-
PDF
- Split View
-
Views
-
Cite
Cite
Annelies Van Rie, Michael G Whitfield, Elise De Vos, Lesley Scott, Pedro Da Silva, Cindy Hayes, Tim H Heupink, Frederick A Sirgel, Wendy Stevens, Robin M Warren, Discordances between molecular assays for rifampicin resistance in Mycobacterium tuberculosis: frequency, mechanisms and clinical impact, Journal of Antimicrobial Chemotherapy, Volume 75, Issue 5, May 2020, Pages 1123–1129, https://doi.org/10.1093/jac/dkz564
Close - Share Icon Share
Abstract
Molecular assays are endorsed for detection and confirmation of rifampicin-resistant TB. The frequency, causal mechanisms and impact of discordant results between molecular tests are not well understood.
The prevalence of discordant results was determined by pairwise comparison of molecular test results in a cohort of 749 rifampicin-resistant TB patients in three South African provinces. Culture isolates were sent to a research laboratory for WGS and rifampicin MIC determination. Clinical information was collected through medical file review.
The prevalence of discordances between Xpert MTB/RIF and MTBDRplus was 14.5% (95% CI 10.9%–18.9%), 5.6% (95% CI 2.2%–13.4%) between two consecutive Xpert assays and 4.2% (95% CI 2.2%–7.8%) between two consecutive MTBDRplus assays. Likely mechanisms of discordances were false rifampicin susceptibility on MTBDRplus (due to variants not included in mutant probes or heteroresistance with loss of minor variants in culture), false resistance on molecular assay in rifampicin-susceptible isolates, and human error. The healthcare worker changed the treatment regimen in 33% of patients with discordant results and requested 232 additional molecular tests after a first confirmatory test was performed in 460 patients. A follow-up Xpert assay would give the healthcare worker the ‘true’ rifampicin-resistant TB diagnosis in at least 73% of discordant cases.
The high rate of discordant results between Xpert and MTBDRplus has important implications for the laboratory, clinician and patient. While root causes for discordant result are multiple, a follow-up Xpert assay could guide healthcare workers to the correct treatment in most patients.
Introduction
In 2018, the WHO estimated that 484 000 people worldwide developed rifampicin-resistant TB, of which 78% had MDR TB, defined as resistance to both rifampicin and isoniazid.1 Rapid diagnosis and timely initiation of effective treatment are essential for optimal outcomes of rifampicin-resistant TB treatment and to prevent ongoing transmission of resistant Mycobacterium tuberculosis strains.
Resistance to rifampicin is predominantly (95% of cases) caused by genetic variants in the rifampicin resistance-determining region (RRDR) of the RNA polymerase β subunit (rpoB) gene.2 To improve the diagnosis of rifampicin-resistant TB, molecular tests that identify variants in the RRDR have been developed. In 2008, the WHO endorsed line probe assays,3 of which Genotype Hain MTBDRplus (Hain Lifescience, Nehren, Germany) is most commonly used. Subsequently, the Xpert MTB/RIF (Xpert) and Xpert MTB/RIF Ultra assays (Cepheid Inc., Sunnyvale, CA, USA) were endorsed in 2010 and 2017, respectively, as the initial diagnostic test for detection of rifampicin-resistant TB.4 The global roll out of the Xpert assay has revolutionized the diagnosis of drug-resistant TB in routine clinical care, with >34 million cartridges procured by the public health sector by the end of 2017.4 , 5
In South Africa, the detection of rifampicin-resistant TB by Xpert is routinely followed by assessment for MDR TB using a line probe assay.6 While the combination of the Xpert and MTBDRplus is currently the fastest way to diagnose rifampicin-resistant TB and MDR TB, this approach can be challenging when there is a discordance in rifampicin resistance results between the two molecular tests. Similarly, the recommendation to repeat the Xpert assay in the case of detection of rifampicin-resistant TB in a person not considered to be at high risk of rifampicin-resistant TB7 can lead to discordant results between the two Xpert assays. Any discordance between test results can lead to confusion, can undermine the clinicians’ confidence in the diagnosis of rifampicin resistance by molecular tests, triggers additional laboratory investigations and presents a challenge to the clinical management.
In this study, we investigated discordant results between Xpert and MTBDRplus, repeat Xpert and repeat MTBDRplus performed for patient management at routine laboratories in three provinces in South Africa and assessed how these discordances impact patient management. Additionally, by linking the routine laboratory results to data on the MIC of rifampicin and rpoB sequencing performed on the same samples at a research laboratory, we aimed to gain insight into the mechanisms that cause discordances between these two molecular tests.
Materials and methods
Study population and data collection
We performed a secondary analysis of data collected by a prospective cohort (EXIT-RIF study) of patients diagnosed with rifampicin-resistant TB between November 2012 and December 2013 in three South African provinces (Free State, Eastern Cape and Gauteng). Adults (≥18 years old) diagnosed with rifampicin-resistant TB by MTBDRplus (version 1) or Xpert in one of these provinces were eligible for study participation. People diagnosed with rifampicin-resistant TB on phenotypic drug susceptibility testing as the initial diagnostic test were excluded from this study. For this analysis, only patients who had interpretable results on more than one molecular test (Xpert or MTBDRplus) were included.
The MTBDRplus and Xpert assays were performed as part of routine care by the provincial National Health Services Laboratories (NHLS). All Xpert assays were performed directly on sputum samples; MTBDRplus was performed directly on decontaminated sputum for smear microscopy-positive specimens or on a culture isolate for smear-negative specimens.
Sputum cultures of patients diagnosed with rifampicin-resistant TB were retrieved from the NHLS laboratory and sent to the South African Medical Research Council Centre for Tuberculosis Research laboratory at Stellenbosch University for sequencing and determination of the MIC of rifampicin. Rifampicin MICs were determined using the BACTEC MGIT 960 System with EpiCenter TB eXiST software according to the 1% proportion method8 at concentration ranges of 1, 5, 10, 20, 50 and 100 mg/L. A sample with an MIC ≤1 mg/L was classified as rifampicin susceptible.9 M. tuberculosis sputum cultures were lysed with lysozyme, treated with proteinase K, and DNA was extracted using the phenol/chloroform extraction and ethanol precipitation method.10 DNA was prepared for WGS following the method of Baym et al. 11 Pooled libraries were sequenced on an Illumina HiSeq 2500 system as per the manufacturer’s protocol. Reads were quality checked using FastQC and aligned to the reference genome H37Rv (GenBank accession no. NC_000962.3) using BWA-MEM.12 Genome coverage was assessed (minimum 40× median coverage), and joint variant calling was conducted by GATK4 using MarkDuplicates, BaseRecalibrator, HaplotypeCaller, GenotypeGVCFs and Variant Quality Score Recalibration tools.13
Clinical information including treatment initiation date, treatment regimen and changes in treatment regimen was collected through medical record review at all primary care facilities and hospitals where the patient received any care for rifampicin-resistant TB.
Ethics
All patients gave informed consent for participation in the EXIT-RIF cohort study. The study was approved by the institutional review board of the University of North Carolina, Chapel Hill, NC, USA and the Human Ethics Research Committee of the University of the Witwatersrand, Johannesburg, South Africa.
Data analysis
We performed pairwise comparisons of Xpert and MTBDRplus results, two consecutive Xpert results and two consecutive MTBDRplus results for all patients in whom more than one molecular assay had been performed for routine clinical care. In the case of discordances between two molecular tests, we evaluated the clinical impact thereof by assessing whether the report of a discordant result led to a change in treatment regimen.
To gain insight into the mechanisms underlying discordances between two molecular tests, we linked the results of the routine Xpert and MTBDRplus assays to the results of the MIC of rifampicin and rpoB sequencing performed on the same samples at the research laboratory.
Assays with a non-interpretable result (M. tuberculosis negative, error, indeterminate, rifampicin resistance result not reported) were excluded from the analyses because information on the presence or absence of rifampicin resistance is not available.
Results
Population
Among 749 cohort study participants, about half were female (49.0%) and 77.6% were HIV positive. At the time of the rifampicin-resistant TB diagnosis, 33.2% of people living with HIV were on ART, 32.1% were ART naive, 7.2% had ART interrupted and 27.3% had unknown ART status. Half (52%) of all participants had a history of TB treatment, 14.8% had a friend or family member with MDR TB and 2.9% of patients reported working at a healthcare facility.
Use of molecular tests to diagnose and manage rifampicin-resistant TB
Rifampicin-resistant TB was initially diagnosed by Xpert in 502 patients and by MTBDRplus in 247 patients (Figure 1). Among the 502 patients initially diagnosed by Xpert, a second molecular test was requested in 342 (68%) patients: an MTBDRplus assay in 264 patients with a median of 7 days (IQR 4–14 days) between the two tests, or an Xpert assay in 78 patients with a median time of 15 days (IQR 6–65 days) between the two assays. Subsequently, another 210 molecular tests were requested in 179 patients, with a median time of 22 days (IQR 7–63 days) between second and subsequent assays. Among the 247 patients initially diagnosed by MTBDRplus, a second molecular test was requested in 118 (48%) patients: an Xpert assay in 17 (7%) patients with a median of 62 days (IQR 28–95 days) between the two samples or an MTBDRplus in 101 patients with the median of 34 days (IQR 9–66 days) between two samples. Subsequently, 22 additional molecular tests were requested in 16 patients, with a median of 51 days between the second and all subsequent assays (IQR 15–157 days).
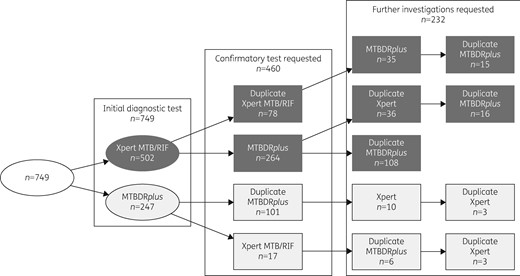
Flow chart of diagnostic and confirmatory molecular tests requested (including failed tests and tests with errors or an indeterminate result) for rifampicin in 749 study cohort participants.
Prevalence of discordances between molecular assays
One or more discordant results occurred in 56 (9.6%, 95% CI 7.5%–12.3%) of 582 paired samples with interpretable results (i.e. M. tuberculosis present and no error) for both tests (Table 1), collected from 394 individual patients. Among the 297 paired Xpert and MTBDRplus assay results, both tests detected rifampicin resistance in 254 isolates (85.5%, 95% CI 81.1%–89.1%). Discordant results were observed in 43 (14.5%, 95% CI 10.9%–18.9%) sample pairs, with almost all (42/43) discordances due to the presence of rifampicin susceptibility by MTBDRplus in the presence of rifampicin resistance by Xpert. Of the 72 paired Xpert assay results, 68 (94.4%, 95% CI 86.6%–97.8%) were concordant and 4 (5.6%, 95% CI 2.2%–13.4%) were discordant. Of the 213 paired MTBDRplus assay results, 204 (95.8%, 95% CI 92.2%–97.8%) were concordant (194 twice resistant and 10 twice susceptible) and 9 (4.2%, 95% CI 2.2%–7.8%) were discordant.
Discordant results between 582 paired molecular tests with interpretable results performed for assessment of rifampicin-resistant TB at routine laboratories in South Africa
| Test and results . | Sample pairsa . | Proportion (95% CI) . |
|---|---|---|
| Xpert and MTBDRplus (n=297) | ||
| concordant RIFR | 254 | 85.5% (81.1%–89.1%) |
| concordant RIFS | 0 | 0% |
| Xpert RIFS MTBDRplus RIFR | 1 | 0.4% (0.06%–1.9%) |
| Xpert RIFR MTBDRplus RIFS | 42 | 14.1% (10.6%–18.6%) |
| Repeat Xpert (n=72) | ||
| concordant RIFR | 68 | 94.4% (86.6%–97.8%) |
| concordant RIFS | 0 | 0% |
| discordant | 4 | 5.6% (2.2%–13.4%) |
| Repeat MTBDRplus (n=213) | ||
| concordant RIFR | 194 | 91.1% (86.5%–94.2%) |
| concordant RIFS | 10 | 4.7% (2.6%–8.4%) |
| discordant | 9 | 4.2% (2.2%–7.8%) |
| Test and results . | Sample pairsa . | Proportion (95% CI) . |
|---|---|---|
| Xpert and MTBDRplus (n=297) | ||
| concordant RIFR | 254 | 85.5% (81.1%–89.1%) |
| concordant RIFS | 0 | 0% |
| Xpert RIFS MTBDRplus RIFR | 1 | 0.4% (0.06%–1.9%) |
| Xpert RIFR MTBDRplus RIFS | 42 | 14.1% (10.6%–18.6%) |
| Repeat Xpert (n=72) | ||
| concordant RIFR | 68 | 94.4% (86.6%–97.8%) |
| concordant RIFS | 0 | 0% |
| discordant | 4 | 5.6% (2.2%–13.4%) |
| Repeat MTBDRplus (n=213) | ||
| concordant RIFR | 194 | 91.1% (86.5%–94.2%) |
| concordant RIFS | 10 | 4.7% (2.6%–8.4%) |
| discordant | 9 | 4.2% (2.2%–7.8%) |
RIFR, rifampicin resistant; RIFS, rifampicin susceptible.
Samples included in the sample pairs were collected on different dates. The number of sample pairs included in the Xpert versus MTBDRplus, repeat Xpert and repeat MTBDRplus analyses differed, depending on which tests were requested by the clinician in charge of patient care.
Discordant results between 582 paired molecular tests with interpretable results performed for assessment of rifampicin-resistant TB at routine laboratories in South Africa
| Test and results . | Sample pairsa . | Proportion (95% CI) . |
|---|---|---|
| Xpert and MTBDRplus (n=297) | ||
| concordant RIFR | 254 | 85.5% (81.1%–89.1%) |
| concordant RIFS | 0 | 0% |
| Xpert RIFS MTBDRplus RIFR | 1 | 0.4% (0.06%–1.9%) |
| Xpert RIFR MTBDRplus RIFS | 42 | 14.1% (10.6%–18.6%) |
| Repeat Xpert (n=72) | ||
| concordant RIFR | 68 | 94.4% (86.6%–97.8%) |
| concordant RIFS | 0 | 0% |
| discordant | 4 | 5.6% (2.2%–13.4%) |
| Repeat MTBDRplus (n=213) | ||
| concordant RIFR | 194 | 91.1% (86.5%–94.2%) |
| concordant RIFS | 10 | 4.7% (2.6%–8.4%) |
| discordant | 9 | 4.2% (2.2%–7.8%) |
| Test and results . | Sample pairsa . | Proportion (95% CI) . |
|---|---|---|
| Xpert and MTBDRplus (n=297) | ||
| concordant RIFR | 254 | 85.5% (81.1%–89.1%) |
| concordant RIFS | 0 | 0% |
| Xpert RIFS MTBDRplus RIFR | 1 | 0.4% (0.06%–1.9%) |
| Xpert RIFR MTBDRplus RIFS | 42 | 14.1% (10.6%–18.6%) |
| Repeat Xpert (n=72) | ||
| concordant RIFR | 68 | 94.4% (86.6%–97.8%) |
| concordant RIFS | 0 | 0% |
| discordant | 4 | 5.6% (2.2%–13.4%) |
| Repeat MTBDRplus (n=213) | ||
| concordant RIFR | 194 | 91.1% (86.5%–94.2%) |
| concordant RIFS | 10 | 4.7% (2.6%–8.4%) |
| discordant | 9 | 4.2% (2.2%–7.8%) |
RIFR, rifampicin resistant; RIFS, rifampicin susceptible.
Samples included in the sample pairs were collected on different dates. The number of sample pairs included in the Xpert versus MTBDRplus, repeat Xpert and repeat MTBDRplus analyses differed, depending on which tests were requested by the clinician in charge of patient care.
Discordant test results were three times more likely between the two different molecular tests compared with a repeat of the same molecular assay (OR 3.1, 95% CI 1.3–8.5). Discordant results were also more likely in patients with low bacterial load, with a longer time to culture positivity among samples with discordant compared with concordant Xpert results [median time to positive (TTP) 24 days (IQR 20–29) versus 16 days (IQR 13–21)].
Management of patients with discordant test results
Of the 394 patients with ≥2 M. tuberculosis positive and interpretable (no error) molecular assay results, 50 had one or more discrepant rifampicin resistance results between two molecular tests. The healthcare worker acted upon the receipt of the result by changing the treatment regimen in one in three (16/48 or 33.3%) patients alive and in care when the discordant test result was reported. Regimen switched comprised a switch from a second-line regimen to a first-line regimen in 12 patients and from a first-line to a second-line regimen in 4 patients.
Mechanism of discordances between molecular assays
Among the 749 participants, a viable sample was sent to the research laboratory in 337 patients. Of the 331 isolates with valid MIC results, 274 were rifampicin resistant (MIC >1 mg/L) and 57 were susceptible to rifampicin (MIC ≤1 mg/L). Of the 328 isolates successfully sequenced, 303 had a genetic variant detected in the RRDR and 25 were rpoB WT. Information on both MIC and rpoB sequence was available for 15 of the 50 patients with discordant results.
The most frequent mechanism causing a discordance was the detection of a genetic variant by the Xpert assay (by one of the five probes), which was not revealed by the MTBDRplus assay due to the presence of an rpoB mutation not represented by a mutant probe on the MTBDRplus assay in seven patients (patients 1–7, Table 2). In two other patients, a false-negative MTBDRplus assay result was most probably due to the presence of heteroresistance with loss of the minor variant during culture (patients 8 and 9, Table 2). In two patients, the likely mechanisms was false resistance by a molecular assay, with rifampicin resistance on two consecutive specimens in the presence of the WT sequence and MIC <1 mg/L (patients 14 and 15, Table 2). The mechanism remained unidentified and could have included human error (administrative error) or a false-positive Xpert result in the remaining four patients (patients 10–13, Table 2).
Likely mechanisms for discordances between molecular assays in patients with one or more discordances between two molecular tests and results available for the MIC of rifampicin (RIF MIC) on liquid culture and WGS
| . | Xpert . | . | MTBDRplus . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 1 . | 2 . | Xpert probea . | 1 . | 2 . | RIF MIC . | rpoB variant (genome position and coverage on WGS) . | E. coli numbering . | Most likely mechanism . | Likely true RIF resistance status . | Expected result if Xpert is used to resolve discrepancy . |
| 1 | R | A | S culture | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 2 | R | E | S sputum | S | 760600 A>C Fixed | L533P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 3 | R | B | S culture | R culture | S | 761155 C>T Near fixed (>98%) | D516Y | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | |
| 4 | A | R culture | S sputum | S | 761095 T>C Near fixed (>98%) | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 5 | A | R culture | S sputum | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 6 | C | R culture | S culture | R | 761128 C>T Fixed | S522L | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 7 | B | R culture | S culture | R | 761110 A>T Near fixed (>98%) | D516A | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 8 | R | B | S culture | R | 761104 TCATGGA>A Low-frequency variant (2%) | 515 6bp del | False-negative MTBDRplus due to absence of mutant probeb or loss of minor variant in culture | Resistant | RIF resistant | ||
| 9 | R | D | R sputum | S culture | R | 761139 C>T Heteroresistance (18%) | H526Y | False-negative MTBDRplus due to loss of minor variant in culture | Resistant | RIF resistant | |
| 10 | R | S | E | R culture | R | 761155 C>T Fixed | S531L | Unknown (possible administrative error) | Resistant | RIF resistant | |
| 11 | R | – | S culture | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 12 | R | – | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 13 | R | – | S culture | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | ||
| 14 | R | R | – | S sputum | S sputum | S | WT | False-positive Xpert | Susceptible | Resistant (?) | |
| 15 | S | – | R | R | S | WT | False-positive MTBDRplus | Susceptible | Susceptible | ||
| . | Xpert . | . | MTBDRplus . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 1 . | 2 . | Xpert probea . | 1 . | 2 . | RIF MIC . | rpoB variant (genome position and coverage on WGS) . | E. coli numbering . | Most likely mechanism . | Likely true RIF resistance status . | Expected result if Xpert is used to resolve discrepancy . |
| 1 | R | A | S culture | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 2 | R | E | S sputum | S | 760600 A>C Fixed | L533P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 3 | R | B | S culture | R culture | S | 761155 C>T Near fixed (>98%) | D516Y | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | |
| 4 | A | R culture | S sputum | S | 761095 T>C Near fixed (>98%) | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 5 | A | R culture | S sputum | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 6 | C | R culture | S culture | R | 761128 C>T Fixed | S522L | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 7 | B | R culture | S culture | R | 761110 A>T Near fixed (>98%) | D516A | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 8 | R | B | S culture | R | 761104 TCATGGA>A Low-frequency variant (2%) | 515 6bp del | False-negative MTBDRplus due to absence of mutant probeb or loss of minor variant in culture | Resistant | RIF resistant | ||
| 9 | R | D | R sputum | S culture | R | 761139 C>T Heteroresistance (18%) | H526Y | False-negative MTBDRplus due to loss of minor variant in culture | Resistant | RIF resistant | |
| 10 | R | S | E | R culture | R | 761155 C>T Fixed | S531L | Unknown (possible administrative error) | Resistant | RIF resistant | |
| 11 | R | – | S culture | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 12 | R | – | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 13 | R | – | S culture | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | ||
| 14 | R | R | – | S sputum | S sputum | S | WT | False-positive Xpert | Susceptible | Resistant (?) | |
| 15 | S | – | R | R | S | WT | False-positive MTBDRplus | Susceptible | Susceptible | ||
Likely mechanisms for discordances between molecular assays in patients with one or more discordances between two molecular tests and results available for the MIC of rifampicin (RIF MIC) on liquid culture and WGS
| . | Xpert . | . | MTBDRplus . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 1 . | 2 . | Xpert probea . | 1 . | 2 . | RIF MIC . | rpoB variant (genome position and coverage on WGS) . | E. coli numbering . | Most likely mechanism . | Likely true RIF resistance status . | Expected result if Xpert is used to resolve discrepancy . |
| 1 | R | A | S culture | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 2 | R | E | S sputum | S | 760600 A>C Fixed | L533P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 3 | R | B | S culture | R culture | S | 761155 C>T Near fixed (>98%) | D516Y | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | |
| 4 | A | R culture | S sputum | S | 761095 T>C Near fixed (>98%) | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 5 | A | R culture | S sputum | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 6 | C | R culture | S culture | R | 761128 C>T Fixed | S522L | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 7 | B | R culture | S culture | R | 761110 A>T Near fixed (>98%) | D516A | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 8 | R | B | S culture | R | 761104 TCATGGA>A Low-frequency variant (2%) | 515 6bp del | False-negative MTBDRplus due to absence of mutant probeb or loss of minor variant in culture | Resistant | RIF resistant | ||
| 9 | R | D | R sputum | S culture | R | 761139 C>T Heteroresistance (18%) | H526Y | False-negative MTBDRplus due to loss of minor variant in culture | Resistant | RIF resistant | |
| 10 | R | S | E | R culture | R | 761155 C>T Fixed | S531L | Unknown (possible administrative error) | Resistant | RIF resistant | |
| 11 | R | – | S culture | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 12 | R | – | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 13 | R | – | S culture | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | ||
| 14 | R | R | – | S sputum | S sputum | S | WT | False-positive Xpert | Susceptible | Resistant (?) | |
| 15 | S | – | R | R | S | WT | False-positive MTBDRplus | Susceptible | Susceptible | ||
| . | Xpert . | . | MTBDRplus . | . | . | . | . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | 1 . | 2 . | Xpert probea . | 1 . | 2 . | RIF MIC . | rpoB variant (genome position and coverage on WGS) . | E. coli numbering . | Most likely mechanism . | Likely true RIF resistance status . | Expected result if Xpert is used to resolve discrepancy . |
| 1 | R | A | S culture | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 2 | R | E | S sputum | S | 760600 A>C Fixed | L533P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 3 | R | B | S culture | R culture | S | 761155 C>T Near fixed (>98%) | D516Y | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | |
| 4 | A | R culture | S sputum | S | 761095 T>C Near fixed (>98%) | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 5 | A | R culture | S sputum | S | 761095 T>C Fixed | L511P | False-negative MTBDRplus in absence of mutant probeb | Low-level resistant (disputed mutation) | RIF resistant | ||
| 6 | C | R culture | S culture | R | 761128 C>T Fixed | S522L | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 7 | B | R culture | S culture | R | 761110 A>T Near fixed (>98%) | D516A | False-negative MTBDRplus in absence of mutant probeb | Resistant | RIF resistant | ||
| 8 | R | B | S culture | R | 761104 TCATGGA>A Low-frequency variant (2%) | 515 6bp del | False-negative MTBDRplus due to absence of mutant probeb or loss of minor variant in culture | Resistant | RIF resistant | ||
| 9 | R | D | R sputum | S culture | R | 761139 C>T Heteroresistance (18%) | H526Y | False-negative MTBDRplus due to loss of minor variant in culture | Resistant | RIF resistant | |
| 10 | R | S | E | R culture | R | 761155 C>T Fixed | S531L | Unknown (possible administrative error) | Resistant | RIF resistant | |
| 11 | R | – | S culture | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 12 | R | – | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | |||
| 13 | R | – | S culture | S sputum | S | WT | Unknown (possible administrative error or false-positive Xpert) | Susceptible | Susceptible if human error; resistant if false-positive Xpert | ||
| 14 | R | R | – | S sputum | S sputum | S | WT | False-positive Xpert | Susceptible | Resistant (?) | |
| 15 | S | – | R | R | S | WT | False-positive MTBDRplus | Susceptible | Susceptible | ||
Taking together the results of all tests performed in each individual patient, we predict that rifampicin resistance was present in most (10/15) patients with a discrepant result, albeit low-level resistance in a substantial proportion, and that a follow-up Xpert assay would guide health workers towards the correct classification of rifampicin resistance in at least 73% (≥11/15) of patients with a discrepant result (Table 2).
Discussion
In this study, we observed a relatively high (9.6%) rate of discordant rifampicin resistance results between molecular tests performed as part of routine management. The discordancy rate was three times higher between the two different molecular tests than when the same molecular test was repeated, with a 14.5% discordancy rate between Xpert and MTBDRplus tests, compared with 5.6% for consecutive Xpert tests and 4.2% for consecutive MTBDRplus assays. Discordances were caused by the presence of a variant not included as a mutant probe in the MTBDRplus assay, heteroresistance with loss of the minor variant during culture, administrative error or a false-positive molecular test. Healthcare workers frequently requested additional molecular tests following a first confirmatory test and only acted upon a discrepant result in one out of three patients with a discordant result.
To date, most studies have focused on discordances between molecular and phenotypic assays, with only five relatively small studies investigating discordances between molecular tests. One study at an Indian reference laboratory observed a high (34%) discordant rate between MTBDRplus and Xpert among 62 rifampicin monoresistant patients.14 Discordances were mainly due to rifampicin susceptibility on Xpert in the presence of rifampicin resistance on MTBDRplus. Sanger sequencing suggested poor detection of the L533P mutation by the Xpert probe E as the main cause of discordance.14 Three studies found an 8% prevalence of discordance between Xpert and MTBDRplus, slightly lower than the 14.5% observed in our cohort. One study was nested within the 2011–13 Bangladesh drug resistance survey, which observed a 7.6% discordance between MTBDRplus and Xpert assays among 92 rifampicin-resistant TB cases.15 Sanger sequencing showed that most false-negative MTBDRplus occurred in the presence of a disputed mutation or rare variant. A small retrospective study in Zimbabwe of 39 samples including 12 rifampicin-resistant samples observed a 7.7% discordance between MTBDRplus and Xpert.16 A study in Johannesburg, South Africa reported an 8.3% prevalence of discordance among 263 patients.17 Similar to our findings, discordance occurred mainly due the presence of rifampicin resistance by Xpert in the presence of detection of a rifampicin-susceptible result by MTBDRplus. 17 In-depth investigation was not possible due to the lack of sequencing results, but the results suggested that discordances were associated with probe delay and rifampicin resistance detected by Xpert probe B. This finding is in contrast to the results of our study and the result of the study in Bangladesh, where in-depth analysis using sequencing showed that none of the Xpert probes was associated with discordant results. Finally, a complete (100%) concordance rate between MTBDRplus and Xpert was observed in two studies: a retrospective study at the Haitian national reference laboratory of 153 samples harbouring rpoB mutations18 and a prospective study in Taiwan of 44 samples with rifampicin-resistant TB detected by Xpert.19
Reports on discordances between repeat Xpert or repeat MTBDRplus assays are even scarcer, even though the 2018 Field Guide for the Management of Drug-Resistant Tuberculosis recommends a repeat Xpert in a new diagnosis of rifampicin-resistant TB in settings with a low rifampicin-resistant TB prevalence.7 We are not aware of any publication reporting discordances between repeat or confirmatory MTBDRplus assays and only one study documented rates of discordance between an initial and confirmatory Xpert assay.20 This study, performed at private clinics in Bangladesh, found a much higher (32% or 22/69 patients) rate of discordant Xpert results when performed as a confirmatory test on a remnant or second specimen. Discordant results occurred mostly (20/22) in specimens with very low bacterial load.20 We also found a longer time to culture positivity, a marker of low bacterial load, in samples with discordant compared with concordant Xpert results [median TTP 24 days (IQR 20–29) versus 16 days (IQR 13–21)].
Few studies have investigated the mechanism causing discordances between two molecular tests. In a recent article, Hofmann-Thiel et al. 21 hypothesized that several mechanisms can cause discordances, but they did not provide evidence for or the relative importance of these mechanisms in routine conditions. The most frequent cause of discordance observed in our cohort was false-negative results by MTBDRplus in the presence of ‘disputed’ mutations, defined as rpoB mutations associated with low-level rifampicin resistance that is missed by rapid phenotypic drug susceptibility tests.22 Disputed mutations have been associated with false-negative Xpert results for older versions of the Xpert assay,23 , 24 but not with the newer version, as confirmed in our study. Disputed mutations have also been associated with a ‘ghost band’ on the MTBDRplus assay resulting in a failure to detect rifampicin resistance,15 , 23 as observed in our cohort. The reason for this ghost band is that resistance by non-canonical mutations on MTBDRplus cannot be detected but only inferred by the lack of binding of the amplicons to one of the WT probes,25 which may be more prone to human error compared with the detection of resistance by hybridization to mutant probes.23 These disputed mutations, which make up a substantial proportion (∼10%) of rpoB mutations in different parts of the world,22 , 26 , 27 are associated with slower growth22 and may be clinically significant, as has been shown in observational studies.26 , 28 , 29 Another cause of discordant results was the presence of heteroresistance, defined as the coexistence of susceptible and resistant organisms in the same sample. Heteroresistance can result in the presence of mixed MTBDRplus banding patterns or false-susceptible rifampicin resistance on MTBDRplus performed on culture isolates as susceptible bacilli grow preferentially on liquid media.15 , 30 , 31 A third cause was a false-positive molecular test for rifampicin resistance detection by either test, as previously reported for Xpert15 , 32 , 33 and MTBDRplus in a few cases.15 Finally, sample mix-up and administrative errors in recording and reporting of results can never be excluded, especially under the busy routine laboratory conditions in high-burden countries.
Discordant results between two molecular tests (especially Xpert and MTBDRplus) can have important implications for clinical care and public health as they can result in additional tests requested and can pose challenges to patient management. Patients wrongly diagnosed with rifampicin-resistant TB may receive a prolonged period of unnecessarily toxic and poorly effective drugs, while those misclassified as having drug-susceptible TB after an initial diagnosis of rifampicin-resistant TB may receive ineffective first-line treatment. Our observation that three in four patients with discordant results can be resolved by a follow-up Xpert assay support the recent guidelines that recommend a confirmatory Xpert assay in patients at ‘low rifampicin-resistant TB risk’ and/or a MTBDRplus assay to assess for MDR TB following an rifampicin-resistant TB diagnosis.7
While this is a large prospective study of discordant results between molecular assays for rifampicin resistance performed at routine laboratories, several limitations should be noted. First, because this was a secondary analysis of existing data, the interpretation of the discordant results by the clinician was not documented in a standardized manner. Not changing a treatment regimen does not necessarily mean that an informed decision to continue the same regimen was not made. Second, not all samples could be retrieved for sequencing and rifampicin MIC testing, limiting our ability to assess the prevalence of the different mechanisms of discordant results. A third limitation is that the Xpert Ultra and MTBDRplus version 2 assays have improved sensitivity compared with the Xpert MTB/RIF and MTBDRplus version 1 assays that were used during the study period. It is, however, likely that discordances between different assay will still occur and that the same mechanisms will be responsible. Lastly, because assays were performed on different clinical specimens, discordances due to sampling of different pulmonary lesions harbouring bacilli with different drug resistance could not be excluded as a potential causal mechanism.
Conclusions
Molecular assays have become the main method for detection and confirmation of rifampicin resistance in M. tuberculosis, but discordances between test results have important implications for the laboratory, clinician and patient. Our findings of a high (10%) prevalence of discordant results between Xpert and MTBDRplus under routine conditions and an array of underlying causes highlight both the importance and complexity of this issue. Future research should assess the value of a repeat Xpert in the case of a discrepant result to guide healthcare workers in their treatment decisions.
Acknowledgements
We thank the participants and the healthcare workers for their dedication to this study. We thank Arnab Pain at King Abdullah University of Science and Technology for performing the whole-genome sequencing.
Funding
This study was made possible by funding by the Division of AIDS, National Institutes of Health (#R01 AI099026) and by the Fonds Wetenschappelijk Onderzoek (FWO) (Centre for Whole Genome Sequencing of Mycobacterium tuberculosis, # G0F8316N). This research was supported by the South African Medical Research Council.
Transparency declarations
None to declare.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the South African Medical Research Council.
References
World Health Organization. Global Tuberculosis Report.
World Health Organization. Molecular Line Probe Assays for Rapid Screening of Patients at Risk of Multidrug-Resistant Tuberculosis (MDR-TB) - Policy Statement.
World Health Organization. Global Tuberculosis Report.
World Health Organization. Monitoring of Xpert MTB/RIF roll-out.
SADOH. Interim clinical guidance for the implementation of injectable-free regimens for rifampicin-resistant tubercuosis in adults, adolescents and children. South African Department of Health.
CLSI. Susceptibility Testing of Mycobacteria, Nocardia, and Other Aerobic Actinomycetes—Approved Standard M24-A2.
Global Laboratory Initiative. Line probe assays for drug-resistant tuberculosis detection: interpretation and reporting guide for laboratory staff and clinicians.



