-
PDF
- Split View
-
Views
-
Cite
Cite
Dàmaris Berbel, Jordi Càmara, Aida González-Díaz, Meritxell Cubero, Guillem López de Egea, Sara Martí, Fe Tubau, M Angeles Domínguez, Carmen Ardanuy, Deciphering mobile genetic elements disseminating macrolide resistance in Streptococcus pyogenes over a 21 year period in Barcelona, Spain, Journal of Antimicrobial Chemotherapy, Volume 76, Issue 8, August 2021, Pages 1991–2003, https://doi.org/10.1093/jac/dkab130
Close - Share Icon Share
Abstract
To phenotypically and genetically characterize the antibiotic resistance determinants and associated mobile genetic elements (MGEs) among macrolide-resistant (MR) Streptococcus pyogenes [Group A streptococci (GAS)] clinical isolates collected in Barcelona, Spain.
Antibiotic susceptibility testing was performed by microdilution. Isolates were emm and MLST typed and 55 were whole-genome sequenced to determine the nature of the macrolide resistance (MR) determinants and their larger MGE and chromosomal context.
Between 1998 and 2018, 142 of 1028 GAS (13.8%) were MR. Among 108 isolates available for molecular characterization, 41.7% had cMLSB, 30.5% iMLSB and 27.8% M phenotype. Eight erm(B)-containing strains were notable in having an MDR phenotype conferred by an MGE encoding several antibiotic resistance genes. MR isolates were comprised of several distinct genetic lineages as defined by the combination of emm and ST. Although most lineages were only transiently present, the emm11/ST403 clone persisted throughout the period. Two lineages, emm9/ST75 with erm(B) and emm77/ST63 with erm(TR), emerged in 2016–18. The erm(B) was predominantly encoded on the Tn916 family of transposons (21/31) with different genetic contexts, and in other MGEs (Tn6263, ICESpHKU372 and one harbouring an MDR cluster called ICESp1070HUB). The erm(TR) was found in ICESp2905 (8/17), ICESp1108-like (4/17), ICESpHKU165 (3/17) and two structures described in this study (IMESp316HUB and ICESp3729HUB). The M phenotype [mef(A)-msr(D)] was linked to phage φ1207.3. Eight integrative conjugative element/integrative mobilizable element (ICE/IME) cluster groups were classified on the basis of gene content within conjugation modules. These groups were found among MGEs, which corresponded with the MR-containing element or the site of integration.
We detected several different MGEs harbouring erm(B) or erm(TR). This is the first known description of Tn6263 in GAS and three MGEs [IMESp316HUB, ICESp3729HUB and ICESp1070HUB] associated with MR. Periods of high MR rates in our area were mainly associated with the expansion of certain predominant lineages, while in low MR periods different sporadic and low prevalence lineages were more frequent.
Introduction
Streptococcus pyogenes [Group A streptococci (GAS)] is a human pathogen causing a range of diseases, most commonly pharyngitis and mild-to-severe skin and soft tissue infections.1 Due to its high susceptibility to β-lactams, penicillins and cephalosporins are the primary antibiotics used to treat GAS infections. In cases of β-lactam allergy, macrolides or lincosamides are the recommended alternatives. There are two main mechanisms of macrolide resistance (MR) in GAS (MR-GAS). The first mechanism is target site (23S rRNA) modification by erm methylases conferring the MLSB phenotype (resistance to macrolide–lincosamide–streptogramin B). The expression of erm genes may be constitutive (cMLSB) or inducible (iMLSB). The second mechanism is active drug export via efflux pumps encoded by mef genes that confer resistance to 14- and 15-membered ring macrolides (M phenotype).2 Point mutations in the 23S rRNA or alterations in the L4 and L22 riboproteins are less frequent, and have different resistance phenotypes depending on the mutations found.3
The erm genes are widespread in streptococci, commonly encoded on mobile genetic elements (MGEs) of the Tn916 family.4–7 While mef genes may be found in prophages [mef(A); φ1207.3, φm46.1] and mega element [mef(E)],4,8–10 most of these elements are characterized by their ability to recombine and to integrate new resistance genes.4 Most epidemiological reports of the frequency of MR in GAS do not provide information as to the specific determinant conferring resistance, their genetic context within associated MGEs, the nature of these elements such as integrative conjugative elements (ICEs) and integrative mobilizable elements (IMEs), and the site of integration of these elements within the chromosome.4,6,7,11,12 ICEs are distinguished from IMEs by the presence of genetic content providing the capacity for self-mobilization; IMEs are not capable of mobilizing themselves. Nevertheless, both IMEs and ICEs contribute to the dissemination of antibiotic resistance.13–16
Changes in the prevalence of MR in S. pyogenes have been associated with antibiotic consumption and the dynamics of GAS clones.17–20 Previously, in Barcelona, we reported an increase and subsequent decline in MR over a 15 year period, in which we observed a shift in MR phenotypes associated with a clonal switch.17 In the present study, we have analysed the dynamics and characteristics of MR-GAS clones causing adult disease in southern Barcelona. Moreover, using WGS, we characterized the MGEs that carry MR genes.
Materials and methods
Hospital setting, bacterial isolates and antibiotic susceptibility testing
This study was conducted at the Hospital Universitari de Bellvitge, an adult hospital that serves a population of nearly 300 000 inhabitants. The study design and methods are summarized in Figure S1 (available as Supplementary data at JAC Online).
GAS isolated from clinical samples were routinely tested for antimicrobial susceptibility by either disc diffusion or microdilution following EUCAST recommendations (breakpoint tables version 10.0, 2020; www.eucast.org). Antibiotic susceptibility to penicillin, ampicillin, cefotaxime, erythromycin, clindamycin, linezolid, tetracycline, chloramphenicol, ciprofloxacin, vancomycin, trimethoprim/sulfamethoxazole and kanamycin was tested by microdilution in all MR-GAS isolates. The macrolide and lincosamide resistance phenotype (iMLSB, cMLSB, or M) was assessed by the D-zone test, as described elsewhere.17 Macrolide consumption was gauged using the ECDC antimicrobial consumption database (https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/trend-country).
WGS analysis
A total of 55 strains were selected for WGS (Figure S1), including all available MR-GAS (42 out of 47) from 2009 to 2018, and 13 representative isolates from the historical MR-GAS series (1998–2008).17 This selection included the oldest available isolate of each different combination of MR-containing element and lineage defined by emm–MLST. Lineages accounting for four or more isolates were designated as high prevalence, two-to-three isolates as low prevalence, and represented by a single isolate as sporadic.
Genomic DNA purification was performed using QIAamp® DNA Mini Kit (Qiagen) and quantified with QuantiFluor® dsDNA System (Promega). Paired-end libraries (2 × 150 bp) were prepared with the Nextera XT kit and sequenced on the MiSeq platform (Illumina). Quality of the sequencing data was assessed through FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Reads were quality trimmed, duplicated reads removed and, finally, assembled with Geneious 9.1.7 (Biomatters) with its own assembler using medium sensitivity default parameters.
Epidemiological GAS markers (emm-type and MLST) were identified using the emm CDC databases and criteria (https://www2.cdc.gov/vaccines/biotech/strepblast.asp)21 and the MLST website (https://pubmlst.org/spyogenes/). Additional analyses to explore the presence of resistance mechanisms, virulence factors and different MGEs were performed using a variety of tools available online (ResFinder 3.0, VFDB and ICEberg).22–24
Inference of phylogeny for the WGS MR-GAS was performed by constructing an assembly-based SNP phylogenetic tree with the default parameters of Parsnp of the Harvest suite25 except for parameter ‘x’, which identifies and removes recent recombination using PhilPack,26 and parameter C, which was adjusted to 2000 to maximize the reference coverage. The GAS reference genome M1GAS (accession number NC_002737) was used as a reference for the phylogenetic analysis.27 Phylogenetic tree visualization was done using Microreact.28
Resistance genes and flanking sequences present in the study isolates but absent from the genome of M1GAS27 were considered candidate MGE content. These putative MGEs were compared with previously described sequences present in public databases. Schematics illustrating nucleotide sequence alignments of the MGEs of this cohort with previously described elements were generated using Easyfig.29 The insertion site and size of the MGEs were deduced after comparing synteny with the M1GAS reference genome by a Blastn alignment between the candidate MGE, with at least 3 kb of chromosomal genome surroundings, and the corresponding region of the M1GAS reference genome. First, we compared, by sequence alignment, the different MGEs found (Figure S9) and divided into different IME/ICE clusters when the sequence without the MR determinant (e.g. without Tn6002 in ICESpHKU397 or without the partial Tn916 in ICESpHKU372) shared an identity lower than 80%. Those IME/ICE clusters encoded genes involved in mobilization functions. Identification and annotation of signature genes/proteins involved in ICE/IME integration/excision, mobilization and replication were accomplished using ICEberg and BlastX comparison to NCBI NR database of the predicted ORFs.14–16,24 Based on this gene content, MGEs were also categorized into distinct conjugation clusters using the criteria and methods described by Ambroset et al.15 Amino acid sequences of coupling protein, virB4, relaxases and integrases were aligned with other streptococcal ICEs and defective ICEs (dICEs) using the Clustal Omega algorithm (https://galaxy.pasteur.fr/) with default parameters. Trees of these alignments were built with MEGA7 using maximum likelihood, as described by Ambroset et al.,15 and branch support of the groupings was estimated using bootstrap 100 replicates.
Statistical analysis
The correlation between macrolide consumption (DDDs per 1000 habitants/day) and the prevalence of MR-GAS (percentage of resistance) was evaluated by calculating Spearman’s bivariate rho correlation coefficient and its statistical significance. The Simpson’s diversity index was calculated to evaluate the lineage diversity in two different periods: those with MR rates higher than 10% and macrolide consumption over 2 DDDs per 1000 habitants/day; and those with MR rates <10% and macrolide consumption <2 DDDs per 1000 habitants/day.
Ethics
This research project was approved by the clinical research ethics committee of the Hospital Universitari de Bellvitge (PR354/20). All confidential information was protected according to national standards. Written informed consent was not considered necessary.
Nucleotide sequence accession number
Sequence data were deposited in the European Nucleotide Archive under the project accession number PRJEB38407. Sample accession numbers ERS4576275 to ERS4576329 (Table S1) available at http://www.ebi.ac.uk/ena/data/view/PRJEB38407.
Results and discussion
Antibiotic susceptibility, macrolide resistance trends, resistance genes and lineages
Of the 1028 GAS collected during 1998–2018, 142 (13.8%) were erythromycin resistant. Eighty-two strains (57.8%) were collected from men, and the mean age was 47.6 years old (SD 19). Among MR-GAS, the overall rates of resistance to other antimicrobials were: 60.6% for tetracycline, 22.2% high-level resistance (HLR) to kanamycin, 12.7% for trimethoprim/sulfamethoxazole, 7.4% chloramphenicol, and 3.8% for ciprofloxacin. All MR-GAS were susceptible to ampicillin, cefotaxime, linezolid and vancomycin. Moreover, eight isolates showed an MDR phenotype with resistance to erythromycin, clindamycin, trimethoprim/sulfamethoxazole, chloramphenicol and HLR kanamycin.
Among the 142 MR-GAS, 108 were available for molecular studies [66 out of 95 from the historical period17 and 42 out of 47 from the 2009–18 period (Figure S1)]. According to the macrolide and lincosamide resistance phenotype, all 30 M phenotype had mef(A) and msr(D); among 45 cMLSB, 42 had erm(B) and 3 had erm(TR); of 33 iMLSB, 19 had erm(TR) and 14 had erm(B).
Changes in MR rates have been associated with the clonal dynamics of GAS lineages carrying MR determinants and also with high levels of antibiotic consumption that may favour the emergence and spread of resistant organisms. Figure 1a shows the macrolide consumption and the rate of MR-GAS. Although there is a parallel between the macrolide consumption and MR-GAS rates, it had a moderate correlation after analysis by Spearman’s rho (rs of 0.607) without statistical significance (P = 0.148). Figure 1b depicts the temporal distribution of MR-GAS lineages and their MR determinants [mef(A), erm(B) and erm(TR)]. In the first years, there was no consistent correspondence between macrolide consumption and MR rates. While macrolide consumption decreased over the first 10 years of the study period (1998–2007), the MR rate initially increased from 17.3% in 1998–2000 to 37.6% in 2001–03 before decreasing to 20.4% in 2004–06. The peak of MR (up to 37.6%) observed in the 2001–03 period (Figure 1a) was associated with an IVDU outbreak caused by emm25/ST350 lineage (Figure 1b) and the expansion of other lineages (emm11/ST403 and emm28/ST52) as previously described.17 The emm11/ST403 and emm28/ST52 clones, mainly associated with erm(B), have been described in Spain and Portugal and less frequently in other European countries and in the USA (Table S2).18,19,30–35 The M phenotype was associated with emm4/ST39, emm6/ST382, emm75/ST49 and emm12/ST36, mainly in the 1998–2008 period. Among them, the emm12/ST36 lineage has been found worldwide harbouring mef(A)-msr(D), although in China and Korea this lineage was also related to erm(B) (Table S2).18,20,30,33,35,36 After 2003, MR rates decreased and remained stable below 8% between 2006 and 2015, associated with the lower macrolide consumption as observed in many European countries.18–20,30,32,33 Isolates harbouring macrolide efflux pump genes were not detected in our setting since 2012, but mef-associated lineages are still present in other countries.30,31,34,36 From 2016 onwards, MR increased slightly (up to 11.7%) in parallel with higher macrolide consumption and the spread of the emm77/ST63 lineage. The emm77/ST63 lineage warrants continued surveillance as it has been found in some European countries and the USA associated with erm(TR) (Table S2) and it is currently the most prevalent among MR-GAS in our area (Figure 1b).18,20,30–35 Low prevalence and sporadic lineages containing erm genes have increased in representation in our cohort since 2007; this shift has been particularly notable from 2016 onwards when erm(B) is found in lineages other than emm11/ST403 or emm9/ST75 (Figure 1b and Table S1).
![Dynamics of macrolide resistance rates, phenotypes, macrolide consumption and lineages among S. pyogenes collected from adults (1998–2018). (a) Frequency of macrolide resistance (MR) and phenotypes. Each colour represents an MR phenotype. M, M phenotype (resistance to 14- and 15-membered ring macrolides); i/cMLSB, induced/constitutive MLSB phenotype (resistance to macrolide–lincosamide–streptogramin B). Macrolide consumption is represented by DDDs per 1000 habitants/day. (b) Number of available isolates (108 from 142 MR-GAS) of lineages. Prevalent (written in red) and low prevalence (written in blue) lineages are depicted separately while sporadic clones (written in green) are grouped together, by period harbouring MR determinants [erm(B) in purple, erm(TR) in pink and mef(A) in green]. Single locus variants are indicated by a triangle [emm28/ST244 isolated in 2004 harbouring erm(TR), emm6/ST37 isolated in 2017 harbouring erm(B) and emm81/ST117 isolated in 2007 harbouring erm(B)] and a different emm/ST combination is indicated by a square [emm141/ST403 isolated in 2017 harbouring erm(B)]. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/76/8/10.1093_jac_dkab130/1/m_dkab130f1.jpeg?Expires=1750419008&Signature=lc5dbFVRMpBUnOpenwCekTDDZ9nSmVgWAmi6~5g62uNYRJqdd0pYMSvrxKroKAtStJppakiM9M7hekzhckaANpezBuSo~B1QgarLG~huJ-Xqlj3T-m-ZSBuMRRH~uPSV7WFYIdIWyHMH-1duJrWa5ZozIrpUHLPQMGYjquTq5V48D5cT1EuyBpu3FFS8inQ507KP8ZasVaeJ-RX~IEwb86556XlDdQ~4Sa83jWcA3UHXnEuINFBsI-UMqyZ3iVM6wqSV2ZVGE50nYx9xfQIWcp1RdOnhilLfD3L7uc6RlLOZ730f-G77QZwPl4BL19SWGeSSnlf8lAzK-9r9VmgP8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Dynamics of macrolide resistance rates, phenotypes, macrolide consumption and lineages among S. pyogenes collected from adults (1998–2018). (a) Frequency of macrolide resistance (MR) and phenotypes. Each colour represents an MR phenotype. M, M phenotype (resistance to 14- and 15-membered ring macrolides); i/cMLSB, induced/constitutive MLSB phenotype (resistance to macrolide–lincosamide–streptogramin B). Macrolide consumption is represented by DDDs per 1000 habitants/day. (b) Number of available isolates (108 from 142 MR-GAS) of lineages. Prevalent (written in red) and low prevalence (written in blue) lineages are depicted separately while sporadic clones (written in green) are grouped together, by period harbouring MR determinants [erm(B) in purple, erm(TR) in pink and mef(A) in green]. Single locus variants are indicated by a triangle [emm28/ST244 isolated in 2004 harbouring erm(TR), emm6/ST37 isolated in 2017 harbouring erm(B) and emm81/ST117 isolated in 2007 harbouring erm(B)] and a different emm/ST combination is indicated by a square [emm141/ST403 isolated in 2017 harbouring erm(B)]. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Rates of MR in GAS vary widely both temporally and geographically, contemporary tending to be lower in northern European countries (below 4%) and higher in Asian countries (China 88.9% in 2010–17 and Japan 41.5% in 2011–13).30,31,36–38 The current MR rate (11.7%, 2016–18) found in this study is similar to that in other European countries (Greece 10.95% and Italy 10.7% in 2013) and the USA (14.4% in 2015).20,32,34
Phylogenetic relationship, virulence factors and MGE
The relationship between MR-GAS genetic lineages, MR determinant encoding MGEs, and a set of known virulence factors was evaluated relative to the phylogeny of the 55 WGS isolates. The core SNP-based phylogeny, along with a virulence factor gene and macrolide resistance determinants in a presence/absence matrix are illustrated in Figure 2. WGS data, accession numbers, acquired resistance determinants and isolate information are summarized in Table S1. Among the 108 MR-GAS, 28 genetic lineages, as defined by the combination of emm-type and MLST (MLST single and double loci variants of a common emm-type were included in single lineages), were identified (Figure 1b); 27 are represented in the 55 WGS isolates (Figure 2). Among the 28 genetic MR-GAS lineages found, nine emm/MLST lineages accounted for 76% of the 108 MR-GAS isolates (prevalent clones). Nine emm/MLST lineages accounted for two or three isolates each (low prevalence clones). The remaining 10 emm/MLST combinations were represented by a single isolate (sporadic clones). Consistent with emm-type and MLST being relevant epidemiological markers of distinct genetic backgrounds, in the tree, MR-GAS isolates branched in groups in concordance with the emm/ST combination, including single and double loci variants. Virulence factor gene content also corresponded with emm/ST lineages; and among the virulence genes screened, the emm1/ST28 lineage isolates had the highest number of detected virulence factors.
![Phylogenetic tree and presence/absence matrix depicting virulence and acquired resistance genes. The strain names on the tree branches are coloured representing the prevalent clones (>4 isolates, red), the low prevalence clones (2–3 isolates, blue) and the sporadic isolates (single isolates, green). The coloured squares in the first column represent the emm-type/ST combinations, specified on the left side. Colours of the squares in the second column represent the time period. The squares in the third column indicate the sample in which the strain was isolated. The blue squares represent a presence/absence matrix of virulence factors related to adherence, invasiveness, immune system evasion and toxin production (represented in different shades of blue). After the virulence factors, the coloured-squares column represents the MGE in which resistance genes were found (if there are variations from what is described, there is a dot inside the square). The purple squares represent the presence of acquired resistance determinants of macrolides and/or lincosamides [erm(B), erm(TR), mef(A), msr(D) and lnu(C)], tetracyclines [tet(M), tet(O), tet(T) and tet(L)], aminoglycosides [ant(6)-Ia and aph(3′)-III] and chloramphenicol [cat(pC194)]. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jac/76/8/10.1093_jac_dkab130/1/m_dkab130f2.jpeg?Expires=1750419008&Signature=hNMS2bWu0W2BkBOhbyOzaRqxIfdp~fpmfbq~hZAy1F0eDG4F6pEmI5ivuxZA5RR1X917tBfJisGt7iStg2sGUtj-p8pSqba~rxcgJEggf67wuIfSf1x9NXB5BDott6Xg7INdJvwuiYmjSwYgojyJl01bL2haPUjB~wsjvwDMrRxLRjD30pv9XHrtaGFRRjXjm9-et9iogFCzF7kM4M1ysu4l5K43KAsBuJhdAycJyPtcIIlC6IFVPjG6OPTPUrwOIajTI33KXIqBI3muTWYDA~vLBeon211XJ6Ph3wJLydazwBNoFP8dLpUfwFOH3Q0WwODtuZBHiEEdAEmxDudPCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Phylogenetic tree and presence/absence matrix depicting virulence and acquired resistance genes. The strain names on the tree branches are coloured representing the prevalent clones (>4 isolates, red), the low prevalence clones (2–3 isolates, blue) and the sporadic isolates (single isolates, green). The coloured squares in the first column represent the emm-type/ST combinations, specified on the left side. Colours of the squares in the second column represent the time period. The squares in the third column indicate the sample in which the strain was isolated. The blue squares represent a presence/absence matrix of virulence factors related to adherence, invasiveness, immune system evasion and toxin production (represented in different shades of blue). After the virulence factors, the coloured-squares column represents the MGE in which resistance genes were found (if there are variations from what is described, there is a dot inside the square). The purple squares represent the presence of acquired resistance determinants of macrolides and/or lincosamides [erm(B), erm(TR), mef(A), msr(D) and lnu(C)], tetracyclines [tet(M), tet(O), tet(T) and tet(L)], aminoglycosides [ant(6)-Ia and aph(3′)-III] and chloramphenicol [cat(pC194)]. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Analysis of MGEs encoding MR genes
As vectors for the transmission of antibiotic resistance, the MGEs encoding MR genes and their mechanisms of mobilization are of infection prevention and treatment importance. We analysed the genetic context of MR genes in depth, as well as the insertion site of the putative MGEs in the GAS genome and compared the MGE with previously described elements. A summary of these results can be found in Table S1 and is shown in Figures 3–6 and Figures S2–S10. The structures identified are further detailed with regard to the MR determinant contained. Among them, three new structures and different rearrangements of previously described MGEs were found.
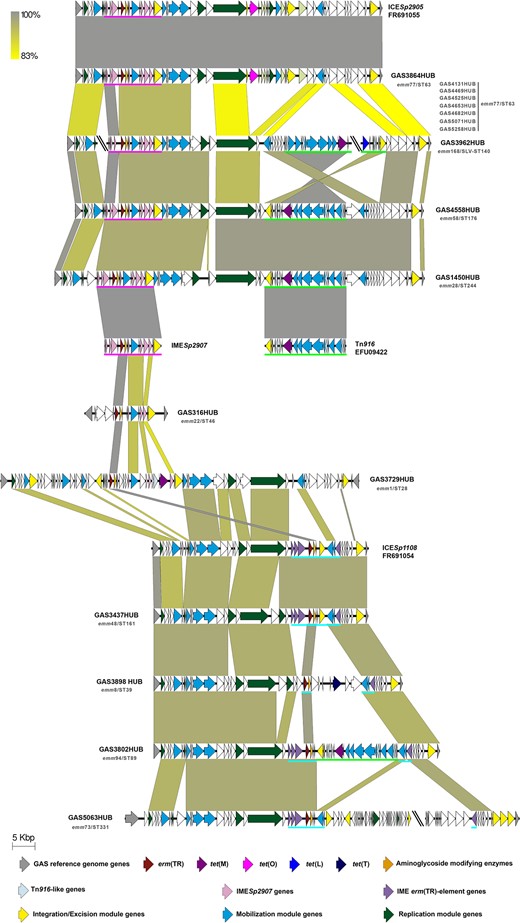
Schematic representation of the erm(TR)-containing elements. This figure shows the genetic elements containing erm(TR) related to three main structures: first, those related to ICESp2905 (accession number FR691055) in the upper part that could contain either the IMESp2907 element with tet(O) (underlined in pink) or the Tn916 (accession number EFU09422) harbouring tet(M) (underlined in green); second, in the bottom those related to ICESp1108 (accession number FR691054) containing the IME erm(TR) element (underlined in blue); third, in the middle of the figure isolates partially related to the previously cited elements. The yellow-to-grey shaded areas connect the regions based on similarity (identity from 83% to 100%). The arrows depict the genes contained in each element; truncated sequences among different contigs are highlighted with a double slash (\\). Coloured arrows represent different genes. Grey, GAS reference genes (M1GAS, accession number NC_002737). Resistance genes: erm(TR) in red, tet(M) in purple, tet(O) in fuchsia, tet(L) in ultramarine-blue, tet(T) in dark blue, and aminoglycoside-modifying enzymes in orange. Genes harboured in previously described MGEs: Tn916-like in light blue, IMESp2907 in light pink, and IME erm(TR) element in light purple. Yellow, integration/excision module genes (integrases, recombinases, excisionases and transposases). Blue, mobilization module genes (T4SS genes and relaxases). Dark green, replication module genes (topoisomerases, regulatory proteins and helicases). Representative isolates are highlighted in boldface and the other isolates with the same structure in grey; under the name is shown the emm/ST type of each isolate. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
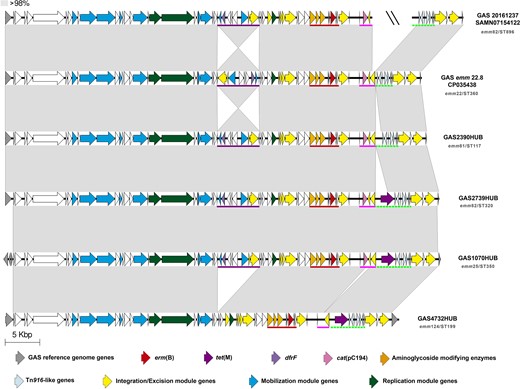
Schematic representation of the multidrug-resistance (MDR) element containing erm(B). This figure shows the structure of the MDR determinants containing elements found in four strains of our collection (HUB) compared with previously sequenced isolates in the literature GAS emm22.8 (accession number CP035438)47 and GAS20161237 (SRA accession number SAMN07154122).34 This structure harbours four resistance-containing regions: dfrF region (underlined in purple), MAS element (underlined in red), cat(pC194) region (underlined in pink), and partial Tn916-containing tet(M) underlined with a green dotted line. The grey-shaded areas connect regions based on similarity (over 98% identity). The sequence interruption among different contigs is represented with a double slash (\\). Arrows represent genes. Colours represent different genes. Grey, GAS reference genes (M1GAS, accession number NC_002737). Resistance genes: erm(B) in red, tet(M) in purple, dfrF in light purple, cat(pC194) in pink, and aminoglycoside-modifying enzymes in orange. Light blue, genes harboured in Tn916-like elements. Yellow, genes related to integration/excision module (integrases, recombinases, excisionases and transposases). Blue, mobilization module genes (T4SS genes and relaxases). Dark green, replication module genes (topoisomerases, regulatory proteins and helicases). The emm/ST type is specified in grey under every isolate name. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
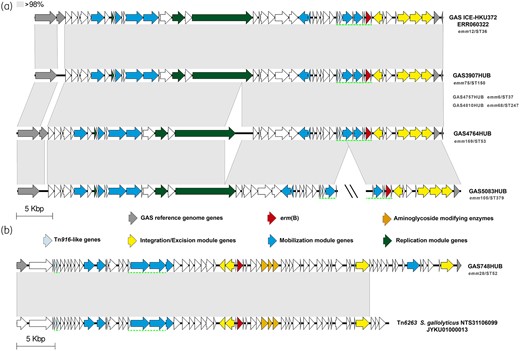
Schematic representation of other erm(B)-containing structures. (a) Representation of the elements found in six strains that harbour only erm(B). This structure is similar to that found in the GAS ICE HKU372 (Run SRA accession number ERR060322).7 (b) Structure found in GAS748HUB that is very similar to Tn6263 previously described in S. gallolyticus NTS31106099 (accession number JYKU01000013)48 harbouring erm(B) and aminoglycoside-modifying enzymes. The regions containing partial Tn916 are underlined with a green dotted line. The grey-shaded areas connect regions based on similarity (over 98% identity). Double slash (\\) represents sequence interruption by different contigs. Genes are represented with arrows; the colours represent different genes. Grey, GAS reference genes (M1GAS, accession number NC_002737). Resistance genes: erm(B) in red and aminoglycoside-modifying enzymes in orange. Light blue, genes harboured in Tn916-like elements. Yellow, genes related to integration/excision module (integrases, recombinases, excisionases and transposases). Blue, mobilization module genes (T4SS genes and relaxases). Dark green, replication module genes (topoisomerases, regulatory proteins and helicases). Representative isolates are highlighted in boldface and the other isolates with the same structure in grey; under the name is showed the emm/ST type of each isolate. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
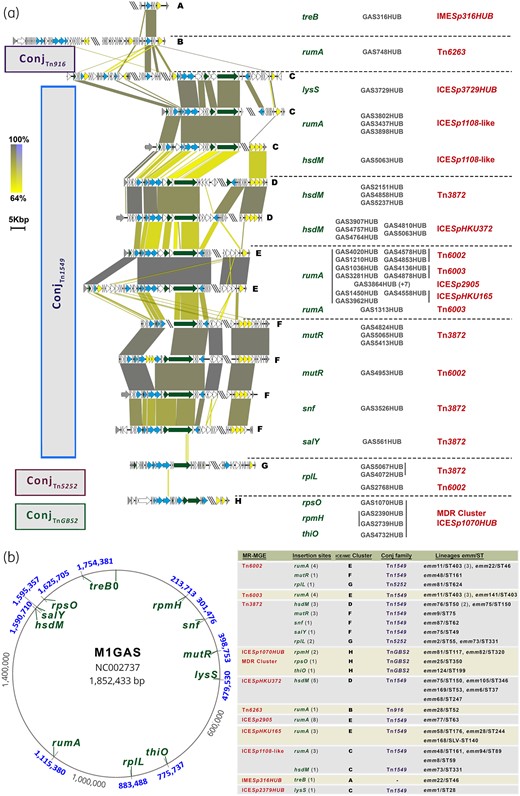
Schematic representation of the association of the mobilization, replication and insertion/excision clusters with the insertion site (a) and the different MGEs insertion sites in the reference genome related to the ICE/IME clusters, conjugation families and lineages (b). (a) This figure represents the ICE/IME clusters of the different elements excluding the MR-containing elements previously described. This emphasizes the association between the mobilization, replication and insertion/excision clusters and the insertion site (E—rumA, D—hsdM, G—rplL) with the MR-containing element itself (C—ICESp1108-like, H—MDR cluster) or both (F—Tn3872 and mutR), depending on the integrase content. In boxes on the left side is shown the conjugation family classification15 of the ICE/IME clusters. The yellow-to-grey-shaded areas connect regions based on similarity (identity 64%–100%). The yellow-to-blue areas represent a reversed sequence. The genes are represented with arrows and the colours represent different genes. Yellow, integration/excision module (integrases, recombinases, excisionases and transposases). Blue, mobilization module genes (T4SS genes and relaxases). Dark green, replication module genes (topoisomerases, regulatory proteins and helicases). Double slash (\\) represents sequence interruption by different contigs and triple slash (\\\) represents the place where the sequences have been split to remove the MR-containing element from the sequence. The insertion-site names are coloured in green, the MR-containing element in red and the names of the different isolates in grey. There are vertical bars in order to group the isolates’ names with their MR-containing element (bar on the right) and with the corresponding insertion site (bar on the left). The different conjugation clusters are named in bold letters from A to H and are separated by a dotted line. (b) The insertion sites of the different resistance-carrying mobile elements referred to the M1GAS genome (accession number NC_002737) are depicted in this figure. The gene names of insertion sites are coloured in green and their positions in the entire genome of the M1GAS are highlighted in blue. The table next to the figure summarizes the number of isolates (in grey between parentheses) of the different macrolide-resistance-containing elements (MR-MGE; written in red) found in the different insertion sites, the ICE/IME cluster associated (bold letters A to H; see Figure S9), the corresponding conjugation family15 (written in purple; see Figure S10) and the lineages harbouring each combination. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
mef(A)-containing elements
M phenotype isolates harboured mef(A) within the phage φ1207.3 inserted in comEC as described (Figure S2).4,10,39 All these isolates had the mef(A)–msr(D) tandem recently described as necessary for the production of an active efflux transport system.40 Although they have been described in GAS, we did not detect mef(E),4,18,41,mef(I) or mef(O).42,43 Two emm12/ST36 strains (GAS3409HUB and GAS3609HUB) had a 164 bp deletion in the holin gene characteristic of prophages.
erm(TR)-containing elements
The erm(TR) has been associated with two previously known IMEs, IMESp2907 and the erm(TR)-element, which are inserted in ICEs, ICESp2905 and ICESp1108, respectively.5 In our study, erm(TR)-containing elements were identified in 17 GAS and, based on gene content and synteny, were divided into three groups: 11 GAS had arrangements related to IMESp2907, four had the erm(TR)-element related to ICESp1108, and the remaining two had miscellaneous structures (Figure 3).
Among 11 GAS with IMESp2907, eight emm77/ST63 isolates (iMLSB phenotype) had this IME inserted into ICESp2905, which also carried tet(O). These results are in agreement with previous observations in Europe linking the emm77/ST63 lineage with the presence of both erm(TR) and tet(O).5,19,20,30–32,35 The remaining three IMESp2907-containing strains also had the tet(M) gene in a Tn916, and both elements were merged into a structure similar to the ICESpHKU165 previously described in an emm12/ST36 isolate in Hong Kong.7 The GAS3962HUB isolate had an ICESpHKU165-like element, while GAS1450HUB and GAS4558HUB differed by having Tn916 in a reversed orientation (Figure S3). Although erythromycin and tetracycline resistance in GAS is usually caused by the combinations of either erm(TR)–tet(O) or erm(B)–tet(M) combinations,4erm(TR)–tet(M) has occasionally been found as well.
The second group accounted for four clonally unrelated isolates, which had the erm(TR) element encoded on elements sharing gene content and synteny with ICESp1108. One of them (GAS3437HUB) had few gene content variations in the ICE while the other three had different insertions and rearrangements within the erm(TR) element: GAS3898HUB had a tet(T)-containing region, GAS3802HUB had a reversed Tn916, and GAS5063HUB had a multi-gene insertion without resistance determinants. ICESp1108-like structures have also been described in Streptococcus agalactie and Streptococcus suis, demonstrating the capacity of these ICESp1108-like structures to spread among different streptococci.44,45
The remaining two erm(TR)-GAS structures were less similar to the ones previously described. The GAS316HUB isolate was partially related to IMESp2907. The last isolate (GAS3729HUB) had a large element including an IMESp2907-like structure together with a partial Tn916 (orf13-tet(M)-orf6-Tn916), and an ICE/IME cluster similar to that found in ICESp1108. This last isolate belongs to the highly invasive clone emm1/ST28, which is rarely associated with macrolide resistance.
erm(B)-containing elements
Among 31 erm(B)-containing strains, based on gene content similarity with previously described erm(B)-encoding elements, three groups of MGEs were found. The first and largest group comprised 21 GAS with erm(B) carried by transposons of the Tn916 family: Tn6002 (n = 6, Figure S4), Tn6003 (n = 5, Figure S5), and Tn3872 (n = 10, Figure S6).
In addition to the gene content of previously described Tn916 family elements,4 to our knowledge, we found new gene content involved in MGE mobilization that has not been previously described linked to these transposons (depicted in their respective figures). Among them, the six strains with Tn6002 had different ICE/IME clusters depending on the insertion site (Figure S4). The three emm11/ST403 GAS had a structure previously described as ICESpHKU397 in 50 isolates from the emm12/ST36 Hong Kong study.7 The Tn6003 resulting after the insertion of the MAS element into Tn60024 was found in five isolates (Figure S5); all but one were emm11/ST403. However, the analysis of this Tn6003 was limited by sequence fragmentation due to flanking repeats around the MAS element, defined previously as an unstable genetic element capable of spontaneous excision and mobilization and complicates the short-read WGS analysis.35,46 In two isolates (GAS3281HUB and GAS1313HUB), the ICE/IME cluster preceding Tn6003 had insertions that did not contain known resistance determinants. Finally, Tn3872 (n = 9), resulting from the insertion of Tn917 into a Tn916, or a Tn3872-like (GAS5067HUB) element, was found in 10 isolates divided in three groups, depending on the ICE/IME cluster and the insertion site (Figure S6).
The second group of erm(B)-containing GAS comprised four isolates that had a new structure (ICESp1070HUB). This structure is similar to ICESp1116,6 but harbours a complete tet(M) gene and also other resistance determinants [dfrF, cat(pC194) and the aminoglycoside-modifying enzymes cluster aph(3′)III-sat4-ant(6)Ia] that confer an MDR phenotype (Figure 4 and Figure S7). These four MDR-GAS isolates were each of a different genetic lineage (emm81/ST117, emm82/ST320, emm25/ST350 and emm124/ST199) consistent with ICESp1070HUB having the capacity to mobilize and transfer between lineages. After a BLAST search in the NCBI database, we identified two strains in two independent studies from the USA harbouring this MDR cluster.34,47 However, the MDR cluster of one of our four MDR isolates (GAS4732HUB) lacked dfrF, cat(pC194) was disrupted and lost the MDR condition. It seems that this MDR cluster can recombine and integrate or excise resistance determinants, which is a cause for concern. Besides this MDR cluster, GAS2390HUB also had a tet(M) gene carried by an Tn916, as occurred in two isolates from the USA (GASemm22.847 and GAS2016123734).
Finally, the third group comprised six erm(B)-containing isolates in which the erm(B) was not included in the classical Tn916 (Figure 5). Among them, five isolates from different lineages had an MGE related to a structure described previously in ICESpHKU372.7 The remaining strain (GAS748HUB) had a structure harbouring the Tn6263 previously characterized in Streptococcus gallolyticus NT31106099 inserted in rumA/pnp.48
lnu(C)-containing element
Besides MR genes, a single isolate also had lnu(C), a resistance gene associated with lincosamide inactivation. The IME MtnSag1, containing lnu(C),49 was found in an ICESpn8140-like structure (Figure S8). Curiously, this isolate (GAS2390HUB) also harboured the ICESp1070HUB and an Tn916. The presence of genes conferring LSAP phenotype is uncommon among GAS: in fact, only two additional isolates harbouring lnu(B)–lsa(E) and lsa(C) have been described to date.34,50
Analysis of the ICE/IME clusters within MGE
The presence of genes involved in integration/excision as well as mobilization and replication is crucial to the process of resistance acquisition mediated by genetic elements. A large diversity of gene content has been found in the MGEs disseminating macrolide resistance genes among S. pyogenes.4–7 This diversity in part stems from the frequent recombination of functional modules among these ICEs/dICEs/IMEs, making the classification of these elements complex/non-trivial. Such classification is essential for developing an understanding of the epidemiological dynamics of these antibiotic resistance-encoding elements in the population. Ambroset et al.15 have developed a method for the classification of these diverse elements based on differences in the genes encoding for integration/excision and conjugative transfer in conjunction with the site of integration. To further characterize the MGEs described herein and to facilitate comparison with the elements described by Ambroset et al.,15 we applied this classification system to our cohort/set of isolates. We analysed the IME/ICE clusters and the putative associations between them with genes mediating conjugation, with resistance determinants, and also with sites of chromosomal integration. Using pairwise sequence alignment, we first analysed the relationship between all the elements containing erm(B) and erm(TR) described in the present study (Figure S9). In the overall analysis, which included both resistance determinants and conjugation cluster genes, we identified eight ICE/IME clusters with less than 80% of homology (named from A to H; Figure 6a). These ICE/IME clusters were further grouped in conjugation families following the Ambroset et al.15 scheme (see below). In general, there was an association between MR elements and ICE/IME clusters, with the exception of Tn6002 and Tn3872 (Figure 6a and b). Nevertheless, isolates having either Tn6002 or Tn3872 and belonging to the same lineage (emm/ST) had the same ICE/IME cluster, suggesting the clonal spread of resistance. For instance, all three emm11/ST403 having Tn6002 had the cluster E.
The composition of ICE/IME clusters includes genes involved in integration/excision, mobilization and replication of MGE. The ICE/IME clusters analysis was performed in order to differentiate ICEs and IMEs among the structures found and to associate them to the conjugation families previously described in streptococcal ICEs and dICEs (Figure S10).15 The ICEs contain all the machinery required for mobilization (relaxase, recombinase and a Type 4 Secretory System, or T4SS) whereas IMEs only contain a relaxase and a recombinase; they are autonomous for excision and integration, but require more conjugation machinery to transfer.13 In Gram-positives, since they lack an external membrane, the full T4SS is not required for transfer. Nevertheless, the conjugative system needs at least a relaxase (rel), a coupling protein (T4CP encoded by cpl gene, formerly virD4), a virB4 T4SS ATPase (tivB4) and the soluble lytic translycosilase (encoded by slt or virB1).16 Ambroset et al.15 classified these components from streptococcal ICEs and dICEs based on the phylogenetic analysis of their amino acid sequences: cpl (cpl-I, -IIa, -IIb), tivB4 (virB4-Ia, -Ib, -Ic), and rel (rel-I to -III). Moreover, they named the different conjugation families found (ConjTn916, ConjICESt3, ConjvanG, ConjTn1549, ConjTn5252, ConjTnGBS2 and ConjTnGBS1). In this study, as genes involved in mobilization, we considered only those orf detected in ICEberg,24 those with a BlastX harbouring motifs that might play a role in this function and those conserved between elements.15,16,51 Among eight ICE/IME clusters, only cluster A was considered as an IME as it contains a mobA/L relaxase and a serine integrase. Cluster B harbours a non-canonical FtsK-derived coupling protein (Cp-I), a tivB6-like gene (orf15-Tn916), a tivB4 (virB4-Ia), a cell wall hydrolase, relaxases (rel-I) and integrases, all features suggesting that this MGE is an ICE despite its differences with respect to C to H clusters.14 This cluster B was associated with the previously described Tn6263 and it belongs to ConjTn916 conjugation family.48 Clusters C to H contain at least the minimum conjugation machinery required, so we consider them to be ICEs; among them, clusters C, D, E and F belong to ConjTn1549 while clusters G and H belong to the conjugation families ConjTn5252 and ConjTnGBS2, respectively (Figure 6a and Figure S10). These conjugation families are grouped into the same superfamily Tn5252 and, as characteristic of this group,15 ICE/IME clusters C to H contain Cp-IIa, Rel-II and virB4-Ic (Figure S10). Moreover, Cluster H is found in the ICESp1070HUB harbouring the MDR cluster and shares most of the mobilization cluster of ICESp1116, which is widespread among Italian GAS and was described as similar to TnGallo1 of S. gallolyticus (Figure S7).6
Finally, we analysed the integration loci of MGEs in the M1GAS27 genome depicted in Figure 6b. Among 11 insertion sites identified (rumA, hsdM, rplL, mutR, salY, snf, rpmH, rpsO, thiO and treB), we found an association between three ICE/IME clusters and a specific insertion site (E−rumA, D−hsdM and G−rplL) (Figure 6a). The remaining ICE/IME clusters were found inserted in different genome regions. The insertion loci depend on the integrase encoded in the conjugation cluster. The integrases found in each cluster related to the insertion site correlated with previous descriptions in streptococcal ICEs studies;15 a single site-specific serine integrase (group I) inserted in rumA (clusters B, E and some C isolates; Figure S10D), the tyrosine site-specific integrases inserted in rplL (cluster G) and lysS (cluster C, GAS3729HUB; Figure S10E), a triplet of site-specific serine integrases (group II) inserted in hsdM (cluster D and cluster C GAS5063HUB; Figure S10D) and a DDE transposase (cluster H; Figure S10F) with low specificity target inserted in rpmH, rpsO and thiO. Moreover, to our knowledge, we also recorded a new finding, a triplet of serine integrases (group II) in cluster F that inserted in different genome regions (low specificity; mutR, snf and salY) and was similar to that described in ConjTn1549 ICESintB196 as a site-specific recombinase (Figure S10D).
In this GAS cohort, we have found a wide variety of ICEs linked to different emm/ST combinations. Despite the huge diversity of MGE and ICE/IME clusters, most of the associations of GAS lineages and MGE remained unchanged over the study period. For instance, the emm11/ST403 isolates, detected during almost the whole period, usually harboured Tn6002 or Tn6003, both of them related to conjugation cluster E and inserted in rumA (Figures 1b and 6b). Furthermore, the genetic background of these isolates was highly similar throughout the entire period (Figure 2). Similar findings are observed in the recently rising endemic lineages emm77/ST63–erm(TR)–ICESp2905–cluster E. On the other hand, other MGEs, like ICEs containing Tn3872, ICESpHKU372, ICESpHKU165 and ICESp1108-like, were associated with different emm/ST combinations; a finding that also supports the involvement of horizontal transfer in MR spread. In periods of high MR rates (up to 10%) and high macrolide consumption (>2 DDDs per 1000 habitants/day), the clonal and IME/ICE diversity defined by Simpson’s diversity index was lower than in periods with low MR rates and low macrolide consumption [0.92, 95% CI (0.89–0.94) versus 0.97, 95% CI (0.94–1.0) for lineages and 0.87, 95% CI (0.83–0.94) versus 0.96, 95% CI (0.93–0.99) for ICE/IME].
Our study has some limitations that should be noted. First, the study focuses on a single centre; however, this allowed us to assess a large historical series that may broaden our understanding of MR clonal dynamics. Second, our study did not include commensal or paediatric isolates. Third, the short-read WGS did not allow the resolution of repetitive sequences in some isolates; nevertheless, they can be assumed when checking the sequence coverage.
Conclusions
In the Barcelona, Spain, geographical area, MR rates have fluctuated from 37.6% to 5.6% over the last two decades, currently standing at 11.7%. The MR-GAS changes in our area could have been driven by both clonal dynamics and increased rates of macrolide consumption. The highest peaks of resistance were associated with higher presence of prevalent MR lineages, while in low MR rate periods a major diversity of MGEs and different emm/ST combinations was detected. While mef(A) was homogeneously harboured by φ1207.3, the erm(B) and erm(TR) genes were detected in 10 and five different MGEs, respectively. Two elements containing erm(TR) [IMESp316HUB and ICESp3729HUB] and one MDR cluster containing erm(B) [ICESp1070HUB] are described in this study for the first time, to our knowledge. Furthermore, we report the first known observation of Tn6263 (originally described in a S. gallolyticus) in a GAS isolate. These findings highlight the importance of studies of MGEs carrying MR determinants in streptococci to improve our understanding of MR spread.
Acknowledgements
This study was partially presented at the XVIII Congreso de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, Valencia, Spain, 2014 (poster P-080), and at the Twenty-Ninth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, the Netherlands, 2019 (oral session O-0994).
We thank the staff at the Microbiology Department of Hospital Universitari de Bellvitge for daily contributions to this project.
Funding
This study was supported by Instituto de Salud Carlos III through the projects from Fondo de Investigaciones Sanitarias (INT15/0186, INT16/0117 and PI18/00339) and CIBER de Enfermedades Respiratorias (CIBERES—CB06/06/0037), co-funded by the European Regional Development Fund/European Social Fund (ERDF/ESF, ‘Investing in your future’), and CERCA Programme/Generalitat de Catalunya for institutional support. D.B. was supported by ‘Formació Post-Residència en Recerca’ predoctoral contract from Hospital Univeristari de Bellvitge.
Transparency declarations
None to declare.
Supplementary data
Tables S1 and S2 and Figures S1 to S10 are available as Supplementary data at JAC Online.



