiotics and antifungal agents (Fig. 5).
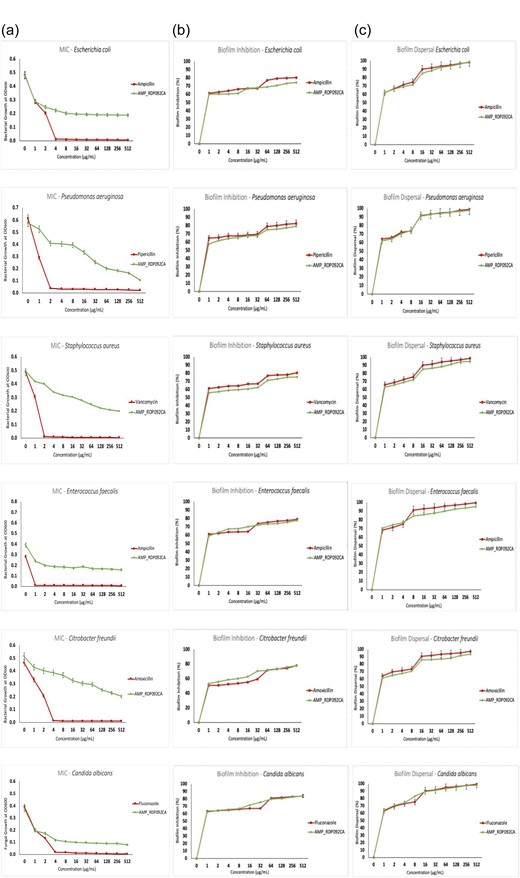
MIC and antibiofilm activities of AMP RDP092CA_120. (A) MIC measurements, (B) biofilm inhibition, and (C) biofilm dispersal.
Discussion
With 12 species currently validated, it is evident that the genus Corallococcus is diverse, being second only to Myxococcus spp. in environmental abundance. Although phenotypic properties including colonial morphology and biochemical profile shares features with other species of Corallococcus, genome sequence analysis clearly shows the distinctiveness of RDP092CA. Genome sequence-based taxonomy has identified novel myxobacterial species, and allowed a complete revamping of myxobacterial taxonomy, bringing insights and clarity into their diversity and evolution (Waite et al. 2020). RDP092CA had ANI values between 84% and 92%, and dGGDH values between 30 and 44.8, when compared against the 12 type species. Being lower than the species thresholds to other type strain genomes, these values indicate that RDP092CA is a novel species close to C. interemptor, C. coralloides, and C. exiguus. 16S rRNA sequence and ANI-based trees show a distinct clade comprising of RDP092CA, C. interemptor, C. coralloides, and C. exiguus. However, looking at their phenotypic characters, there are substantial differences between the four species.
Most studies have used E. coli as bait when isolating myxobacteria from soil. We used C. albicans as the prey in anticipation of selectively isolating myxobacteria that will predate on yeast-like organisms. Candida and non-Candida yeast cause a variety of infections in immunocompromised individuals (Leaw et al. 2007). This complicates treatment if multidrug resistant species like C. auris are present (Pallotta et al. 2023). Predation assays were performed against eight yeast type species of medical importance. RDP092CA showed good activity against M. guillermondii DSM 6381 (34 mm diameter clearance of prey lawns—Table 7) but poor activity against C. auris (17.5 mm). Infections of M. guillermondii, although rarer than C. albicans, can become challenging to treat due to their recalcitrant nature in complicated scenarios such as candidemia (Francisco et al. 2023). Interestingly, RDP092CA exhibited strong antibiofilm properties against all Candida spp.
Against bacterial prey, strain RDP092CA produced larger zones of predation against Staph. aureus and Cit. freundii but did not do well against Ent. faecalis. However, previous studies on myxobacterial predation have shown good activity against Gram-negative bacteria such as K. pneumoniae and E. coli and but less activity against Staph. aureus (Livingstone et al. 2017). Looking at antibiofilm activities such as biofilm inhibition and biofilm dispersal, strain RDP092CA performed very well against all five bacteria tested. There are limited studies on antibiofilm properties of myxobacteria, and studies have shown pathogenic bacteria like E. coli utilize biofilm formation as a potential strategy to evade the killing activity of Myxococcus xanthus (DePas et al. 2014). Therefore, strain RDP092CA exhibiting good antibiofilm activity offers an intriguing mechanism to counter this defence. There was little correlation between predation and antibiofilm activities. This may be because predation assays are carried out in a solid phase (agar growth) while antibiofilm activities are in liquid broth in multiwell plates, where killing mechanisms may differ due to varied growth conditions. We further tested the predatory activity in a complex biofilm-wound flow system comprising a five-species polymicrobial biofilm. RDP092CA and the five bacterial prey species were embedded in agarose plugs and then placed in a flow device immersed in SWF. There was no significant reduction in viable counts of five-species polymicrobial biofilm in the wound flow system. This may be attributed to the complex growth conditions in the wound flow system, providing additional challenge to the establishment and predatory activity of RDP092CA within the biofilm community. Previous studies with the same flow device showed the five bacterial species biofilm was inhibited by topical chemical agents such as HOCl and Neosporin, but the addition of live organisms offers another level of complexity that warrants further investigation (Khalid et al. 2023).
The genome of RDP092CA harboured BGCs, but fewer than in the genomes of other species such as C. soli, C. terminator, and C. silvisoli (Table 6). Our previous study on AMPs from eight type species of myxobacteria showed promising candidates with antimicrobial activities (Arakal et al. 2022). AMPs are increasingly being explored for antimicrobial properties due to the exponential rise of antimicrobial resistance (Huan et al. 2020). Therefore, we screened for AMPs in the genome of RDP092CA. Of the 152 AMPs predicted, 21 were predicted to be ‘potent’ with antimicrobial properties against two or more bacteria, fungi, and viruses. We chose one AMP, RDP092CA_120, to characterize its antimicrobial activity in vitro. The synthesized AMP showed high MICs of >512 μg/ml for all the five bacteria tested and C. albicans. However, when comparing the growth reduction in the MIC assays, the AMP seemed to inhibit C. albicans more than the bacteria (Fig. 4A). Interestingly, the AMP inhibited biofilm formation and killed preformed biofilms of the five bacteria and C. albicans similarly to the traditional antibiotics and antifungal agents used as positive controls (Fig. 4B and C), which warrants further study.
Description of Corallococcus senghenyddensis sp. nov
Corallococcus senghenyddensis (seng.hen.ydd.en'sis. N.L. masc. adj. senghenyddensis, indicating the isolation of the type strain from the town Senghenydd in South Wales, UK (51°35′43″N, 003°15′54″W).
Vegetative cells are nonmotile, Gram-negative bacilli measuring 1.2–1.4 μm by 3–6 μm. On VY-2 agar they appear as pale orange swarming colonies. Fruiting bodies are yellow to orange forming cartilaginous, hard horns, and coral-shaped structures. Myxospores within the fruiting bodies measure 0.8–1.5 μm in diameter. Cells grow at temperatures ranging between 20°C and 37°C, optimum temperature being 37°C. Growth is seen at pH between 5 and 9, optimum pH being 7. Cells produce catalase, gelatinase, pyrazinamidase esterase, lipase, leucine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, ⍺-glucosidase, and N-acetyl-β-glucosaminidase. Cells hydrolyse gelatin and esculin, ferment glucose, ribose, xylose, and maltose but not mannitol, lactose, glycogen, or sucrose. Cells are susceptible to cefotaxime and ceftazidime but resistant to gentamicin and trimethoprim sulfamethoxazole. The strain exhibited very good predatory activity (>30 mm) against Cit. freundii, Staph. aureus, and M. guillermondii and, showed strong antibiofilm activity (>95%) against all bacteria, C. lusitaniae, and C. tropicalis. Fatty acid analysis showed the presence of unusual amino acids such as C16:1 w11c and C16:1 w5c. Major fatty acids are iso-C15:0 (13-methyltetradecanoic), iso-C14:0 3-OH, and iso-C17:0 (isomargaric). DNA G + C content is 71.4 mol% and the genome size is 8 482 601 bp. The type strain is RDP092CA (=NBRC 116490T = CCOS 2109T), which was isolated in 2021 from soil collected from Senghenydd, Wales, UK. The sequenced genome is available in the NCBI database under the accession number JAYESI000000000.
Conflict of interest
The authors declare no conflict of interest.
Funding
New Lecturer Research Grant, Applied Microbiology International.
Author contributions
Benita S. Arakal (Formal analysis, Methodology, Writing – original draft), Richard S. Rowlands (Methodology), Michael McCarthy (Methodology), David E. Whitworth (Supervision, Writing – review & editing), Sarah E. Maddocks (Supervision, Writing – review & editing), Philip E. James (Supervision, Writing – review & editing), and Paul G. Livingstone (Conceptualization, Supervision, Writing – review & editing).
Data availability
Most data generated or analysed during this study are included in this published article and its supplementary information files. Those datasets generated during and/or analysed during the current study that are not published here are available from the corresponding author on reasonable request.



