-
PDF
- Split View
-
Views
-
Cite
Cite
Amanda Vaccalluzzo, Alessandra Pino, Raffaela Luisa Grimaldi, Cinzia Caggia, Stefano Cianci, Cinzia Lucia Randazzo, Lacticaseibacillus rhamnosus TOM 22.8 (DSM 33500) is an effective strategy for managing vaginal dysbiosis, rising the lactobacilli population, Journal of Applied Microbiology, Volume 135, Issue 5, May 2024, lxae110, https://doi.org/10.1093/jambio/lxae110
Close - Share Icon Share
Abstract
The present study is a single-centre, randomized, controlled clinical trial aimed to evaluate the effectiveness of the probiotic Lacticaseibacillus rhamnosus TOM 22.8 (DSM 33500) strain, orally administrated, to treat vaginal dysbiosis.
Overall, 80 women, with signs and symptoms of vaginal dysbiosis, were enrolled and allocated to the treatment group (A, n=60), who took 1 capsule of the probiotic strain for 10 consecutive days, or the non-treatment group (B, n=20), who did not receive any treatment. Clinical (vaginal signs and symptoms; pH of the vaginal fluid; Amsel criteria; Nugent score; Lactobacillary grade) and microbiological examinations were performed at baseline (T0), 10 days (T1), and 30 (T2) days after the oral administration of the probiotic TOM 22.8 strain. The latter resulted in a restoration of the physiological pH, accompanied by remission or attenuation of clinical signs and symptoms as well as the improvement of the quality of life (QoL). Microbiological data revealed a significant reduction of potentially pathogenic bacteria.
The administration of the L. rhamnosus TOM 22.8 probiotic strain could be proposed as an effective strategy for the treatment of vaginal dysbiosis.
The present study supports evidence on the effectiveness of the L. rhamnosus TOM 22.8 probiotic strain to balance the vaginal microbiota improving the QoL of women under reproductive age.
Introduction
The vaginal microbiota is a complex ecosystem, characterized by a community of microorganisms, that regulate several aspects of the women's health, such as fertility and susceptibility to infections (Smith and Ravel 2017). Among the vaginal microbiota population, lactobacilli have been suggested to be essential for establishing the homeostasis of the vaginal microbiome (Ravel et al. 2011; Vitale et al. 2021; Consortium HMP 2012). The health-promoting role of lactobacilli is related to the production of metabolites able to inhibit pathogens (e.g. D/L-lactate, hydrogen peroxide, and bacteriocins). In addition, lactic acid released by lactobacilli promotes low vaginal pH (pH ≤ 4.5) and protects against pathogens invasion (Hearps et al. 2017; Consortium HMP 2012). Finally, some strains create biofilms on the vaginal epithelium, allowing an effective colonization and contrasting the growth of pathogens (Barzegari et al. 2020; Chee et al. 2020; Meroni et al. 2021).
The term vaginal dysbiosis indicates a condition characterized by a reduction of lactobacilli and an overgrowth of pathogens, leading to a dysregulation of the vaginal homeostasis. Although specific clinical and microbiological features allow the definition of distinct entities, such as bacterial vaginosis (BV), anaerobic vaginitis (AV), and vulvovaginal candidiasis (VVC), different species of pathogens, both aerobic, anaerobic, and fungi, usually coexist leading to a multitude of clinical traits (Donders 2007, 2010). The estimated prevalence of BV ranges from 23% to 29% (Peebles et al. 2019), whereas 70%–75% of women worldwide experienced at least one episode of VVC during life (Blostein et al. 2017). Currently, antibiotic treatments are considered as the standard of care. However, although antibiotics are highly effective in relieving symptoms associated with acute infections, they are not selective against pathogens and often unable to provide a long-term protection against recurrences (Bradshaw et al. 2006; Workowski and Bolan 2015). Indeed, recurrence rates for BV and VVC are 83% and 60%, respectively (Marrazzo et al. 2019).
Several clinical studies showed that probiotic administration has better long-term effects, enhancing vaginal homeostasis and suppressing the overgrowth of harmful microorganisms (Wang et al. 2019; Han and Ren 2021). In addition, oral probiotics have also a positive impact on the vaginal microbiota through both immune regulation (Cohen et al. 2012; Eslami et al. 2016; Ho et al. 2016; Recine et al. 2016; Wijgert et al. 2020; Pino et al. 2021; 2023) and the ability to translocate from the gastrointestinal tract to the vagina (Nader-Macías et al. 2021).
The Lacticaseibacillus rhamnosus TOM 22.8 strain (DSM 33500) (patent number 102020000016666) was recently isolated by Pino and co-workers (Pino et al. 2019, 2021) from a healthy vaginal ecosystem and was developed, as a formulation of dietary supplement, by Uriach Italy (Assago, Milan, Italy). The TOM 22.8 strain, along with satisfying safety requirements (absence of DNAse, gelatinase, mucin degradation ability, haemolytic and bile salt hydrolase activities, as well as susceptibility to antibiotics suggested by the European Food Safety Authority), survives during the gastrointestinal transit as suggested by ex vivo experiments, adheres to both Caco-2 and VK2/E6E7 cell lines, and exerts antioxidant and anti-inflammatory activities (Pino et al. 2021). Interestingly, by using the agar spot test, the TOM 22.8 strain shows the ability to inhibit a plethora of pathogens responsible of urogenital tract dysbiosis, such as Escherichia coli, Gardnerella vaginalis, Candida spp., and Staphylococcus aureus (Pino et al. 2021). The TOM 22.8 probiotic strain was also administrated for 10 days on 30 patients, under reproductive age, with signs and symptoms of vaginal dysbiosis in an observation pilot study. A significant reduction of several vaginal pathogens was revealed on patients receiving oral or vaginal administration of the probiotic TOM 22.8 strain. In addition, the eubiosis of the vaginal ecosystem was maintained till 30 days after the end of the treatment (Pino et al. 2021).
Based on the data mentioned above, the present study aimed to evaluate the efficacy of the TOM 22.8 strain to restore the physiological conditions of the vaginal microbiota in a wider range of women with vaginal dysbiosis.
Materials and methods
Study population
A single-centre, randomized, controlled clinical trial was performed at the Department of General Surgery and Medical Surgical Specialties, University of Catania (Italy). All healthy women, who voluntarily accepted to participate in the study and who met specific inclusion criteria [age between 18 and 45 years; presence of leucorrhoea, burning, itching, or subjective vaginal discomfort; at least 3 Amsel criteria (Amsel et al. 1983); Nugent score ≥ 7 (Nugent et al. 1991); lactobacillary grade (LBG) ≥ II (Donders 2007)], were enrolled. All the women presenting one or more of the following criteria were excluded: presence of sexually transmitted disease due to Chlamydia, Neisseria gonorrhoeae, and Trichomonas vaginalis; herpes simplex, human papillomavirus or human immunodeficiency virus infection; confirmed diagnosis of pelvic inflammatory disease; use of vaginal contraceptives, antibiotic, antifungal, probiotic or immunosuppressive drugs in the previous month and any others physiological or pathological conditions that could potentially interfere with the results of the study (e.g. pregnancy or breastfeeding, chronic or neoplastic diseases). Participants were informed about the purpose of the study and the opportunity to freely leave the study at any time. Personal data were anonymously treated according to the Italian law guaranteeing privacy. Each woman who participated in the study was informed regarding the procedures she underwent and signed an informed consent form for data collection. Participation in the study was strictly voluntary and no remuneration was offered.
Study design
The study design was in accordance with the Helsinki Declaration, conforms to the Committee on Publication Ethics guidelines (Wager 2012) and was approved by the Institutional Review Board of the university hospital in which it was performed (registration number 157/2019/PO, 27/04/2022). All the design, analysis, interpretation of data, drafting, and revisions followed the CONSORT (Consolidated Standards of Reporting Trials) and SPIRIT (Standard Protocol Items: Recommendation for Interventional Trials) Statements, available through the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) network. The sample size was determined based on feasibility considerations. However, tolerating a type I error of 0.05 and setting 1-β = 0.80, we estimate that a total of 60 patients, meeting the inclusion and exclusion criteria, described above, allowed us to confidently assess medium effect sizes (∼0.5) when comparing continuous or categorical measures of the designed experimental groups.
As reported in Fig. 1, after the preliminary evaluation, 114 women satisfied the inclusion criteria. However, after the baseline assessment, a total of 10 subjects were excluded for unconfirmed microbiological diagnosis of vaginal dysbiosis. Recruited patients were randomly allocated, based on a 3:1 ratio, to the treatment group (A, n = 78) or the non-treatment group (B, n = 26). Out of the 104 participants, 80 completed all the steps designed for the study by adhering to the therapeutic regime and undergoing scheduled follow-up visits. The remaining 24 patients (18 from the treatment group and 6 from the non-treatment group) left the study for the following reasons: lost to follow-up, fever for seasonal flu, pregnancy, worsening of symptoms, and others.
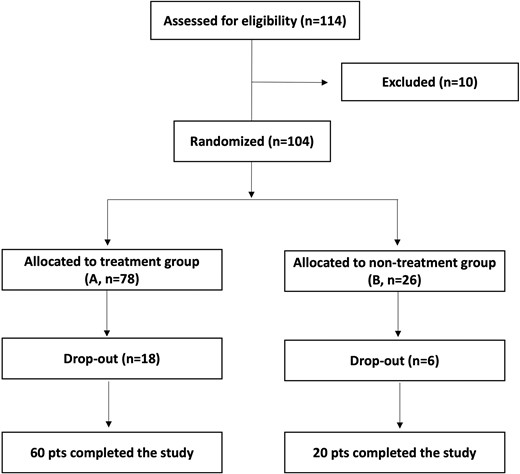
Patients allocated to treatment group (A) took 1 capsule of the probiotic L. rhamnosus TOM 22.8 strain for 10 consecutive days. Each active capsule contained 10 × 109 cfu of the TOM 22.8 strain, dried starch (118.9 mg), magnesium stearate (4.05 mg), and silicon dioxide (4.05 mg). Patients allocated to the non-treatment group (B) did not undergo any treatment. Examinations of each woman were scheduled in three appointments: at baseline (T0), 10 days (T1), and 30 days (T2) after the start of the oral administration of the probiotic TOM 22.8 strain. Each woman reported in a personal diary, any eventual adverse reaction, worsening of symptomatology, or any use of medication, which were carefully documented at each follow-up.
Clinical evaluation
Information about age, smoking habit, relationship status, body mass index, sexual activity, contraceptive use, allergies, and chronic diseases were collected before the treatment. At baseline (T0), 10 days (T1), and 30 days (T2) after the start of the treatment, both vaginal signs and symptoms were evaluated using a 3-point severity score (0, absent or normal; 3, severe), whereas the pH of the vaginal fluid was measured using pH test strips (McKesson, San Francisco, CA, USA). For the purposes of results interpretation, the presence of at least three Amsel criteria was assumed for BV. A Nugent score of 0–3 was interpreted as Lactobacillus-predominant normal vaginal microbiota, a score of 4–6 was considered as intermediate, and a score of 7–10 was assumed as BV-like condition, with the dominance of small Gram-negative and Gram-variable straight and curved rods (Nugent et al. 1991). The LBG was evaluated according to Donders classification. In detail, LBG I was assumed for a normal flora with predominantly lactobacilli, LBG II corresponded to a diminished lactobacillary flora mixed with other bacteria, and grade III was defined as an abnormal flora with several other bacteria and absence of lactobacilli (Donders 2007). In addition, the Short Form-36 (SF-36) questionnaire was administered to assess the quality of life (QoL) at baseline (T0) and 30 days after the start of the treatment (T2) (Ware et al. 1998). The SF-36 questionnaire contains 36 questions grouped into eight categories: physical functioning (10 items), physical role functioning (4 items), bodily pain (2 items), general health (6 items), vitality (4 items), social functioning (2 items), emotional role functioning (3 items), and emotional well-being (5 items). Women were instructed to place a mark, based on their feelings, using a scale from 0 (worst) to 100 (best). For each category, the mean score value was calculated.
The primary endpoints for resolution of the pathological condition were defined as: negative results for at least 2 Amsel criteria, Nugent score lower than 7, change in the microbiota composition with a significant reduction of potential pathogenic bacteria and increase of lactobacilli (at least 3 log cfu/swab).
The secondary endpoints were absence of vaginal symptoms, by subjective evaluation, and improvement of QoL.
Vaginal discharge samples collection and analysis
Vaginal discharge samples were obtained from the lateral vaginal wall and the posterior vaginal fornix using sterile cotton-tipped swabs and immediately transferred, under refrigerated conditions, to the Laboratory of ProBioEtna, spinoff of the University of Catania (Catania, Italy), for the examination. Three swabs were used for clinical criteria evaluation, microbial count, and DNA isolation, respectively. For microbiological analysis, vaginal discharge, collected using swabs filled with transport medium (Transystem Amies Medium Clear, Biolife, Milan, Italy) was treated as previously reported (Pino et al. 2021). Microbiological count was performed in triplicate and results were reported as mean log cfu ml−1 and standard deviation.
Detection of lactobacilli through qPCR
To quantify the Lactobacillus population, the DNA isolated from vaginal specimens were subjected to quantitative PCR (qPCR) analysis. In detail, ice-thawed vaginal swabs preserved in PowerBead Solution (Qiagen GmbH, Hilden, Germany) were used to total genomic DNA isolation. To assure the release of vaginal discharge, the swab was aseptically pressed and rotated several times on the inner wall of the sampling tube. DNA was isolated using the QIAamp DNA Minikit (Qiagen GmbH, Hilden, Germany) and following the Repeated Bead Beating method reported by Virtanen and co-workers (2017) and Randazzo and co-workers (2015). DNA concentration was evaluated by using the fluorimeter Qubit 4.0 (Invitrogen, Carlsbad, CA, USA). Lactobacilli were quantified by qPCR using the primer pairs and conditions reported by Bornes and co-workers (2021). A standard curve for qPCR assays was constructed as follow. The L. rhamnosus TOM 22.8 strain was cultured in the De Man, Rogosa and Sharpe medium and subjected to total DNA isolation as reported by Pino and co-workers (2021). qPCR standard curves were obtained by serially dilution of the isolated DNA with a concentration ranging from 8.7 to 2.7 log cfu ml−1. To ensure the amplification specificity, a melting curve analysis was performed considering a range of temperature between 70°C and 95°C (Vaccalluzzo et al. 2023). To perform the qPCR reactions, the QuantiFast SYBR Green qPCR kit (Qiagen GmbH, Hilden, Germany) was used on a Rotor Gene Q instrument (Qiagen GmbH, Hilden, Germany). Each reaction was repeated at least three times.
Statistical analysis
Categorical and continuous data were represented by frequencies and means ± standard deviations. Normality of data was assessed through the Shapiro–Wilk test. Skewed variables were logarithmically transformed and then standardized. Comparison between the groups was assessed by Fisher’s Exact Test for Count Data, Student t-test or Wilcoxon rank sum test with continuity correction for categorical and continuous variables, respectively. Quality of life and microbiological data were subjected to ANOVA. Differences were considered statistically significant at P < 0.05.
Results
The present study was two arms (treatment and non-treatment groups), single-centre, randomized, controlled clinical trial aimed to evaluate the effectiveness of the oral administration of the probiotic TOM 22.8 strain on volunteers with vaginal dysbiosis. For this reason, Amsel criteria, Nugent score, and LBG were chosen as diagnostic parameters. In addition, a 3-point severity score (0, absent or normal; 3, severe) was used for vaginal signs and symptoms and the SF-36 questionnaire was administrated to evaluate changes in the perceived QoL. The composition and dynamic of the vaginal microbial population were achieved by applying a culture-dependent approach and the Lactobacillus population was quantified by performing a qPCR analysis.
Baseline characteristics of the enrolled patients
Overall, 80 patients with clinical signs and symptoms of vaginal dysbiosis, allocated in the treatment group (A, n = 60) and in the non-treatment group (B, n = 20), completed the study. The demographic and clinical characteristics (sexual activity; smoking habits; body mass index; and contraceptive use), of the population under study, are shown in Table 1. Concerning demographic characteristics, enrolled patients had a mean age of 34.35 ± 6.96 years and the majority of them were sexualy active (75%), with a body mass index ranging from 18.5 to 24.9 (85%), and contraceptive user (61.25%). Based on clinical characteristics revealed at baseline (T0), all enrolled women (100%) reported leucorrhoea and subjective vulvar discomfort, while burning and itching were reported in 37.50% and 46.25% of cases, respectively (Table 1). Homogenous vaginal discharge, presence of clue cells, positive amine test, and vaginal pH > 4.5 were observed in more than 90% of the population under study. In addition, 98.75% of patients had Nugent score ranging between 7 and 10, whereas the LBG was II and III in 45% and 55% of the population, respectively (Table 1).
Baseline demographics and clinical characteristics of the study population (n = 80).
| Demographic characteristics (n = 80) . | |
|---|---|
| Age | 34.35 ± 6.96 |
| Sexual activity | 60 (75%) |
| Smoking | 23 (28.75%) |
| Body mass index (kg/m2) | 22.21 ± 2.75 |
| <18.5 | 1 (1.25%) |
| 18.5–24.9 | 68 (85%) |
| 25–29.9 | 11 (13.75%) |
| Contraceptive use | 49 (61.25%) |
| Oral | 15 (30.61%) |
| Barrier | 16 (32.65%) |
| Others | 18 (36.74%) |
| Clinical characteristics (n = 80) | |
| Vulvovaginal signs and symptoms | |
| Leucorrhoea | 80 (100%) |
| Burning | 30 (37.50%) |
| Itching | 37 (46.25%) |
| Subjective vulvar discomfort | 80 (100%) |
| Amsel Criteria | |
| Homogenous vaginal discharge | 79 (98.75%) |
| Clue cell presence | 75 (93.75%) |
| Positive amine test | 78 (97.50%) |
| Vaginal pH > 4.5 | 77 (96.25%) |
| Nugent score | |
| 0–3 | 0 (0%) |
| 4–6 | 1 (1.25%) |
| 7–10 | 79 (98.75%) |
| Lactobacillary grade | |
| I | 0 (0%) |
| II | 36 (45%) |
| III | 44 (55%) |
| Demographic characteristics (n = 80) . | |
|---|---|
| Age | 34.35 ± 6.96 |
| Sexual activity | 60 (75%) |
| Smoking | 23 (28.75%) |
| Body mass index (kg/m2) | 22.21 ± 2.75 |
| <18.5 | 1 (1.25%) |
| 18.5–24.9 | 68 (85%) |
| 25–29.9 | 11 (13.75%) |
| Contraceptive use | 49 (61.25%) |
| Oral | 15 (30.61%) |
| Barrier | 16 (32.65%) |
| Others | 18 (36.74%) |
| Clinical characteristics (n = 80) | |
| Vulvovaginal signs and symptoms | |
| Leucorrhoea | 80 (100%) |
| Burning | 30 (37.50%) |
| Itching | 37 (46.25%) |
| Subjective vulvar discomfort | 80 (100%) |
| Amsel Criteria | |
| Homogenous vaginal discharge | 79 (98.75%) |
| Clue cell presence | 75 (93.75%) |
| Positive amine test | 78 (97.50%) |
| Vaginal pH > 4.5 | 77 (96.25%) |
| Nugent score | |
| 0–3 | 0 (0%) |
| 4–6 | 1 (1.25%) |
| 7–10 | 79 (98.75%) |
| Lactobacillary grade | |
| I | 0 (0%) |
| II | 36 (45%) |
| III | 44 (55%) |
Baseline demographics and clinical characteristics of the study population (n = 80).
| Demographic characteristics (n = 80) . | |
|---|---|
| Age | 34.35 ± 6.96 |
| Sexual activity | 60 (75%) |
| Smoking | 23 (28.75%) |
| Body mass index (kg/m2) | 22.21 ± 2.75 |
| <18.5 | 1 (1.25%) |
| 18.5–24.9 | 68 (85%) |
| 25–29.9 | 11 (13.75%) |
| Contraceptive use | 49 (61.25%) |
| Oral | 15 (30.61%) |
| Barrier | 16 (32.65%) |
| Others | 18 (36.74%) |
| Clinical characteristics (n = 80) | |
| Vulvovaginal signs and symptoms | |
| Leucorrhoea | 80 (100%) |
| Burning | 30 (37.50%) |
| Itching | 37 (46.25%) |
| Subjective vulvar discomfort | 80 (100%) |
| Amsel Criteria | |
| Homogenous vaginal discharge | 79 (98.75%) |
| Clue cell presence | 75 (93.75%) |
| Positive amine test | 78 (97.50%) |
| Vaginal pH > 4.5 | 77 (96.25%) |
| Nugent score | |
| 0–3 | 0 (0%) |
| 4–6 | 1 (1.25%) |
| 7–10 | 79 (98.75%) |
| Lactobacillary grade | |
| I | 0 (0%) |
| II | 36 (45%) |
| III | 44 (55%) |
| Demographic characteristics (n = 80) . | |
|---|---|
| Age | 34.35 ± 6.96 |
| Sexual activity | 60 (75%) |
| Smoking | 23 (28.75%) |
| Body mass index (kg/m2) | 22.21 ± 2.75 |
| <18.5 | 1 (1.25%) |
| 18.5–24.9 | 68 (85%) |
| 25–29.9 | 11 (13.75%) |
| Contraceptive use | 49 (61.25%) |
| Oral | 15 (30.61%) |
| Barrier | 16 (32.65%) |
| Others | 18 (36.74%) |
| Clinical characteristics (n = 80) | |
| Vulvovaginal signs and symptoms | |
| Leucorrhoea | 80 (100%) |
| Burning | 30 (37.50%) |
| Itching | 37 (46.25%) |
| Subjective vulvar discomfort | 80 (100%) |
| Amsel Criteria | |
| Homogenous vaginal discharge | 79 (98.75%) |
| Clue cell presence | 75 (93.75%) |
| Positive amine test | 78 (97.50%) |
| Vaginal pH > 4.5 | 77 (96.25%) |
| Nugent score | |
| 0–3 | 0 (0%) |
| 4–6 | 1 (1.25%) |
| 7–10 | 79 (98.75%) |
| Lactobacillary grade | |
| I | 0 (0%) |
| II | 36 (45%) |
| III | 44 (55%) |
As reported in Table 2, the randomly allocation (3:1 ratio) of patients in in treatment group (A, n = 60) or non-treatment group (B, n = 20) allowed to gain, at baseline, homogeneous groups for all the demographic parameters (P > 0.05).
Statistical comparison among baseline demographic data between treatment group (A) and non-treatment group (B).
| Demographic characteristics . | Total (n = 80) . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | P-value * . |
|---|---|---|---|---|
| Age | 34.35 ± 6.96 | 34.7 ± 6.96 | 33.2 ± 7.02 | 0.3823 |
| Sexual activity | 60 (75%) | 44 (73.3%) | 16 (80%) | 0.7667 |
| Smoking | 23 (28.75%) | 19 (31.7%) | 4 (20%) | 0.4006 |
| Body mass index (kg/m2) | 22.21 ± 2.75 | 22.2 ± 2.76 | 22.2 ± 2.82 | 1.0000 |
| <18.5 | 1 (1.25%) | 1 (1.66%) | 0 (0%) | 1.0000 |
| 18.5–24.9 | 68 (85%) | 50 (83.3%) | 18 (90%) | 0.7203 |
| 25–29.9 | 11 (13.75%) | 9 (15%) | 2 (10%) | 0.7216 |
| Contraceptive use | 49 (61.25%) | 37 (61.7%) | 12 (60%) | 1.0000 |
| Oral | 15 (30.61%) | 10 (27.03%) | 5 (41.67%) | 0.4727 |
| Barrier | 16 (32.65%) | 12 (32.43%) | 4 (33.33%) | 1.0000 |
| Others | 18 (36.74%) | 15 (40.54%) | 3 (25%) | 0.4942 |
| Demographic characteristics . | Total (n = 80) . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | P-value * . |
|---|---|---|---|---|
| Age | 34.35 ± 6.96 | 34.7 ± 6.96 | 33.2 ± 7.02 | 0.3823 |
| Sexual activity | 60 (75%) | 44 (73.3%) | 16 (80%) | 0.7667 |
| Smoking | 23 (28.75%) | 19 (31.7%) | 4 (20%) | 0.4006 |
| Body mass index (kg/m2) | 22.21 ± 2.75 | 22.2 ± 2.76 | 22.2 ± 2.82 | 1.0000 |
| <18.5 | 1 (1.25%) | 1 (1.66%) | 0 (0%) | 1.0000 |
| 18.5–24.9 | 68 (85%) | 50 (83.3%) | 18 (90%) | 0.7203 |
| 25–29.9 | 11 (13.75%) | 9 (15%) | 2 (10%) | 0.7216 |
| Contraceptive use | 49 (61.25%) | 37 (61.7%) | 12 (60%) | 1.0000 |
| Oral | 15 (30.61%) | 10 (27.03%) | 5 (41.67%) | 0.4727 |
| Barrier | 16 (32.65%) | 12 (32.43%) | 4 (33.33%) | 1.0000 |
| Others | 18 (36.74%) | 15 (40.54%) | 3 (25%) | 0.4942 |
Statistical significance at P < 0.05 comparing treatment and non-treatment groups.
Statistical comparison among baseline demographic data between treatment group (A) and non-treatment group (B).
| Demographic characteristics . | Total (n = 80) . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | P-value * . |
|---|---|---|---|---|
| Age | 34.35 ± 6.96 | 34.7 ± 6.96 | 33.2 ± 7.02 | 0.3823 |
| Sexual activity | 60 (75%) | 44 (73.3%) | 16 (80%) | 0.7667 |
| Smoking | 23 (28.75%) | 19 (31.7%) | 4 (20%) | 0.4006 |
| Body mass index (kg/m2) | 22.21 ± 2.75 | 22.2 ± 2.76 | 22.2 ± 2.82 | 1.0000 |
| <18.5 | 1 (1.25%) | 1 (1.66%) | 0 (0%) | 1.0000 |
| 18.5–24.9 | 68 (85%) | 50 (83.3%) | 18 (90%) | 0.7203 |
| 25–29.9 | 11 (13.75%) | 9 (15%) | 2 (10%) | 0.7216 |
| Contraceptive use | 49 (61.25%) | 37 (61.7%) | 12 (60%) | 1.0000 |
| Oral | 15 (30.61%) | 10 (27.03%) | 5 (41.67%) | 0.4727 |
| Barrier | 16 (32.65%) | 12 (32.43%) | 4 (33.33%) | 1.0000 |
| Others | 18 (36.74%) | 15 (40.54%) | 3 (25%) | 0.4942 |
| Demographic characteristics . | Total (n = 80) . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | P-value * . |
|---|---|---|---|---|
| Age | 34.35 ± 6.96 | 34.7 ± 6.96 | 33.2 ± 7.02 | 0.3823 |
| Sexual activity | 60 (75%) | 44 (73.3%) | 16 (80%) | 0.7667 |
| Smoking | 23 (28.75%) | 19 (31.7%) | 4 (20%) | 0.4006 |
| Body mass index (kg/m2) | 22.21 ± 2.75 | 22.2 ± 2.76 | 22.2 ± 2.82 | 1.0000 |
| <18.5 | 1 (1.25%) | 1 (1.66%) | 0 (0%) | 1.0000 |
| 18.5–24.9 | 68 (85%) | 50 (83.3%) | 18 (90%) | 0.7203 |
| 25–29.9 | 11 (13.75%) | 9 (15%) | 2 (10%) | 0.7216 |
| Contraceptive use | 49 (61.25%) | 37 (61.7%) | 12 (60%) | 1.0000 |
| Oral | 15 (30.61%) | 10 (27.03%) | 5 (41.67%) | 0.4727 |
| Barrier | 16 (32.65%) | 12 (32.43%) | 4 (33.33%) | 1.0000 |
| Others | 18 (36.74%) | 15 (40.54%) | 3 (25%) | 0.4942 |
Statistical significance at P < 0.05 comparing treatment and non-treatment groups.
Diagnostic parameters
Diagnostic parameters (Amsel criteria, Nugent score, and LBG), detected in treatment (n = 60) and non-treatment (n = 20) groups, are shown in Table 3. In detail, the presence of three out of four Amsel criteria (presence of homogeneous, thin, greyish-white vaginal discharge; vaginal pH > 4.5; positivity to the whiff–amine test evaluated by the release of fishy odor after adding 10% potassium hydroxide solution to wet mount; and presence of clue cells on a wet mount microscopy of the vaginal fluid) is necessary for the clinical diagnosis of BV. The Nugent score, considered as a landmark for the microscopic examination of the vaginal smear, is a laboratory-based method used to assess alterations in vaginal microbial population. The Nugent score is assessed on a 10-point scale by optical microscopic observation, under oil immersion, of Gram-stained vaginal smear and the enumeration of the observed morphotypes. A score of 0–3 is interpreted as Lactobacillus-predominant normal vaginal microbiota, a score of 4–6 is considered as intermediate, whereas a score of 7–10, with the dominance of small Gram-negative and Gram-variable straight and curved rods, is assumed as BV-like condition. The LBG was evaluated according to Donders classification as follow: LBG I was assumed for a normal microbiota with predominant lactobacillary morphotypes, LBG II corresponded to a reduction of the lactobacillary population and a concomitant presence of other bacteria, whereas LBG III was defined as an abnormal flora consisting of several other bacteria with the absence of the lactobacillary population.
Diagnostic parameters detected in treatment (A) and non-treatment (B) groups at baseline (T0), 10 days (T1), and 30 days after the start of the treatment (T2).
| Diagnostic parameters . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | ||||
|---|---|---|---|---|---|---|
| . | T0 . | T1 . | T2 . | T0 . | T1 . | T2 . |
| Amsel criteria | ||||||
| Homogenous vaginal discharge | 60 | 5 | 6 | 19 | 16 | 17 |
| Clue cell presence | 58 | 3 | 0 | 17 | 14 | 13 |
| Positive amine test | 60 | 2 | 0 | 18 | 13 | 17 |
| Vaginal pH > 4.5 | 60 | 2 | 0 | 17 | 16 | 18 |
| Nugent score | ||||||
| 0–3 | 0 | 58 | 60 | 0 | 0 | 0 |
| 4–6 | 0 | 2 | 0 | 1 | 11 | 4 |
| 7–10 | 60 | 0 | 0 | 19 | 9 | 16 |
| Lactobacillary grade | ||||||
| I | 0 | 59 | 60 | 0 | 0 | 0 |
| II | 0 | 1 | 0 | 0 | 2 | 0 |
| III | 60 | 0 | 0 | 20 | 18 | 20 |
| Diagnostic parameters . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | ||||
|---|---|---|---|---|---|---|
| . | T0 . | T1 . | T2 . | T0 . | T1 . | T2 . |
| Amsel criteria | ||||||
| Homogenous vaginal discharge | 60 | 5 | 6 | 19 | 16 | 17 |
| Clue cell presence | 58 | 3 | 0 | 17 | 14 | 13 |
| Positive amine test | 60 | 2 | 0 | 18 | 13 | 17 |
| Vaginal pH > 4.5 | 60 | 2 | 0 | 17 | 16 | 18 |
| Nugent score | ||||||
| 0–3 | 0 | 58 | 60 | 0 | 0 | 0 |
| 4–6 | 0 | 2 | 0 | 1 | 11 | 4 |
| 7–10 | 60 | 0 | 0 | 19 | 9 | 16 |
| Lactobacillary grade | ||||||
| I | 0 | 59 | 60 | 0 | 0 | 0 |
| II | 0 | 1 | 0 | 0 | 2 | 0 |
| III | 60 | 0 | 0 | 20 | 18 | 20 |
Diagnostic parameters detected in treatment (A) and non-treatment (B) groups at baseline (T0), 10 days (T1), and 30 days after the start of the treatment (T2).
| Diagnostic parameters . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | ||||
|---|---|---|---|---|---|---|
| . | T0 . | T1 . | T2 . | T0 . | T1 . | T2 . |
| Amsel criteria | ||||||
| Homogenous vaginal discharge | 60 | 5 | 6 | 19 | 16 | 17 |
| Clue cell presence | 58 | 3 | 0 | 17 | 14 | 13 |
| Positive amine test | 60 | 2 | 0 | 18 | 13 | 17 |
| Vaginal pH > 4.5 | 60 | 2 | 0 | 17 | 16 | 18 |
| Nugent score | ||||||
| 0–3 | 0 | 58 | 60 | 0 | 0 | 0 |
| 4–6 | 0 | 2 | 0 | 1 | 11 | 4 |
| 7–10 | 60 | 0 | 0 | 19 | 9 | 16 |
| Lactobacillary grade | ||||||
| I | 0 | 59 | 60 | 0 | 0 | 0 |
| II | 0 | 1 | 0 | 0 | 2 | 0 |
| III | 60 | 0 | 0 | 20 | 18 | 20 |
| Diagnostic parameters . | Treatment group (A, n = 60) . | Non-treatment group (B, n = 20) . | ||||
|---|---|---|---|---|---|---|
| . | T0 . | T1 . | T2 . | T0 . | T1 . | T2 . |
| Amsel criteria | ||||||
| Homogenous vaginal discharge | 60 | 5 | 6 | 19 | 16 | 17 |
| Clue cell presence | 58 | 3 | 0 | 17 | 14 | 13 |
| Positive amine test | 60 | 2 | 0 | 18 | 13 | 17 |
| Vaginal pH > 4.5 | 60 | 2 | 0 | 17 | 16 | 18 |
| Nugent score | ||||||
| 0–3 | 0 | 58 | 60 | 0 | 0 | 0 |
| 4–6 | 0 | 2 | 0 | 1 | 11 | 4 |
| 7–10 | 60 | 0 | 0 | 19 | 9 | 16 |
| Lactobacillary grade | ||||||
| I | 0 | 59 | 60 | 0 | 0 | 0 |
| II | 0 | 1 | 0 | 0 | 2 | 0 |
| III | 60 | 0 | 0 | 20 | 18 | 20 |
At baseline (T0) all the enrolled women showed at least 3 Amsel criteria, a Nugent score ≥ 7, and an abnormal lactobacillary grade (LBG III).
Concerning treatment group (A), the administration of the probiotic L. rhamnosus TOM 22.8 strain for 10 days (T1) determined the reduction of cases of homogenous vaginal discharge, presence of clue cells, positivity to amine test, and vaginal pH > 4.5. In addition changes in the Nugent score were revealed. In fact, at T1 sampling time, 58 patients, out of the 60 allocated in the treatment group (A), showed a Nugent score ranging from 0 to 3. Moreover, a Lactobacillus-dominant vaginal microbiota (LBG I) was detected in 59 women. These results were confirmed 30 days after the start of the treatment (T2). In fact, all patients (n = 60) showed a Nugent score between 0 and 3 and a normal lactobacillary grade (LBG I) (Table 3). Different results were observed in patients allocated in the non-treatment group (B), who did not receive any treatment. In fact, at both T1 and T2 sampling times, the majority of the patients showed homogenous vaginal discharge, clue cells, positivity to amine test, and vaginal pH higher than 4.5. In addition, no patients showed a Nugent score between 0 and 3 as well as normal lactobacillary grade (LBG I) (Table 3).
The administration of the L. rhamnosus TOM 22.8 strain determined the improvement of all the dignostic parameters taken into account.
Vaginal signs and symptoms, and QoL
The therapeutic effect of the probiotic TOM 22.8 strain was evaluated by taking into account changes in vaginal signs and sympoms (leucorrhoea, burning, itching, vulvo-vaginal erytema/edema, and subjective vaginal discomfort) as well as in the perceived QoL. In particular, the improvement of vaginal signs and symptoms was evaluated by using a 3-point severity score (0, absent or normal; 3, severe), whereas changes in the QoL were evaluated through the administration of the SF-36 questionnaire.
Figure 2 provides the mean values related to the score obtained by the assessment of the intensity of vaginal signs and symptoms (leucorrhoea, burning, itching, vulvovaginal erythema/edema, and subjective vaginal discomfort) recorded at T0, T1, and T2 sampling times. Using a 3-point severity score (0, absent or normal; 3, severe), no statistically significant differences were recorded at baseline (T0) between treatment group (A) and non-treatment group (B). Compared with baseline (T0), at both T1 (10 days after the start of the treatment) and T2 (30 days after the start of the treatment) sampling times, a significant improvement of vaginal signs and symptoms was observed only in the treatment group (A) (Fig. 2).
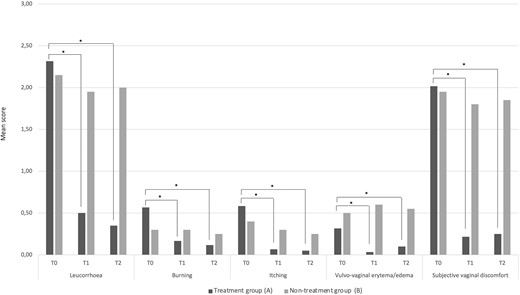
Vaginal signs and symptoms based on a 3-point severity score (0, absent or normal; 3, severe). Data are reported as mean values related to the scores obtained by the assessment of the intensity of clinical signs and symptoms at T0, T1, and T2 sampling times in both treatment group (A) and non-treatment group (B). *Statistical significance at P < 0.05 within each group, at T1 and T2 sampling times compared with baseline (T0).
Results of QoL questionnaire are summarized in Fig. 3. Patients allocated in treatment group (A) showed an improvement of physical and emotional limitations as well as of social functioning. Differently, no variations were observed among the different domains in non-treatment group (B).
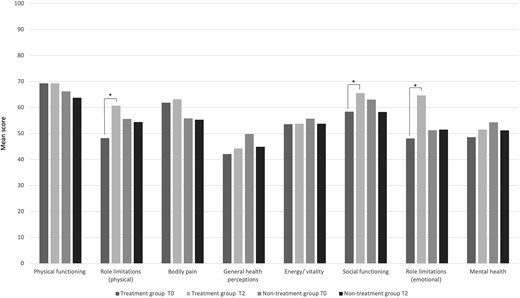
The Short Form-36 (SF-36) questionnaire scores at baseline (T0) and 30 days after the start of the treatment (T2, follow-up). *Statistical significance at P < 0.05 within each group, at T2 sampling time compared with baseline (T0).
The L. rhamnosus TOM 22.8 strain improved the clinical signs and symptoms as well as the QoL of the enrolled women.
Vaginal microbiota composition
The ability of the probiotic TOM 22.8 strain to balance the vaginal microbiota was evaluated by applying a culture dependent method. According to that, Lactobacillus spp., Enterococcus spp., Staphylococcus spp., Streptococcus spp., Gardnerella spp., Candida glabrata, C. albicans, C. krusei, and E. coli were recorded on selective media.
The vaginal microbiota composition, investigated in patients allocated in treatment group (A) and non-treatment group (B) at T0, T1, and T2 sampling times is displayed in Fig. 4. In addition, microbial counts, expressed as mean log cfu ml−1 and standard deviation, of the main microbial groups detected in vaginal discharge samples collected thought the study are reported in Supplementary Table S1. At baseline (T0), all enrolled patients showed a complex microbiota dominated by potentially pathogenic bacteria whereas a low cell density of lactobacilli was detected. In the treatment group (A), the probiotic L. rhamnosus TOM 22.8 strain administration for 10 days (T1) determined a statistically significant reduction of all the microbial groups investigated with the exception for lactobacilli, which significant increased (P < 0.05). Similar trend was revealed 30 days after the start of the treatment (T2). In fact, no significant differences in the vaginal microbiota composition were detected comparing the data obtained at T1 and T2 sampling times (Fig. 4 and Supplementary Table S1). On the contrary, the non-treatment group (B) showed a quite stable microbial composition and no statistically significant difference were detected through the study.
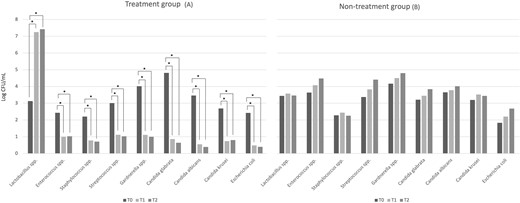
Vaginal microbiota composition. Data are reported as log mean values at baseline (T0), 10 days (T1), and 30 days after the start of the treatment (T2) from patients allocated in the treatment group (A) and the non-treatment group (B). *Statistical significance at P < 0.05 within each group, at T1 and T2 sampling times compared with baseline (T0).
Based on microbiological data the administration of the probiotic TOM 22.8 strain supported a balanced microbiota dominated by lactobacilli.
Lactobacilli population through qPCR
The increase of the lactobacilli population was revealed by qPCR analysis.
The detection threshold allowed to obtain a coefficient of determination (R2) equal to 99.7%. According to the obtained slope value (E = 10^(1-/slope), the amplification efficiency (E) was 102%. The used strandard allowed to obtain a detection threshold ranging from 8.8 copies log units cfu ml−1 to 2.7 copies log units cfu ml−1 with cycle threshold values (Ct) of 7.76 and 26.20, respectively. Figure 5 shows the qPCR data expressed as mean copies log units cfu/swab and standard deviation. In detail, at baseline (T0) patients allocated in both the treatment group (A) and the non-treatment group (B) showed a low number of lactobacilli. In the treatment group (A), the administration of the probiotic L. rhamnosus TOM 22.8 strain for 10 days (T1) determined an increase, in the lactobacilli population, of about 4 log units, reaching a mean value of 8.30 copies log cfu/swab. Compared to T1 sampling time, no significant changes in the lactobacilli population were recorded 30 days after the start of the treatment (T2) (7.63 copies log units cfu/swab). Different behaviour was observed in the non-treatment group (B), characterized by no significant changes in the lactobacilli population during the whole study (average valued of 4.39 copies log units cfu/swab) (Fig. 5).
The probiotic TOM 22.8 strain increased the lactobacilli population in the vaginal ecosystem.
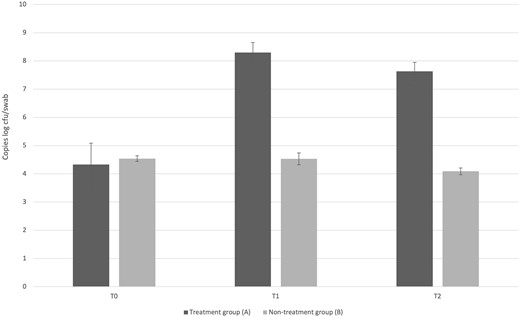
Quantification by qPCR of Lactobacillus spp. among vaginal samples collected at baseline (T0), 10 days (T1), and 30 days after the start of the treatment (T2) from patients allocated in the treatment group (A) and the non-treatment group (B). Data are reported as average log units cfu/swab and standard deviation.
Discussion
Probiotic administration has recently emerged as a valuable treatment of vaginal dysbiosis, showing promising results in several clinical trials (van de Wijgert and Verwijs 2020). Although the antimicrobial drugs represent the standard of care for both BV and VVC treatment, several side effects are associated to their administration. In fact, apart from the well-known spread of antibiotic resistance, antimicrobial drugs are unable to restore the lactobacilli-dominated vaginal bacterial biota and did not selectively act on pathogens, causing the eradication of healthy vaginal microbes. Recently, several meta-analyses revealed that the treatment with probiotics alone is an effective and safe strategy in the therapy of vaginal dysbiosis for both short- and long-term, reducing the antibiotic reliance (Wang et al. 2019; Chen et al. 2022; Chieng et al. 2022). Differently, the probiotics used after antibiotic treatment are effective only for a short term (Wang et al. 2019). In this context, lactobacilli, with proven probiotic properties, have been considered effective to restore and maintain a normal urogenital bacterial biota, improving the cure rate of infections and preventing recurrence (Han and Ren 2021). Despite the strong rationale for using lactobacilli in women with vaginal dysbiosis, most of previous clinical trials have evaluated the probiotic efficacy when associated with antibiotics. In contrast, only few studies have assessed the clinical value of a probiotic regimen alone (van de Wijgert and Verwijs 2020). The present study aimed to confirm the effectiveness of the oral administration of the probiotic L. rhamnosus TOM 22.8 (DSM 33500) strain to reduce both clinical signs and symptoms of vaginal dysbiosis, to colonize the vaginal microbiota, to restore the homeostasis of the vaginal bacterial biota, and to improve the QoL of women with dysbiosis.
Overall, our findings confirm the ability of the probiotic L. rhamnosus TOM 22.8 (DSM 33500) strain, orally administrated, to treat and prevent vaginal dysbiosis (Pino et al. 2021). The oral intake of the L. rhamnosus TOM 22.8 probiotic strain resulted in an effective restoration of the physiological pH, accompanied by remission or attenuation of clinical signs and symptoms, as well as the improvement of the QoL of the enrolled patients. Microbiological data revealed that oral administration of the probiotic strain significantly reduced potentially pathogens, both bacteria and yeasts, responsible for vaginal dysbiosis. This effect was observed 10 days after the start of the treatment (T1) and it was maintained 30 days after the start of the treatment (T2). Moreover, although several evidence have demonstrated the ability of lactobacilli to treat and prevent BV, few studies were focused on VVC (Cianci et al. 2020; López-Moreno and Aguillera 2021). Based on the available literature, a similar incidence of VVC in probiotic and placebo groups or no difference in women with VVC before and after probiotic use were reported (Czaja et al. 2007). Differently, the present study revealed that the probiotic L. rhamnosus TOM 22.8 strain significantly reduced the cell density of Candida spp. in the analyzed vaginal samples. Indeed, the probiotic TOM 22.8 strain was able to antagonize different Candida species (e.g. C. glabrata, C. albicans, and C. krusei) not only during the administration but also during the follow-up period, albeit short, suggesting a possible protective role against relapses. Based on both microbial cell count and quantification by qPCR, we demonstrated that the oral administration of the probiotic TOM 22.8 strain increased, in the vaginal microenvironment, the abundance of lactobacilli. Those findings confirm the assumption that the oral supplementation of specific probiotics can restore the vaginal microbiota (Huang et al. 2014; Pino et al. 2020; Vaccalluzzo et al. 2020; Pino et al. 2022), also supporting the hypothesis of bacterial translocation from the colon to the vagina (Reid et al. 2001; Morelli et al. 2004).
The main limitations of the present study are related to the absence of blinding. In addition, a follow-up period of 30 days could be short to assess the ability of the TOM 22.8 strain to protect against relapses. A randomized, double-blind, controlled trial, with a larger number of patients, is now ongoing to confirm the ability of the TOM 22.8 strain to treat vaginal dysbiosis. In addition, the ability of the probiotic strain, to prevent relapses will be evaluated for a follow-up period longer than 30 days.
Conclusions
Our findings indicate that the probiotic strain L. rhamnosus TOM 22.8 represents an effective and safe treatment for vaginal dysbiosis. It highlights the need for a more thoughtful approach in selecting probiotic strains for both urogenital and intestinal health. This study underscores the significance of employing microbiological methodologies to choose lactobacilli strains carefully to enhance women’s health. Future research will focus on assessing the in vivo vaginal colonization potential of L. rhamnosus TOM 22.8 (DSM 33500).
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the of Catania 1, Azienda Ospedaliero-Universitaria “Policlinico-Vittorio Emanuele” Catania (registration number 157/2019/PO, 27/04/2022). The study was registered to clinicaltrial.gov (ID: NCT05817272, 04/04/2023).
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgement
The work was partially supported by Uriach Italy S.r.l.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors have no relevant financial or non-financial interests to disclose.
Funding
This research received no external funding.
Author contributions
Amanda Vaccalluzzo (Formal analysis, Data curation), Alessandra Pino (Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing—original draft), Raffaela Luisa Grimaldi (Data curation, Formal analysis, Writing—original draft), Cinzia Caggia (Supervision, Writing—review & editing), Stefano Cianci (Conceptualization, Supervision, Writing—review & editing), and Cinzia Lucia Randazzo (Conceptualization, Supervision, Writing—review & editing)
Data availability
All data are incorporated into the article and its online supplementary material.



