-
PDF
- Split View
-
Views
-
Cite
Cite
Richard E. Haaland, Jeffrey Fountain, Chuong Dinh, L. Davis Lupo, Amy Martin, Christopher Conway-Washington, LaShonda Hall, Colleen F. Kelley, J. Gerardo Garcia-Lerma, Walid Heneine, Antiretroviral drug exposure in urethral and glans surface sampling of the penis, Journal of Antimicrobial Chemotherapy, Volume 76, Issue 9, September 2021, Pages 2368–2374, https://doi.org/10.1093/jac/dkab155
Close - Share Icon Share
Abstract
HIV exposure to penile tissues provides a risk of acquisition among men, yet studies evaluating penile antiretroviral (ARV) drug distribution have been lacking. We measured ARVs on urethral and glans surface swabs collected following a dose of tenofovir alafenamide, emtricitabine, elvitegravir, darunavir and cobicistat.
Thirty-five HIV-negative male participants provided urethral swabs, glans swabs, rectal swabs, blood and urine up to 96 h following a single dose of tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat and darunavir. ARVs were measured by liquid chromatography–mass spectrometry with a lower limit of detection (LOD) of 1 ng/swab for swabs and 10 ng/mL for plasma and urine. Concentrations are reported as median and range.
Urethral swab emtricitabine and darunavir concentrations peaked at 4 h for emtricitabine (36 ng/swab; 3–307 ng/swab) and 8 h for darunavir (25 ng/swab; 2–52 ng/swab). Glans swab emtricitabine and darunavir concentrations peaked 24 h after dosing (emtricitabine 14 ng/swab, <LOD–328 ng/swab; darunavir 6 ng/swab, <LOD–149 ng/swab). Estimated peak urethral secretion emtricitabine and darunavir concentrations are between 10 and 20 μg/mL, similar to rectal secretions, 4-fold greater than in plasma, but 2-fold lower than in urine. Tenofovir and elvitegravir were detected on less than 20% of urethral or glans swabs collected within 24 h of dosing.
We document ARV dosing in the urethra and on the glans surface with high drug concentrations noted for emtricitabine and darunavir and lower tenofovir and elvitegravir concentrations. Data suggest a potential protective role of urethral emtricitabine or darunavir against penile HIV acquisition.
Introduction
An estimated 1.7 million people are infected with HIV each year, with approximately half of new infections occurring in men.1 HIV infection though penile tissues is the primary route of HIV acquisition among heterosexual men, but also provides a route of exposure for MSM who practice insertive anal intercourse.2 Virus exposure to mucosal penile tissues allows virus to directly infect HIV target cells located in the mucosa or access cells that facilitate virus infection and dissemination. The inner surface of the male foreskin has resident macrophages, Langerhans, dendritic and CD4+ T cells that all contribute to HIV acquisition.3 Additionally, macrophages resident in the urethra have been shown to be HIV targets that likely facilitate virus dissemination and establish viral reservoirs in the penis.4 HIV prevention options for men currently include condoms, medical male circumcision and daily oral pre-exposure prophylaxis (PrEP). Condom usage is imperfect and estimated to be less than 80% effective at reducing HIV infection among serodiscordant couples.5 Medical male circumcision reduces the risk of HIV infection by 50%–60%, highlighting the contribution of the penile foreskin to HIV acquisition among men and suggesting additional factors contribute to HIV susceptibility following circumcision.6–8 Daily oral PrEP with an antiretroviral (ARV) drug combination containing the NRTIs tenofovir disoproxil fumarate and emtricitabine is highly effective at preventing HIV acquisition among heterosexual men as well as MSM who are highly adherent to daily dosing.9–11 Likewise, dosing with the daily oral tenofovir alafenamide and emtricitabine combination was shown to be effective at preventing HIV acquisition among MSM.12
While detailed pharmacological studies are routinely performed using biopsies and mucosal swabs of the female genital and anorectal tracts to evaluate drug penetration into those compartments and potential contribution to HIV prevention, similar studies in the male genital tract have been lacking. An evaluation of the CCR5 antagonist maraviroc found maraviroc exposure in urethral secretions is greater than that observed in plasma and similar to that in rectal secretions.13 However, these findings have not been extended to other ARVs and drug classes. Thus, PrEP modalities often advance to clinical efficacy testing without information on mucosal drug exposure in the penis. A better understanding of penile drug distribution for approved and candidate PrEP regimens will help define the contribution of mucosal ARVs to PrEP protection and expedite development of novel PrEP regimens and delivery systems being explored for HIV prevention.14–21 In this study, we examined penile distribution of four ARVs from three different drug classes by testing urethral and glans surface swabs collected following a single oral dose of tenofovir alafenamide and emtricitabine in combination with the integrase inhibitor elvitegravir, the PI darunavir and boosted with the pharmacoenhancer cobicistat. We also compared penile and rectal drug exposures and measured urine ARV concentrations to assess their implications on penile tissue dosing.
Methods
Study design
This study involved analysis of specimens collected during a trial registered at ClinicalTrials.gov (NCT03472963) and conducted between April 2018 and December 2018 at the Emory Hope Clinic in Atlanta, GA, USA. The study was funded by the CDC and approved by Emory University and CDC Institutional Review Boards. All participants gave written informed consent and the trial conforms to the US Federal Policy for the Protection of Human Subjects. Thirty-five HIV-negative male participants who reported receptive anal intercourse with another man in the previous 6 months were recruited from existing Emory University study databases. Participants were sequentially assigned to study arms to provide urethral swabs, glans swabs, blood and urine at three clinic visits following a single directly observed oral dose of tenofovir alafenamide/emtricitabine/cobicistat/elvitegravir and darunavir. Participants were confirmed HIV-negative using the Chembio Sure Check HIV 1/2 test (Chembio Diagnostics Systems, Inc., Hauppauge, NY, USA) prior to dosing. Participants provided specimens 2, 24 and 72 h post-dose (n = 15); 4, 48 and 96 h post-dose (n = 15); or 8, 24 and 48 h post-dose (n = 5). Two participants did not complete their final study visit at 72 and 96 h, respectively. Rectal secretion specimens were collected at one of the three post-dose clinic visits to ensure five participants provided rectal specimens at each of the seven post-dose collection times indicated above.
Urethral secretions were collected by inserting a polyester Puritan miniature applicator (Puritan Medical Products, Guilford, ME, USA) 2–4 cm into the urethra and slowly rotating clockwise for 2–3 s. One urethral swab was not analysed. Glans surfaces were sampled by pre-wetting a polyester Puritan applicator (Puritan Medical Products) in PBS and rolling the applicator around the head of the penis and underneath the foreskin, if present. Peripheral blood specimens were collected in sodium citrate cell preparation tubes (CPTs) (Becton Dickinson, Franklin Lakes, NJ, USA). Plasma aliquots were collected from CPTs following centrifugation. First void urine was collected in sterile specimen containers (Thermo Fisher Scientific, Waltham, MA, USA) and analysed to provide insight into the contribution of urine dosing penile tissues as urine frequently passes through the urethra. Rectal secretions were collected via rigid sigmoidoscopy by inserting a polyester Puritan applicator through the scope and rotating clockwise around the bowel wall for 3–5 s. An enema was not used prior to collection of rectal secretions. An anorectal swab and urine were collected at one timepoint per participant to test for Neisseria gonorrhoeae and Chlamydia trachomatis by nucleic acid amplification (Aptima 2 Combo Assay, Marlborough, MA, USA). No urine specimens tested positive for N. gonorrhoeae or C. trachomatis, and one anorectal swab tested positive for C. trachomatis. All specimens were stored at –70°C prior to analysis.
Laboratory measurements
Tenofovir, emtricitabine, elvitegravir, darunavir and tenofovir alafenamide concentrations were measured using HPLC–MS/MS (Sciex, Foster City, CA, USA, Shimadzu Scientific Instruments, Durham, NC, USA). For plasma, 500 μL of methanol containing isotopically labelled versions as internal standards for each analyte (Toronto Research Chemicals Inc., Toronto, Canada) was added to 100 μL of each plasma specimen, vortexed briefly and centrifuged at 3500 g to remove protein precipitates. The supernatant was transferred to a 96-well plate, evaporated to near dryness and resuspended in 100 μL of mobile phase A (0.2% formic acid in water). Urine specimens were diluted 1:10 in mobile phase A containing isotopically labelled internal standards. For urethral, glans and rectal swabs, 500 μL of 80% methanol containing isotopically labelled internal standards was added to each swab and the swab was centrifuged at 3500 g for 15 min. The eluate was centrifuged again at 10 000 g for 5 min to remove protein precipitates. The supernatant was transferred to a 96-well plate, evaporated to near dryness and resuspended in 100 μL of mobile phase A.
Ten microlitres of the final solution was injected onto a UK-C18 column (100 × 1 mm, Imtakt, Portland, OR, USA) connected to a Shimadzu HPLC attached to a Sciex triple quad mass analyzer. A linear aqueous-acetonitrile mobile-phase gradient was used to elute drugs from the column and into the analyzer. Mass transitions (Q1 to Q3) were monitored in positive Multiple Reaction Monitoring (MRM) mode following two transitions for each analyte [tenofovir (288.0/176.31 m/z and 288.0/159.11 m/z), emtricitabine (248.0/130.11 m/z and 248.0/113.11 m/z), elvitegravir (448.2/344.11 m/z and 448.2/143.11 m/z), darunavir (548.1/392.01 m/z and 548.1/436.11 m/z) and tenofovir alafenamide (477.30/270.20 m/z and 477.30/176.20 m/z)]. Drug concentrations were estimated from a standard curve with a range of 0.5–2000 ng/mL and analysed using Analyst software version 1.6.3 (Sciex). Standard curves for plasma and urine specimens were generated in spiked normal human plasma and urine, respectively. Standard curves for swabs were generated by spiking onto dry applicators in a range of 0.5–2000 ng/swab. Standard samples were treated in a similar manner to each specimen matrix. The lower limit of quantification (LLOQ) and limit of detection (LOD) for this study was 1 ng/swab for urethral, glans and rectal swabs, and 10 ng/mL for plasma and urine.
Estimated average material collected on swabs was performed by determining the average difference between post-collection and pre-collection weight of 25 swabs. Statistical calculations were performed using Prism 8 software (GraphPad Software, San Diego, CA, USA). Measurements below the LLOQ were assigned a value of one-half the LLOQ for statistical calculations.
Results
Study participants
Thirty-five HIV-negative MSM with median age 24 years old (range 20–46 years) provided specimens for this study. Participants were predominantly black (60%) or white (23%).
Urethral swab ARV measurements
Emtricitabine and darunavir were detected on 40/54 (74%) and 37/54 (69%), respectively, urethral swabs collected within 24 h after a single oral dose of tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat and darunavir (Figure 1). In contrast, tenofovir and elvitegravir were only detected on 10/54 (19%) and 2/54 (4%) urethral swabs, respectively (data not shown). Tenofovir alafenamide was not measurable on urethral swabs. Tenofovir and elvitegravir were only measurable on urethral swabs in the presence of concordant emtricitabine detection, while darunavir was detectable on four urethral swabs independent of emtricitabine detection. Urethral swab emtricitabine concentrations peaked at 4 h (median 36 ng/swab; range 3–307 ng/swab) and 11/20 (55%) urethral swabs did not contain measurable emtricitabine by 48 h post-dose (Figure 1a). Darunavir concentrations peaked at 8 h (median 25 ng/swab; range 2–52 ng/swab) and 11/20 (55%) urethral swabs did not contain measurable darunavir by 24 h after dosing (Figure 1b). Detectable emtricitabine and darunavir concentrations on urethral swabs were correlated (r = 0.647; 95% CI 0.396–0.808; P < 0.001, Spearman Rank Order).
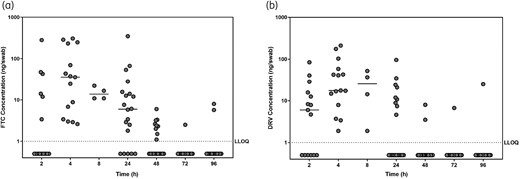
Individual antiretroviral (ARV) drug concentrations on male urethral swabs. Urethral swab concentrations are presented for (a) emtricitabine (FTC) and (b) darunavir (DRV). Horizontal lines indicate median ARV concentrations at each collection time after dosing. The dotted line indicates the lower limit of quantification (LLOQ) for ARV measurement (1 ng/swab).
Glans swab ARV measurements
Emtricitabine and darunavir were detected on 37/55 (67%) and 24/55 (44%), respectively, glans swabs collected within 24 h of dosing (Figure 2). Tenofovir and elvitegravir were detected on 8/55 (15%) and 1/55 (2%), respectively, glans swabs collected within 24 h of dosing (data not shown). Tenofovir alafenamide was not measurable on glans swabs. In the absence of emtricitabine detection, tenofovir and darunavir were detected on one glans swab each, and elvitegravir was detected on two glans swabs. Median glans swab emtricitabine concentrations peaked 24 h after dosing (14 ng/swab, range <LLOQ–328 ng/swab) and 12/20 (60%) glans swabs did not contain measurable emtricitabine by 48 h post-dose (Figure 2a). Darunavir was detectable on more than 50% of glans swabs collected 24 h after dosing (median 6 ng/swab; range <LLOQ–149 ng/swab; Figure 2b), but on less than 50% of glans swabs collected at all other timepoints. Detectable emtricitabine and darunavir concentrations on glans swabs were correlated (r = 0.641; 95% CI 0.356–0.817; P < 0.001, Spearman Rank Order). Detectable emtricitabine, but not darunavir, glans swab concentrations correlated with those measured on corresponding urethral swabs (r = 0.546; 95% CI 0.256–0.7462; P = 0.001, Spearman Rank Order). Six uncircumcised participants provided glans swabs at a total of 17 clinic visits. Among this limited set of specimens from uncircumcised participants, median emtricitabine and darunavir concentrations at 24 h were slightly greater among glans swabs collected from uncircumcised men (n = 5) (emtricitabine 45 ng/swab; darunavir 80 ng/swab) compared with circumcised men (n = 15) (emtricitabine 13 ng/swab, P = 0.095; darunavir 2 ng/swab, P = 0.091, Mann–Whitney U-test) (Figure 2a and b).

Individual antiretroviral (ARV) drug concentrations on male glans surface swabs. Urethral swab concentrations are presented for (a) emtricitabine (FTC) and (b) darunavir (DRV). Filled circles indicate specimens collected from circumcised men and open circles indicate specimens collected from uncircumcised men. Horizontal lines indicate median ARV concentrations at each collection time after dosing. The dotted line indicates the lower limit of quantification (LLOQ) for ARV measurement (1 ng/swab).
Comparison of penile ARV measurements to rectal swab specimens
Rectal swab emtricitabine concentrations were not significantly greater than those measured on urethral or glans swabs collected up to 24 h after dosing (Figure 3a). At 48 h after dosing, emtricitabine concentrations remained measurable on all rectal swabs (median 38 ng/swab; range 2–89 ng/swab) while urethral and glans swab emtricitabine concentrations declined to undetectable levels (Figure 3a). Median rectal swab darunavir concentrations were at least 5-fold greater than those measured on urethral or glans swabs on all specimens collected at least 8 h after dosing (P < 0.005, Mann–Whitney U-test) (Figure 3b). Additionally, darunavir was detectable on 3/5 (60%) rectal swabs collected 96 h after dosing, while darunavir was only detectable on 1/28 urethral and glans swab specimens. Tenofovir was measurable on 4/20 (20%) rectal swabs collected within 24 h of dosing, similar to the proportion of urethral and glans swabs with measurable tenofovir (17%) collected within 24 h of dosing (data not shown). While elvitegravir was detected on less than 3% of urethral and glans swabs collected within 24 h of dosing, elvitegravir was measurable on 14/20 (70%) rectal swabs collected up to 24 h post-dose (data not shown). Median rectal swab elvitegravir concentrations peaked 8 h after dosing (median 14 ng/swab; range 4–880 ng/swab).
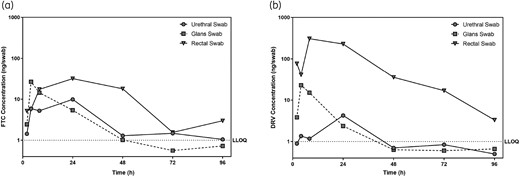
Mean antiretroviral (ARV) drug concentrations on urethral, glans and rectal swabs following a single oral dose of tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat and darunavir. Geometric mean concentrations for urethral swab, glans swabs and rectal swabs are presented for (a) emtricitabine (FTC) and (b) darunavir (DRV) from 2 to 96 h following dosing. The dotted line indicates the lower limit of quantification (LLOQ) for ARV measurement on swabs (1 ng/swab).
Comparison of penile ARV measurements to systemic drug concentrations
Mean emtricitabine and darunavir concentrations peaked 4 h after dosing on urethral swabs, later than peak concentrations in plasma and urine for emtricitabine, but at the same time as peak darunavir concentrations in plasma (Table 1). Similarly, mean emtricitabine and darunavir concentrations peaked 24 h after dosing on glans swabs, later than peak concentrations in plasma and urine. We were unable to reliably detect differences between individual swab pre- and post-collection weights, making it difficult to accurately estimate the volume or weight of material collected from the urethra or glans surface and normalize measurements for each swab individually. However, we estimate urethral and glans swabs collected an average of 2 and 5 mg of material, respectively, which is 20- and 8-fold lower than the average amount of material collected on rectal swabs. Using the average weight of material collected, estimates of peak urethral and glans surface emtricitabine and darunavir are likely similar to those observed on rectal swabs (Table 1). Using these estimates, we calculate peak emtricitabine and darunavir concentrations in urethral secretions to be between 10 000 and 20 000 ng/mL, which are 4- to 8-fold greater than plasma concentrations, but up to 8-fold lower than peak urine concentrations (Table 1). Peak emtricitabine and darunavir concentrations in material collected on glans swabs are estimated to be between 800 and 2000 ng/mL, similar to plasma concentrations, and 10- to 100-fold lower than urine concentrations.
Maximal emtricitabine and darunavir drug concentrations following a single oral dose
| . | Cmax (ng/swab or ng/mL) . | 95% CI . | Tmax (h) . |
|---|---|---|---|
| Emtricitabine | |||
| urethral swab | 27 | 10–72 | 4 |
| glans swab | 10 | 4–24 | 24 |
| rectal swab | 32 | 4–231 | 24 |
| plasma | 1268 | 1050–1531 | 2 |
| urine | 82 776 | 33 736–203 102 | 2 |
| Darunavir | |||
| urethral swab | 23 | 10–50 | 4 |
| glans swab | 4 | 2–11 | 24 |
| rectal swab | 307 | 28–3373 | 8 |
| plasma | 2548 | 1914–3392 | 4 |
| urine | 25 831 | 15 233–43 802 | 8 |
| . | Cmax (ng/swab or ng/mL) . | 95% CI . | Tmax (h) . |
|---|---|---|---|
| Emtricitabine | |||
| urethral swab | 27 | 10–72 | 4 |
| glans swab | 10 | 4–24 | 24 |
| rectal swab | 32 | 4–231 | 24 |
| plasma | 1268 | 1050–1531 | 2 |
| urine | 82 776 | 33 736–203 102 | 2 |
| Darunavir | |||
| urethral swab | 23 | 10–50 | 4 |
| glans swab | 4 | 2–11 | 24 |
| rectal swab | 307 | 28–3373 | 8 |
| plasma | 2548 | 1914–3392 | 4 |
| urine | 25 831 | 15 233–43 802 | 8 |
Maximal emtricitabine and darunavir drug concentrations following a single oral dose
| . | Cmax (ng/swab or ng/mL) . | 95% CI . | Tmax (h) . |
|---|---|---|---|
| Emtricitabine | |||
| urethral swab | 27 | 10–72 | 4 |
| glans swab | 10 | 4–24 | 24 |
| rectal swab | 32 | 4–231 | 24 |
| plasma | 1268 | 1050–1531 | 2 |
| urine | 82 776 | 33 736–203 102 | 2 |
| Darunavir | |||
| urethral swab | 23 | 10–50 | 4 |
| glans swab | 4 | 2–11 | 24 |
| rectal swab | 307 | 28–3373 | 8 |
| plasma | 2548 | 1914–3392 | 4 |
| urine | 25 831 | 15 233–43 802 | 8 |
| . | Cmax (ng/swab or ng/mL) . | 95% CI . | Tmax (h) . |
|---|---|---|---|
| Emtricitabine | |||
| urethral swab | 27 | 10–72 | 4 |
| glans swab | 10 | 4–24 | 24 |
| rectal swab | 32 | 4–231 | 24 |
| plasma | 1268 | 1050–1531 | 2 |
| urine | 82 776 | 33 736–203 102 | 2 |
| Darunavir | |||
| urethral swab | 23 | 10–50 | 4 |
| glans swab | 4 | 2–11 | 24 |
| rectal swab | 307 | 28–3373 | 8 |
| plasma | 2548 | 1914–3392 | 4 |
| urine | 25 831 | 15 233–43 802 | 8 |
Discussion
ARV exposure in mucosal tissues of the rectum and vagina have been well studied to evaluate ARVs for HIV prophylaxis; however, exposure in penile tissues has been largely lacking. To our knowledge, this is one of the first studies to measure penile drug distribution and address a key pharmacological gap for HIV prevention among men. We examined the distribution of four ARVs from three drug classes in the urethra and on the glans surface—two sites providing potential routes of HIV infection among heterosexual men and MSM. We report a range of ARV exposures with high emtricitabine and darunavir concentrations, yet low tenofovir and elvitegravir concentrations, on urethral and glans swab specimens following an oral dose of tenofovir alafenamide/emtricitabine/cobicistat/elvitegravir and darunavir. Darunavir, tenofovir and elvitegravir measurements were noticeably lower in penile compared with rectal sampling. We also estimate emtricitabine and darunavir concentrations in urethral secretions to be greater than in plasma, yet lower than in urine.
Emtricitabine and darunavir were readily detectable on urethral and glans surface swabs collected within 24 h of dosing and estimated peak urethral secretion concentrations are at least 50 and 500 times greater than the reported in vitro IC90 for emtricitabine and darunavir, respectively.22–24 Estimated emtricitabine and darunavir concentrations in urethral secretions exceed those in plasma and are similar to those in the rectum, which is consistent with a previous study of urethral sampling of men receiving maraviroc.13 Therefore, our results suggest a potential role for emtricitabine and darunavir in protecting penile tissues from HIV acquisition among men receiving prophylactic ARV regimens, and emtricitabine may have contributed to the success of HIV prophylaxis trials among men.9–12 Urethral swabs sampled non-keratinized epithelium where emtricitabine and darunavir are likely to enter the vascularized mucosa from circulation.25 High emtricitabine and darunavir concentrations observed in urine are consistent with previous studies and raise the possibility of additional penile tissue dosing when transiently passing through the urethra.26–29 The extent of such dosing is not clear and urine flow could both flush extracellular drug from and deposit drug on urethral surfaces. Of note, one participant provided a urine specimen immediately prior to urethral swab sampling 4 h after dosing and his urethral swab emtricitabine (25 ng/swab) and darunavir (41 ng/swab) concentrations were near the median concentrations of 36 ng/swab and 18 ng/swab, respectively, for participants sampled 4 h post-dose. The source of ARVs collected on glans surface swabs is unclear and could originate from the urethra or transudation through the keratinized glans epithelium. Tissue biopsies can ascertain the mechanism of ARV distribution as is routinely done for the rectal and vaginal compartments. However, penile tissue biopsies cannot be routinely obtained from men. Data from macaque studies of cabotegravir document drug distribution in penile secretions and show similar drug concentrations in the glans, urethra and foreskin tissues.30 These data suggest tissue transudation as a likely mechanism of drug distribution on the glans. Similar macaque studies can illuminate the mechanism of emtricitabine and darunavir distribution on the glans surface. Results from six uncircumcised men suggest potentially higher drug measurements in glans surface sampling of uncircumcised men compared with circumcised men. ARVs in the inner mucosal foreskin may provide additional protection from HIV infection for uncircumcised men receiving HIV prophylaxis. It is also unclear how behavioural practices such as cleaning the glans surface and inner foreskin surface may affect glans surface drug measurements. Additional glans surface ARV data collected from a larger number of uncircumcised men may help determine the contribution of ARVs towards protecting the inner foreskin where uncircumcised men are susceptible to HIV infection.
Elvitegravir was largely undetectable on urethral and glans surface swabs examined here. This result is surprising as elvitegravir was readily measured on rectal swabs in this study and achieved high mucosal concentrations in previous studies of rectal elvitegravir exposure in non-human primates and humans.15,19 This differential dosing of mucosal tissues may be the result of specific differences of elvitegravir distribution to mucosal sites as vaginal elvitegravir exposure was approximately 6-fold lower than rectal exposure in a non-human primate study.15 Alternatively, elvitegravir excretion through faeces may increase drug concentrations in rectal tissue samples compared with other mucosal sites.31 Low urine elvitegravir concentrations have been previously reported and may reflect the limited renal clearance of elvitegravir.27,31
Low tenofovir concentrations in this study likely result from oral dosing with tenofovir alafenamide, instead of tenofovir disoproxil fumarate, which produces higher concentrations of the intracellular active metabolite tenofovir diphosphate in lymphoid cells while maintaining low systemic tenofovir concentrations compared with dosing with tenofovir disoproxil fumarate.32 Efficient processing of tenofovir alafenamide into tenofovir diphosphate in lymphoid cells suggests low tenofovir measurements in penile sampling may not reflect active tenofovir diphosphate in HIV target cells. Previous results demonstrated tenofovir alafenamide produces low tenofovir concentrations in vaginal tissues as well as low tenofovir diphosphate concentrations in vaginal and rectal tissues compared with dosing with tenofovir disoproxil fumarate.33 Non-human primate studies indicated reduced tenofovir and tenofovir diphosphate concentrations in macaques dosed with tenofovir alafenamide compared with tenofovir disoproxil fumarate, while maintaining moderate protection from vaginal virus challenge.34 Together with measurements presented here, these results suggest that, for tenofovir alafenamide, systemic tenofovir diphosphate concentrations are likely contributing to PrEP protection.
Interpretation of these results is limited by a few factors. Drug concentrations reported here were measured on swabs collected from 35 participants at three timepoints after dosing; however, results from this limited sample size may not accurately reflect concentrations in larger or more specific demographic populations. Mucosal drug concentrations needed for protection in vivo for oral drugs are not known, limiting inference of the degree of protection for the detectable drugs in the penile samples. While swabs of the urethra and glans surface provide a convenient way to sample the male penis, these measurements may not accurately reflect tissue drug concentrations. Pharmacological studies of the vagina and rectum frequently collect tissue biopsies to gain valuable information regarding tissue distribution; however, biopsies are not practical from the urethra and glans to provide detailed pharmacological information. Previous studies in vaginal and rectal tissues report mucosal secretion concentrations often correlate with those found in tissues.35 Together, these results suggest ARV measurements from urethral and glans surface swabs reported here likely reflect tissue ARV distribution and penetration into penile tissues. Several ARVs are highly protein bound in plasma, including darunavir and elvitegravir, and the proportion of protein-unbound versus -bound ARVs can differ between mucosal secretions and plasma.36 This analysis focused on total ARV measurements and studies of ARV protein binding may provide additional information regarding the pharmacologically active ARV concentration in penile tissues. Concentrations described in this study were measured following a single oral dose of four ARVs and a pharmacoenhancer and daily dosing regimens may result in ARV accumulation in penile tissues above the measurements reported here. Furthermore, the pharmacoenhancer cobicistat increases the bioavailability of several ARVs, including tenofovir alafenamide, elvitegravir and darunavir, and cobicistat effects on penile ARV distribution should be evaluated separately.31,32,37,38
While penile ARV exposure has been understudied in the context of HIV prevention, we provide one of the first known reports of sampling and measurement of ARVs in the human male urethra and on the glans surface. Emtricitabine and darunavir readily distribute into penile tissues reaching concentrations likely to contribute to protection of men from HIV infection following exposure by insertive sexual intercourse. The methods presented here will facilitate the evaluation of new HIV prevention regimens to address this understudied but critical pharmacological compartment.
Acknowledgements
We would like to thank the study participants as well as the staff at the Emory University Hope Clinic for their time and commitment to this study. We would also like to thank Charles Dobard and David Garber for helpful discussions, and Monica Morris, Evelyn Nash and Bridgett Herrod for technical assistance.
Funding
This study was supported by internal funding from the United States Centers for Disease Control and Prevention.
Transparency declarations
J.G.G.-L. and W.H. are named in a US Government patent application related to methods for HIV prophylaxis. All other authors: none to declare.
Disclaimer
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the United States Centers for Disease Control and Prevention or the Department of Health and Human Services.
References
UNAIDS. AIDS Epidemic Update. Geneva,



