-
PDF
- Split View
-
Views
-
Cite
Cite
Constance Delaugerre, Marie-Laure Nere, Sabrina Eymard-Duvernay, Alix Armero, Laura Ciaffi, Sinata Koulla-Shiro, Adrien Sawadogo, Ndaye Fatou Ngom Gueye, Cheik Tidiane Ndour, Mireille Mpoudi Ngolle, Ali Amara, Marie-Laure Chaix, Jacques Reynes, the ANRS 12286/MOBIDIP study group, Deep sequencing analysis of M184V/I mutation at the switch and at the time of virological failure of boosted protease inhibitor plus lamivudine or boosted protease inhibitor maintenance strategy (substudy of the ANRS-MOBIDIP trial), Journal of Antimicrobial Chemotherapy, Volume 76, Issue 5, May 2021, Pages 1286–1293, https://doi.org/10.1093/jac/dkab002
Close - Share Icon Share
Abstract
The ANRS12286/MOBIDIP trial showed that boosted protease inhibitor (bPI) plus lamivudine dual therapy was superior to bPI monotherapy as maintenance treatment in subjects with a history of M184V mutation.
We aimed to deep analyse the detection of M184V/I variants at time of switch and at the time of virological failure (VF).
Ultra-deep sequencing (UDS) was performed on proviral HIV-DNA at inclusion among 265 patients enrolled in the ANRS 12026/MOBIDIP trial, and on plasma from 31 patients experiencing VF. The proportion of M184V/I variants was described and the association between the M184V/I mutation at 1% of threshold and VF was explored with logistic regression models.
M184V and I mutations were detected in HIV-DNA for 173/252 (69%) and 31/252 (12%) of participants, respectively. Longer duration of first-line treatment, higher plasma viral load at first-line treatment failure and higher baseline HIV-DNA load were associated with the archived M184V. M184I mutation was always associated with a STOP codon, suggesting defective virus. The 48 week estimated probability of remaining free from VF was comparable with or without the M184V/I mutation for dual therapy. At failure, M184V and major PI mutations were detected in 1/17 and 5/15 patients in the bPI arm and in 2/2 and 0/3 in the bPI+lamivudine arm, respectively.
Using UDS evidenced that archiving of M184V in HIV-DNA is heterogeneous despite past historical M184V in 96% of cases. The antiviral efficacy of lamivudine-based dual therapy regimens is mainly due to the residual lamivudine activity.
Introduction
Despite the high efficacy of long-term HIV treatment with a combination of three antiretroviral drugs, there remains a major need for simplified, cheap and less-toxic maintenance strategies. Simplified strategies have been sought to improve the convenience of ART while maintaining effectiveness. Several randomized studies in high-income studies have demonstrated non-inferiority of lamivudine (3TC) plus boosted PI (bPI) maintenance regimens in individuals displaying virological suppression on triple therapy.1 However, these studies excluded patients with previous virological failure (VF) and resistance to study drugs.
MOBIDIP ANRS 12286 was a randomized trial in Sub-Saharan Africa on HIV-1-infected participants displaying virological control on a bPI second-line regimen with multiple previous resistance-associated mutations (RAMs), including the M184V mutation, accumulated during first-line treatment failure on lamivudine.2 This trial demonstrated the high virological efficacy of maintenance on dual therapy with lamivudine plus one bPI (bPI + 3TC), and the superiority of this treatment over monotherapy with bPI in subjects.
The M184V/I mutation of the HIV-1 reverse transcriptase (RT) gene emerges rapidly in patients on NRTI-based treatment regimens containing lamivudine and a third agent with low genetic barrier. It confers strong resistance to lamivudine, both in vitro and in vivo. However, The M184V/I mutation reduces the viral replicative capacity (imposing a fitness cost) and may protect against the emergence of other mutations through changes in RT function.3,4 Together, these factors may account for the antiviral effect observed in patients on continuous treatment with lamivudine plus bPI, despite the presence of the M184V/I mutation.
Our principal objective was to investigate the frequency (by deep sequencing) and the impact of the archived M184V and M184I on virological outcome on bPI monotherapy and bPI + 3TC dual therapy. We also performed an in-depth analysis of RAMs of the RT and protease genes in the plasma of patients experiencing VF.
Patients and methods
Study and participants
The ANRS 12026/MOBIDIP included patients who had previously experienced RT drug resistance due to multiple mutations accumulated during first-line NNRTI-based regimens, presenting virological control [plasma viral load (pVL) <200 copies/mL] on a second-line bPI combined with two NRTIs regimen and switched to monotherapy with the same bPI (n = 133) or on dual therapy (bPI + 3TC; n = 132) after at least 48 weeks.2 The bPI used was once-daily darunavir (800 mg) plus ritonavir (100 mg) or twice-daily lopinavir (400 mg) plus ritonavir (100 mg). At W48, dual therapy was found to be superior to monotherapy, with treatment failure occurring in 4 participants on bPI + 3TC (3%; 95% CI: 0.8%–7.6%) and 33 (24.8%; 95% CI: 17.7%–33.0%) on bPI (difference between groups of 21.8%, 95% CI 13.9%–29.7%; P < 0.0001). VF, defined as a confirmed pVL >500 copies/mL, occurred in 28/133 (21%) patients on bPI and 3/132 (2%) on bPI + 3TC.
Ethics
This study was performed in accordance with good clinical practice with the approval of the institutional ethics committee of the Institut de Recherche pour le Développement in France and the national ethics committees of the other participating countries. All participants provided written informed consent (ClinicalTrials.gov, number NCT01905059).
Samples
Buffy coats were collected on day 0 (D0) of switch to simplified maintenance therapy, for all participants and were stored at −80°C for drug resistance analysis on HIV-1 proviral DNA. Total HIV-1 proviral DNA load was determined with the qPCR Generic HIV-1 DNA Cell kit (Biocentric, Bandol, France) and expressed in log10 copies per million CD4 cells.
Frozen plasma (−80°C) for drug resistance analysis on HIV-1 RNA was available for patients experiencing VF at the time of the second pVL >500 copies/mL.
HIV-1 sequencing
Ultradeep sequencing (UDS) of HIV-1 genes was performed at the Virology Laboratory, Saint Louis Hospital, Paris, France. DNA was extracted from buffy coats with the DSP DNA Mini Kit and a QiaSymphony instrument (Qiagen, Courtaboeuf, France). HIV RNA was extracted from 1 mL of plasma with the Nuclisens kit on an easyMAG® machine (bioMérieux Clinical Diagnostics).
We amplified the RT gene from D0 HIV proviral DNA, for all participants. For patients experiencing VF, the RT and protease genes were also amplified from plasma at the time of VF. Primers and procedures were in accordance with the protocols of the ANRS study group (www.hivfrenchresistance.org). Briefly, amplified proviruses after normalization on HIV DNA level, were prepared with the Nextera XT DNA Library Preparation Kit (Illumina) for UDS of the RT amplicon, with the dual-indexing of 96 samples per run. For the protease amplicon, Illumina adapter overhang nucleotide sequences were added to the gene-specific sequences during the second PCR. All steps were performed in accordance with the manufacturer’s instructions, except that the reagent and input DNA volumes were halved, and libraries were normalized manually.
Bioinformatic identification of RAMs
The PASeq tool (www.paseq.org) was used to identify the RAMs at baseline and at the time of virological failure. The baseline samples were analysed with the peripheral blood mononuclear cell (PBMC) protocol, whereas samples from patients experiencing VF were analysed with the DeepVariants subtypeB protocol. The PBMC protocol uses the consensus B (DQ127534) sequence as the reference sequence, whereas the DeepVariants subtypeB protocol uses HXB2 (K03455) as reference sequence. We also used the reference HIV-1 A1 subtype because most of the samples belonged to HIV-1 non-B subtypes. In both cases, PASeq was parameterized to recover only mutations with a frequency of at least 1%, and APOBEC-3G-induced mutations (STOP codon and M184I) were not filtered out. The RT and protease RAMs of the 2018 HIV resistance algorithm v29 (www.hivfrenchresistance.org) were identified.
Statistical analysis
Categorical variables are expressed as frequencies and proportions. Continuous variables are expressed as the median, IQR, and range.
The RT gene analysis on HIV proviral DNA focused on sequences harbouring lamivudine mutations at codon 184 (M184V or M184I) and STOP codons. Mutation proportions were compared between groups using the Pearson's chi-squared tests.
Univariate and multivariate logistic regression models were used to identify factors associated with archived M184V/I detected at 1% in proviral DNA at D0. The covariates included were sex, nadir CD4+ cell counts, WHO stage 3 or 4 events, HIV DNA levels at D0, duration of first-line and second-line treatments, tenofovir/lamivudine backbone in the second-line regimen, pVL at first-line failure and HIV-1 viral subtype (being CRF02 or not). Factors associated with a P value <0.25 in the univariable model were selected for the final multivariable models. A sensitivity analysis was also performed at 5% threshold as this is suggested as a clinically relevant threshold for plasma HIV RNA.5
Failure-free survival probabilities, with or without archived M184V/I, were estimated using the Kaplan–Meier method. Logistic regression models were also performed to analyse the association of archived M184V/I and virological outcome at W48 in the monotherapy arm because of a low number of VF in the dual-therapy arm (n = 3). The same factors were included, plus the baseline pVL (<50 copies/mL or between 50 and 200 copies/mL) and treatment adherence.
Results
Patient characteristics at baseline
The baseline characteristics of the 252 patients for whom resistance data were available at D0 are presented in Table 1 and were similar to the whole population enrolled in MOBIDIP trial.2 Median duration on ART was 91 months (IQR 72–108), with a median of 50 months (IQR 33–69) on first-line and 37 months (IQR 30–47) on second-line treatment. Median HIV proviral DNA load was 3.5 log10 copies/million CD4 cells. At first-line treatment failure, 96% of patients had the M184V mutation and 64% had at least one thymidine-associated mutation (TAM) in plasma genotypes.
| Characteristic . | Monotherapy with bPI (n = 126) . | Dual therapy bPI + 3TC (n = 126) . | Total (n = 252) . |
|---|---|---|---|
| Women n (%) | 96 (76%) | 88 (70%) | 184 (73%) |
| Age (years), median (IQR) | 41 (36–49) | 43 (37–50) | 42 (36–50) |
| At inclusion | |||
| CD4 count (cell/mm3), median (IQR) | 489 (382–667) | 472 (360–633) | 474 (379–652) |
| VL <50 copies/mL, n (%) | 100 (79%) | 104 (83%) | 204 (81%) |
| HIV DNA (Log copies/106 CD4), n (%) | 3.5 (3.1–3.8) | 3.5 (3–3.8) | 3.5 (3.1–3.8) |
| HIV history | |||
| Nadir CD4 < 100 cells/mm3, n (%) | 72 (57%) | 64 (51%) | 136 (54%) |
| CD4 first failure, cells/mm3, median (IQR) | 191 (77–293) | 190 (116–283) | 190 (95–288) |
| VL at first failure ≥100 000 copies/mL, n (%) | 31 (25%) | 23 (18%) | 54 (21%) |
| Antiretroviral history | |||
| Duration of first-line treatment (months), median (IQR) | 51 (33–69) | 50 (36–68) | 50 (33–69) |
| Duration of second-line treatment (months), median (IQR) | 37 (30–47) | 38 (30–47) | 37 (30–47) |
| Drug-resistance at first-line treatment failure, n (%) | |||
| ≥1 TAMs | 88 (70%) | 72 (58%) | 160 (64%) |
| ≥3 TAMs | 40 (32%) | 34 (27%) | 74 (30%) |
| M184V mutation | 118 (94%) | 121 (97%) | 239 (96%) |
| Characteristic . | Monotherapy with bPI (n = 126) . | Dual therapy bPI + 3TC (n = 126) . | Total (n = 252) . |
|---|---|---|---|
| Women n (%) | 96 (76%) | 88 (70%) | 184 (73%) |
| Age (years), median (IQR) | 41 (36–49) | 43 (37–50) | 42 (36–50) |
| At inclusion | |||
| CD4 count (cell/mm3), median (IQR) | 489 (382–667) | 472 (360–633) | 474 (379–652) |
| VL <50 copies/mL, n (%) | 100 (79%) | 104 (83%) | 204 (81%) |
| HIV DNA (Log copies/106 CD4), n (%) | 3.5 (3.1–3.8) | 3.5 (3–3.8) | 3.5 (3.1–3.8) |
| HIV history | |||
| Nadir CD4 < 100 cells/mm3, n (%) | 72 (57%) | 64 (51%) | 136 (54%) |
| CD4 first failure, cells/mm3, median (IQR) | 191 (77–293) | 190 (116–283) | 190 (95–288) |
| VL at first failure ≥100 000 copies/mL, n (%) | 31 (25%) | 23 (18%) | 54 (21%) |
| Antiretroviral history | |||
| Duration of first-line treatment (months), median (IQR) | 51 (33–69) | 50 (36–68) | 50 (33–69) |
| Duration of second-line treatment (months), median (IQR) | 37 (30–47) | 38 (30–47) | 37 (30–47) |
| Drug-resistance at first-line treatment failure, n (%) | |||
| ≥1 TAMs | 88 (70%) | 72 (58%) | 160 (64%) |
| ≥3 TAMs | 40 (32%) | 34 (27%) | 74 (30%) |
| M184V mutation | 118 (94%) | 121 (97%) | 239 (96%) |
| Characteristic . | Monotherapy with bPI (n = 126) . | Dual therapy bPI + 3TC (n = 126) . | Total (n = 252) . |
|---|---|---|---|
| Women n (%) | 96 (76%) | 88 (70%) | 184 (73%) |
| Age (years), median (IQR) | 41 (36–49) | 43 (37–50) | 42 (36–50) |
| At inclusion | |||
| CD4 count (cell/mm3), median (IQR) | 489 (382–667) | 472 (360–633) | 474 (379–652) |
| VL <50 copies/mL, n (%) | 100 (79%) | 104 (83%) | 204 (81%) |
| HIV DNA (Log copies/106 CD4), n (%) | 3.5 (3.1–3.8) | 3.5 (3–3.8) | 3.5 (3.1–3.8) |
| HIV history | |||
| Nadir CD4 < 100 cells/mm3, n (%) | 72 (57%) | 64 (51%) | 136 (54%) |
| CD4 first failure, cells/mm3, median (IQR) | 191 (77–293) | 190 (116–283) | 190 (95–288) |
| VL at first failure ≥100 000 copies/mL, n (%) | 31 (25%) | 23 (18%) | 54 (21%) |
| Antiretroviral history | |||
| Duration of first-line treatment (months), median (IQR) | 51 (33–69) | 50 (36–68) | 50 (33–69) |
| Duration of second-line treatment (months), median (IQR) | 37 (30–47) | 38 (30–47) | 37 (30–47) |
| Drug-resistance at first-line treatment failure, n (%) | |||
| ≥1 TAMs | 88 (70%) | 72 (58%) | 160 (64%) |
| ≥3 TAMs | 40 (32%) | 34 (27%) | 74 (30%) |
| M184V mutation | 118 (94%) | 121 (97%) | 239 (96%) |
| Characteristic . | Monotherapy with bPI (n = 126) . | Dual therapy bPI + 3TC (n = 126) . | Total (n = 252) . |
|---|---|---|---|
| Women n (%) | 96 (76%) | 88 (70%) | 184 (73%) |
| Age (years), median (IQR) | 41 (36–49) | 43 (37–50) | 42 (36–50) |
| At inclusion | |||
| CD4 count (cell/mm3), median (IQR) | 489 (382–667) | 472 (360–633) | 474 (379–652) |
| VL <50 copies/mL, n (%) | 100 (79%) | 104 (83%) | 204 (81%) |
| HIV DNA (Log copies/106 CD4), n (%) | 3.5 (3.1–3.8) | 3.5 (3–3.8) | 3.5 (3.1–3.8) |
| HIV history | |||
| Nadir CD4 < 100 cells/mm3, n (%) | 72 (57%) | 64 (51%) | 136 (54%) |
| CD4 first failure, cells/mm3, median (IQR) | 191 (77–293) | 190 (116–283) | 190 (95–288) |
| VL at first failure ≥100 000 copies/mL, n (%) | 31 (25%) | 23 (18%) | 54 (21%) |
| Antiretroviral history | |||
| Duration of first-line treatment (months), median (IQR) | 51 (33–69) | 50 (36–68) | 50 (33–69) |
| Duration of second-line treatment (months), median (IQR) | 37 (30–47) | 38 (30–47) | 37 (30–47) |
| Drug-resistance at first-line treatment failure, n (%) | |||
| ≥1 TAMs | 88 (70%) | 72 (58%) | 160 (64%) |
| ≥3 TAMs | 40 (32%) | 34 (27%) | 74 (30%) |
| M184V mutation | 118 (94%) | 121 (97%) | 239 (96%) |
Archived drug-resistance in HIV proviral DNA at switch onto simplified maintenance therapy
The M184V and I mutation frequency distributions are shown for both groups in Figure 1. Overall, 173/252 (69%) and 31/252 (12%) participants harboured the M184V and I mutations at a frequency of at least 1%, respectively, with no difference between treatment groups. The median frequency of M184V variants per patient was 59% (IQR 30%–97%), with no evidence of M184V (<1%) for 79 subjects (31%). Using the relevant threshold of 5% for plasma UDS genotype, the M184V mutation was reported for 162/252 (64%) patients and M184I for 20/252 (8%). The M184V and M184I mutations were detected concomitantly in 19 patients, with M184V predominating in 13 cases and M184I in the other 6 cases.

Frequencies of archived M184V/I mutations in HIV DNA before treatment simplification to bPI monotherapy or bPI + 3TC dual therapy. The frequency of the M184V and I mutations was presented in red for bPI + 3TC arm and in blue for the bPI arm. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
M184I as STOP codon is APOBEC-3G-mediated hypermutations. Consistently, we found that 90% of samples with M184I mutations also harboured at least one STOP codon and the frequencies of both were correlated [r = 0.86 (0.74–0.93), Figure S1, available as Supplementary data at JAC Online]. With or without APOBEC filtration, the median (IQR) of M184I mutation was 17.5% (2.8%–56.8%).
We analysed the factors associated with archived M184V/I in HIV DNA at inclusion (Table 2). In a multivariate analysis, longer duration of first-line treatment, higher pVL at first-line treatment failure and higher baseline HIV DNA load were associated with archived M184V. Of note, the duration of effective second-line treatment (corresponding to the duration of viral suppression) was not associated with the archived M184V mutation detection. Second-line treatment without tenofovir/lamivudine had a trend to be associated with the presence of M184I in HIV-DNA.
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| M184V | ||||||
| Women | 0.73 | 0.39–1.35 | 0.31 | |||
| Age (years) | 1.01 | 0.98–1.03 | 0.72 | |||
| WHO stage 3 or 4 | 1.32 | 0.76–2.28 | 0.33 | |||
| Nadir CD4 (/100 cells/mm3) | 0.79 | 0.56–1.10 | 0.16 | 0.86 | 0.58–1.27 | 0.45 |
| DNA/million CD4 (log) | 2.66 | 1.58–4.48 | <0.001 | 2.57 | 1.49–4.44 | 0.001 |
| First line duration (years) | 1.20 | 1.04–1.39 | 0.015 | 1.25 | 1.06–1.47 | 0.007 |
| Second line duration (years) | 0.93 | 0.66–1.30 | 0.65 | |||
| pVL at first-line failure (log copies/mL) | 2.19 | 1.40–3.44 | 0.001 | 2.10 | 1.27–3.46 | 0.004 |
| TDF/3TC second line | 0.94 | 0.54–1.63 | 0.81 | |||
| CRF02 | 0.73 | 0.42–1.28 | 0.27 | |||
| M184I | ||||||
| Women | 0.89 | 0.39–2.04 | 0.78 | |||
| Age (years) | 1.00 | 0.96–1.04 | 0.97 | |||
| WHO stage 3 or 4 | 1.44 | 0.63–3.28 | 0.38 | |||
| Nadir CD4 (/100 cells/mm3) | 1.01 | 0.63–1.62 | 0.98 | |||
| DNA/million CD4 (log) | 1.48 | 0.72–3.04 | 0.28 | |||
| First line duration (years) | 1.05 | 0.87–1.28 | 0.59 | |||
| Second line duration (years) | 0.91 | 0.57–1.48 | 0.71 | |||
| pVL at first-line failure (log copies/mL) | 1.09 | 0.61–1.94 | 0.78 | |||
| TDF/3TC second line | 0.49 | 0.23–1.05 | 0.066 | 0.48 | 0.23–1.04 | 0.063 |
| CRF02 | 0.54 | 0.25–1.15 | 0.11 | 0.53 | 0.25–1.15 | 0.11 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| M184V | ||||||
| Women | 0.73 | 0.39–1.35 | 0.31 | |||
| Age (years) | 1.01 | 0.98–1.03 | 0.72 | |||
| WHO stage 3 or 4 | 1.32 | 0.76–2.28 | 0.33 | |||
| Nadir CD4 (/100 cells/mm3) | 0.79 | 0.56–1.10 | 0.16 | 0.86 | 0.58–1.27 | 0.45 |
| DNA/million CD4 (log) | 2.66 | 1.58–4.48 | <0.001 | 2.57 | 1.49–4.44 | 0.001 |
| First line duration (years) | 1.20 | 1.04–1.39 | 0.015 | 1.25 | 1.06–1.47 | 0.007 |
| Second line duration (years) | 0.93 | 0.66–1.30 | 0.65 | |||
| pVL at first-line failure (log copies/mL) | 2.19 | 1.40–3.44 | 0.001 | 2.10 | 1.27–3.46 | 0.004 |
| TDF/3TC second line | 0.94 | 0.54–1.63 | 0.81 | |||
| CRF02 | 0.73 | 0.42–1.28 | 0.27 | |||
| M184I | ||||||
| Women | 0.89 | 0.39–2.04 | 0.78 | |||
| Age (years) | 1.00 | 0.96–1.04 | 0.97 | |||
| WHO stage 3 or 4 | 1.44 | 0.63–3.28 | 0.38 | |||
| Nadir CD4 (/100 cells/mm3) | 1.01 | 0.63–1.62 | 0.98 | |||
| DNA/million CD4 (log) | 1.48 | 0.72–3.04 | 0.28 | |||
| First line duration (years) | 1.05 | 0.87–1.28 | 0.59 | |||
| Second line duration (years) | 0.91 | 0.57–1.48 | 0.71 | |||
| pVL at first-line failure (log copies/mL) | 1.09 | 0.61–1.94 | 0.78 | |||
| TDF/3TC second line | 0.49 | 0.23–1.05 | 0.066 | 0.48 | 0.23–1.04 | 0.063 |
| CRF02 | 0.54 | 0.25–1.15 | 0.11 | 0.53 | 0.25–1.15 | 0.11 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| M184V | ||||||
| Women | 0.73 | 0.39–1.35 | 0.31 | |||
| Age (years) | 1.01 | 0.98–1.03 | 0.72 | |||
| WHO stage 3 or 4 | 1.32 | 0.76–2.28 | 0.33 | |||
| Nadir CD4 (/100 cells/mm3) | 0.79 | 0.56–1.10 | 0.16 | 0.86 | 0.58–1.27 | 0.45 |
| DNA/million CD4 (log) | 2.66 | 1.58–4.48 | <0.001 | 2.57 | 1.49–4.44 | 0.001 |
| First line duration (years) | 1.20 | 1.04–1.39 | 0.015 | 1.25 | 1.06–1.47 | 0.007 |
| Second line duration (years) | 0.93 | 0.66–1.30 | 0.65 | |||
| pVL at first-line failure (log copies/mL) | 2.19 | 1.40–3.44 | 0.001 | 2.10 | 1.27–3.46 | 0.004 |
| TDF/3TC second line | 0.94 | 0.54–1.63 | 0.81 | |||
| CRF02 | 0.73 | 0.42–1.28 | 0.27 | |||
| M184I | ||||||
| Women | 0.89 | 0.39–2.04 | 0.78 | |||
| Age (years) | 1.00 | 0.96–1.04 | 0.97 | |||
| WHO stage 3 or 4 | 1.44 | 0.63–3.28 | 0.38 | |||
| Nadir CD4 (/100 cells/mm3) | 1.01 | 0.63–1.62 | 0.98 | |||
| DNA/million CD4 (log) | 1.48 | 0.72–3.04 | 0.28 | |||
| First line duration (years) | 1.05 | 0.87–1.28 | 0.59 | |||
| Second line duration (years) | 0.91 | 0.57–1.48 | 0.71 | |||
| pVL at first-line failure (log copies/mL) | 1.09 | 0.61–1.94 | 0.78 | |||
| TDF/3TC second line | 0.49 | 0.23–1.05 | 0.066 | 0.48 | 0.23–1.04 | 0.063 |
| CRF02 | 0.54 | 0.25–1.15 | 0.11 | 0.53 | 0.25–1.15 | 0.11 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| M184V | ||||||
| Women | 0.73 | 0.39–1.35 | 0.31 | |||
| Age (years) | 1.01 | 0.98–1.03 | 0.72 | |||
| WHO stage 3 or 4 | 1.32 | 0.76–2.28 | 0.33 | |||
| Nadir CD4 (/100 cells/mm3) | 0.79 | 0.56–1.10 | 0.16 | 0.86 | 0.58–1.27 | 0.45 |
| DNA/million CD4 (log) | 2.66 | 1.58–4.48 | <0.001 | 2.57 | 1.49–4.44 | 0.001 |
| First line duration (years) | 1.20 | 1.04–1.39 | 0.015 | 1.25 | 1.06–1.47 | 0.007 |
| Second line duration (years) | 0.93 | 0.66–1.30 | 0.65 | |||
| pVL at first-line failure (log copies/mL) | 2.19 | 1.40–3.44 | 0.001 | 2.10 | 1.27–3.46 | 0.004 |
| TDF/3TC second line | 0.94 | 0.54–1.63 | 0.81 | |||
| CRF02 | 0.73 | 0.42–1.28 | 0.27 | |||
| M184I | ||||||
| Women | 0.89 | 0.39–2.04 | 0.78 | |||
| Age (years) | 1.00 | 0.96–1.04 | 0.97 | |||
| WHO stage 3 or 4 | 1.44 | 0.63–3.28 | 0.38 | |||
| Nadir CD4 (/100 cells/mm3) | 1.01 | 0.63–1.62 | 0.98 | |||
| DNA/million CD4 (log) | 1.48 | 0.72–3.04 | 0.28 | |||
| First line duration (years) | 1.05 | 0.87–1.28 | 0.59 | |||
| Second line duration (years) | 0.91 | 0.57–1.48 | 0.71 | |||
| pVL at first-line failure (log copies/mL) | 1.09 | 0.61–1.94 | 0.78 | |||
| TDF/3TC second line | 0.49 | 0.23–1.05 | 0.066 | 0.48 | 0.23–1.04 | 0.063 |
| CRF02 | 0.54 | 0.25–1.15 | 0.11 | 0.53 | 0.25–1.15 | 0.11 |
Impact of archived M184V/I on treatment outcome
In the dual therapy arm, the 48 week estimated probability of remaining free from VF was comparable in the 2 groups: 97.6% (95% CI 91.8%–99.7%) with M184V and 97.6% (95% CI 87.1%–99.9%) without M184V (Figure 2a). This was also found for M184I, with 100% (95% CI 75.3%–100%) with M184I and 97.3% (95% CI 92.4%–99.4%) without M184I.
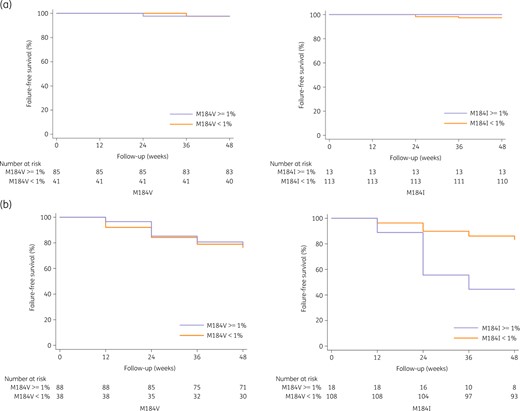
Estimated probability of remaining free from virological failure (VF) with and without the presence of the M184V/I mutation. (a) In the dual therapy group. (b) In the monotherapy group. The left panel is for the M184V mutation and the right panel for the M184I mutation. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In the monotherapy arm, the 48 week estimated probability of remaining free from VF was also comparable in both groups: 78.4% (95% CI 68.4%–86.5%) with M184V and 76.3% (95% CI 59.8%–88.6%) without M184V (Figure 2b). In contrast, the 48 week estimated probability of remaining free from VF was 44.4% (95% CI 21.5%–69.2%) with M184I and 83.3% (95% CI 4.9%–89.8%) without M184I. In the univariate and multivariate analysis performed only for patients on bPI monotherapy because the number of VF was too small in bPI + 3TC (Table 3), only the M184I mutation at 1% threshold had a trend to be associated with VF [OR 4.48 (95% CI 0.97–20.80); P = 0.055]. Similar results were confirmed for the sensitivity analysis using a threshold of 5% for the detection of the mutation M184V or I, without any association with virological failure (Table S1, Figure S2).
Factors associated with virological failure in patients on bPI monotherapy (n = 126)
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| Women | 0.58 | 0.23–1.46 | 0.24 | 0.52 | 0.17–1.52 | 0.23 |
| Adherence always ≥95% (pill count) | 0.49 | 0.20–1.22 | 0.13 | 0.84 | 0.29–2.45 | 0.75 |
| Baseline VL <50 copies/mL | 0.56 | 0.21–1.48 | 0.24 | 0.49 | 0.16–1.53 | 0.22 |
| DNA/million CD4 (log) | 0.89 | 0.41–1.93 | 0.77 | |||
| First line duration (years) | 0.82 | 0.66–1.03 | 0.082 | 0.83 | 0.64–1.08 | 0.16 |
| Second line duration (years) | 0.66 | 0.37–1.18 | 0.16 | 0.72 | 0.37–1.41 | 0.34 |
| pVL at first-line failure (log copies/mL) | 1.12 | 0.59–2.14 | 0.72 | |||
| M184V ≥ 1% | 0.89 | 0.36–2.19 | 0.80 | |||
| M184V ≥ 20% | 0.65 | 0.28–1.51 | 0.32 | |||
| M184V ≥ 75% | 0.24 | 0.07–0.84 | 0.026 | 0.53 | 0.13–2.20 | 0.39 |
| M184I ≥ 1% | 6.25 | 2.17–18.01 | 0.001 | 4.48 | 0.97–20.80 | 0.055 |
| At least 3 TAMs ≥ 1% | 0.14 | 0.02–1.13 | 0.065 | 0.18 | 0.02–1.68 | 0.13 |
| At least one STOP codon | 3.18 | 1.31–7.70 | 0.011 | 1.66 | 0.43–6.45 | 0.46 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| Women | 0.58 | 0.23–1.46 | 0.24 | 0.52 | 0.17–1.52 | 0.23 |
| Adherence always ≥95% (pill count) | 0.49 | 0.20–1.22 | 0.13 | 0.84 | 0.29–2.45 | 0.75 |
| Baseline VL <50 copies/mL | 0.56 | 0.21–1.48 | 0.24 | 0.49 | 0.16–1.53 | 0.22 |
| DNA/million CD4 (log) | 0.89 | 0.41–1.93 | 0.77 | |||
| First line duration (years) | 0.82 | 0.66–1.03 | 0.082 | 0.83 | 0.64–1.08 | 0.16 |
| Second line duration (years) | 0.66 | 0.37–1.18 | 0.16 | 0.72 | 0.37–1.41 | 0.34 |
| pVL at first-line failure (log copies/mL) | 1.12 | 0.59–2.14 | 0.72 | |||
| M184V ≥ 1% | 0.89 | 0.36–2.19 | 0.80 | |||
| M184V ≥ 20% | 0.65 | 0.28–1.51 | 0.32 | |||
| M184V ≥ 75% | 0.24 | 0.07–0.84 | 0.026 | 0.53 | 0.13–2.20 | 0.39 |
| M184I ≥ 1% | 6.25 | 2.17–18.01 | 0.001 | 4.48 | 0.97–20.80 | 0.055 |
| At least 3 TAMs ≥ 1% | 0.14 | 0.02–1.13 | 0.065 | 0.18 | 0.02–1.68 | 0.13 |
| At least one STOP codon | 3.18 | 1.31–7.70 | 0.011 | 1.66 | 0.43–6.45 | 0.46 |
Factors with P value <0.25 in the univariable analysis were included in the multivariable model.
Factors associated with virological failure in patients on bPI monotherapy (n = 126)
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| Women | 0.58 | 0.23–1.46 | 0.24 | 0.52 | 0.17–1.52 | 0.23 |
| Adherence always ≥95% (pill count) | 0.49 | 0.20–1.22 | 0.13 | 0.84 | 0.29–2.45 | 0.75 |
| Baseline VL <50 copies/mL | 0.56 | 0.21–1.48 | 0.24 | 0.49 | 0.16–1.53 | 0.22 |
| DNA/million CD4 (log) | 0.89 | 0.41–1.93 | 0.77 | |||
| First line duration (years) | 0.82 | 0.66–1.03 | 0.082 | 0.83 | 0.64–1.08 | 0.16 |
| Second line duration (years) | 0.66 | 0.37–1.18 | 0.16 | 0.72 | 0.37–1.41 | 0.34 |
| pVL at first-line failure (log copies/mL) | 1.12 | 0.59–2.14 | 0.72 | |||
| M184V ≥ 1% | 0.89 | 0.36–2.19 | 0.80 | |||
| M184V ≥ 20% | 0.65 | 0.28–1.51 | 0.32 | |||
| M184V ≥ 75% | 0.24 | 0.07–0.84 | 0.026 | 0.53 | 0.13–2.20 | 0.39 |
| M184I ≥ 1% | 6.25 | 2.17–18.01 | 0.001 | 4.48 | 0.97–20.80 | 0.055 |
| At least 3 TAMs ≥ 1% | 0.14 | 0.02–1.13 | 0.065 | 0.18 | 0.02–1.68 | 0.13 |
| At least one STOP codon | 3.18 | 1.31–7.70 | 0.011 | 1.66 | 0.43–6.45 | 0.46 |
| . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| Characteristic . | OR . | 95% CI . | P . | aOR . | 95% CI . | P . |
| Women | 0.58 | 0.23–1.46 | 0.24 | 0.52 | 0.17–1.52 | 0.23 |
| Adherence always ≥95% (pill count) | 0.49 | 0.20–1.22 | 0.13 | 0.84 | 0.29–2.45 | 0.75 |
| Baseline VL <50 copies/mL | 0.56 | 0.21–1.48 | 0.24 | 0.49 | 0.16–1.53 | 0.22 |
| DNA/million CD4 (log) | 0.89 | 0.41–1.93 | 0.77 | |||
| First line duration (years) | 0.82 | 0.66–1.03 | 0.082 | 0.83 | 0.64–1.08 | 0.16 |
| Second line duration (years) | 0.66 | 0.37–1.18 | 0.16 | 0.72 | 0.37–1.41 | 0.34 |
| pVL at first-line failure (log copies/mL) | 1.12 | 0.59–2.14 | 0.72 | |||
| M184V ≥ 1% | 0.89 | 0.36–2.19 | 0.80 | |||
| M184V ≥ 20% | 0.65 | 0.28–1.51 | 0.32 | |||
| M184V ≥ 75% | 0.24 | 0.07–0.84 | 0.026 | 0.53 | 0.13–2.20 | 0.39 |
| M184I ≥ 1% | 6.25 | 2.17–18.01 | 0.001 | 4.48 | 0.97–20.80 | 0.055 |
| At least 3 TAMs ≥ 1% | 0.14 | 0.02–1.13 | 0.065 | 0.18 | 0.02–1.68 | 0.13 |
| At least one STOP codon | 3.18 | 1.31–7.70 | 0.011 | 1.66 | 0.43–6.45 | 0.46 |
Factors with P value <0.25 in the univariable analysis were included in the multivariable model.
Drug resistance mutations in plasma at VF
We analysed the presence of RAMs in the RT and protease genes at the time of VF, by performing UDS on plasma from 28 patients on bPI and 3 on bPI + 3TC. Median (IQR) HIV-1 RNA load was 1947 (1141–12 952) copies/mL at VF. The RT and protease genes were successfully sequenced for 19/31 (61%) and 18/31 (58%) subjects, respectively (Table 4 and Table S2).
| Characteristic . | Monotherapy bPI (n = 133) . | Dual therapy bPI + 3TC (n = 132) . |
|---|---|---|
| Virological failure | 28 (21%) | 3 (2%) |
| Resistance analysis | ||
| RT | 17 | 2 |
| PR | 15 | 3 |
| Developed resistance to ART | 10 | 2 |
| Any NRTI resistance mutations | 3 (18%) | 2 (100%) |
| M184V/I alone | 0 | 1 |
| At least 1 TAM alone | 2 | 0 |
| M184V + TAMs | 1 | 1 |
| Any NNRTI resistance mutations | 9 (53%) | 2 (100%) |
| K103N | 5 | 1 |
| Others ± K103N (V106A/M, K101E, E138K, Y181C, Y188C, G190A/E, H221Y, P225H) | 4 | 1 |
| Any major PI resistance mutations | 5 (34%) | 0 (0%) |
| M46L | 2 | |
| I47A/V | 1 | |
| I54V | 1 | |
| L90M | 3 |
| Characteristic . | Monotherapy bPI (n = 133) . | Dual therapy bPI + 3TC (n = 132) . |
|---|---|---|
| Virological failure | 28 (21%) | 3 (2%) |
| Resistance analysis | ||
| RT | 17 | 2 |
| PR | 15 | 3 |
| Developed resistance to ART | 10 | 2 |
| Any NRTI resistance mutations | 3 (18%) | 2 (100%) |
| M184V/I alone | 0 | 1 |
| At least 1 TAM alone | 2 | 0 |
| M184V + TAMs | 1 | 1 |
| Any NNRTI resistance mutations | 9 (53%) | 2 (100%) |
| K103N | 5 | 1 |
| Others ± K103N (V106A/M, K101E, E138K, Y181C, Y188C, G190A/E, H221Y, P225H) | 4 | 1 |
| Any major PI resistance mutations | 5 (34%) | 0 (0%) |
| M46L | 2 | |
| I47A/V | 1 | |
| I54V | 1 | |
| L90M | 3 |
Major PI mutations emerged in patients experienced VF on lopinavir/ritonavir monotherapy.
| Characteristic . | Monotherapy bPI (n = 133) . | Dual therapy bPI + 3TC (n = 132) . |
|---|---|---|
| Virological failure | 28 (21%) | 3 (2%) |
| Resistance analysis | ||
| RT | 17 | 2 |
| PR | 15 | 3 |
| Developed resistance to ART | 10 | 2 |
| Any NRTI resistance mutations | 3 (18%) | 2 (100%) |
| M184V/I alone | 0 | 1 |
| At least 1 TAM alone | 2 | 0 |
| M184V + TAMs | 1 | 1 |
| Any NNRTI resistance mutations | 9 (53%) | 2 (100%) |
| K103N | 5 | 1 |
| Others ± K103N (V106A/M, K101E, E138K, Y181C, Y188C, G190A/E, H221Y, P225H) | 4 | 1 |
| Any major PI resistance mutations | 5 (34%) | 0 (0%) |
| M46L | 2 | |
| I47A/V | 1 | |
| I54V | 1 | |
| L90M | 3 |
| Characteristic . | Monotherapy bPI (n = 133) . | Dual therapy bPI + 3TC (n = 132) . |
|---|---|---|
| Virological failure | 28 (21%) | 3 (2%) |
| Resistance analysis | ||
| RT | 17 | 2 |
| PR | 15 | 3 |
| Developed resistance to ART | 10 | 2 |
| Any NRTI resistance mutations | 3 (18%) | 2 (100%) |
| M184V/I alone | 0 | 1 |
| At least 1 TAM alone | 2 | 0 |
| M184V + TAMs | 1 | 1 |
| Any NNRTI resistance mutations | 9 (53%) | 2 (100%) |
| K103N | 5 | 1 |
| Others ± K103N (V106A/M, K101E, E138K, Y181C, Y188C, G190A/E, H221Y, P225H) | 4 | 1 |
| Any major PI resistance mutations | 5 (34%) | 0 (0%) |
| M46L | 2 | |
| I47A/V | 1 | |
| I54V | 1 | |
| L90M | 3 |
Major PI mutations emerged in patients experienced VF on lopinavir/ritonavir monotherapy.
Overall, 12 patients had RAMs, 10 in the bPI arm and 2 in the bPI + 3TC arm. M184V emerged in plasma for one patient on bPI (#17) and two patients on 3TC+bPI (#19, #20). TAMs and NNRTI RAMs were observed overall in 4 and 11 patients, respectively. Five (34%) patients in the bPI monotherapy group (all treated with lopinavir/ritonavir) harboured viruses with major protease RAMs at codons M46V/I, I47A, I54V and L90M, whereas no protease RAMs emerged in the three patients on dual therapy. Of note, the L90M mutation was archived in baseline HIV DNA of two patients. Resistance to lopinavir/ritonavir was evidenced for three among the five cases and all remained susceptible to darunavir/ritonavir.
The comparison of RAMs between DNA at switch and RNA at failure are detailed in Supplementary data (Table S2 and Figure S3). In the bPI arm, M184V was archived in HIV-DNA at D0 in 13 patients (among 17 with NGS data) but the emergence of M184V in plasma was observed in only one patient (#17). In the bPI + 3TC arm, one of the two patients (#19, #20) with M184V in plasma already had this mutation in archived HIV-DNA at baseline (# 20).
Discussion
Using deep sequencing, we showed that the frequency of the archived M184V variants was very heterogeneous (median frequency variants per patient of 59%) despite the history of M184V mutation in plasma at first-line failure in 96% of patients. Unexpectedly, M184V variants were not detected in HIV-DNA at the 1% threshold in 31% of patients.
The factors associated with archived M184V (at 1% threshold) were a longer duration on first-line ART, a higher pVL at first-line treatment failure and a higher HIV DNA reservoir at switch to second-line maintenance ART. Virological monitoring is limited in low-income countries, and patients may, therefore, accumulate drug resistance mutations over time during suboptimal first-line ART.6–9 Interestingly, we reported that previous failures are more important for the archiving of the M184V mutation than factors during viral suppression (antiretroviral drugs pressure or duration of virological suppression). This could be different for patients with regular monitoring of virological failure with rapid change for optimal treatment. Factors associated with the detection of the M184I in HIV-DNA were not the same as those found for the M184V mutation. Only a second-line regimen without lamivudine was associated with the M184I archive, suggesting a possible reversion of M184V-to-I when the lamivudine is stopped. This reversion suggests an ongoing evolution that may occur during residual replication or viral reactivation.
In previous studies, we showed a higher number of RAMs in cumulative HIV RNA genotype than in HIV DNA genotype, even using UDS, in patients with a long experience of ART in whom the virus was controlled.10,11 Assuming that the RAMs detected in previous plasma samples are always maintained might result in an overestimation of drug resistance, because some RAMs may be cleared to levels undetectable with standard techniques, after a long period of virological control, particularly if the selection pressure for these RAMs exerted by the drug is interrupted.10,12 Even though we found no association between archived M184V and the duration of effective second-line treatment, we know from deep sequencing that the M184V mutation is cleared from the HIV reservoir after 3 years of suppressive second-line therapy in one-third of patients. It could be different for other mutations such as NRTI or NNRTI RAMs
Despite the detection of M184V in 69% of patients, the virological efficacy of bPI + 3TC dual therapy was superior to bPI alone for maintenance purposes. We confirmed for each treatment group that the 48 week estimated probability of remaining free from VF was comparable with or without the M184V mutation. The residual activity of lamivudine was demonstrated more than a decade ago in a study showing a viral load increase of 0.5 log10 after discontinuing lamivudine despite the presence of the M184V mutation.13 The remaining susceptibility to lamivudine could also be due to the low proportion (<20%) of the M184V mutation reported among 44% of subjects. In addition to the antiviral effect of lamivudine, the maintenance of the M184V mutation might contribute to the success of bPI + 3TC dual therapy by sustaining a diminished viral fitness and preventing the emergence of resistance mutations against the bPI.3 Recently, Gregson et al.14 reported a similar level of viral load with or without M184V/I in persons experiencing virological failure of first-line therapy, probably because of compensatory mutations, such as L74I, to the fitness default of M184V/I variants.
Our results confirm previous findings for treatment-experienced patients with drug resistance who switched to a lamivudine-based regimen.11,15,16 In the DoLULAM study, in which 27 multi-treatment-experienced patients were switched onto lamivudine plus dolutegravir as a maintenance therapy, no VF was reported at 48 weeks, despite the detection by UDS of M184V/I in 63% of patients before the switch.11 In two large retrospective cohorts, the 48 week estimated probability of remaining free from VF under lamivudine-based dual therapy (protease inhibitor or dolutegravir) or triple therapy (abacavir/lamivudine/dolutegravir) was comparable with or without a history of M184V/I mutations.15,16 Of note in these both studies, no deep sequencing of HIV DNA was performed before the simplification of maintenance ART. In a recent prospective pilot study, dolutegravir plus lamivudine was effective in maintaining virological control in 27 patients despite past historical lamivudine resistance and presence of archived lamivudine RAMs detected by UDS.17
Interestingly, the presence of M184I mutation in 12% of patients was always associated with a STOP codon at similar frequencies, suggesting that the presence of M184I in proviral DNA is a marker of a defective virus due to APOBEC enzyme rather than a lamivudine-resistant mutant, as previously described.11,18 Notably, the detection of the M184I mutation was associated with VF in the monotherapy group at 1% threshold but not at 5%. With the lack of lamivudine pressure, viral rebound could be more rapid when the competition between defective viruses and more fit viruses (even with resistance mutations) occurs. Our result suggests that the potential role of M184I defective viruses in treatment responses should be further investigated.
The second part of this study focused on drug resistance at VF. The main result was the selection of major PI RAMs in 34% of patients, all on lopinavir/ritonavir monotherapy. Resistance to bPI was not observed in previous studies on bPI monotherapy for maintenance, in contrast with bPI monotherapy used at second line, most patients develop lopinavir resistance within 1 year of ongoing viral replication.19 We showed in studies performed in vivo and in vitro phenotype experiments on the full gag-protease region that non-B HIV-1 subtype influenced susceptibility and response to boosted lopinavir/ritonavir monotherapy in ART-naive patients.20–22 In the dual-therapy arm, the M184V mutation was found in two patients, confirming the low rate of VF and the absence of PI resistance.1,23–27 Interestingly, one of those participants had reactivation of archived M184V during VF which illustrates the relevance of archived resistance.
This study has several limitations. First, Illumina UDS technology generates short reads, precluding the quantification of linked RAMs and defective viruses, particularly for large deletions. Second, rates of amplification/sequencing failure are high for plasma samples, due to the lower PCR amplification rates of non-B viruses, lower pVL and/or plasma storage problems during shipment. Third, the current limitation of proviral DNA testing could be a sampling problem with sample cells that do not contain archived mutations. This contrasts with plasma genotyping, which tests for mutations in the setting of active viral replication uniformly distributed in a sample. Finally, the low number of VF evidenced in the dual therapy group did not allow a sensitive analysis of the impact of archived mutations on virological response.
In conclusion, the MOBIDIP trial showed that the bPI plus lamivudine treatment strategy was highly effective in patients with a history of M184V mutation using population-based sequencing. Using deep sequencing, the M184V mutation variants were present at very different levels suggesting that bPI plus lamivudine antiviral efficacy is due mainly to the residual lamivudine activity. We also identified that prolonged virological failure without close monitoring (by viral load and genotype) was associated with the archive of M184V mutation. Importantly, all the results demonstrating the residual effect of lamivudine with historical lamivudine resistance have not been evidenced for other antiretroviral drugs and resistance mutations.
Funding
This work was supported by the French National Agency for Research on AIDS and Viral Hepatitis (ANRS) (ANRS 12286/MOBIDIP trial).
Transparency declarations
C.D. and J.R. declare grant receipt/advisory board membership with Gilead, ViiV, MSD and Janssen. The remaining authors have none to declare.
Author contributions
C.D., M.L.C, J.R. designed the study; L.C., S.K.S., A.S., N.F.N.G., C.T.N., M.N.M., J.R. provided medical care to the participants and collected clinical and biological data; M.L.N. performed experiments; C.D., Alix Armero, M.L.C., Ali Amara, S.E.D. and J.R. analysed the results and made the figures; S.E.D. performed statistical analyses; C.D., M.L.C., M.P. and J.R. drafted the initial version of the paper. All authors revised the manuscript and approved the final version.
Supplementary data
Tables S1 and S2 and Figures S1 to S3 are available as Supplementary data at JAC Online.
References
TenoRes Study Group.



