-
PDF
- Split View
-
Views
-
Cite
Cite
Jens Becker, Javier E Fernandez, Alexandra Rossano, Mireille Meylan, Vincent Perreten, Clonal dissemination of MDR Pasteurella multocida ST79 in a small Swiss veal calf farm with high use of antibiotics, Journal of Antimicrobial Chemotherapy, Volume 77, Issue 10, October 2022, Pages 2886–2888, https://doi.org/10.1093/jac/dkac270
Close - Share Icon Share
Pasteurella multocida is a commensal bacterium of the upper respiratory tract of calves and may cause respiratory infections in a multifactorial aetiopathogenesis.1 To control diseases, antimicrobial treatment has been commonly applied using standard antimicrobial agents (β-lactams, tetracyclines, sulphonamides, diaminopyrimidines, phenicols, aminoglycosides) and highest priority critically important antimicrobial agents (macrolides, fluoroquinolones, higher-generation cephalosporins).2 However, limiting the use of the latter is already required by law in the EU (since January 2022).3
In the framework of a large field study where prevalence of and antimicrobial resistance in P. multocida in calves was investigated in 38 Swiss farms,4 we observed that 20 isolates exhibited an MDR profile. The isolates were isolated between February and December 2017 from 17 individual calves of mixed breeds (dairy breeds, dual-purpose breeds, cross-breeds) in one single farm producing on average 50 calves a year. MICs were determined using Thermo Scientific™ Sensititre™ EUST2 plates for tetracycline, streptomycin, sulfamethoxazole and trimethoprim, and BOPO6F plates for the other tested antimicrobial agents, following CLSI recommendations.5 The isolates exhibited resistance to ampicillin (MIC, 0.5 mg/L), tetracycline (≥16 mg/L), spectinomycin (≥128 mg/L) and tulathromycin (≥128 mg/L). They also showed high MICs of oxytetracycline (≥16 mg/L), tylosin (≥64 mg/L), tilmicosin (≥128 mg/L), tiamulin (≥32 mg/L), clindamycin (≥32 mg/L), gentamicin (≥4 mg/L), neomycin (≥64 mg/L), streptomycin (≥64 mg/L) and sulfamethoxazole (≥1024 mg/L), and were susceptible to enrofloxacin (≤0.12 mg/L), danofloxacin (≤0.12 mg/L), ceftiofur (≤0.25 mg/L), trimethoprim (≤1 mg/L) and florfenicol (≤1 mg/L). This MDR phenotype, which includes resistance to the macrolide tulathromycin, is rarely observed in Europe6,7 and has not been observed in P. multocida in Switzerland thus far. This prompted us to characterize these isolates using WGS analysis and to explore their occurrence in calves over time with regard to the antimicrobial treatment history on the farm.
The complete genome of the P. multocida isolates was obtained from Nextera DNA Flex libraries sequenced on Illumina MiSeq (2×150 bp paired-end). To obtain a closed reference genome, strain PS3536-1p was additionally sequenced with MinION Oxford Nanopore Technologies (ONT). Both Illumina and ONT reads were de novo assembled using Unicycler software (v0.4.4), generating a complete plasmid-free circular genome of 2 335 429 bp (GenBank acc. no. CP061052). MLST was performed using the RIRDC MLST scheme from the P. multocida PubMLST database (https://pubmlst.org/organisms/pasteurella-multocida), indicating that all the isolates belonged to ST79. A core-genome SNP (cgSNP) analysis was performed using all additional ST79 strains available in GenBank (n = 83) (accessed 18 June 2021). Recombination events were removed using Gubbins (v2.4.1) and a phylogenetic tree was created based on the resulting cgSNPs using IQ-Tree (v2.1.2) (Figure 1a). SNP calling using Snippy (v4.6.0) against the reference genome PS3536-1p showed that the isolates from the Swiss farm differed from each other by 0 to 3 SNPs and by 43 SNPs from the closest related genome of GenBank (acc. no. CP015570, strain USDA-ARS-USMARC-59962 from cattle), emphasizing clonal dissemination of P. multocida ST79 between calves on the same farm over an 11 month period (Figure 1a).
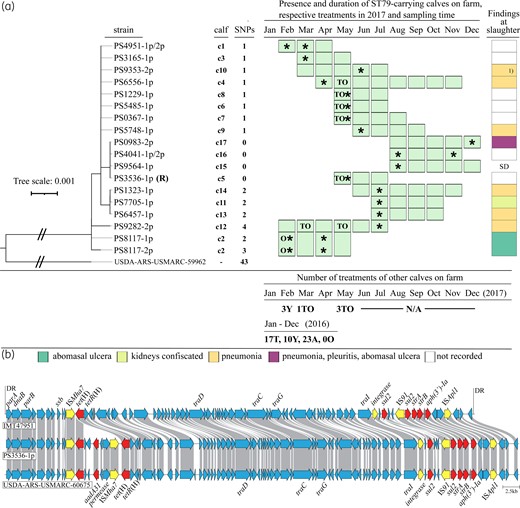
Phylogenetic tree obtained from cgSNP analysis of MDR P. multocida ST79 isolated from 17 calves on a Swiss farm over an 11 month period and antimicrobial treatments recorded on the farm in 2016 and 2017 (a), as well as comparative analysis of the chromosomal ICEs from P. multocida IMT47951 (GenBank acc. no. CP087380), P. multocida USDA-ARS-USMARC-60675 (GenBank acc. no. CP015567) and P. multocida PS3536-1p (GenBank acc. no. CP061052) (b). (a) The genome of P. multocida ST79 strain PS3536-1p (GenBank acc. no. CP061052) was used as a reference (R) for SNP calculation. The tree was rooted with the closest related P. multocida ST79 strain USDA-ARS-USMARC-59962 from GenBank (acc. no. CP015570). Antimicrobial treatments: A, ampicillin; T, tulathromycin; O, oxytetracycline; Y, tylosin; TO, tulathromycin and oxytetracycline (not applied concomitantly, but within the same month). Asterisks indicate the sampling time of the investigated calves. Post-mortem findings at the slaughterhouse are indicated with different colours. 1) In addition to pneumonia, the calf had a knee joint infection, deficient hindquarter and low carcass value. N/A, treatment records not available; SD, sudden death on the farm. (b) Arrows indicate the different ORFs of the ICEs with resistance genes shown in red, transposase genes in yellow and others in blue; grey-shaded lines indicate the homologous regions between ICEs; DR, direct repeats (ATTCAAAA); the ICE comparison illustration was created using clinker v0.0.23. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
The isolates contained an adenine to guanine substitution in each of the six copies of the 23S rRNA gene at position 2058, which confers resistance to macrolides in P. multocida.8 They also carried the tetracycline efflux gene tet(H), the sulphonamide-resistant dihydropteroate synthase gene sul2, the spectinomycin/streptomycin adenylyltransferase gene aadA31, the streptomycin phosphotransferase genes strA and strB, and the kanamycin/neomycin phosphotransferase gene aph(3’)-Ia (detected using ResFinder 4.1). The genes were located on a chromosomal integrative and conjugative element (ICE), which shared 99.99% DNA identity (2 SNPs) with the ICE of a bovine P. multocida isolated in the USA in 2013 and 91.09% identity with the ICE (designated Tn7407) of a bovine P. multocida isolated in Germany in 2019.9 The ICE of PS3536-1p differed from Tn7407 by the integration of a 6782 bp fragment containing aadA31 (Figure 1b). Despite resistance to β-lactams, the BBL DrySlide Nitrocefin test did not reveal β-lactamase production. However, the isolate contained an ftsI gene mutation, generating an alanine to serine substitution at amino acid position 525 in PBP3. The association of this mutation with β-lactam resistance in P. multocida has not been reported and investigated with regard to its mechanism of action,7 but a lysin-526 substitution is known to confer decreased susceptibility to ampicillin in β-lactamase-negative Haemophilus influenzae, another member of Pasteurellaceae.10 Furthermore, the capsular protein of the isolates matched type A and lipopolysaccharide L3 of serovar Heddleston 3 (GenBank acc. no. KF314825).11
Several parenteral treatments with tulathromycin, tylosin and oxytetracycline, and oral administration of amoxicillin were recorded during the study year and the previous year (Figure 1a). Calves were slaughtered at 159.3 ± 20.2 days (mean ± SD). Clonal dissemination of P. multocida ST79 was observed throughout a period that was distinctly longer than the lifespan of a calf, indicating that P. multocida ST79 was maintained within the herd by circulating among calves that were present on the farm at the same time (Figure 1a). The frequent use of macrolides, tetracyclines and β-lactams and the presence of P. multocida ST79 exhibiting resistance to these antimicrobial agents in different calves over several months indicate that this bacterium has been selected and maintained on the farm over time under antimicrobial selective pressure. Macroscopic organ lesions indicative of pneumonia or other diseases at slaughter indicated prolonged periods of illness (Figure 1a). The farmer also fattens pigs alongside calves and runs a livestock trading company on-site, which presents the risk of disseminating the MDR P. multocida ST79 to other animals and farms. Antimicrobial resistance in animal pathogens and the use of antimicrobial agents, particularly of the most critically important ones, need to be strictly monitored to identify farms acting as reservoirs to limit the further spread of such MDR bacteria in livestock.
Acknowledgements
We are grateful to the farmer for providing access to his farm and animals.
Funding
This work was supported by the Migros Genossenschaftsbund, IP-SUISSE, the Federal Office of Agriculture and the National Research Programme ‘Antimicrobial Resistance’ (NRP 72) of the Swiss National Science Foundation (407240_167083) and by internal funds (REF-660-50) of the Institute of Veterinary Bacteriology, University of Bern, Bern, Switzerland.
Transparency declarations
None to declare.



