-
PDF
- Split View
-
Views
-
Cite
Cite
António Teixeira Rodrigues, Fátima Roque, Maria Piñeiro-Lamas, Amílcar Falcão, Adolfo Figueiras, Maria Teresa Herdeiro, Effectiveness of an intervention to improve antibiotic-prescribing behaviour in primary care: a controlled, interrupted time-series study, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 9, September 2019, Pages 2788–2796, https://doi.org/10.1093/jac/dkz244
Close - Share Icon Share
Abstract
High rates of antibiotic misprescribing in primary care, with alarming clinical and economic consequences, highlight the urgent need for interventions to improve antibiotic prescribing in this setting.
To assess the effectiveness on antibiotic prescribing quality indicators of a multifaceted intervention targeting health professionals’ and patients’ behaviour regarding antibiotic use.
We conducted a pragmatic cluster-randomized controlled trial in the catchment area covered by Portugal’s Central Regional Health Administration. The intervention consisted of a multidisciplinary, multifaceted programme involving physicians, pharmacists and patients, and comprising outreach visits for physicians and pharmacists, and educational materials for health professionals and patients. The following were assessed: relative ratios of prescription of penicillins sensitive to β-lactamase, penicillin combinations including β-lactamase inhibitors, third- and fourth-generation cephalosporins and fluoroquinolones; and the ratio of broad- to narrow-spectrum antibiotics. An interrupted time-series analysis for multiple-group comparisons was performed. The study protocol was registered on Clinical.trials.gov (NCT02173509).
The participation rate in the educational intervention was 64% (197/309 GPs) in a total of 25 counties. Statistically significant improvements were obtained, not only in the relative prescription of penicillins sensitive to β-lactamase (overall relative change of +896%) and penicillin combinations including β-lactamase inhibitors (−161%), but also in the ratio of broad- to narrow-spectrum antibiotics (−200%). Statistically significant results were also obtained for third- and fourth-generation cephalosporins, though only in the immediate term.
This study showed that quality indicators of antibiotic prescribing can be improved by tackling influences on behaviour including knowledge and attitudes surrounding physicians’ clinical practice. Accordingly, these determinants must be considered when implementing interventions aimed at improving antibiotic prescribing.
Introduction
Increasing misuse and overuse of antibiotics worldwide1 has led to escalating rates of resistance2 and harmful risks for individual patients,3 with little or no clinical benefit.4 As most antibiotics are prescribed in primary care,5 interventions to tackle this global concern are thus needed in this setting. Portugal is no exception: the consumption of antibacterials for systemic use in primary care remains high [in 2012, compared with the country scoring best in the EU/EEA, 22.7 DDDs per 1000 inhabitants per day (DID) versus 11.3 DID in the Netherlands/2.33 packages per 1000 inhabitants per day (PID) versus 1.14 in Sweden].6 The rate of hospital-acquired infection is significantly higher than the European mean7 and Portugal has one of the highest incidence rates of infection due to MRSA (47%) and vancomycin-resistant Enterococcus faecium (20.1%).7 Consequently, the association between the development of resistance and increasing rates of morbidity, mortality, length of hospitalization and cost of healthcare8 has become a cause for alarm among patients, healthcare providers and policymakers alike.
Gonzales et al.9 estimated a rate of excessive use of antibiotics in acute respiratory infections in ambulatory practice of 55% and Schroeck et al.10 reported a figure of ∼64% of patients inadequately treated, mainly because of antibiotics being prescribed when not indicated.10 More recently, in an estimation of outpatient oral antibiotic prescribing, Fleming-Dutra et al.11 stated that approximately one-third of all antibiotics prescribed were unnecessary and emphasized the urgent need to tackle antibiotic misprescribing and overprescribing in primary care. In Portugal, like in other European countries, antibiotics may only be dispensed by community pharmacies under medical prescription. Hence, there is need for multifaceted, multidisciplinary programmes that are designed on the basis of the characteristics of each practice and across settings12,13 and take both clinical and behavioural determinants into account.14
We developed a joint multidisciplinary, multifaceted intervention targeting health professionals’ and patients’ behaviour regarding antibiotic use, which significantly decreased overall antibiotic use by 3.71%.15 Since inappropriate antibiotic prescribing involves a combination of overprescribing and misprescribing factors, the European Surveillance of Antibiotic Consumption (ESAC) adopted the quality indicators for antibiotic consumption validated by Coenen et al.16 These quality indicators are used by ESAC to compare antibiotic consumption over European countries and are fundamental to characterize, improve and guide the development of interventions. This study sought to assess the effectiveness of the intervention on quality indicators of antibiotic-prescribing behaviour.
Methods
Ethics
We conducted a pragmatic, cluster-randomized, controlled trial in the catchment area covered by Portugal's Central Regional Health Administration. Authorization was obtained from Portugal's Central Regional Health Administration (Permit Number 015650) and the Portuguese Data Protection Authority (Comissão Nacional de Proteção de Dados/CNPD) (Permit Number 2886). The study protocol was registered on Clinical.trials.gov (NCT02173509).
Study setting and population
In the outpatient setting of the Portuguese Public Health Service, most people are registered with an outpatient primary care centre and/or GP and, under normal circumstances, consult their GP on their primary care needs. Portugal's Central Regional Health Administration, the setting for this study, covers a total of 77 counties. The primary care service has a pyramidal structure made up of Health Centre Groups (Agrupamentos de Centros de Saúde) (n = 6) and Local Healthcare Units (Unidades Locais de Saúde) (n = 2) organized around the country’s referral hospitals. The above 77 counties had a total catchment population of 1.7 million in 2012.17
Randomization
A randomized cluster design was chosen to avoid contamination within control and intervention groups. Based on the above-mentioned pyramidal structure and taking the referral hospitals serving the respective outpatient centres into account, we proceeded to create eight spatial clusters. Cluster randomization was then performed by two of the authors (A. Figueiras and M. T. H.) using computer generation of random numbers (EPIDAT® program). Of the total of eight clusters, four were allocated to the intervention and four to the control group.
The random distribution of the clusters to the intervention group and control group was unbalanced by the number of municipalities, health professionals and population. However, there is a valid method of random distribution (unequal randomization)18,19 that forces the groups to have different sizes.
Intervention
The intervention was designed as the ultimate goal of the following sequential steps targeted at improving antibiotic prescribing in primary care: (i) systematic review to identify factors that influence physicians’ behaviour;20 (ii) questionnaire development and validation to assess the determinants of antibiotic prescribing;21 and (iii) a cohort study to assess the impact of each factor on the quality indicators of antibiotic-prescribing behaviour.22 This cohort study and the controlled intervention were developed in the same area.
The intervention consisted of a multidisciplinary, multifaceted programme involving physicians, pharmacists and patients, and using a combination of learning methods, topics and strategies to improve clinical behaviour. It included active (outreach visits targeting physicians and pharmacists; ∼2 h each) and passive (educational materials targeting general population) features. The intervention was expressly designed to address gaps in knowledge and attitudes that had been previously identified in the same target population. Figure 1 depicts a schematic representation of the intervention’s features.
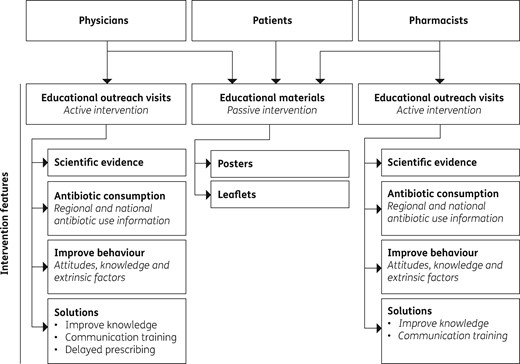
The physician educational outreach visit presentation, available as Supplementary data at JAC Online, included: (i) a characterization of antibiotic use and resistance in Europe, Portugal and the study setting; (ii) the relationship between consumption and resistance; (iii) rates of antibiotic use in primary care; (iv) factors previously identified as underlying inappropriate antibiotic prescribing20,22 (both intrinsic, such as attitudes, and extrinsic, such as patient- or health system-related factors); and (v) a detailed explanation of strategies to improve antibiotic use (simulated dialogues of physician–patient communication focused on attitudes previously identified, in the same target population, as affecting antibiotic prescribing,22 and evidence of delayed prescribing methods).23
A letter was circulated to all clinical directors of primary care facilities in the intervention group, informing and inviting all physicians to participate in the study. Each intervention was booked in collaboration with the clinical director, usually to coincide with the weekly staff meeting, and was performed by one of the authors (A. T. R.). Participation in the intervention session was voluntary and no financial benefit was received by study participants. The intervention took place from 31 May 2013 to 3 October 2013. During the same period, outreach visits to community pharmacists were made by one of the authors (F. R.), with the dual aim of improving pharmacists’ antibiotic dispensing and enlisting them to play a role in the distribution of flyers and posters and elucidation of the included information, thus contributing to improved awareness of the rational use of antibiotics by the general population . The pharmacists’ intervention was designed with data obtained from previous studies about knowledge and attitudes in the same target pharmacists.24
Regarding the educational materials (available as Supplementary data at JAC Online), posters and flyers were distributed to the general population in primary care centres and community pharmacies of the intervention counties. These materials included messages regarding the excessive and inadequate use of antibiotics and their impact on health, emphasizing that microbial resistances are putting future generations at risk.
The control group did not receive the intervention and continued with the usual practice.
Scientific support was obtained from the Official College of Physicians, the Portuguese Association of Family Medicine and the Official College of Pharmacists.
Outcome measures
We used the quality indicators validated by Coenen et al.16 (2007) to calculate monthly prescribing rates (from September 2010 to September 2014) both for indicators reflecting relative ratios of prescribing and for the ratio of broad- to narrow-spectrum antibiotics:
percentage prescription of penicillins sensitive to β-lactamase (J01CE_%)
percentage prescription of penicillin combinations including β-lactamase inhibitors (J01CR_%)
percentage prescription of third- and fourth-generation cephalosporins (J01DD+DE_%)
percentage prescription of fluoroquinolones (J01MA_%)
the ratio of consumption of broad-spectrum penicillins to narrow-spectrum penicillins, cephalosporins and macrolides (J01B/N_%)
This set of indicators was chosen because they are periodically published by ESAC5 and Portugal’s national authorities, allowing the interpretation of intervention effectiveness in a national and international context.
Statistical analysis
Yt is the outcome variable
Tt is time since the start of the study (months)
Xt is a dummy variable representing the intervention (pre-intervention = 0; post-intervention = 1)
XtTt, ZTt, ZXt and ZXtTt are the interaction terms
Z represents the group assignment (intervention = 1; control = 0)
β0 is the starting level of the outcome variable for the control group
β1 is the slope of the outcome variable until the intervention
β2 is the change in the outcome in the period immediately following the intervention
β3 is the difference between pre- and post-intervention slopes for the control group
β4 is the difference in the level (intercept) of the outcome between the intervention and control groups prior to the intervention
β5 is the difference in the level (trend) of the outcome between the intervention and control groups prior to the intervention
β6 indicates the difference between treatment and controls prior to the intervention
β7 is the difference in the level (trend) of the outcome between the intervention and control groups post- as compared with pre-intervention
et is the error estimation.
seasonality assumes different values according to the winter quarters (January–March and October–December) and summer quarters (April–June and July–September)
Based on Zhang et al.,30 we calculated the relative change brought about by the intervention, by combining immediate (level) and non-immediate (trend) changes into a single estimate. Relative change expressed as a percentage was also assessed. Relative change was calculated for the first month after the start of the intervention (T = intervention month + 1), exclusively including significant terms.27
Linear post-intervention trends in the intervention (β1Tt + β5ZTt + β3XtTt + β7ZXtTt) and control (β1Tt + β3XtTt) groups and the difference between them (β5ZTt + β7ZXtTt) were assessed,25 where:
β1Tt is the slope of the outcome variable until the intervention
β3XtTt is the difference between pre- and post-intervention slopes for the control group
β5ZTt is the difference in the level (trend) of the outcome between the intervention and control groups prior to the intervention
β7ZXtTt is the difference in the level (trend) of the outcome between the intervention and control groups post- as compared with pre-intervention
Results
The participation rate was 64% (197/309 GPs) in a total of 25 counties (100%). Figure 2 shows the process of randomization, allocation, participation, follow-up and analysis.

Impact on the quality of penicillin prescribing
In terms of the quality of penicillin prescribing, there were statistically significant improvements in the quality indicators evaluated in both the immediate (first month after the intervention) and the non-immediate (after the first month of the intervention) term (Table 1).
| Prescribing quality indicator . | Period . | Coefficient (β) . | 95% CI . | P . | Relative change . | Relative change (%) . |
|---|---|---|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | immediate effect | 1.142 | 0.842–1.442 | <0.001a | 9.963 | +896 |
| non-immediate effect | 0.034 | 0.005–0.062 | 0.023b | |||
| Penicillin combinations including β-lactamase inhibitors (% of total) | immediate effect | −5.220 | −9.223 to −1.216 | 0.011b | −0.608 | −161 |
| non-immediate effect | −0.494 | −0.879 to −0.110 | 0.012b | |||
| Third- and fourth-generation cephalosporins (% of total) | immediate effect | −0.459 | −0.785 to −0.132 | 0.006a | 0.002 | −100 |
| non-immediate effect | 0.028 | −0.003 to 0.060 | 0.077 | |||
| Fluoroquinolones (% of total) | immediate effect | −0.464 | −3.219 to 2.290 | 0.738 | 0.000 | −100 |
| non-immediate effect | 0.024 | −0.241 to 0.289 | 0.858 | |||
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | immediate effect | 2.300 | −0.213 to 4.814 | 0.072 | −1.000 | −200 |
| non-immediate effect | −0.454 | −0.695 to −0.212 | <0.001a |
| Prescribing quality indicator . | Period . | Coefficient (β) . | 95% CI . | P . | Relative change . | Relative change (%) . |
|---|---|---|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | immediate effect | 1.142 | 0.842–1.442 | <0.001a | 9.963 | +896 |
| non-immediate effect | 0.034 | 0.005–0.062 | 0.023b | |||
| Penicillin combinations including β-lactamase inhibitors (% of total) | immediate effect | −5.220 | −9.223 to −1.216 | 0.011b | −0.608 | −161 |
| non-immediate effect | −0.494 | −0.879 to −0.110 | 0.012b | |||
| Third- and fourth-generation cephalosporins (% of total) | immediate effect | −0.459 | −0.785 to −0.132 | 0.006a | 0.002 | −100 |
| non-immediate effect | 0.028 | −0.003 to 0.060 | 0.077 | |||
| Fluoroquinolones (% of total) | immediate effect | −0.464 | −3.219 to 2.290 | 0.738 | 0.000 | −100 |
| non-immediate effect | 0.024 | −0.241 to 0.289 | 0.858 | |||
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | immediate effect | 2.300 | −0.213 to 4.814 | 0.072 | −1.000 | −200 |
| non-immediate effect | −0.454 | −0.695 to −0.212 | <0.001a |
P < 0.01.
P < 0.05.
| Prescribing quality indicator . | Period . | Coefficient (β) . | 95% CI . | P . | Relative change . | Relative change (%) . |
|---|---|---|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | immediate effect | 1.142 | 0.842–1.442 | <0.001a | 9.963 | +896 |
| non-immediate effect | 0.034 | 0.005–0.062 | 0.023b | |||
| Penicillin combinations including β-lactamase inhibitors (% of total) | immediate effect | −5.220 | −9.223 to −1.216 | 0.011b | −0.608 | −161 |
| non-immediate effect | −0.494 | −0.879 to −0.110 | 0.012b | |||
| Third- and fourth-generation cephalosporins (% of total) | immediate effect | −0.459 | −0.785 to −0.132 | 0.006a | 0.002 | −100 |
| non-immediate effect | 0.028 | −0.003 to 0.060 | 0.077 | |||
| Fluoroquinolones (% of total) | immediate effect | −0.464 | −3.219 to 2.290 | 0.738 | 0.000 | −100 |
| non-immediate effect | 0.024 | −0.241 to 0.289 | 0.858 | |||
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | immediate effect | 2.300 | −0.213 to 4.814 | 0.072 | −1.000 | −200 |
| non-immediate effect | −0.454 | −0.695 to −0.212 | <0.001a |
| Prescribing quality indicator . | Period . | Coefficient (β) . | 95% CI . | P . | Relative change . | Relative change (%) . |
|---|---|---|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | immediate effect | 1.142 | 0.842–1.442 | <0.001a | 9.963 | +896 |
| non-immediate effect | 0.034 | 0.005–0.062 | 0.023b | |||
| Penicillin combinations including β-lactamase inhibitors (% of total) | immediate effect | −5.220 | −9.223 to −1.216 | 0.011b | −0.608 | −161 |
| non-immediate effect | −0.494 | −0.879 to −0.110 | 0.012b | |||
| Third- and fourth-generation cephalosporins (% of total) | immediate effect | −0.459 | −0.785 to −0.132 | 0.006a | 0.002 | −100 |
| non-immediate effect | 0.028 | −0.003 to 0.060 | 0.077 | |||
| Fluoroquinolones (% of total) | immediate effect | −0.464 | −3.219 to 2.290 | 0.738 | 0.000 | −100 |
| non-immediate effect | 0.024 | −0.241 to 0.289 | 0.858 | |||
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | immediate effect | 2.300 | −0.213 to 4.814 | 0.072 | −1.000 | −200 |
| non-immediate effect | −0.454 | −0.695 to −0.212 | <0.001a |
P < 0.01.
P < 0.05.
The effect (relative change) on the relative prescription of penicillins sensitive to β-lactamase was +896%, with an increase in the first month after the start of the intervention (P < 0.001) plus a statistically significant increase in trend (P = 0.023). Inversely, the prescription of penicillin combinations including β-lactamase inhibitors (percentage of total) displayed an immediate and non-immediate reduction effect, with an overall relative change of −161%.
Additionally, monthly post-intervention trends (Table 2) in the quality indicators showed that the difference (intervention minus control) was positive for the prescription of penicillins sensitive to β-lactamase and negative for the prescription of penicillin combinations.
| Prescribing quality indicator . | Control (coefficient) . | Intervention (coefficient) . | Difference . |
|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | 0.010 | 0.043 | 0.033 |
| Penicillin combinations including β-lactamase inhibitors (% of total) | 0.083 | −0.250 | −0.333 |
| Third- and fourth-generation cephalosporins (% of total) | 0.006 | 0.037 | 0.032 |
| Fluoroquinolones (% of total) | −0.072 | −0.049 | 0.023 |
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | 0.336 | −0.054 | −0.390 |
| Prescribing quality indicator . | Control (coefficient) . | Intervention (coefficient) . | Difference . |
|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | 0.010 | 0.043 | 0.033 |
| Penicillin combinations including β-lactamase inhibitors (% of total) | 0.083 | −0.250 | −0.333 |
| Third- and fourth-generation cephalosporins (% of total) | 0.006 | 0.037 | 0.032 |
| Fluoroquinolones (% of total) | −0.072 | −0.049 | 0.023 |
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | 0.336 | −0.054 | −0.390 |
| Prescribing quality indicator . | Control (coefficient) . | Intervention (coefficient) . | Difference . |
|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | 0.010 | 0.043 | 0.033 |
| Penicillin combinations including β-lactamase inhibitors (% of total) | 0.083 | −0.250 | −0.333 |
| Third- and fourth-generation cephalosporins (% of total) | 0.006 | 0.037 | 0.032 |
| Fluoroquinolones (% of total) | −0.072 | −0.049 | 0.023 |
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | 0.336 | −0.054 | −0.390 |
| Prescribing quality indicator . | Control (coefficient) . | Intervention (coefficient) . | Difference . |
|---|---|---|---|
| Penicillins sensitive to β-lactamase (% of total) | 0.010 | 0.043 | 0.033 |
| Penicillin combinations including β-lactamase inhibitors (% of total) | 0.083 | −0.250 | −0.333 |
| Third- and fourth-generation cephalosporins (% of total) | 0.006 | 0.037 | 0.032 |
| Fluoroquinolones (% of total) | −0.072 | −0.049 | 0.023 |
| Ratio of broad- to narrow-spectrum prescription of penicillins, cephalosporins and macrolides | 0.336 | −0.054 | −0.390 |
Impact on the quality of cephalosporin prescribing
Whereas a significant relative decrease was seen in the prescribing of third- and fourth-generation cephalosporins in the first month after the start of the intervention, no statistically significant results were observed in the non-immediate term (relative change of 0.002) (Table 1).
Impact on the quality of fluoroquinolone prescribing
No statistically significant effect was found for the relative prescription of fluoroquinolones (Table 1; P > 0.05 for both the immediate and the non-immediate effect). Monthly trends in the post-intervention period were negative for both the intervention and control groups (−0.049 and −0.072, respectively).
Impact on the quality of broad- to narrow-spectrum antibiotic prescribing
There was an estimated change of −200% in the ratio of broad- to narrow-spectrum antibiotics. Statistically significant results were only obtained for the non-immediate term effect (P < 0.001). As can be seen in Table 2, the difference of −0.390 in the post-intervention period indicates that the ratio decreased more in the intervention group than in the control group.
Discussion
The results reported here reflect the effectiveness of a joint multifaceted, multidisciplinary intervention on the appropriateness of antibiotic prescribing. Tackling antibiotic misprescribing behaviour is a necessary response that recognizes the importance of both reducing antibiotic overuse and improving the quality indicators of the antibiotics prescribed. With respect to misprescribing practices, our results indicate significant improvements in the values of quality indicators of penicillins, the most widely used class of antibiotics,31 and cephalosporins, and in the ratio of broad- to narrow-spectrum antibiotics, which all goes to reflect the improvement in prescription practices.
Making decisions about antibiotic prescribing is influenced by clinical, social and behavioural determinants.32,33 By combining the different elements pinpointed as underlying physicians’ antibiotic-prescribing behaviour (attitudes, knowledge and perceptions regarding antibiotic use, socio-demographic factors and communication issues), this intervention adds evidence on how to tackle this global concern in primary care. Furthermore, the use of a pragmatic randomized design combined with the inclusion of simulated physician–patient dialogues, a multidisciplinary and multifaceted approach and a high level of participation served to strengthen the results obtained.
Impact on the quality indicators of antibiotic prescribing
Improvements were obtained in the immediate and non-immediate term for the relative prescription of penicillins and broad- to narrow-spectrum antibiotics and in the immediate term for the prescription of cephalosporins. No impact was observed for fluoroquinolone prescribing or for changes in cephalosporin prescribing trends (non-immediate).
Firstly, the statistically significant results obtained for the reduction in the prescribing of penicillins with β-lactamase inhibitors, plus the increase in penicillins sensitive to β-lactamase, in both the immediate and non-immediate term, revealed a positive effect of the educational outreach visit on the appropriate use of this subclass of antibiotics. Penicillins are the most widely consumed subclass of antibiotics (∼50%)31 and using them wisely is fundamental in terms of preserving their effectiveness. The high estimated increase in the relative prescription of penicillins sensitive to β-lactamase (+896%) might be related to the fact that Portuguese rates for this indicator are much lower than the European mean (0.1% versus 5.29%).31
Secondly, in view of the fact that no statistically significant change in penicillin consumption was observed as a result of the intervention (−2.55%, P = 0.191; published elsewhere15), the increased prescription of penicillins sensitive to β-lactamase would suggest a behavioural change in antibiotic-prescribing patterns, characterized by the prescription of narrower- rather than wider-spectrum antibiotics, and the promotion and implementation of strategies, such as delayed prescribing, resulting in a lack of impact on overall antibiotic use.
Thirdly, the relative reduction in the prescription of penicillins with β-lactamase inhibitors, combined with the statistically significant reduction in the monthly ratio of broad- to narrow-spectrum antibiotics, also points to an extremely important resulting improvement in physicians’ prescribing behaviour. The intervention’s multifaceted design, i.e. combining clinical and social aspects of the prescribing process with a multidisciplinary approach that included physicians, pharmacists and educational materials for patients, may have had an impact on this ratio, which is of major importance in primary care.34
With regard to the quality indicators of cephalosporin prescribing, the intervention led to a statistically significant reduction in the first month after onset of the intervention but no changes in trend (non-immediate). To comprehend these results, it is essential to know that in the period from 2010 to 2015 (with the single exception of 2011), the relative prescribing rate of third- and fourth-generation cephalosporins in Portugal was lower than the average for European countries reporting to ESAC (in 2013, the year in which the intervention took place, this figure was 34.26% versus 38.25%).31
No changes were found in the relative prescription of fluoroquinolones. As the prescribing of these antibiotics in primary care is associated with the severity and duration of the disease and with the patient’s age,35 it might be more difficult for physicians to change their behaviour in such cases.
Impact on absolute rates of antibiotic use
This study did not seek to assess the impact on absolute rates of prescribing, since sales data are a better outcome for the purpose of measuring the global impact of an intervention on such indicators.36 In this regard, results of the effectiveness of the intervention as a whole (published elsewhere15) showed a significant reduction in overall antibiotic sales (−3.71%, P = 0.0459). One central feature of the intervention that might have had an impact on sales, though not prescription data, is the delayed prescribing strategy, whereby a patient is provided with a prescription but advised to delay using it in the expectation that the symptoms will resolve spontaneously.23 In line with the literature, delayed prescribing might have a substantial effect on antibiotic use (one-third of antibiotic prescribing when compared with immediate prescribing).37
Study strengths
The results reflect important changes in the behavioural aspects of antibiotic-prescribing patterns,20,22 together with the promotion of strategies such as delayed prescribing38 and communication skills,39 which have shown themselves to be effective in terms of improving antibiotic prescribing in primary care.
The effectiveness of the intervention might be related to its multidisciplinary, multifaceted approach, which: (i) included health professionals and patients; (ii) was designed to reflect physicians’ own practice;22 (iii) adopted different features (such as educational visits, information on regional and national data and discussion of possible improvement strategies); and (iv) addressed clinical and behavioural factors underlying the antibiotic prescribing process. As shown by the literature, social determinants are of pivotal importance in making decisions about antibiotic prescribing and behavioural approaches can be effective in primary care practice to reduce rates of use36,40 and improve the quality of antibiotic prescribing.41 Our results add to existing evidence of the impact on prescribing quality indicators, which is of the greatest importance in terms of improving antibiotic use in primary care.
Another important aspect of the intervention, and one that might have further contributed to its effectiveness, was the passive approach, focused on patients and implemented at outpatient centres and community pharmacies. This passive educational and awareness campaign, using posters and leaflets, might have had an influence on factors such as patient pressure to prescribe antibiotics, which has been referred to both in the literature20,42 and in previous research done on this population22 as having a significant impact on antibiotic prescribing.
Important methodological strengths of the study include its clustered design, which reduced cross-contamination, randomization, which avoided selection bias, and the use of a control arm, which served to eliminate biases such as seasonal variation, behavioural changes over time and external interventions, thus enabling causality between the intervention and the result measured to be assessed. Similarly important was the trial’s pragmatic design, ensuring intervention effectiveness, which might have been greater with a higher participation rate.
Insofar as analysis was concerned, an ITT analysis was performed. Bearing in mind that control clusters are much larger than intervention clusters, if contamination did in fact occur there would be a higher probability of this having an impact in the form of diminishing the effect, thus resulting in an underestimate rather than an overestimate.
Study limitations
Firstly, the study conclusions reported here are limited by the absence of diagnostic information, thereby impeding an in-depth evaluation. Since all antibiotics prescribed for systemic use were included in the analysis, this might have led to an underestimate of the intervention effect.
Secondly, the results presented did not reflect the impact of some intervention features, such as passive patient education or delayed prescribing, which might affect the consumption but not the prescription of antibiotics.
Thirdly, the analysis did not consider demographic or clinical differences in the populations attended by the physician, which, if present and different between study arms, might have had an impact on the final result.
Fourthly, the fact that the random distribution of the cluster to the intervention group and control group was unbalanced by the number of municipalities, health professionals and population could be considered a limitation. However, we believe this is not a limitation since this is a very similar situation to that of an unequal randomization,18,19 which forces the groups to have different sizes. Our group has carried out studies with a methodology similar to this in which the proportions of clusters in each group was 1:3.43,44 In cluster-randomized trials the data were adjusted for baseline values of the same variable so that only the changes are compared, not absolute values.45
Future perspectives
From a research perspective, conducting a longer-term follow-up analysis to assess the intervention’s effectiveness in terms of prescribing and resistance should be very useful in gaining an in-depth understanding of its real effect on clinical practice. The literature shows that these types of interventions can have a positive effect46,47 on antibiotic prescribing and use, a fact that must be considered when conducting clinical and economic evaluations of such interventions.
This research could also prove very useful in policy making. The study’s pragmatic design enhances its applicability as empirical support for future local, national and international initiatives aimed at tackling this global concern.
Improving the quality indicators of antibiotic prescribing is one of the main challenges facing efforts to halt antibiotic misuse. This study shows that these indicators can be improved by addressing knowledge and behavioural aspects surrounding physicians’ clinical practice. Accordingly, this intervention’s features and the results obtained should be considered when implementing countrywide or multi-country interventions. All the stakeholders, policy makers, health professionals, scientific societies and universities should be involved in the design and implementation of such interventions.
Acknowledgements
We would like to thank all the physicians who agreed to participate in this study and the following organizations that collaborated in the study: the Portuguese Central Regional Health Administration, the Official College of Physicians and the Portuguese Association of Family Medicine. Similarly, thanks must go to the Institute for Biomedicine/iBiMED (UID/BIM/04501/2013 - POCI-01-0145-FEDER-007628), which is supported by the Portuguese Foundation for Science and Technology (FCT/MCTES) through national funds (PIDDAC), and to FEDER for co-funding under the PT2020 Partnership Agreement.
Funding
This work was supported by a Foundation for Science and Technology (Fundação para a Ciência e Tecnologia/FCT) grant (PTDC/SAU-ESA/105530/2008) from the Portuguese Ministry of Science and Education, and co-financed by European Regional Development Fund (Fundo Europeu para o Desenvolvimento Regional/FEDER) through the Operational Competitiveness Programme (Programa Operacional Fatores de Competitividade/COMPETE Program).
Transparency declarations
None to declare.
Author contributions
A. Teixeira Rodrigues, F. Roque, A. Falcão, A. Figueiras and M. T. Herdeiro designed the study. A. Teixeira Rodrigues and F. Roque prepared the educational materials and the presentation, which were revised by all the authors. A. Teixeira Rodrigues led the physician educational outreach visit and F. Roque led the pharmacist educational outreach visit. A. Teixeira Rodrigues, M. Piñeiro-Lamas, A. Figueiras and M. T. Herdeiro analysed the data. A. Teixeira Rodrigues wrote the manuscript. All authors revised and approved the final version.
References
ECDC. Antimicrobial Consumption Database (ESAC-Net): Country Overview. https://ecdc.europa.eu/en/antimicrobial-consumption/database/country-overview.
ECDC. Summary of the Latest Data on Antibiotic Consumption in the European Union: 2016. https://ecdc.europa.eu/sites/portal/files/documents/antibiotics-ESAC-Net%20Summary%202016_0.pdf.
Programa de Prevenção e Controlo de Infeções e de Resistência aos Antimicrobianos. Prevenção e Controlo de Infeções e de Resistência aos Antimicrobianos em números – 2015. Direção-Geral da Saúde,
PORDATA. Resident Population, Estimates at December 31st. https://www.pordata.pt/en/Municipalities/Resident+population++estimates+at+December+31st-120.
ECDC. Antimicrobial Consumption Database (ESAC-Net): Quality Indicators for Antibiotic Consumption in the Community. https://ecdc.europa.eu/en/antimicrobial-consumption/database/quality-indicators.
John M. Eisenberg Center for Clinical Decisions and Communications Science. Interventions to improve antibiotic prescribing for uncomplicated acute respiratory tract infections, 6 April 2016. In:



