-
PDF
- Split View
-
Views
-
Cite
Cite
Łukasz Grabowski, Karolina Pierzynowska, Lidia Gaffke, Zuzanna Cyske, Grzegorz Mincewicz, Grzegorz Węgrzyn, The use of phage display systems to combat infectious diseases in poultry: diagnostic, vaccine, and therapeutic approaches, Journal of Applied Microbiology, Volume 134, Issue 1, January 2023, lxac012, https://doi.org/10.1093/jambio/lxac012
Close - Share Icon Share
Abstract
Development of molecular biology and understanding structures and functions of various biological molecules and entities allowed to construct various sophisticated tools for different biotechnological, medical, and veterinary applications. One of them is the phage display technology, based on the possibility to create specific bacteriophages bearing fusion genes, which code for fusion proteins consisting of a phage coat protein and a peptide of any amino acid sequence. Such proteins retain their biological functions as structural elements of phage virions while exposing foreign peptide sequences on their surfaces. Genetic manipulations allow to construct phage display libraries composed of billions of variants of exposed peptides; such libraries can be used to select peptides of desired features. Although the phage display technology has been widely used in biotechnology and medicine, its applications in veterinary and especially in poultry science were significantly less frequent. Nevertheless, many interesting discoveries have been reported also in the latter field, providing evidence for a possibility of effective applications of phage display-related methods in developing novel diagnostic tools, new vaccines, and innovative potential therapies dedicated to poultry. Especially, infectious diseases caused by avian viruses, bacteria, and unicellular eukaryotic parasites were investigated in this field. These studies are summarized and discussed in this review, with presentation of various possibilities provided by different phage display systems in development of useful and effective products facilitating management of the problem of infectious diseases of poultry.
Introduction
Phage display technology is based on the presentation of various recombinant peptides on the surface of virions of bacteriophages (Jaroszewicz et al. 2022). The flexibility of phage coat proteins allows to clone a desired DNA sequence fused to a selected gene encoding one of proteins forming bacteriophage capsid, and in many cases, a product of the fusion gene retains its activity in building the virion while presenting corresponding peptide at the viral surface (Fig. 1). Extensive studies led to indication that some of proteins of various phages can function in building of infective virions in the presence of any attached amino acid sequence while others are still functional only if relatively short peptides are fused to them. This led to selection of specific phage proteins and their genes, which can be effectively employed to prepare recombinant phages exposing products of fusion genes (for details, see Jaroszewicz et al. 2022). Therefore, it is possible to construct bacteriophages presenting virtually any peptides on their virions, thus providing systems for both effective production of chosen specific peptides and construction of large libraries composed of millions or even billions of phages, each carrying a different peptide on its surface.
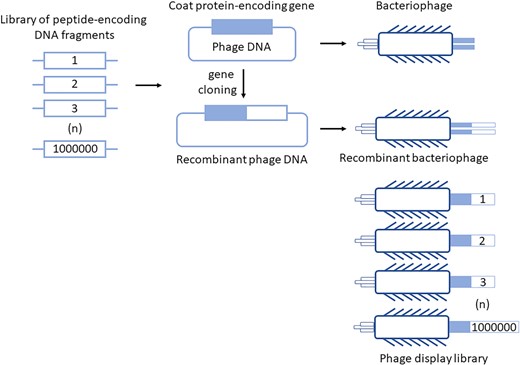
The principle of the phage display technology. Peptide-encoding DNA fragments can be cloned into phage genome to make fusions with a gene coding for one of coat proteins of phage virions. Then, the fusion genes are expressed during bacteriophage development and fusion proteins are incorporated into the virions. The recombinant peptides are thus exposed at virion surface. This technology allows to construct libraries of peptides consisting of millions or billions of different clones, which can be further used to select peptides of specific, desired features.
As presented schematically in Fig. 1, the construction of phage display library starts from preparation of a large series of DNA fragments, constructed by either chemical synthesis of oligonucleotides with random nucleotide sequences or amplification of DNA regions employing samples of mRNAs isolated from specific cells (spleen cells) and reverse transcriptase. Such a library of peptide-encoding DNA fragments is cloned into phage vector to obtain billions of variants of the fusion gene consisting of the sequence encoding a selected virion protein and the foreign coding sequence. Following introduction of such phage-derived DNA molecules into bacterial cells, recombinant bacteriophages are multiplied in their hosts and liberated from them, forming a phage display library composed of billions of virion variants, each (in principle) exposing a different foreign peptide on its surface.
The importance of the phage display technology has been recognized a few years ago by awarding the 2018 Nobel Prize ‘for the phage display of peptides and antibodies’ (as reviewed recently (Jaroszewicz et al. 2022). Many different phage display systems, employing different bacteriophages, are available, and they were reviewed, summarized, and discussed recently (Jaroszewicz et al. 2022). Possible applications of such systems in constructing diagnostic tests, vaccines, and therapeutic molecules are huge, and numerous examples of various medical approaches of phage display can be found in recent review articles (Alfaleh et al. 2020, Ali et al. 2020, Lai and Lim 2020, Aloisio et al. 2021, Anand et al. 2021, Li et al. 2021; Foglizzo and Marchiò, 2021; Li et al. 2021, Roth et al. 2021, Suárez et al. 2021, Sun et al. 2021, Zhao et al. 2021, Alizadeh et al. 2022, Ch’ng et al. 2022, Mahdavi et al. 2022, Yeoh et al. 2022, Zambrano et al. 2022, Zhang et al. 2022).
Among various phage display systems, those based on filamentous (M13-like) and tailed (T4-, T7-, and λ-based) bacteriophages are the most commonly used ones. Their advances and limitations were discussed thoroughly in the recent review article on molecular principles of the phage display technology (Jaroszewicz et al. 2022), but they can be summarized briefly as follows. Filamentous phages can be propagated very effectively in cultures of susceptible Escherichia coli cells, reaching titers as high as 1013–1014 visions (precisely: plaque forming units, PFU) per ml. This allows rapid and efficient production of large libraries of phages exposing different peptides on their surfaces. The major limitations of filamentous phage display systems are the restriction in the sizes of exposed peptides or their abundance. Namely, although pIII and pVIII coat proteins are generally capable of accepting foreign peptides fused to them without losing their functions, there are specific restriction in this matter. The pVIII protein (occurring in about 2700 copies per virion) is useful for displaying small foreign peptides (several amino acid residues), while being ineffective for the display of larger proteins due to steric hinderances. The pIII protein occurs in three to five copies per virion and can be employed for displaying large peptides, but their abundance on the virion surface is low. Moreover, larger phages are less stable and propagate significantly slower than wild-type M13. Another drawback of these systems is related to the fact that filamentous phages are liberated from bacterial cells without disrupting the cell membrane. Since proteins building the capsid have to cross the membrane, fusion peptides with sequences preventing effective transition from the cell to environment can be retained in the cytoplasm together with the whole virion. On the other hand, tailed bacteriophages can display relatively large proteins on their surfaces, not only because of bigger sizes of their visions, but also due to their ability to accept of larger fragments of foreign DNA in their genomes. Moreover, these phages are released from the host bacteria through cell lysis, thus, there are no restrictions for the biochemical properties of peptides exposed on the virion surface. However, titers of lysates of tailed phages are considerably (a few orders of magnitude) lower than those of filamentous phages, thus, phage display libraries prepared with the systems based on former bacteriophages are significantly smaller than those obtained with the latter ones.
For the practical purposes of the use of phage display systems in diagnostic, vaccine, and therapeutic approaches, employing libraries of exposed antibodies, the fragment antigen-binding (Fab) regions of antibodies, and single-chain variable fragments (scFvs) (Fig. 2a) is of special interest. A scheme for construction of such phage display libraries is shown in Fig. 2b. Following immunization of animas with specific antigen, total mRNA is isolated from lymphocytes (such a sample is especially rich in mRNAs encoding antibodies), and cDNA pools are synthesized using reverse transcriptase, followed by PCR-mediated amplification of DNA fragments coding for either Fab regions or VH and VL parts of antibodies. In the latter case, both fragments are joined by a linker. Such libraries of DNA sequences are then cloned into genomes of phage vectors to obtain large sets of recombinant bacteriophages, which after propagation in bacterial cells form phage display libraries exposing Fab or scFvs.

Antibody, and Fab and scFv fragments (panel a), and a scheme for construction of phage display libraries for selection of specific Fab and scFv molecules (panel b).
Despite poultry science is a large area of study and applications, and aviculture and bird breeding are extremely important in nutrition and economy, the use of phage display in these fields is drastically less frequent relative to medicine and biotechnology. When searching the PubMed database (https://pubmed.ncbi.nlm.nih.gov/last accessed on May 6, 2022), the number of review article records, which appeared after using the query ‘phage display and review’ was 1066 whereas when the query ‘phage display and poultry and review’ was applied, only four records appeared. Moreover, three out of four these reviews concerned chickens as alternative immune sources (Finlay et al. 2017), the use of chicken avidin to develop novel ligand-binding compounds (Hytönen 2020) and employment of chicken egg yolk antibodies (Somasundaram et al. 2020). Only one review article, published in 2013, discussed the use of phage display in studies on the hemagglutinin of the avian influenza H5N1 virus and its antigenic architecture (Velkov et al. 2013). However, even the latter paper did mention poultry only as a reservoir of the viruses, which can infect humans. Therefore, in the light of this evident gap in review articles, here we aimed to summarize and discuss attempts to employ the phage display technology in poultry science and specific possible applications in this field.
Phage display in studies on avian viruses
The most intensive studies in which the phage display methods were used in the field of poultry science concerned avian viruses. Published works described development of diagnostic methods, vaccines, and potential therapeutics for detecting and/or combating viral infections in poultry. Details of these works are reviewed and discussed in this chapter.
Avian influenza virus
Avian influenza causes one the most severe infection problems in poultry, as outbreaks lead usually to complete slaughtering of the bird population, which results in huge economic loss (Blagodatski et al. 2021). Even more serious problems are caused by the fact that some strains of the avian influenza virus are able to effectively infect mammals, including pigs and humans (Moore et al. 2021). Therefore, there are many approaches to develop novel, effective methods for diagnosis and treatment of this disease. The phage display technology provides a powerful platform for innovative applications in both these fields.
The possibility of construction of phage display libraries, exposing millions of variants of peptides on virions, provides an excellent tool for searching specific molecules able to interact with selected targets. This possibility includes the use of libraries exposing Fab regions. Such libraries can be employed to select specific antibody fragments, which recognize chosen proteins occurring at the surface of pathogenic microbes, thus, providing tools for construction of specific diagnostic tests. This kind of approach was used to select Fab regions specifically recognizing hemagglutinin protein of the H5N1 avian influenza virus (A/Vietnam/1203/04) (Pitaksajjakul et al. 2010). To construct the most useful library, chickens were immunized with the recombinant hemagglutinin protein. Following isolation of transcripts from immune system cells and preparation of cDNA by reverse transcription, the library was constructed and scanned for Fab molecules strongly and specifically recognizing the hemagglutinin from the H5N1 virus. In this way, a kind of monoclonal antibody could be obtained, which could be subsequently used for construction of diagnostic tests allowing specific detection of the H5N1 avian influenza virus (Pitaksajjakul et al. 2010). By analyzing this example, one should stress that the choice of the optimal source of the genetic material for construction of phage display library is crucial for further selection of specific peptides, which bind to the desired target molecule. In this case, immunization of chickens with the recombinant hemagglutinin protein and isolation of RNA from selected types of cells led to significant enrichment of transcripts encoding Fab variants recognizing the protein used for this immunization. A similar method was used to identify Fab variants recognizing a multi-basic cleavage site in the hemagglutinin protein derived from a highly pathogenic H5N1 avian influenza virus but not a corresponding epitope present in a less virulent strain. Such Fab might be used to distinguish viruses of different virulence. In this case, the phage libraries were constructed after immunizing mice with the protein encoded by the highly virulent virus (Dong et al. 2013).
Another option employed in studies devoted to construct specific diagnostic tests is to selected scFvs, which are fusion proteins consisting of variable regions of the heavy and light chains of immunoglobulins with a linker containing several amino acid residues. Construction of phage display libraries exposing scFvs requires additional cloning steps; however, its advantage is a possibility to increase the efficiency of identification of specific antibody-like peptides interacting with desired antigens/epitopes. Using such a method, an effective selection of scFvs specifically recognizing the hemagglutinin of the H5N1 avian influenza virus strain A/Hubei/1/2010 was possible (Wu et al. 2014). A similar approach was used to obtain scFvs recognizing the NP nucleoprotein of the H5N1 virus (Sengupta et al. 2014). That work was an example of elegant experiments in which mice were immunized with a recombinant NP antigen, and the genetic material from spleen cells was used to construct the library of phages exposing variable regions of the heavy and light chains of antibodies connected through a linker. In this case, to prepare such a useful phage display library, the scFv-encoding DNA was inserted into a phagemid. Interestingly, a modified ELISA test was constructed using the overproduced scFv specific for the NP protein, which revealed 100% sensitivity and 98.7%–100% specificity in detection of the H5N1 avian influenza virus in several hundred chicken sera (Sengupta et al. 2014).
Quite different or ‘reverse’ (relative to those described above) approach, employing the phage display technology, can be used for identification of specific antibodies in tested sera. Whole-genome-fragment phage display libraries can be constructed using random fragments of genomes of investigated pathogens (like bacteria or viruses) to obtain bacteriophages exposing relatively long fragments of peptides (Beghetto and Gargano 2019). When such libraries of peptides derived from the H5N1 influenza virus were constructed and tested using antibody repertoire from sera of survivors of infections with this virus, specific peptides could be identified, which appeared useful in construction of a rapid, ELISA-based test for the virus detection (Khurana et al. 2011). The same method was used in studies on the H7N7 virus (Khurana et al. 2016) to identify antibodies that might be potentially useful not only in diagnostics but also in protection against or treatment of viral infections. The phage display libraries based on the whole-genome-fragments were also employed to design H5N1-specific vaccines. Namely, when analyses of neutralizing antibodies and sera from individuals who had recovered from H5N1 viral infection were performed, specific virus-derived peptides, exposed on phage virions, could be identified. These viral peptides were recognized very effectively by antibodies/sera of those who survived the H5N1 infection (Khurana et al. 2009). Therefore, the idea is that such peptides might be used in further studies to develop an effective vaccine, specific to the virus which genome was used to construct the phage display library. In fact, this method might be recognized as a potentially effective approach for searching the most immunogenic peptides, assuming that the natural verification of efficiency of production of specific antibodies can be effectively tested using whole-genome-fragment phage display libraries and sera from survivors of previous viral infection.
The phage display technology can be used not only in searching for specific antibodies, Fab fragments, or scFvs, but also for exposing known antigens on the bacteriophage capsid surface. Phage-exposed antigens may reveal increased immunogenicity because virions can acts as adjuvants. Therefore, since enhanced immune response may occur in organisms treated with such constructs, phages may be employed as carriers of vaccines to immunize animals or humans. This method has been used to construct a prototype of a vaccine against avian influenza virus A, where a specific 8-amin-acid-long fragment of the virus-encoded transmembrane protein M2 was exposed on the surface of the M13-derived phage after cloning of the corresponding DNA fragment into the phage genome to form a fusion with the M13 gene VIII (Lotfi et al. 2019). When such a modified phage was used to immunize chickens, the birds effectively produced antibodies against the M2 protein. These experiments indicated that bacteriophages can be effective carriers of antigens in the process of development of specific vaccines.
Apart from developing diagnostic tests and vaccines, the phage display-mediated search for specific scFvs could be used to produce neutralizing antibodies which might be employed in therapeutic procedures after infection by viruses, like avian influenza virus. This strategy can be exemplified by the work in which phage display was used to select scFvs that recognized recombinant hemagglutinin (Li et al. 2016). Interestingly, when neutralizing scFvs were identified, overproduced in E. coli cells, and applied to chickens, they suppressed infections caused by avian influenza H5N1 and H1N1, but not N3N2, viruses (Li et al. 2016). Therefore, this technology allows to select very specific scFvs, active against some but not all subtypes of the virus.
Finally, it is worth mentioning that to develop either vaccines or therapeutic antibodies against the avian influenza virus, not only phage display, but also other peptide display systems have been used. For example, the bacterial display system with Lactobacillus plantarum (Jiang et al. 2017) and yeast display system with Saccharomyces cerevisiae (Lei et al. 2020) have been successfully used. Although useful in production of recombinant peptides displayed on surfaces of cells, these systems are nevertheless more complicated and preparation of the libraries is significantly more laborious and time consuming than in the case of phage display systems. Moreover, number of possible variants of peptides exposed on phage virions, and number of possible clones obtained in constructing phage display libraries far exceed those obtained in the bacterial of yeast display systems.
Newcastle disease virus
Newcastle disease is an infectious disease of many avian species. It is caused by the Newcastle disease virus (NDV), and the infected birds develop various symptoms resulting from dysfunctions of respiratory, digestive, and nervous systems. The disease occurs worldwide and in most cases the infection of birds is lethal (Ul-Rahman et al. 2022).
Early work on the use of the phage display technology in studies on the NDV was focused on searching for antibodies that might be employed in pathotyping of the virus isolates (Li et al. 2002). Rabbits were immunized with the C-terminal fragment of the F2 protein of the virus, and RNA material from rabbit cells was used to construct phage display library exposing scFvs. Specific clones were isolated, which were recognized as promising in further construction of a test for pathotyping of the NDV (Li et al. 2002). The pathotyping of this virus with the use of a phage display library was continued subsequently (Ramanujam et al. 2004). This line of studies led to identification of peptides, which partially inactivated the virus in in vitro tests (Ozawa et al. 2005). In more recent studies, the yeast surface display system was used to analyze interactions between specific antibodies and linear antigenic domains of the hemagglutinin-neuraminidase protein from the NDV genotype VII (Li et al. 2015).
Duck hepatitis virus and other avian hepatitis viruses
Various viruses can cause avian hepatitis. This disease, depending on the kind of infecting virus, results in severe or milder symptoms related to liver dysfunctions, while in some cases the disease is fatal, especially for poultry (Yugo et al. 2016). Among viruses responsible for avian hepatitis, duck hepatitis viruses (various types), avian hepatitis E virus, turkey hepatitis virus, and fowl adenoviruses are the most abundant and the best studied ones (Yugo et al. 2016).
To select specific scFvs against the VP3 protein of the duck hepatitis A virus type 1, mice were immunized with this protein and following isolation of total RNA from spleen, the phage display library was constructed (Wang et al. 2018). During the procedures similar to those described above for avian influenza viruses, specific clones were selected, producing scFvs of high sensitivity and specificity for the virus (Wang et al. 2018). They might be potentially used in the future to develop procedures of prevention or treatment of ducks endangered by the duck hepatitis A virus type 1.
Another approach was to develop a diagnostic test for the same virus. However, instead of constructing libraries for selection of scFvs, a phage display library was prepared which exposed nanobodies (defined as recombinant variable domains of heavy-chain-only antibodies) on bacteriophage virions (Xue et al. 2019). The selected nanobodies against the VP1 protein were not neutralizing (Xue et al. 2019), however they can be used in developing specific tests for identification of the duck hepatitis A virus type 1.
Interestingly, the phage display method was also used to identify epitopes, which are common to avian, swine, and human hepatitis E viruses (Wang et al. 2015). Therefore, it is evident that the spectrum of the possible uses of the phage display technology in poultry science is broad, and definitely not restricted to simple searching for antibody-like molecules useful in diagnosis or treatment of viral diseases.
Avian infectious bronchitis virus
Infectious bronchitis is one of the major respiratory diseases of birds, causing significant loses in the poultry industry. This disease is caused by a specific coronavirus, called the avian infectious bronchitis virus. Although currently used vaccines have attenuated the problem, the emergence of novel virus variants did not allow to eliminate it (de Wit et al. 2021). Therefore, further studies are still required to find new vaccines and diagnostic tools which might facilitate to manage this disease in poultry.
The spike protein (called also the S protein) plays an important role in pathogenicity of the avian infectious bronchitis virus and it is one of the most immunogenic proteins of this virus. Using a phage display library, it was possible to identify epitopes, which are common to most (if not all) variants of the virus, as well as those revealing high variability (Zou et al. 2015). Therefore, one might suggest that both of these kinds of epitopes and corresponding antibodies could be used in construction of diagnostic tools for detection of the avian infectious bronchitis virus and for identification of its specific variants.
Since one of the virus receptors on avian cells is aminopeptidase N, a library of phages exposing 12-amino-acid-long peptides was screened to identify those interacting with this enzyme. Importantly, the selected peptides interacted efficiently with antibodies against the S protein of the avian infectious bronchitis virus (Sun et al. 2021). Then, an interesting approach was developed to use phage clones exposing such peptides as vaccines in chickens, and production of the virus-neutralizing antibodies was detected (Sun et al. 2021). This is a potentially perspective method of combination of the use of phage display to identify specific peptides interacting with the virus receptor, and then to use selected phages, carrying such peptides, as vaccines. This approach might be of particular interest as production of bacteriophages in relatively large amounts is effective and relatively cheap, thus, production of phage-based vaccines could be effective both biotechnologically and economically. Moreover, bacteriophages are interesting carriers of antigens, which might also act as a kind of adjuvant.
Infectious bursal disease virus
When immature B lymphocytes of the bursa of Fabricius are attacked by infectious bursal disease virus, a severe immunosuppression may develop in birds. Such a disease causes serious problems in the poultry industry (Alkie and Rautenschlein 2016). The virus is highly variable, which is one of characteristic features of viruses having double-stranded RNA as a genetic material.
To investigate virus-host cell interactions, total mRNA was isolated from cells of the chicken bursa of Fabricius, cDNA was obtained, and phage display library was constructed with the T7 bacteriophage-derived vector (Luo et al. 2010). When this library was used to search for virus-binding peptides, a specific surface immunoglobulin M (sIgM) could be identified, and corresponding monoclonal antibodies could be produced. In the in vitro tests, these antibodies reduced infectivity of the infectious bursal disease virus (Luo et al. 2010). Hence, potential therapeutic methods might be suggested with the use of such antibodies.
Another work was conducted to produce a specific vaccine with the use of a different phage display system, based on the T4 bacteriophage. The DNA sequence coding for the VP2 protein of the infectious bursal disease virus was fused with the SOC gene of the phage, and the fusion protein SOC-VP2 was exposed on the surface of the phage virion (Cao et al. 2005). Such recombinant protein was immunogenic, and more importantly, when chickens were immunized with the phages exposing the SOC-VP2 recombinant protein, protection against infection by the infectious bursal disease virus was evident (Cao et al. 2005). This is another example of effectiveness of the use of bacteriophages as carriers of recombinant viral proteins in vaccines which efficiently protected birds against virus-caused diseases.
Other avian viruses
The use of the phage display technology was not restricted to the viruses mentioned in preceding subsections. Instead, diagnostic and vaccine/therapeutic approaches were investigated for several other viruses with employing bacteriophages exposing peptides on their virions. The examples of such studies include the use of phage display-related methods to (i) identify the B-cell epitopes in the glycoprotein E of the duck Tembusu virus, a flavivirus infecting ducks (Li et al. 2016a), and in the VP3 protein of the waterfowl parvovirus, infecting geese and ducks (Li et al. 2016b), which might be useful in the development of diagnostic tools and therapeutic targets, and (ii) obtain scFvs against the foot-and-mouth disease virus (Foord et al. 2007) and Usutu virus (Schoenenwald et al. 2020). Interestingly, phage display was also used in studies on human viral or virus-mediated diseases where chicken models were employed, e.g. during investigations of rhinoviruses (Hodits et al. 1995) or Rous sarcoma virus (Bloemendal et al. 2004).
In summary, the phage display technology is a powerful method, which can be used in various studies on avian viruses. Nevertheless, vast majority of research performed to date in this field was focused on development of diagnostic tools, vaccines, or potential therapeutics. In fact, phage display-based methods provide a great opportunity to identify and isolate specific Fab, scFvs, nanobodies, or antibodies after a relatively quick and effective screening of millions of clones exposing peptides of various sequences. Moreover, bacteriophages exposing recombinant viral proteins could be used as effective vaccines. In this case, the major advantages are simplicity and rapidness of production of specific phage clones in large amounts, and adjuvant-like properties of such recombinant phages.
Phage display in studies on bacterial pathogens infecting birds
Among many bacterial pathogens infecting birds, the most problematic for the poultry industry (due to either causing severe avian diseases or a possibility of transmission of bacteria from poultry or poultry-derived products to humans) appear Salmonella enterica (Jajere 2019) and Campylobacter jejuni (Awad et al. 2018, Lopes et al. 2021, Śmiałek et al. 2021). Although the number of published studies in which the phage display technology was used to investigate bacterial infections of poultry is lower than that of papers describing analogous studies with viral infections, still important discoveries have been reported in the former field. They are summarized and discussed briefly below.
Salmonella enterica
Although vaccination is an important procedure to protect poultry against infections with S. enterica, in the poultry industry practice, there is a significant problem in distinguishing vaccinated and infected animals. To overcome this problem, a phage display-based method was used to distinguish peptides, which can be bound by antibodies produced by Salmonella-infected chickens from those produced by vaccinated ones (Naqid et al. 2016b). Indeed, this method appeared to specifically identify both kinds of peptides. In the specific tests, using the discriminatory peptides it was possible to identify the infection with 100% sensitivity and specificity (Naqid et al. 2016b). These results showed again the power of the phage display methods, as otherwise it would be extremely difficult to determine what antibodies are specific for infected and what for vaccinated chickens. The possibility to screen large numbers of variants of peptide sequences allowed to overcome such a difficulty and to develop the specific differentiating method relatively quickly.
Interestingly, quite similar approach was used to discriminate between poultry infected with S. enterica of various serovars. Following phage display experiments, it was possible to identify peptides, which allowed to discriminate between infections with S. Enteritidis and those with other serovars (S. Hadar and S. Typhimurium) (Naqid et al. 2016a). Therefore, the usefulness of the phage display technology in finding discriminatory peptides has been confirmed.
Campylobacter jejuni
As in the case of viral infections of poultry, the use of the phage display method in studies on C. jejuni was focused on development of novel diagnostic procedures and new therapies. The former task is represented by the work on generation and characterization of scFvs, which were specific for C. jejuni. The phage display library was constructed using mRNA (subsequently reverse transcribed to cDNA) isolated from spleens of rabbits immunized with attenuated cells of this bacterium (Nzuma et al. 2018). Following the screening procedures, it was possible to select variants revealing specific binding to C. jejuni in mixtures containing other bacteria (Nzuma et al. 2018).
Different approaches were employed to find potential therapeutics against C. jejuni. When a C. jejuni strain isolated from poultry was used, it was possible to find several peptides in a phage display library that reduced growth of cells of this bacterium with the efficiency of 99.9% in vitro (Bishop-Hurley et al. 2010). Further selection allowed to find a peptide reducing the viability of C. jejuni while not affecting other bacterial species (Bishop-Hurley et al. 2010). Such a peptide might be a promising molecule for further studies on potential therapeutics controlling infections of chickens by C. jejuni. In another work, llama was immunized with flagella derived from C. jejuni, and following construction of the phage display library, single-domain antibodies (characteristic for camelids) specific for C. jejuni were isolated (Riazi et al. 2013). These antibodies significantly reduced motility of bacterial cells and, more importantly, considerably decreased efficiency of colonization of chicken ceca when administered orally after infection with C. jejuni (Riazi et al. 2013). Therefore, various potential therapeutic methods can be developed against C. jejuni when the phage display methods are used.
Other bacteria
Avibacterium paragallinarum is a pathogenic bacterium causing infectious coryza in birds (Nhung et al. 2017). An attempt to develop a vaccine against this microorganism involved the use of phage library and identification of peptides interacting strongly with polyclonal antibodies against A. paragallinarum serovar A (Wang et al. 2007). Then, the selected sequence was used to construct an E. coli clone expressing a recombinant gene coding for a fusion protein, containing the selected peptide joined to one of surface proteins of this bacterium. Interestingly, when such bacterial cells were injected into muscles of chickens, a specific serological response to A. paragallinarum serovar A was detected (Wang et al. 2007). Hence, this was the first step to develop a vaccine effectively neutralizing this pathogen.
To produce antibodies against Staphylococcus aureus, specific scFvs were identified after construction of the bacteriophage T7-derived phage display library using mRNA isolated from the spleen of chickens, which were immunized with inactivated S. aureus cells (Li et al. 2016). In this way, specific scFvs against S. aureus could be found, opening further studies on development of potential therapeutics.
Phage display in studies on avian coccidiosis
Avian coccidiosis is a severe disease of birds caused by protozoan parasites belonging to the genus Eimeria (Mesa-Pineda et al. 2021). These unicellular eukaryotic parasites severely influence functions of the host intestine, causing impairment of the absorption of nutrients and subsequent reduction in growth and lowering general physiological performance. These disorders can lead to the host death, and in the case of poultry farms, coccidiosis leads to significant economical loses (Lee et al. 2022). Therefore, prevention and treatment of this disease are important issues, though they are not simple tasks due to difficulties in finding effective drugs against eukaryotic parasites. In fact, the phage display technology might be helpful in searching for effective peptides acting as either vaccines or biological drugs. Despite the high potential of this technology in developing vaccines, as demonstrated in the previous chapters on avian viral and bacterial diseases, similar works leading to construction of potential vaccines against Eimeria were conducted using yeast display, rather than phage display, systems. In those studies, the parasite’s microneme-2 protein was used as the antigen while optimizations of amino acid sequences were performed by mutagenesis and selection of the most effective clones (Sun et al. 2014.; Chen et al. 2018). An interesting idea was to use yeast cells for exposing selected peptides as an oral vaccine.
In the literature, there are more reports on the use of the phage display method in developing specific anti-coccidiosis drugs. An early report in this field described a phage display-mediated identification of a peptide that disrupted the pellicle of sporozoites of E. acervulina and E. tenella (da Silva et al. 2002). This peptide, called PW2, revealed an antibiotic-like activity against these parasites and appeared a promising candidate for a drug. Another work aimed to develop a passive immune therapy by selecting different IgA fragments that were able to specifically bind sporozoites and oocysts of E. acervulina (Wieland et al. 2006). Similar specificity of binding to sporozoites of E. tenella was achieved by selecting scFvs; however, several rounds of the panning procedure, performed after constructing of the phage display library, allowed to enrich the mixture of phages with clones revealing the strong binding to the desired antigens, and then to obtain promising recombinant antibodies (Abi-Ghanem et al. 2008). Interestingly, such a line of research led also to discovery of a previously unknow protein of E. tenella, called EtGAM22, with the gene coding for this protein expressed at the gametocyte stage (Krücken et al. 2008). This protein might be a potential therapeutical target or an antigen for vaccination. The passive immune therapy approach has been further developed by phage display-mediated selection of scFvs and subsequent construction of transgenic crop seeds exposing antibodies, which reveal neutralization of Eimeria oocysts (Zimmermann et al. 2009).
Recent studies indicated a new strategy to combat avian coccidiosis. The phage display libraries were screened to find regular peptides, rather than antibody-like molecules, which can strongly and specifically interact with either protein AMA1 (Ma et al. 2019) or microneme protein 3 (Chen et al. 2021) of E. tenella. Following successful identification of such peptides, in vitro effects were tested on Madin-Darby Bovine Kidney (MDBK) cells to indicate that tested peptides inhibited the invasion of sporozoites. Moreover, when bacteriophages exposing these peptides were administered orally to chickens, protective effects against infection with E. tenella have been observed (Ma et al. 2019, Chen et al. 2021). Therefore, bacteriophage virions bearing specific peptides fused to one of capsid proteins can be used not only as vaccines (as indicated in preceding chapters) but also as carriers of drugs when administered orally and acting as anti-parasitic therapeutics.
Conclusion
The phage display technology provides a series of powerful methods to select various kinds of peptides revealing specific and precisely desired features. Although it was used quite moderately in poultry science (a summary of such studies is presented in Table 1), the employment of this technology provided evidence for its successful applications in developing sophisticated tools for diagnosis, vaccination, and treatment of poultry. The specific examples include works connected to viral, bacterial, and parasitic diseases of birds, which are recognized as major veterinary and economic problems in the poultry industry. In fact, the use of phage display technology led to development of various therapeutics approved for the clinical use in human medicine. The examples are Durvalumab (Imfinzi), specific antibody against programmed death ligand-1, for treatment of urothelial carcinoma and non-small cell lung cancer, Ramucirumab (Cyramza) which inactivates vascular endothelial growth factor receptor 2 (VEGFR2), for treatment of gastric cancer and gastro-esophageal junction adenocarcinoma, Raxibacumab (ABthrax), which neutralizes the protective antigen component of Bacillus anthracis, for prophylaxis and treatment of anthrax, and others. Moreover, various companies offer services for selection of specific antibodies, Fab or scFvs, which are then used for preparation of diagnostic kits (see, e.g. https://www.hybribody.com/; https://www.proteogenix.science/antibody-production/phage-display-services/; https://www.pepscan.com/; https://phagenbio.creative-biolabs.com/production-and-purification-of-phage; https://www.oakbiosciences.com/services/antibodyengineering/phage-display-screening; https://www.bio-bench.com/phagemab.html). In fact, in many such kits, these antigen-recognizing molecules were developed using the phage display technology. Nevertheless, most of these therapeutics and diagnostic tools are employed to treat or diagnose human diseases, with veterinary medicine (and especially poultry science) remaining largely untapped field for the development of such methods based on phage display. This might be considered surprising as phage display technology offers a broad spectrum of methods for selection of specific peptides, interacting with various molecules which might be important therapeutic targets or diagnostic markers. Their usefulness has been confirmed in studies discussed in this paper and summarized in Table 1. The only disadvantages are related to limitations of specific phage display systems, indicated in the introductory chapter of this paper; however, they can be overcome by choosing different bacteriophage vectors, appropriate for specific purposes. Perhaps one of the major barriers are procedures focused on terminating infected or sick birds rather than on effective diagnostic and therapeutic methods, and concentrated predominantly on detection of pathogens potentially dangerous to humans as consumers of poultry-derived products. Nevertheless, it seems likely that development of effective diagnosis and treatment of infectious diseases of poultry might not only be beneficial to birds but also lead to economic profits. Therefore, we suggest that further studies on the use of the phage display-related methods in the poultry science are important and should bring new fascinating discoveries leading to development of novel diagnostic tools, efficient vaccines, and effective drugs for avian diseases caused by viruses, bacteria, and unicellular eukaryotic parasites.
| Type of phage/display . | Origin of the library . | Application . | Target pathogen . | Finding(s) . | Reference . |
|---|---|---|---|---|---|
| Filamentous phage | cDNA derived from chickens immunized with recombinant hemagglutinin protein constructed from H5N1 avian influenza virus | Diagnostic, therapeutic | Avian influenza virus H5N1 | (i) Three clones highly specific to the virus (ii) One clone inhibited target virus infection in Madin-Darby canine kidney (MDCK) cells (iii) One clone showed strong reactivity with several influenza A virus subtypes | Pitaksajjakul et al. 2010 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDL) expressing fragments of 15–350 amino acids, covering all the proteins of H5N1 | Diagnostic, vaccine development | Avian influenza virus H5N1 | (i) Identification of several potentially protective H5N1-specific human antibody epitopes (ii) Identification of the strong reactivity against PB1-F2, a putative virulence factor, following H5N1 infection | Khurana et al. 2009 |
| Filamentous phage | Mice immunized with the hemagglutinin protein (HA) containing multi-basic cleavage site (CS) | Diagnostic | Avian influenza virus H5N1 | (i) Bounding of the CS peptide and the H5N1 HA protein (ii) Detection of the CS peptide and H5N1 HA protein by open sandwich ELISA | Dong et al. 2013 |
| Filamentous phage | Phage display libraries (GFPDLs) expressing peptides of 15 to 350 amino acids across the complete genome of the HPAI H7N7 | Prophylactic, therapeutic | Avian influenza virus H7N7 | (i) In vivo expression of PA-X and its recognition by the immune system during human influenza A virus infection | Khurana et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Therapeutic | Avian influenza viruses H5N1 and H1N1 | (i) Obtaining an antibody and its derived peptide that suppressed infection with H5N1 and H1N1 viruses (ii) Demonstration that scFv selected from rHA2 can have neutralizing activity by interfering with the function of the HA stem region during virus entry into target cells | Li et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Vaccine development | Influenza A virus subtypes | (i) Demonstration of immunogenicity and antibody production by the SLLTEVET peptide | Lotfi et al. 2016 |
| Yeast display | S. cerevisiae EBY100 expressing hemagglutinin (HA) of A/Anhui/1/2013 (AH-H7N9) | Vaccine development | Avian influenza virus H7N9 | (i) Oral administration of transformed yeast resulted in the production of IgG antibodies and the cytokines IFN-γ and IL-4 | Lei et al. 2020 |
| Filamentous phage | Phage-display library applied to select antibody fragments for HPAI strain A/Hubei/1/2010 | Diagnostic | Avian influenza H5N1 virus | (i) Three scFvs showed high binding affinity to several HPAI H5N1 strains | Wu et al. 2014 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDLs) | Diagnostic | Avian influenza H5N1 virus | (ii) Both antibodies showed high levels of sensitivity and specificity in detecting H5N1 infections | Khurana et al. 2011 |
| Filamentous phage | 1594-bp DNA fragment of the NP gene amplified from the Indian isolate of H5N1 virus (A/chicken/Jalgaon/India/8824/2006) | Diagnostic | Avian influenza H5N1 virus | (i) Production of antibodies with 100% sensitivity with HI and AGID and specificity of 98.7% with HI and 100% with AGID. | Sengupta et al. 2014 |
| Bacterial display | Recombinant Lactobacillus plantarum (L. plantarum) strains with surface exposure of hemagglutinin (HA2) subunit 2, alone or together with thermal toxin subunit B (LTB) from enterotoxigenic E. coli | Therapeutic, vaccine development | Avian influenza virus H9N2 | (i) Administered HA2-LTB expression strain effectively protected mice from Avian influenza virus (ii) Significant increase in the percentage of CD3 + CD4 + IL-4+, CD3 + CD4 + IFN-γ+ and CD3 + CD4 + IL-17 + T cells and CD3 + CD8 + IFN-γ+ T cells in the spleen (iii) Increased serum IFN-γ production | Jiang et al. 2017 |
| Filamentous phage (M13K07) | Phage display library derived from a rabbit immunized with a peptide conjugate | Diagnostic | NDV | (i) Obtaining antibodies capable of highly specific and sensitive detection of virulent NDV isolates | Li et al. 2002 |
| Yeast display | Yeast strain S. cerevisiae EBY-100 | Prophylactic | NDV | (i) Demonstrating that the P2 domain of HN has dominant immunogenic potential against NDV | Li et al. 2015 |
| Filamentous phage | DNA for scFvs cloned into the pCANTAB5E vector | Prophylactic, therapeutic | Duck hepatitis virus type 1 (DHAV-1) | (i) The action of 7 VP3-specific scFvs with high sensitivity and specificity for DHAV-1 binding was demonstrated | Wang et al. 2018 |
| Filamentous phage | Phage display VHHs library | Diagnostic | Duck hepatitis virus type 1 (DHAV-1) | (i) Obtaining an Nb25 nanoprotein that could react with the conserved linear B cell epitope 174PAPTST179 in DHAV-1 VP1 | Xue et al. 2019 |
| Filamentous phage | Phage display MAbs library | Diagnostic, prophylactic | Avian hepatitis E virus (HEV) | (i) Two monoclonal antibodies (MAbs) against the avian HEV capsid protein, i.e. 3E8 and 1B5, cross-reacted with the swine HEV capsid protein (ii) The I/VPHD motif was an necessary core sequence, and P and H were two key amino acids for recognition by MAb 3E8 | Wang et al. 2015 |
| Filamentous phage | Commercially available, M13 gene 3-based, random peptide phage display library | Diagnostic | Avian infectious bronchitis virus (IBV)–1 | (i) Obtaining antibodies that could be recognized by 10 IBV strains (IBVs) with five different genotypes (ii) Identification of two linear B cell epitopes that were recognized by the obtained antibodies | Zou et al. 2015 |
| Filamentous phage | Commercially available Ph.D™-12 Phage Display Peptide Library Kit | Prophylactic, treatment | Avian infectious bronchitis virus (IBV) | (i) The neutralizing effect of two peptides (H and T) against IBV in chicken embryo kidney cells (ii) High-affinity peptides reduced IBV proliferation in the trachea, lungs and kidneys of chickens and alleviated virus-induced lesions | Sun et al. 2021 |
| Phage T7 | cDNA T7 phage display library | Other | Infectious bursal disease virus (IBDV) | (i) The λ light chain of surface immunoglobulin M (sIgM) was shown to specifically interact with IBDV in an in vitro virulence-independent manner | Luo et al. 2010 |
| Phage T4 | T4-displayed VP2 protein | Vaccine development | Infectious bursal disease virus (IBDV) | (i) The recombinant VP2 protein was immunogenic and induced specific antibodies in immunized chickens which protected them from IBDV virus infection | Cao et al. 2005 |
| Filamentous phage | Semisynthetic phage display library | Therapeutic | Rous sarcoma virus | (i) The obtained antibodies recognized activated vitronectin only in tumor tissues, making vitronectin a new target for anti-cancer strategies | Bloemendal et al. 2004 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library Kit (New England BioLabs) | Diagnostic, therapeutic | Duck Tembusu Virus (DTMUV) | (i) Identification of a minimal B-cell epitope, 374EXE/DPPFG380, that mediates binding to a non-neutralizing monoclonal antibody and is a cross-reactive epitope in most flaviviruses, including Zika, West Nile, Yellow fever, dengue, and Japanese encephalitis viruses | Li et al. 2016a |
| Filamentous phage | Phagemid vector pCANTAB-link | Diagnostic | Foot-and-Mouth Disease Virus (FMDV) | (i) Thirty-two clones were obtained that represented three distinct genetic sequences: 26, FM27 and FM29; each of them bound the 3B region of the 3ABC protein | Foord et al. 2007 |
| Filamentous phage | The DIII-immune phage display scFv library | Diagnostic | USUTU virus | (i) Four scFvs were obtained that bound Usutu virus domain III in the nanomolar range (ii) The usefulness of the obtained antibodies in laboratory diagnostics was confirmed | Schoenenwald et al. 2020 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library | Diagnostic, vaccine development | Waterfowl parvovirus (WPV) | (i) The neutralizing epitope on VP3 of GPV was identified and the general antigenic domain was determined | Li et al. 2016b |
| Filamentous phage | cDNA for the chicken OVR | Therapeutic | Rhinoviruses (HRVs) | (i) Obtained antibodies strongly bound to the chicken OVR receptor from membrane extracts of vesicles and COS-7 cells transfected with a plasmid encoding OVR | Hodits et al. 1995 |
| Filamentous phage | Phage-peptide library panned against pure IgY from nine chickens | Diagnostic, vaccine development | S. enterica, various serovars | (i) Application to differentiate antibody responses in poultry to infections with distinct serovars of S. enterica (ii) Twenty seven anti-IgY-specific peptides were identified from multiple animals infected with S. Enteritidis | Naqid et al. 2016a |
| Filamentous phage | Nine amino acid peptides on coat protein VIII (next generation phage-display (NGPD)) | Diagnostic, vaccine development | Salmonella Typhimurium and Salmonella Entiritidis | (i) Obtaining peptides that identified Salmonella infection with 100% sensitivity and specificity | Naqid et al. 2016b |
| Filamentous phage | Phage display library generated from the heavy chain IgG variable domain repertoire of a llama immunized with C. jejuni flagella | Therapeutic | C. jejuni | (i) Obtained antibodies reduced C. jejuni colonization in the cecum (ii) The mechanism of action of the antibodies was shown to involve specific binding of anti-flagellin antibodies to C. jejuni flagellin | Riazi et al. 2013 |
| Filamentous phage | The f88-4/15-mer peptide phage-display library | Prophylactic, therapeutic | C. jejuni | (i) Twenty seven phage-displayed peptides, representing 11 unique clones, were shown to inhibit the growth of C. jejuni up to 99.9% in vitro; the obtained peptides were highly specific | Bishop-Hurley et al. 2010 |
| Filamentous phage | The scFv antibody phage-display library constructed using spleen mRNA derived from a rabbit immunized with γ-irradiated C. jejuni cells | Diagnostic | C. jejuni | (i) scFv80 was shown to bind specifically and strongly to C. jejuni cells (ii) The IMS-qPCR method was developed that enabled to specifically and sensitively detect C. jejuni in mixed cultures within 3 hours | Nzuma et al. 2018 |
| Filamentous phage | Ph.D TM phage peptide library kit | Diagnostic, vaccine development | A. paragallinarum | (i) Specific serological response to serotype A of A. paragallinarum was elicited (ii) The obtained antibodies protected the chickens from infection with A. paragallinarum 0083 | Wang et al. 2007 |
| Phage T7 | scFv antibodies against S. aureus from the spleen T7 phage display system | Therapeutic | S. aureus | (i) The spleen-derived SFV6 protein showed a high reactivity against S. aureus | J et al. 2016 |
| Filamentous phage | cDNAs encoding VH and VL domains displayed after using the phagemid pEXHAM1 | Therapeutic, prophylactic | Eimeria species | (i) Antibodies were expressed in tobacco leaves infiltrated by agrobacteria or in seeds of transgenic pea plants (ii) Oral administration of flour prepared from transgenic pea seeds had a high parasite neutralizing activity in vivo (iii) Feeding poultry with shreds prepared from pea seeds with expressed antibodies led to significant reduction in Eimeria oocyst infection | Zimmermann et al. 2009 |
| Phage R408 | Genomic DNA isolated from oocysts, ligated into SnaBI-digested pG8SAET and transferred into E. coli TG1 cells | Therapeutic, vaccine development | E. tenella | (i) The antibody bound to the stieda body of sporocysts and significantly impaired in vitro excystation of sporozoites | Krücken et al. 2008 |
| Filamentous phage | The Ph.D. Peptide Library Kits Ph.D.-7, Ph.D.-C7C, and Ph.D.-12 | Therapeutic | E. acervulina and E. tenella | (i) The newly discovered peptide, PW2, showed in vitro activity against the sporozoites of E. acervulina and E. tenella (ii) Anti-fungal activity of PW2 peptide and low activity against tachyzoites was demonstrated | da Silva et al. 2002 |
| Yeast display | EtMic2 pYSD-EtMIC2 expression plasmid transferred into the S. cerevisiae HAO strain | Vaccine development | E. tenella | (i) The EtMic2 protein, administered as a vaccine, could improve weight gain, reduced cecal pathology and lowered fecal oocyst excretion (ii) The oral vaccine stimulated humoral and cellular immune responses | Sun et al. 2014 |
| Yeast display | pCTCON2-EtMIC2 recombinant plasmids, transferred into the S. cerevisiae EBY100 strain | Vaccine development | E. tenella | (i) Two protein variants (1130 and 2119) showed high immunogenicity and protective efficacy against E. tenella infections, increasing weight gain and significantly reducing lesion scores and fecal oocyst excretion, as well as increasing sIgA antibody production and lymphocyte proliferation | Chen et al. 2018 |
| Filamentous phage | Specific EtAMA1-binding peptide selected by using Phage Display Peptide Library Kit | Therapeutic | E. tenella | (i) Specific EtAMA1-binding peptides significantly inhibited sporozoite invasion of MDBK cells | Ma et al. 2019 |
| Filamentous phage | Peptides binding to EtMIC3-bc1 protein prepared based on phage display peptide library kit | Therapeutic | E. tenella | (i) Three tested peptides A, D and W, effectively inhibited MDBK cell invasion by sporozoites | Chen et al. 2021 |
| Filamentous phage | Standard phage display library | Therapeutic, vaccine developmnet | E. tenella | (i) The recombinant antibody showed specific binding to E. tenella sporozoites | Abi-Ghanem et al. 2008 |
| Filamentous phage (M13-KO7) | The chicken IgA Fab phagemid vector pChick3 | Vaccine development | E. acervulina | (i) A phagemid vector (pChick3) for the display and selection of chicken IgA antibodies in Fab format was developed | Wieland et al. 2006 |
| Type of phage/display . | Origin of the library . | Application . | Target pathogen . | Finding(s) . | Reference . |
|---|---|---|---|---|---|
| Filamentous phage | cDNA derived from chickens immunized with recombinant hemagglutinin protein constructed from H5N1 avian influenza virus | Diagnostic, therapeutic | Avian influenza virus H5N1 | (i) Three clones highly specific to the virus (ii) One clone inhibited target virus infection in Madin-Darby canine kidney (MDCK) cells (iii) One clone showed strong reactivity with several influenza A virus subtypes | Pitaksajjakul et al. 2010 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDL) expressing fragments of 15–350 amino acids, covering all the proteins of H5N1 | Diagnostic, vaccine development | Avian influenza virus H5N1 | (i) Identification of several potentially protective H5N1-specific human antibody epitopes (ii) Identification of the strong reactivity against PB1-F2, a putative virulence factor, following H5N1 infection | Khurana et al. 2009 |
| Filamentous phage | Mice immunized with the hemagglutinin protein (HA) containing multi-basic cleavage site (CS) | Diagnostic | Avian influenza virus H5N1 | (i) Bounding of the CS peptide and the H5N1 HA protein (ii) Detection of the CS peptide and H5N1 HA protein by open sandwich ELISA | Dong et al. 2013 |
| Filamentous phage | Phage display libraries (GFPDLs) expressing peptides of 15 to 350 amino acids across the complete genome of the HPAI H7N7 | Prophylactic, therapeutic | Avian influenza virus H7N7 | (i) In vivo expression of PA-X and its recognition by the immune system during human influenza A virus infection | Khurana et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Therapeutic | Avian influenza viruses H5N1 and H1N1 | (i) Obtaining an antibody and its derived peptide that suppressed infection with H5N1 and H1N1 viruses (ii) Demonstration that scFv selected from rHA2 can have neutralizing activity by interfering with the function of the HA stem region during virus entry into target cells | Li et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Vaccine development | Influenza A virus subtypes | (i) Demonstration of immunogenicity and antibody production by the SLLTEVET peptide | Lotfi et al. 2016 |
| Yeast display | S. cerevisiae EBY100 expressing hemagglutinin (HA) of A/Anhui/1/2013 (AH-H7N9) | Vaccine development | Avian influenza virus H7N9 | (i) Oral administration of transformed yeast resulted in the production of IgG antibodies and the cytokines IFN-γ and IL-4 | Lei et al. 2020 |
| Filamentous phage | Phage-display library applied to select antibody fragments for HPAI strain A/Hubei/1/2010 | Diagnostic | Avian influenza H5N1 virus | (i) Three scFvs showed high binding affinity to several HPAI H5N1 strains | Wu et al. 2014 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDLs) | Diagnostic | Avian influenza H5N1 virus | (ii) Both antibodies showed high levels of sensitivity and specificity in detecting H5N1 infections | Khurana et al. 2011 |
| Filamentous phage | 1594-bp DNA fragment of the NP gene amplified from the Indian isolate of H5N1 virus (A/chicken/Jalgaon/India/8824/2006) | Diagnostic | Avian influenza H5N1 virus | (i) Production of antibodies with 100% sensitivity with HI and AGID and specificity of 98.7% with HI and 100% with AGID. | Sengupta et al. 2014 |
| Bacterial display | Recombinant Lactobacillus plantarum (L. plantarum) strains with surface exposure of hemagglutinin (HA2) subunit 2, alone or together with thermal toxin subunit B (LTB) from enterotoxigenic E. coli | Therapeutic, vaccine development | Avian influenza virus H9N2 | (i) Administered HA2-LTB expression strain effectively protected mice from Avian influenza virus (ii) Significant increase in the percentage of CD3 + CD4 + IL-4+, CD3 + CD4 + IFN-γ+ and CD3 + CD4 + IL-17 + T cells and CD3 + CD8 + IFN-γ+ T cells in the spleen (iii) Increased serum IFN-γ production | Jiang et al. 2017 |
| Filamentous phage (M13K07) | Phage display library derived from a rabbit immunized with a peptide conjugate | Diagnostic | NDV | (i) Obtaining antibodies capable of highly specific and sensitive detection of virulent NDV isolates | Li et al. 2002 |
| Yeast display | Yeast strain S. cerevisiae EBY-100 | Prophylactic | NDV | (i) Demonstrating that the P2 domain of HN has dominant immunogenic potential against NDV | Li et al. 2015 |
| Filamentous phage | DNA for scFvs cloned into the pCANTAB5E vector | Prophylactic, therapeutic | Duck hepatitis virus type 1 (DHAV-1) | (i) The action of 7 VP3-specific scFvs with high sensitivity and specificity for DHAV-1 binding was demonstrated | Wang et al. 2018 |
| Filamentous phage | Phage display VHHs library | Diagnostic | Duck hepatitis virus type 1 (DHAV-1) | (i) Obtaining an Nb25 nanoprotein that could react with the conserved linear B cell epitope 174PAPTST179 in DHAV-1 VP1 | Xue et al. 2019 |
| Filamentous phage | Phage display MAbs library | Diagnostic, prophylactic | Avian hepatitis E virus (HEV) | (i) Two monoclonal antibodies (MAbs) against the avian HEV capsid protein, i.e. 3E8 and 1B5, cross-reacted with the swine HEV capsid protein (ii) The I/VPHD motif was an necessary core sequence, and P and H were two key amino acids for recognition by MAb 3E8 | Wang et al. 2015 |
| Filamentous phage | Commercially available, M13 gene 3-based, random peptide phage display library | Diagnostic | Avian infectious bronchitis virus (IBV)–1 | (i) Obtaining antibodies that could be recognized by 10 IBV strains (IBVs) with five different genotypes (ii) Identification of two linear B cell epitopes that were recognized by the obtained antibodies | Zou et al. 2015 |
| Filamentous phage | Commercially available Ph.D™-12 Phage Display Peptide Library Kit | Prophylactic, treatment | Avian infectious bronchitis virus (IBV) | (i) The neutralizing effect of two peptides (H and T) against IBV in chicken embryo kidney cells (ii) High-affinity peptides reduced IBV proliferation in the trachea, lungs and kidneys of chickens and alleviated virus-induced lesions | Sun et al. 2021 |
| Phage T7 | cDNA T7 phage display library | Other | Infectious bursal disease virus (IBDV) | (i) The λ light chain of surface immunoglobulin M (sIgM) was shown to specifically interact with IBDV in an in vitro virulence-independent manner | Luo et al. 2010 |
| Phage T4 | T4-displayed VP2 protein | Vaccine development | Infectious bursal disease virus (IBDV) | (i) The recombinant VP2 protein was immunogenic and induced specific antibodies in immunized chickens which protected them from IBDV virus infection | Cao et al. 2005 |
| Filamentous phage | Semisynthetic phage display library | Therapeutic | Rous sarcoma virus | (i) The obtained antibodies recognized activated vitronectin only in tumor tissues, making vitronectin a new target for anti-cancer strategies | Bloemendal et al. 2004 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library Kit (New England BioLabs) | Diagnostic, therapeutic | Duck Tembusu Virus (DTMUV) | (i) Identification of a minimal B-cell epitope, 374EXE/DPPFG380, that mediates binding to a non-neutralizing monoclonal antibody and is a cross-reactive epitope in most flaviviruses, including Zika, West Nile, Yellow fever, dengue, and Japanese encephalitis viruses | Li et al. 2016a |
| Filamentous phage | Phagemid vector pCANTAB-link | Diagnostic | Foot-and-Mouth Disease Virus (FMDV) | (i) Thirty-two clones were obtained that represented three distinct genetic sequences: 26, FM27 and FM29; each of them bound the 3B region of the 3ABC protein | Foord et al. 2007 |
| Filamentous phage | The DIII-immune phage display scFv library | Diagnostic | USUTU virus | (i) Four scFvs were obtained that bound Usutu virus domain III in the nanomolar range (ii) The usefulness of the obtained antibodies in laboratory diagnostics was confirmed | Schoenenwald et al. 2020 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library | Diagnostic, vaccine development | Waterfowl parvovirus (WPV) | (i) The neutralizing epitope on VP3 of GPV was identified and the general antigenic domain was determined | Li et al. 2016b |
| Filamentous phage | cDNA for the chicken OVR | Therapeutic | Rhinoviruses (HRVs) | (i) Obtained antibodies strongly bound to the chicken OVR receptor from membrane extracts of vesicles and COS-7 cells transfected with a plasmid encoding OVR | Hodits et al. 1995 |
| Filamentous phage | Phage-peptide library panned against pure IgY from nine chickens | Diagnostic, vaccine development | S. enterica, various serovars | (i) Application to differentiate antibody responses in poultry to infections with distinct serovars of S. enterica (ii) Twenty seven anti-IgY-specific peptides were identified from multiple animals infected with S. Enteritidis | Naqid et al. 2016a |
| Filamentous phage | Nine amino acid peptides on coat protein VIII (next generation phage-display (NGPD)) | Diagnostic, vaccine development | Salmonella Typhimurium and Salmonella Entiritidis | (i) Obtaining peptides that identified Salmonella infection with 100% sensitivity and specificity | Naqid et al. 2016b |
| Filamentous phage | Phage display library generated from the heavy chain IgG variable domain repertoire of a llama immunized with C. jejuni flagella | Therapeutic | C. jejuni | (i) Obtained antibodies reduced C. jejuni colonization in the cecum (ii) The mechanism of action of the antibodies was shown to involve specific binding of anti-flagellin antibodies to C. jejuni flagellin | Riazi et al. 2013 |
| Filamentous phage | The f88-4/15-mer peptide phage-display library | Prophylactic, therapeutic | C. jejuni | (i) Twenty seven phage-displayed peptides, representing 11 unique clones, were shown to inhibit the growth of C. jejuni up to 99.9% in vitro; the obtained peptides were highly specific | Bishop-Hurley et al. 2010 |
| Filamentous phage | The scFv antibody phage-display library constructed using spleen mRNA derived from a rabbit immunized with γ-irradiated C. jejuni cells | Diagnostic | C. jejuni | (i) scFv80 was shown to bind specifically and strongly to C. jejuni cells (ii) The IMS-qPCR method was developed that enabled to specifically and sensitively detect C. jejuni in mixed cultures within 3 hours | Nzuma et al. 2018 |
| Filamentous phage | Ph.D TM phage peptide library kit | Diagnostic, vaccine development | A. paragallinarum | (i) Specific serological response to serotype A of A. paragallinarum was elicited (ii) The obtained antibodies protected the chickens from infection with A. paragallinarum 0083 | Wang et al. 2007 |
| Phage T7 | scFv antibodies against S. aureus from the spleen T7 phage display system | Therapeutic | S. aureus | (i) The spleen-derived SFV6 protein showed a high reactivity against S. aureus | J et al. 2016 |
| Filamentous phage | cDNAs encoding VH and VL domains displayed after using the phagemid pEXHAM1 | Therapeutic, prophylactic | Eimeria species | (i) Antibodies were expressed in tobacco leaves infiltrated by agrobacteria or in seeds of transgenic pea plants (ii) Oral administration of flour prepared from transgenic pea seeds had a high parasite neutralizing activity in vivo (iii) Feeding poultry with shreds prepared from pea seeds with expressed antibodies led to significant reduction in Eimeria oocyst infection | Zimmermann et al. 2009 |
| Phage R408 | Genomic DNA isolated from oocysts, ligated into SnaBI-digested pG8SAET and transferred into E. coli TG1 cells | Therapeutic, vaccine development | E. tenella | (i) The antibody bound to the stieda body of sporocysts and significantly impaired in vitro excystation of sporozoites | Krücken et al. 2008 |
| Filamentous phage | The Ph.D. Peptide Library Kits Ph.D.-7, Ph.D.-C7C, and Ph.D.-12 | Therapeutic | E. acervulina and E. tenella | (i) The newly discovered peptide, PW2, showed in vitro activity against the sporozoites of E. acervulina and E. tenella (ii) Anti-fungal activity of PW2 peptide and low activity against tachyzoites was demonstrated | da Silva et al. 2002 |
| Yeast display | EtMic2 pYSD-EtMIC2 expression plasmid transferred into the S. cerevisiae HAO strain | Vaccine development | E. tenella | (i) The EtMic2 protein, administered as a vaccine, could improve weight gain, reduced cecal pathology and lowered fecal oocyst excretion (ii) The oral vaccine stimulated humoral and cellular immune responses | Sun et al. 2014 |
| Yeast display | pCTCON2-EtMIC2 recombinant plasmids, transferred into the S. cerevisiae EBY100 strain | Vaccine development | E. tenella | (i) Two protein variants (1130 and 2119) showed high immunogenicity and protective efficacy against E. tenella infections, increasing weight gain and significantly reducing lesion scores and fecal oocyst excretion, as well as increasing sIgA antibody production and lymphocyte proliferation | Chen et al. 2018 |
| Filamentous phage | Specific EtAMA1-binding peptide selected by using Phage Display Peptide Library Kit | Therapeutic | E. tenella | (i) Specific EtAMA1-binding peptides significantly inhibited sporozoite invasion of MDBK cells | Ma et al. 2019 |
| Filamentous phage | Peptides binding to EtMIC3-bc1 protein prepared based on phage display peptide library kit | Therapeutic | E. tenella | (i) Three tested peptides A, D and W, effectively inhibited MDBK cell invasion by sporozoites | Chen et al. 2021 |
| Filamentous phage | Standard phage display library | Therapeutic, vaccine developmnet | E. tenella | (i) The recombinant antibody showed specific binding to E. tenella sporozoites | Abi-Ghanem et al. 2008 |
| Filamentous phage (M13-KO7) | The chicken IgA Fab phagemid vector pChick3 | Vaccine development | E. acervulina | (i) A phagemid vector (pChick3) for the display and selection of chicken IgA antibodies in Fab format was developed | Wieland et al. 2006 |
| Type of phage/display . | Origin of the library . | Application . | Target pathogen . | Finding(s) . | Reference . |
|---|---|---|---|---|---|
| Filamentous phage | cDNA derived from chickens immunized with recombinant hemagglutinin protein constructed from H5N1 avian influenza virus | Diagnostic, therapeutic | Avian influenza virus H5N1 | (i) Three clones highly specific to the virus (ii) One clone inhibited target virus infection in Madin-Darby canine kidney (MDCK) cells (iii) One clone showed strong reactivity with several influenza A virus subtypes | Pitaksajjakul et al. 2010 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDL) expressing fragments of 15–350 amino acids, covering all the proteins of H5N1 | Diagnostic, vaccine development | Avian influenza virus H5N1 | (i) Identification of several potentially protective H5N1-specific human antibody epitopes (ii) Identification of the strong reactivity against PB1-F2, a putative virulence factor, following H5N1 infection | Khurana et al. 2009 |
| Filamentous phage | Mice immunized with the hemagglutinin protein (HA) containing multi-basic cleavage site (CS) | Diagnostic | Avian influenza virus H5N1 | (i) Bounding of the CS peptide and the H5N1 HA protein (ii) Detection of the CS peptide and H5N1 HA protein by open sandwich ELISA | Dong et al. 2013 |
| Filamentous phage | Phage display libraries (GFPDLs) expressing peptides of 15 to 350 amino acids across the complete genome of the HPAI H7N7 | Prophylactic, therapeutic | Avian influenza virus H7N7 | (i) In vivo expression of PA-X and its recognition by the immune system during human influenza A virus infection | Khurana et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Therapeutic | Avian influenza viruses H5N1 and H1N1 | (i) Obtaining an antibody and its derived peptide that suppressed infection with H5N1 and H1N1 viruses (ii) Demonstration that scFv selected from rHA2 can have neutralizing activity by interfering with the function of the HA stem region during virus entry into target cells | Li et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Vaccine development | Influenza A virus subtypes | (i) Demonstration of immunogenicity and antibody production by the SLLTEVET peptide | Lotfi et al. 2016 |
| Yeast display | S. cerevisiae EBY100 expressing hemagglutinin (HA) of A/Anhui/1/2013 (AH-H7N9) | Vaccine development | Avian influenza virus H7N9 | (i) Oral administration of transformed yeast resulted in the production of IgG antibodies and the cytokines IFN-γ and IL-4 | Lei et al. 2020 |
| Filamentous phage | Phage-display library applied to select antibody fragments for HPAI strain A/Hubei/1/2010 | Diagnostic | Avian influenza H5N1 virus | (i) Three scFvs showed high binding affinity to several HPAI H5N1 strains | Wu et al. 2014 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDLs) | Diagnostic | Avian influenza H5N1 virus | (ii) Both antibodies showed high levels of sensitivity and specificity in detecting H5N1 infections | Khurana et al. 2011 |
| Filamentous phage | 1594-bp DNA fragment of the NP gene amplified from the Indian isolate of H5N1 virus (A/chicken/Jalgaon/India/8824/2006) | Diagnostic | Avian influenza H5N1 virus | (i) Production of antibodies with 100% sensitivity with HI and AGID and specificity of 98.7% with HI and 100% with AGID. | Sengupta et al. 2014 |
| Bacterial display | Recombinant Lactobacillus plantarum (L. plantarum) strains with surface exposure of hemagglutinin (HA2) subunit 2, alone or together with thermal toxin subunit B (LTB) from enterotoxigenic E. coli | Therapeutic, vaccine development | Avian influenza virus H9N2 | (i) Administered HA2-LTB expression strain effectively protected mice from Avian influenza virus (ii) Significant increase in the percentage of CD3 + CD4 + IL-4+, CD3 + CD4 + IFN-γ+ and CD3 + CD4 + IL-17 + T cells and CD3 + CD8 + IFN-γ+ T cells in the spleen (iii) Increased serum IFN-γ production | Jiang et al. 2017 |
| Filamentous phage (M13K07) | Phage display library derived from a rabbit immunized with a peptide conjugate | Diagnostic | NDV | (i) Obtaining antibodies capable of highly specific and sensitive detection of virulent NDV isolates | Li et al. 2002 |
| Yeast display | Yeast strain S. cerevisiae EBY-100 | Prophylactic | NDV | (i) Demonstrating that the P2 domain of HN has dominant immunogenic potential against NDV | Li et al. 2015 |
| Filamentous phage | DNA for scFvs cloned into the pCANTAB5E vector | Prophylactic, therapeutic | Duck hepatitis virus type 1 (DHAV-1) | (i) The action of 7 VP3-specific scFvs with high sensitivity and specificity for DHAV-1 binding was demonstrated | Wang et al. 2018 |
| Filamentous phage | Phage display VHHs library | Diagnostic | Duck hepatitis virus type 1 (DHAV-1) | (i) Obtaining an Nb25 nanoprotein that could react with the conserved linear B cell epitope 174PAPTST179 in DHAV-1 VP1 | Xue et al. 2019 |
| Filamentous phage | Phage display MAbs library | Diagnostic, prophylactic | Avian hepatitis E virus (HEV) | (i) Two monoclonal antibodies (MAbs) against the avian HEV capsid protein, i.e. 3E8 and 1B5, cross-reacted with the swine HEV capsid protein (ii) The I/VPHD motif was an necessary core sequence, and P and H were two key amino acids for recognition by MAb 3E8 | Wang et al. 2015 |
| Filamentous phage | Commercially available, M13 gene 3-based, random peptide phage display library | Diagnostic | Avian infectious bronchitis virus (IBV)–1 | (i) Obtaining antibodies that could be recognized by 10 IBV strains (IBVs) with five different genotypes (ii) Identification of two linear B cell epitopes that were recognized by the obtained antibodies | Zou et al. 2015 |
| Filamentous phage | Commercially available Ph.D™-12 Phage Display Peptide Library Kit | Prophylactic, treatment | Avian infectious bronchitis virus (IBV) | (i) The neutralizing effect of two peptides (H and T) against IBV in chicken embryo kidney cells (ii) High-affinity peptides reduced IBV proliferation in the trachea, lungs and kidneys of chickens and alleviated virus-induced lesions | Sun et al. 2021 |
| Phage T7 | cDNA T7 phage display library | Other | Infectious bursal disease virus (IBDV) | (i) The λ light chain of surface immunoglobulin M (sIgM) was shown to specifically interact with IBDV in an in vitro virulence-independent manner | Luo et al. 2010 |
| Phage T4 | T4-displayed VP2 protein | Vaccine development | Infectious bursal disease virus (IBDV) | (i) The recombinant VP2 protein was immunogenic and induced specific antibodies in immunized chickens which protected them from IBDV virus infection | Cao et al. 2005 |
| Filamentous phage | Semisynthetic phage display library | Therapeutic | Rous sarcoma virus | (i) The obtained antibodies recognized activated vitronectin only in tumor tissues, making vitronectin a new target for anti-cancer strategies | Bloemendal et al. 2004 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library Kit (New England BioLabs) | Diagnostic, therapeutic | Duck Tembusu Virus (DTMUV) | (i) Identification of a minimal B-cell epitope, 374EXE/DPPFG380, that mediates binding to a non-neutralizing monoclonal antibody and is a cross-reactive epitope in most flaviviruses, including Zika, West Nile, Yellow fever, dengue, and Japanese encephalitis viruses | Li et al. 2016a |
| Filamentous phage | Phagemid vector pCANTAB-link | Diagnostic | Foot-and-Mouth Disease Virus (FMDV) | (i) Thirty-two clones were obtained that represented three distinct genetic sequences: 26, FM27 and FM29; each of them bound the 3B region of the 3ABC protein | Foord et al. 2007 |
| Filamentous phage | The DIII-immune phage display scFv library | Diagnostic | USUTU virus | (i) Four scFvs were obtained that bound Usutu virus domain III in the nanomolar range (ii) The usefulness of the obtained antibodies in laboratory diagnostics was confirmed | Schoenenwald et al. 2020 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library | Diagnostic, vaccine development | Waterfowl parvovirus (WPV) | (i) The neutralizing epitope on VP3 of GPV was identified and the general antigenic domain was determined | Li et al. 2016b |
| Filamentous phage | cDNA for the chicken OVR | Therapeutic | Rhinoviruses (HRVs) | (i) Obtained antibodies strongly bound to the chicken OVR receptor from membrane extracts of vesicles and COS-7 cells transfected with a plasmid encoding OVR | Hodits et al. 1995 |
| Filamentous phage | Phage-peptide library panned against pure IgY from nine chickens | Diagnostic, vaccine development | S. enterica, various serovars | (i) Application to differentiate antibody responses in poultry to infections with distinct serovars of S. enterica (ii) Twenty seven anti-IgY-specific peptides were identified from multiple animals infected with S. Enteritidis | Naqid et al. 2016a |
| Filamentous phage | Nine amino acid peptides on coat protein VIII (next generation phage-display (NGPD)) | Diagnostic, vaccine development | Salmonella Typhimurium and Salmonella Entiritidis | (i) Obtaining peptides that identified Salmonella infection with 100% sensitivity and specificity | Naqid et al. 2016b |
| Filamentous phage | Phage display library generated from the heavy chain IgG variable domain repertoire of a llama immunized with C. jejuni flagella | Therapeutic | C. jejuni | (i) Obtained antibodies reduced C. jejuni colonization in the cecum (ii) The mechanism of action of the antibodies was shown to involve specific binding of anti-flagellin antibodies to C. jejuni flagellin | Riazi et al. 2013 |
| Filamentous phage | The f88-4/15-mer peptide phage-display library | Prophylactic, therapeutic | C. jejuni | (i) Twenty seven phage-displayed peptides, representing 11 unique clones, were shown to inhibit the growth of C. jejuni up to 99.9% in vitro; the obtained peptides were highly specific | Bishop-Hurley et al. 2010 |
| Filamentous phage | The scFv antibody phage-display library constructed using spleen mRNA derived from a rabbit immunized with γ-irradiated C. jejuni cells | Diagnostic | C. jejuni | (i) scFv80 was shown to bind specifically and strongly to C. jejuni cells (ii) The IMS-qPCR method was developed that enabled to specifically and sensitively detect C. jejuni in mixed cultures within 3 hours | Nzuma et al. 2018 |
| Filamentous phage | Ph.D TM phage peptide library kit | Diagnostic, vaccine development | A. paragallinarum | (i) Specific serological response to serotype A of A. paragallinarum was elicited (ii) The obtained antibodies protected the chickens from infection with A. paragallinarum 0083 | Wang et al. 2007 |
| Phage T7 | scFv antibodies against S. aureus from the spleen T7 phage display system | Therapeutic | S. aureus | (i) The spleen-derived SFV6 protein showed a high reactivity against S. aureus | J et al. 2016 |
| Filamentous phage | cDNAs encoding VH and VL domains displayed after using the phagemid pEXHAM1 | Therapeutic, prophylactic | Eimeria species | (i) Antibodies were expressed in tobacco leaves infiltrated by agrobacteria or in seeds of transgenic pea plants (ii) Oral administration of flour prepared from transgenic pea seeds had a high parasite neutralizing activity in vivo (iii) Feeding poultry with shreds prepared from pea seeds with expressed antibodies led to significant reduction in Eimeria oocyst infection | Zimmermann et al. 2009 |
| Phage R408 | Genomic DNA isolated from oocysts, ligated into SnaBI-digested pG8SAET and transferred into E. coli TG1 cells | Therapeutic, vaccine development | E. tenella | (i) The antibody bound to the stieda body of sporocysts and significantly impaired in vitro excystation of sporozoites | Krücken et al. 2008 |
| Filamentous phage | The Ph.D. Peptide Library Kits Ph.D.-7, Ph.D.-C7C, and Ph.D.-12 | Therapeutic | E. acervulina and E. tenella | (i) The newly discovered peptide, PW2, showed in vitro activity against the sporozoites of E. acervulina and E. tenella (ii) Anti-fungal activity of PW2 peptide and low activity against tachyzoites was demonstrated | da Silva et al. 2002 |
| Yeast display | EtMic2 pYSD-EtMIC2 expression plasmid transferred into the S. cerevisiae HAO strain | Vaccine development | E. tenella | (i) The EtMic2 protein, administered as a vaccine, could improve weight gain, reduced cecal pathology and lowered fecal oocyst excretion (ii) The oral vaccine stimulated humoral and cellular immune responses | Sun et al. 2014 |
| Yeast display | pCTCON2-EtMIC2 recombinant plasmids, transferred into the S. cerevisiae EBY100 strain | Vaccine development | E. tenella | (i) Two protein variants (1130 and 2119) showed high immunogenicity and protective efficacy against E. tenella infections, increasing weight gain and significantly reducing lesion scores and fecal oocyst excretion, as well as increasing sIgA antibody production and lymphocyte proliferation | Chen et al. 2018 |
| Filamentous phage | Specific EtAMA1-binding peptide selected by using Phage Display Peptide Library Kit | Therapeutic | E. tenella | (i) Specific EtAMA1-binding peptides significantly inhibited sporozoite invasion of MDBK cells | Ma et al. 2019 |
| Filamentous phage | Peptides binding to EtMIC3-bc1 protein prepared based on phage display peptide library kit | Therapeutic | E. tenella | (i) Three tested peptides A, D and W, effectively inhibited MDBK cell invasion by sporozoites | Chen et al. 2021 |
| Filamentous phage | Standard phage display library | Therapeutic, vaccine developmnet | E. tenella | (i) The recombinant antibody showed specific binding to E. tenella sporozoites | Abi-Ghanem et al. 2008 |
| Filamentous phage (M13-KO7) | The chicken IgA Fab phagemid vector pChick3 | Vaccine development | E. acervulina | (i) A phagemid vector (pChick3) for the display and selection of chicken IgA antibodies in Fab format was developed | Wieland et al. 2006 |
| Type of phage/display . | Origin of the library . | Application . | Target pathogen . | Finding(s) . | Reference . |
|---|---|---|---|---|---|
| Filamentous phage | cDNA derived from chickens immunized with recombinant hemagglutinin protein constructed from H5N1 avian influenza virus | Diagnostic, therapeutic | Avian influenza virus H5N1 | (i) Three clones highly specific to the virus (ii) One clone inhibited target virus infection in Madin-Darby canine kidney (MDCK) cells (iii) One clone showed strong reactivity with several influenza A virus subtypes | Pitaksajjakul et al. 2010 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDL) expressing fragments of 15–350 amino acids, covering all the proteins of H5N1 | Diagnostic, vaccine development | Avian influenza virus H5N1 | (i) Identification of several potentially protective H5N1-specific human antibody epitopes (ii) Identification of the strong reactivity against PB1-F2, a putative virulence factor, following H5N1 infection | Khurana et al. 2009 |
| Filamentous phage | Mice immunized with the hemagglutinin protein (HA) containing multi-basic cleavage site (CS) | Diagnostic | Avian influenza virus H5N1 | (i) Bounding of the CS peptide and the H5N1 HA protein (ii) Detection of the CS peptide and H5N1 HA protein by open sandwich ELISA | Dong et al. 2013 |
| Filamentous phage | Phage display libraries (GFPDLs) expressing peptides of 15 to 350 amino acids across the complete genome of the HPAI H7N7 | Prophylactic, therapeutic | Avian influenza virus H7N7 | (i) In vivo expression of PA-X and its recognition by the immune system during human influenza A virus infection | Khurana et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Therapeutic | Avian influenza viruses H5N1 and H1N1 | (i) Obtaining an antibody and its derived peptide that suppressed infection with H5N1 and H1N1 viruses (ii) Demonstration that scFv selected from rHA2 can have neutralizing activity by interfering with the function of the HA stem region during virus entry into target cells | Li et al. 2016 |
| Filamentous phage | The Tomlinson I and J scFv phage display libraries | Vaccine development | Influenza A virus subtypes | (i) Demonstration of immunogenicity and antibody production by the SLLTEVET peptide | Lotfi et al. 2016 |
| Yeast display | S. cerevisiae EBY100 expressing hemagglutinin (HA) of A/Anhui/1/2013 (AH-H7N9) | Vaccine development | Avian influenza virus H7N9 | (i) Oral administration of transformed yeast resulted in the production of IgG antibodies and the cytokines IFN-γ and IL-4 | Lei et al. 2020 |
| Filamentous phage | Phage-display library applied to select antibody fragments for HPAI strain A/Hubei/1/2010 | Diagnostic | Avian influenza H5N1 virus | (i) Three scFvs showed high binding affinity to several HPAI H5N1 strains | Wu et al. 2014 |
| Filamentous phage | Whole-genome-fragment phage display libraries (GFPDLs) | Diagnostic | Avian influenza H5N1 virus | (ii) Both antibodies showed high levels of sensitivity and specificity in detecting H5N1 infections | Khurana et al. 2011 |
| Filamentous phage | 1594-bp DNA fragment of the NP gene amplified from the Indian isolate of H5N1 virus (A/chicken/Jalgaon/India/8824/2006) | Diagnostic | Avian influenza H5N1 virus | (i) Production of antibodies with 100% sensitivity with HI and AGID and specificity of 98.7% with HI and 100% with AGID. | Sengupta et al. 2014 |
| Bacterial display | Recombinant Lactobacillus plantarum (L. plantarum) strains with surface exposure of hemagglutinin (HA2) subunit 2, alone or together with thermal toxin subunit B (LTB) from enterotoxigenic E. coli | Therapeutic, vaccine development | Avian influenza virus H9N2 | (i) Administered HA2-LTB expression strain effectively protected mice from Avian influenza virus (ii) Significant increase in the percentage of CD3 + CD4 + IL-4+, CD3 + CD4 + IFN-γ+ and CD3 + CD4 + IL-17 + T cells and CD3 + CD8 + IFN-γ+ T cells in the spleen (iii) Increased serum IFN-γ production | Jiang et al. 2017 |
| Filamentous phage (M13K07) | Phage display library derived from a rabbit immunized with a peptide conjugate | Diagnostic | NDV | (i) Obtaining antibodies capable of highly specific and sensitive detection of virulent NDV isolates | Li et al. 2002 |
| Yeast display | Yeast strain S. cerevisiae EBY-100 | Prophylactic | NDV | (i) Demonstrating that the P2 domain of HN has dominant immunogenic potential against NDV | Li et al. 2015 |
| Filamentous phage | DNA for scFvs cloned into the pCANTAB5E vector | Prophylactic, therapeutic | Duck hepatitis virus type 1 (DHAV-1) | (i) The action of 7 VP3-specific scFvs with high sensitivity and specificity for DHAV-1 binding was demonstrated | Wang et al. 2018 |
| Filamentous phage | Phage display VHHs library | Diagnostic | Duck hepatitis virus type 1 (DHAV-1) | (i) Obtaining an Nb25 nanoprotein that could react with the conserved linear B cell epitope 174PAPTST179 in DHAV-1 VP1 | Xue et al. 2019 |
| Filamentous phage | Phage display MAbs library | Diagnostic, prophylactic | Avian hepatitis E virus (HEV) | (i) Two monoclonal antibodies (MAbs) against the avian HEV capsid protein, i.e. 3E8 and 1B5, cross-reacted with the swine HEV capsid protein (ii) The I/VPHD motif was an necessary core sequence, and P and H were two key amino acids for recognition by MAb 3E8 | Wang et al. 2015 |
| Filamentous phage | Commercially available, M13 gene 3-based, random peptide phage display library | Diagnostic | Avian infectious bronchitis virus (IBV)–1 | (i) Obtaining antibodies that could be recognized by 10 IBV strains (IBVs) with five different genotypes (ii) Identification of two linear B cell epitopes that were recognized by the obtained antibodies | Zou et al. 2015 |
| Filamentous phage | Commercially available Ph.D™-12 Phage Display Peptide Library Kit | Prophylactic, treatment | Avian infectious bronchitis virus (IBV) | (i) The neutralizing effect of two peptides (H and T) against IBV in chicken embryo kidney cells (ii) High-affinity peptides reduced IBV proliferation in the trachea, lungs and kidneys of chickens and alleviated virus-induced lesions | Sun et al. 2021 |
| Phage T7 | cDNA T7 phage display library | Other | Infectious bursal disease virus (IBDV) | (i) The λ light chain of surface immunoglobulin M (sIgM) was shown to specifically interact with IBDV in an in vitro virulence-independent manner | Luo et al. 2010 |
| Phage T4 | T4-displayed VP2 protein | Vaccine development | Infectious bursal disease virus (IBDV) | (i) The recombinant VP2 protein was immunogenic and induced specific antibodies in immunized chickens which protected them from IBDV virus infection | Cao et al. 2005 |
| Filamentous phage | Semisynthetic phage display library | Therapeutic | Rous sarcoma virus | (i) The obtained antibodies recognized activated vitronectin only in tumor tissues, making vitronectin a new target for anti-cancer strategies | Bloemendal et al. 2004 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library Kit (New England BioLabs) | Diagnostic, therapeutic | Duck Tembusu Virus (DTMUV) | (i) Identification of a minimal B-cell epitope, 374EXE/DPPFG380, that mediates binding to a non-neutralizing monoclonal antibody and is a cross-reactive epitope in most flaviviruses, including Zika, West Nile, Yellow fever, dengue, and Japanese encephalitis viruses | Li et al. 2016a |
| Filamentous phage | Phagemid vector pCANTAB-link | Diagnostic | Foot-and-Mouth Disease Virus (FMDV) | (i) Thirty-two clones were obtained that represented three distinct genetic sequences: 26, FM27 and FM29; each of them bound the 3B region of the 3ABC protein | Foord et al. 2007 |
| Filamentous phage | The DIII-immune phage display scFv library | Diagnostic | USUTU virus | (i) Four scFvs were obtained that bound Usutu virus domain III in the nanomolar range (ii) The usefulness of the obtained antibodies in laboratory diagnostics was confirmed | Schoenenwald et al. 2020 |
| Filamentous phage | Ph.D-12 TM Phage Display Peptide Library | Diagnostic, vaccine development | Waterfowl parvovirus (WPV) | (i) The neutralizing epitope on VP3 of GPV was identified and the general antigenic domain was determined | Li et al. 2016b |
| Filamentous phage | cDNA for the chicken OVR | Therapeutic | Rhinoviruses (HRVs) | (i) Obtained antibodies strongly bound to the chicken OVR receptor from membrane extracts of vesicles and COS-7 cells transfected with a plasmid encoding OVR | Hodits et al. 1995 |
| Filamentous phage | Phage-peptide library panned against pure IgY from nine chickens | Diagnostic, vaccine development | S. enterica, various serovars | (i) Application to differentiate antibody responses in poultry to infections with distinct serovars of S. enterica (ii) Twenty seven anti-IgY-specific peptides were identified from multiple animals infected with S. Enteritidis | Naqid et al. 2016a |
| Filamentous phage | Nine amino acid peptides on coat protein VIII (next generation phage-display (NGPD)) | Diagnostic, vaccine development | Salmonella Typhimurium and Salmonella Entiritidis | (i) Obtaining peptides that identified Salmonella infection with 100% sensitivity and specificity | Naqid et al. 2016b |
| Filamentous phage | Phage display library generated from the heavy chain IgG variable domain repertoire of a llama immunized with C. jejuni flagella | Therapeutic | C. jejuni | (i) Obtained antibodies reduced C. jejuni colonization in the cecum (ii) The mechanism of action of the antibodies was shown to involve specific binding of anti-flagellin antibodies to C. jejuni flagellin | Riazi et al. 2013 |
| Filamentous phage | The f88-4/15-mer peptide phage-display library | Prophylactic, therapeutic | C. jejuni | (i) Twenty seven phage-displayed peptides, representing 11 unique clones, were shown to inhibit the growth of C. jejuni up to 99.9% in vitro; the obtained peptides were highly specific | Bishop-Hurley et al. 2010 |
| Filamentous phage | The scFv antibody phage-display library constructed using spleen mRNA derived from a rabbit immunized with γ-irradiated C. jejuni cells | Diagnostic | C. jejuni | (i) scFv80 was shown to bind specifically and strongly to C. jejuni cells (ii) The IMS-qPCR method was developed that enabled to specifically and sensitively detect C. jejuni in mixed cultures within 3 hours | Nzuma et al. 2018 |
| Filamentous phage | Ph.D TM phage peptide library kit | Diagnostic, vaccine development | A. paragallinarum | (i) Specific serological response to serotype A of A. paragallinarum was elicited (ii) The obtained antibodies protected the chickens from infection with A. paragallinarum 0083 | Wang et al. 2007 |
| Phage T7 | scFv antibodies against S. aureus from the spleen T7 phage display system | Therapeutic | S. aureus | (i) The spleen-derived SFV6 protein showed a high reactivity against S. aureus | J et al. 2016 |
| Filamentous phage | cDNAs encoding VH and VL domains displayed after using the phagemid pEXHAM1 | Therapeutic, prophylactic | Eimeria species | (i) Antibodies were expressed in tobacco leaves infiltrated by agrobacteria or in seeds of transgenic pea plants (ii) Oral administration of flour prepared from transgenic pea seeds had a high parasite neutralizing activity in vivo (iii) Feeding poultry with shreds prepared from pea seeds with expressed antibodies led to significant reduction in Eimeria oocyst infection | Zimmermann et al. 2009 |
| Phage R408 | Genomic DNA isolated from oocysts, ligated into SnaBI-digested pG8SAET and transferred into E. coli TG1 cells | Therapeutic, vaccine development | E. tenella | (i) The antibody bound to the stieda body of sporocysts and significantly impaired in vitro excystation of sporozoites | Krücken et al. 2008 |
| Filamentous phage | The Ph.D. Peptide Library Kits Ph.D.-7, Ph.D.-C7C, and Ph.D.-12 | Therapeutic | E. acervulina and E. tenella | (i) The newly discovered peptide, PW2, showed in vitro activity against the sporozoites of E. acervulina and E. tenella (ii) Anti-fungal activity of PW2 peptide and low activity against tachyzoites was demonstrated | da Silva et al. 2002 |
| Yeast display | EtMic2 pYSD-EtMIC2 expression plasmid transferred into the S. cerevisiae HAO strain | Vaccine development | E. tenella | (i) The EtMic2 protein, administered as a vaccine, could improve weight gain, reduced cecal pathology and lowered fecal oocyst excretion (ii) The oral vaccine stimulated humoral and cellular immune responses | Sun et al. 2014 |
| Yeast display | pCTCON2-EtMIC2 recombinant plasmids, transferred into the S. cerevisiae EBY100 strain | Vaccine development | E. tenella | (i) Two protein variants (1130 and 2119) showed high immunogenicity and protective efficacy against E. tenella infections, increasing weight gain and significantly reducing lesion scores and fecal oocyst excretion, as well as increasing sIgA antibody production and lymphocyte proliferation | Chen et al. 2018 |
| Filamentous phage | Specific EtAMA1-binding peptide selected by using Phage Display Peptide Library Kit | Therapeutic | E. tenella | (i) Specific EtAMA1-binding peptides significantly inhibited sporozoite invasion of MDBK cells | Ma et al. 2019 |
| Filamentous phage | Peptides binding to EtMIC3-bc1 protein prepared based on phage display peptide library kit | Therapeutic | E. tenella | (i) Three tested peptides A, D and W, effectively inhibited MDBK cell invasion by sporozoites | Chen et al. 2021 |
| Filamentous phage | Standard phage display library | Therapeutic, vaccine developmnet | E. tenella | (i) The recombinant antibody showed specific binding to E. tenella sporozoites | Abi-Ghanem et al. 2008 |
| Filamentous phage (M13-KO7) | The chicken IgA Fab phagemid vector pChick3 | Vaccine development | E. acervulina | (i) A phagemid vector (pChick3) for the display and selection of chicken IgA antibodies in Fab format was developed | Wieland et al. 2006 |
Conflict of interest
The authors declare no conflict of interest.
Funding
This work was supported by the National Center for Research and Development, Poland (grant number TECHMATSTRATEG2/410747/11/NCBR/2019).
Author contributions
Ł.G. and K.P. conceptualized the work, search and select literature, prepared table and figures, and revised the manuscript; L.G., Z.C., and G.M. participated in literature review and revision of the manuscript; G.W. supervised the work, drafted manuscript, and prepared the final version of the paper.



