-
PDF
- Split View
-
Views
-
Cite
Cite
Masahiro Abe, Shigeki Nakamura, Yuki Kinjo, Yuka Masuyama, Junichi Mitsuyama, Mitsuo Kaku, Yoshitsugu Miyazaki, Efficacy of T-2307, a novel arylamidine, against ocular complications of disseminated candidiasis in mice, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 5, May 2019, Pages 1327–1332, https://doi.org/10.1093/jac/dkz020
Close - Share Icon Share
Abstract
T-2307, a novel arylamidine, shows broad-spectrum activity against pathogenic fungi, including Candida albicans. Ocular candidiasis is one of the serious complications associated with Candida bloodstream infection and is known to be refractory to conventional antifungal agents. The aim of the present study was to clarify the effectiveness of T-2307 against ocular candidiasis using a mouse model.
We evaluated ocular fungal burden in mice infected with C. albicans that received treatment with antifungal agents [T-2307, liposomal amphotericin B (LAMB) or fluconazole] for 3 consecutive days. We also assessed survival rates of mice after C. albicans infection followed by treatment for 7 consecutive days. In addition, ocular T-2307 concentrations and in vitro effectiveness against C. albicans biofilm formation were evaluated.
The ocular fungal burdens were significantly reduced after T-2307 treatment compared with the control group (no treatment received) and were comparable with those observed following treatment with LAMB or fluconazole in both early- and late-phase treatment experiments. In addition, all of the mice treated with antifungal agents survived for 3 weeks after infection, whereas mice in the control group died within 3 days. The ocular T-2307 trough concentration was maintained above the MIC in the infected mice. An in vitro biofilm inhibition experiment showed that T-2307 suppressed C. albicans biofilm formation at the sub-MIC level, which was comparable with amphotericin B.
Given these results in experimental disseminated candidiasis, T-2307 may be an effective treatment against the complication of ocular candidiasis.
Introduction
Candida species are among the most common causative pathogens of bloodstream infections in hospitals and ocular candidiasis is a potentially severe complication that may lead to visual field defects or blindness if appropriate therapy is delayed.1,2 Previously, it has been shown that all Candida species may cause ocular complications and that ocular candidiasis occurs in ∼10%–25% of Candida bloodstream infections.3,4 Ocular candidiasis is known to be refractory to conventional antifungal drugs because of their poor ocular permeability, although the guidelines of the IDSA recommend that the appropriate antifungal drugs, especially triazoles, should be administered after the diagnosis.5
T-2307 is a novel arylamidine developed by Toyama Chemical Co., Ltd (Tokyo, Japan) that exhibits broad-spectrum antifungal activity against the majority of pathogenic fungi in vitro and in vivo.6–9 This drug selectively disrupts yeast mitochondrial function, which is a different antifungal mechanism compared with conventional drugs.10 Furthermore, MICs of T-2307 have been shown to be remarkably low, especially for yeast-like fungi such as Candida species, implying that this drug may be effective against ocular candidiasis, even though the ocular permeability of T-2307 is low. This study evaluates the efficacy of T-2307 against Candida albicans systemic infections and ocular candidiasis in comparison with that of conventional antifungal agents, fluconazole and liposomal amphotericin B (LAMB).
Materials and methods
Mice
Female C57BL/6J mice, 7–8 weeks old, were purchased from Japan SLC, Inc. (Shizuoka, Japan) and maintained under specific pathogen-free conditions at the National Institute of Infectious Diseases in Japan. All experiments were reviewed and approved by the Animal Care and Use Committee of the National Institute of Infectious Diseases. Protocols were designed to minimize animal suffering and limit the number used for the experiments (approval number: 117050). In each experiment, we used five or six mice for each antifungal treatment or no-treatment control group.
Yeast strain and infection models
A previously described ocular candidiasis mouse model was used with a minor modification.11 A reference strain of C. albicans (SC5314) was used to cause ocular candidiasis. C. albicans was grown on yeast extract peptone dextrose (YPD) agar at 30°C for 2 days, then in YPD broth at 30°C for 18–24 h. After incubation, yeasts were collected using sterile loops, washed with sterile PBS and resuspended in sterile PBS at the appropriate concentrations mentioned below. For the organ fungal burden evaluation experiments, a suspension of C. albicans was prepared at ∼5.0 × 106 cfu/mL; for the survival analysis experiments, a suspension was prepared at ∼3.0 × 105 cfu/mL. Mice were injected with 200 μL via the lateral tail vein to cause Candida dissemination.
Antifungal agents and treatment schedules
T-2307 was kindly provided by Toyama Chemical Co., Ltd. LAMB and fluconazole were purchased from Dainippon Sumitomo Pharma (Tokyo, Japan) and Sigma–Aldrich (St Louis, MO, USA), respectively. T-2307 was dissolved in sterile 0.9% sodium chloride and mice were injected subcutaneously with 100 μL of the resulting solution. LAMB was first dissolved in sterile water and then diluted in a 5% glucose solution and mice were injected intravenously with 200 μL of the resulting solution via the lateral tail vein. Fluconazole was first dissolved in DMSO and then suspended in sterile 0.9% sodium chloride and each mouse received 100 μL intragastrically. The dose of T-2307, LAMB or fluconazole administered per mouse was 4, 3 or 75 mg/kg, respectively. In the control groups, each mouse was subcutaneously injected with 100 μL of sterile 0.9% sodium chloride. Treatment administration began 2 h after infection in the early-phase treatment experiments and 12 h after infection in the late-phase treatment experiments. In each experiment, antifungal agents were administered once daily for 3 consecutive days. For survival analysis, these antifungal treatments began 2 h after infection and were administered once daily for 7 consecutive days.
Evaluation of organ fungal burden
Three days after infection, the mice were euthanized and the kidneys and eyeballs were collected aseptically, homogenized in sterile PBS and the homogenates of the kidneys were serially diluted using sterile PBS. Kidney homogenate was plated on YPD agar and eyeball homogenate on YPD agar with added antibiotics (penicillin/streptomycin), both at 100 μL per plate. The fungal burden was determined by counting cfu after incubation for 2 days at 30°C.
Antifungal drug susceptibility tests
The susceptibility of the reference strain of C. albicans (SC5314) to amphotericin B and fluconazole was determined using a commercial Frozen Plate Kit (Eiken Chemical Co., Ltd, Tokyo, Japan). This Frozen Plate Kit was based on microdilution methods and the results were interpreted according to the CLSI method M27-A4. The MIC of fluconazole was scored as the IC50 and that for amphotericin B was scored as the lowest drug concentration that resulted in complete growth inhibition. Quality control was performed using Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019. The drug susceptibility test using T-2307 was performed using broth microdilution methods in accordance with CLSI method M27-A4. The MIC of T-2307 was scored as the IC50.
Evaluation of ocular T-2307 concentration
For infection of mice, C. albicans was prepared at ∼5.0 × 106 cfu/mL and 200 μL was injected via the lateral tail vein to cause Candida dissemination. For uninfected control mice, 200 μL of sterile PBS was similarly injected. Two hours after injection, 4 mg/kg T-2307 was subcutaneously injected into each mouse and these mice were euthanized 24 h after treatment, followed by aseptic collection of the eyeballs. The T-2307 concentration in the eyeballs was measured by LC-tandem MS in electrospray positive-ionization mode on an AB Sciex API 5000 triple-quadrupole mass spectrometer combined with an ACQUITY UPLC using the Analyst software and multiple-reaction monitoring (MRM) of precursor/product transitions. The MRM transitions used were 438.2/302.2 for T-2307 and 452.2/316.2 for the internal T-23318 standard. Chromatography was performed using an ACQUITY UPLC BEH C18 Column (1.7 μm, 2.1 mm × 50 mm, Waters) using a reverse-phase gradient elution. The aqueous mobile phase was composed of 0.2% formic acid/5% acetonitrile (MeCN)/5 mmol/L ammonium acetate/distilled water and the organic mobile phase was composed of 0.2% formic acid/95% acetonitrile/5 mmol/L ammonium acetate/distilled water. The eyeballs were homogenized prior to extraction by combining 19 parts MeCN containing 1.3% trichloroacetic acid in methanol to 1 part eyeball homogenate. One milligram per millilitre 50% MeCN stock was serially diluted in 50% MeCN containing 0.1% formic acid to create standard curves and quality control spiking solutions. Ten microlitres of neat spiking solutions was added to 200 μL of eyeball homogenate and extraction was performed by adding protein precipitation solvent containing 100 ng/mL T-23318. Extracts were loaded onto protein precipitation plates and analysed via LC-tandem MS. Sample analysis was accepted if the concentrations of the quality control samples were within 20% of the nominal concentration.
Biofilm inhibition assay
The reference strain of C. albicans (SC5314) was grown on YPD agar at 30°C for 2 days, then in YPD broth at 30°C for 18–24 h. After incubation, yeasts were collected using sterile loops, washed with sterile PBS, resuspended in YPD broth and diluted to 1.0 × 107 cells/mL. First, 100 μL of this inoculum was added to each well of a 96-well plate and incubated for 4 h to allow the cells to adhere to the plastic surface, as previously described.12,13 In negative control wells, 100 μL of YPD broth was added. After the adhesion period, each well was washed three times with 200 μL sterile PBS to remove planktonic C. albicans cells. After washing, ¼× MIC of each of the antifungal drugs in 100 μL YPD broth was added to each well. YPD broth without any antifungal drugs was added to the positive and negative control wells. The plate was then incubated for 24 h to allow C. albicans to form a biofilm. After 24 h of incubation, the supernatant in each well was carefully removed so as not to disturb the biofilms and 100 μL of methanol was added to fix the biofilm. After fixation, each well was washed three times with PBS and stained with 0.1% crystal violet for 60 min. After crystal violet staining, each well was washed several times with PBS. Finally, crystal violet bound to the C. albicans biofilms was dissolved with 200 μL of methanol and OD595 was measured using a microtitre plate reader.
Statistical analysis
Continuous variables were compared between groups with equal variance using analysis of variance, followed by Tukey–Kramer honestly significant difference post hoc tests for pair-wise comparisons. If the standard deviations differed between groups, means were compared using Kruskal–Wallis tests and Dunn’s post hoc tests for pair-wise comparisons. All P values <0.05 were considered significantly different. GraphPad Prism, version 7 (GraphPad Software), was used for all analyses.
Results
Organ fungal burden analysis
To investigate the efficacy of T-2307, we performed in vivo experiments using our experimental C. albicans ocular candidiasis mouse model and compared the performance of T-2307 with that of other antifungal agents. The MICs of the antifungal agents for the C. albicans reference strain were as follows: T-2307, 0.001 mg/L; amphotericin B, 1 mg/L; and fluconazole, 0.5 mg/L. In the early-phase treatment experiments, the ocular fungal burdens of the mice were significantly reduced after treatment with T-2307 compared with mice in the no-treatment control group (Figure 1a). In addition, there were no significant differences in the ocular fungal burdens between the T-2307, LAMB and fluconazole treatment groups. The fungal burden of the kidneys was significantly reduced in the T-2307 treatment group compared with the no-treatment control group (Figure 1b). There were no significant differences between the T-2307 and fluconazole treatment groups, although the fungal burden in the kidneys was significantly reduced in the LAMB treatment group.
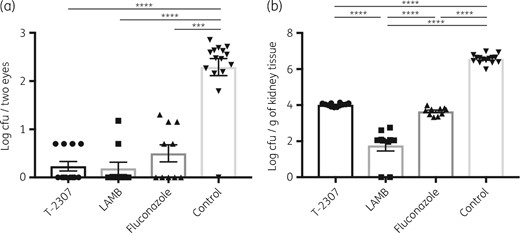
Fungal burden in the eyes (a) and the kidneys (b) of mice after infection with C. albicans and antifungal agent treatments in the early-phase treatment experiments. Ocular and kidney fungal burden is shown as log cfu/two eyes and log cfu/g of kidney tissue, respectively. All results are expressed as mean ± SEM and data were pooled from three independent experiments (T-2307 group, n = 12; LAMB group, n = 10; fluconazole group, n = 10; and control group, n = 15). ***P < 0.001 and ****P < 0.0001.
In addition, we performed late-phase treatment experiments to evaluate whether the efficacy of T-2307 is owing to drug penetration and control of the fungus in the eyes or to control of the fungus in the bloodstream. Even in the late-phase treatment experiments, the ocular fungal burden was significantly reduced after treatment with T-2307 compared with mice in the no-treatment control group and there were no significant differences in the ocular fungal burden between T-2307, LAMB and fluconazole treatment groups (Figure 2a), which was similar to the results of the early-phase treatment experiments. The fungal burden of the kidneys was also significantly reduced in the T-2307 treatment group compared with the no-treatment control group (Figure 2b) and was also significantly reduced in the LAMB treatment group. These results suggested that T-2307 is similar in potency to the other antifungal agents in ocular candidiasis and that the decline in ocular fungal burden was caused by penetration of T-2307 into the eyes, not from control of fungal load in the bloodstream.
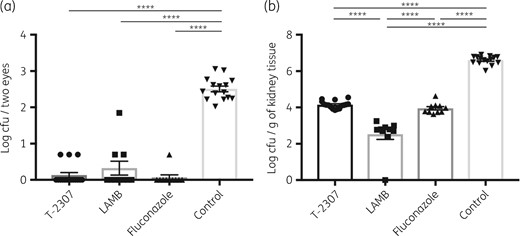
Fungal burden in the eyes (a) and the kidneys (b) of mice after infection with C. albicans and antifungal agent treatments in the late-phase treatment experiments. Ocular and kidney fungal burden is shown as log cfu/two eyes and log cfu/g of kidney tissue, respectively. All results are expressed as mean ± SEM and data were pooled from three independent experiments (T-2307 group, n = 16; LAMB group, n = 10; fluconazole group, n = 10; and control group, n = 15). ****P < 0.0001.
Survival analysis
To evaluate the efficacy of T-2307 against disseminated candidiasis, we compared the survival rates of untreated mice with those of T-2307-, LAMB- or fluconazole-treated mice. In this analysis, all of the untreated mice died within 3 days after C. albicans injection, whereas T-2307-, LAMB- or fluconazole-treated mice survived for 3 weeks after injection (Figure 3). We also assessed weight loss in each treatment group, which showed transient body weight loss after infection (average maximum weight loss: 7.8%, 3.0% and 5.0% in the T-2307, LAMB and fluconazole treatment groups, respectively). However, the body weights of the mice in the T-2307 treatment group recovered within 7 days after infection and none of the treated mice showed apparent weight loss after discontinuation of treatment. These results showed that T-2307 could be used for treatment against lethal disseminated candidiasis.
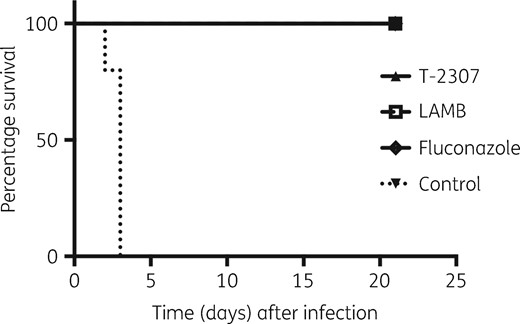
Survival rates of untreated and T-2307-, LAMB- or fluconazole-treated mice after infection. T-2307 group, n = 6; LAMB group, n = 5; fluconazole group, n = 5; and no-treatment control group, n = 5.
Measurement of ocular T-2307 concentration
Given the in vivo efficacy of T-2307 against C. albicans systemic infection, we measured the ocular trough concentration of T-2307, comparing the concentrations between infected mice and uninfected mice. Ocular T-2307 concentration was maintained 24 h after administration (Figure 4). In addition, the ocular concentration was significantly higher in the infected mice compared with that in the uninfected group [infected group versus uninfected group: 270.6 ± 8.744 ng/g versus 245.2 ± 6.208 ng/g (24 h); n = 5 for each group; P = 0.045]. This result demonstrated that T-2307 could have an extended inhibitory effect on C. albicans, especially in the eyes under inflammatory conditions, indicating that T-2307 may be a useful antifungal agent against ocular candidiasis.
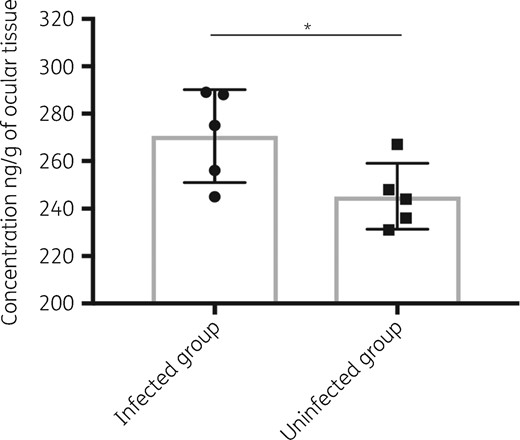
Ocular T-2307 trough concentration. Ocular T-2307 concentration is shown as ng/g of ocular tissue. All results are expressed as mean ± SD (n = 5 for each group). *P < 0.05.
In vitro efficacy of T-2307 against the formation of C. albicans biofilm
Based on the results of the in vivo experiments and ocular drug concentrations, we investigated the effects of T-2307 on the formation of C. albicans biofilms. We compared the efficacy of T-2307 with that of amphotericin B and fluconazole at the ¼× MIC level. As shown in Figure 5, T-2307 and amphotericin B suppressed biofilm formation to a similar extent. This inhibitory effect on biofilm formation could lead not only to suppression of cellular adhesion to medical devices, such as catheters, but also to the prevention of chronic infection because of clearance effects against Candida species.
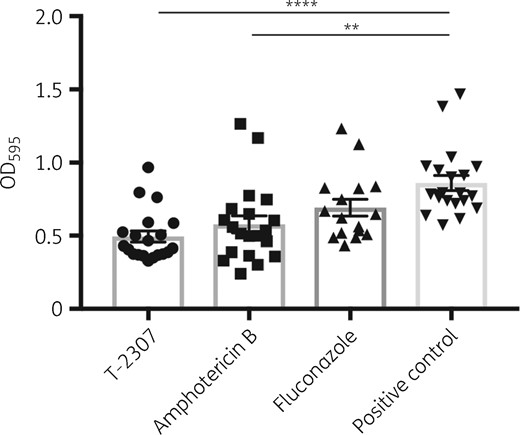
C. albicans biofilm stained with crystal violet. Biofilm values are shown as OD595. All results are expressed as mean ± SEM and data were pooled from three independent experiments (T-2307 group, n = 20; amphotericin B group, n = 20; no-treatment control group, n = 20; and fluconazole group, n = 16). **P < 0.01 and ****P < 0.0001.
Discussion
Our investigations demonstrated that T-2307 was effective against C. albicans systemic infection and suppressed ocular C. albicans growth. The fungal burden in both the eyeballs and kidneys was drastically reduced in mice following T-2307 treatment compared with those receiving no treatment in both early- and late-phase treatment experiments. Ocular candidiasis is known to be refractory to conventional antifungal agents, as these drugs have poor ocular permeability. Although the IDSA guidelines recommend that echinocandins should be used as the first-line agent against candidaemia, they are not recommended in cases of ocular candidiasis because of poor intraocular penetration.5,14 Fluconazole and voriconazole have been reported to have better intraocular penetration rates and could therefore be used in cases of ocular candidiasis.15–17 However, azole-resistant Candida species may also cause ocular candidiasis, in which case these triazoles may not be effective. In addition, LAMB may also be used for treatment against ocular candidiasis, although long-term treatment is not recommended because of its toxicity. On the other hand, it was previously shown that T-2307 exhibited potent activity against non-albicans Candida and fluconazole-resistant C. albicans.6 Our experiments showed that intraocular trough concentrations of T-2307 could be maintained above the MIC level and its ocular permeability may be elevated under inflammatory conditions, resulting in T-2307-suppressed intraocular fungal growth. Given these results, T-2307 may be a new therapeutic agent against systemic candidiasis, especially ocular candidiasis.
In organ fungal burden analysis, the kidney fungal burden of mice treated with LAMB was significantly less than for those treated with T-2307 or fluconazole. This result may be derived from differences in the mechanism of action between T-2307, LAMB and fluconazole. LAMB is known to be fungicidal against C. albicans, whereas fluconazole usually has fungistatic activity.18 According to our results, T-2307 may have fungistatic activity comparable with fluconazole. Although fungal burden in the kidneys was significantly different between treatment groups, all of the mice in the treatment groups had good overall conditions and survived for 3 weeks. Given these results, T-2307 could suppress fungal infection in a fungistatic manner and treat C. albicans systemic infection regardless of the kidney fungal burden.
T-2307 suppressed the formation of C. albicans biofilms, even though the drug was added after the adhesion period. Amphotericin B has been shown to be effective against C. albicans biofilms;19,20 the effect of T-2307 on C. albicans biofilm formation was similar to that of amphotericin B and both drugs significantly suppressed biofilm formation compared with the negative control. T-2307 effectively suppressed biofilm formation at the sub-MIC level and even the ocular T-2307 trough concentrations were above the MIC level, indicating that T-2307 could have extended inhibitory effects after administration because of its extremely low MIC for C. albicans.
The present study encountered a number of limitations. First, we presented only a series of results obtained using a reference strain of C. albicans. However, we also evaluated the ocular and kidney fungal burden using a clinical isolate of C. albicans, which showed results similar to those of the reference strain (data not shown). Therefore, the experimental results using the reference strain may represent those obtained using clinical isolated strains. Second, histopathological analysis was not conducted in our mouse model. However, the ocular fungal burden was significantly reduced after T-2307 treatment and, according to our previous investigations, ocular inflammation was not detected if ocular fungal loads were low.11 Hence, it is possible that ocular candidiasis may not be detected in antifungal agent-treated mice histopathologically.
In summary, to the best of our knowledge, this is the first report describing the effectiveness of T-2307 against ocular candidiasis using a mouse model. T-2307 suppressed the growth of C. albicans in murine eyes in both the early- and late-phase treatment experiments and was also effective against disseminated candidiasis. T-2307 has an extremely low MIC for C. albicans and the ocular concentration can be maintained above the MIC for an extended period following administration, even though ocular drug penetration is generally poor. Our investigations indicate that T-2307 may be a useful candidate against ocular candidiasis.
Funding
This work was supported in part by the Research Program on Emerging and Re-emerging Infectious Diseases of the Japan Agency for Medical Research and Development (AMED) (grant numbers JP18fk0108045, JP18fk0108008, JP18fk0108049) and the Japan Agency for Scientific Research (grant numbers 17K10040, 66K09939, 67K10030) of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Transparency declarations
Y. M. is an employee of FUJIFILM Corporation and J. M. is an employee of Toyama Chemical Co., Ltd. No authors own stock or options in the company. All other authors: none to declare.



