-
PDF
- Split View
-
Views
-
Cite
Cite
Kévin Alexandre, Anaïs Soares, Françoise Chau, Bruno Fantin, François Caron, Manuel Etienne, Temocillin breakpoints in pyelonephritis: evaluation in a murine model due to ESBL-producing Escherichia coli clinical isolates, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 5, May 2019, Pages 1323–1326, https://doi.org/10.1093/jac/dky569
Close - Share Icon Share
Abstract
Due to a spectrum restricted to Enterobacteriaceae and stability against ESBL and AmpC enzymes, temocillin is of major interest for the treatment of pyelonephritis. But there are still uncertainties about the optimal regimen and clinical breakpoints.
To study in a murine model of pyelonephritis the activity of temocillin against Escherichia coli isolates with different MICs in order to evaluate clinical breakpoints.
Four clinical uropathogenic E. coli isolates with temocillin MICs of 8 mg/L (Ec8), 16 mg/L (Ec16), 32 mg/L (Ec32) and 64 mg/L (Ec64) were evaluated. Antibiotic 24 h T>MIC achieved in humans was reproduced in mice with either intravenous temocillin (2 g q12h or 2 g q8h) or intravenous imipenem (1 g q8h). Efficacy was assessed by bacterial count in kidneys.
Compared with controls, temocillin at 2 g q12h was highly efficient against Ec8 (−3.32 log10 cfu/g and negative cultures in 93% of mice; P < 0.001); imipenem gave similar results. Temocillin at 2 g q12h also induced high reduction of bacterial count against Ec16 (−2.92 log10 cfu/g; P < 0.001), albeit cultures were negative in only 48% of mice. In contrast, no significant effect was observed in mice infected by Ec32 (−0.01 log10 cfu/g; P = 0.981) or Ec64 (−0.55 log10 cfu/g; P = 0.523). Even temocillin at 2 g q8h failed to control Ec32 infection (−1.55 log10 cfu/g; P = 0.197).
This model suggests a clinical breakpoint up to 16 mg/L for non-severe pyelonephritis treated with temocillin at 2 g q12h, a value consistent with the few previous available data.
Introduction
Urinary tract infections (UTIs) are the most common human infections due to ESBL-producing Escherichia coli. Temocillin has emerged as an attractive carbapenem-sparing option because of a spectrum mainly restricted to Enterobacteriaceae, a stability against numerous β-lactamases, including ESBL and AmpC enzymes, and minimal risk of Clostridium difficile infection.1
Debate is, however, still ongoing about the optimal temocillin therapeutic schedule. Current recommended regimens are 2 g q12h or 2 g q8h depending on infection severity and strain susceptibility.1,2
As a consequence, there is still no international consensus regarding temocillin breakpoints, with three different values for susceptible isolates, i.e. MIC ≤8, ≤16 and ≤32 mg/L, depending on the country.3–5
Two previous animal studies have suggested that a humanized temocillin regimen of 2 g q12h retains activity against E. coli strains with MICs up to 16 mg/L.6,7 Both studies used genetically modified bacteria. One study used a unique isolate with a temocillin MIC of 8 mg/L, the results being derived from pharmacokinetic calculations. The other study was not conducted in UTI, hence antibiotic concentrations at the site of infection might differ notably. Therefore, we aimed to further study in a murine model of pyelonephritis the in vivo activity of temocillin against different ESBL-producing E. coli clinical isolates, in order to contribute to better definition of clinical breakpoints for systemic UTI.
Materials and methods
Bacterial strains
Four clinical uropathogenic E. coli isolates belonging to phylogroup B2 (i.e. the most urovirulent phylogroup)8 were selected. All produced the most common ESBL currently encountered (i.e. CTX-M-15)9 with a minimal fitness cost estimated by a maximum growth rate (MGR) ≥1.4 h−1 (calculated as previously described).10 Their temocillin MICs (determined by the CLSI-approved broth microdilution method in triplicate) were 8, 16, 32 and 64 mg/L. They were named Ec8, Ec16, Ec32 and Ec64, respectively, according to their temocillin MIC.
Murine model of pyelonephritis
Experiments were approved by the national ethics committees (agreement number 05214.02). All invasive procedures were performed under anaesthesia. Animals were housed in regulation cages (five animals per cage) with free access to food and water. Pyelonephritis was induced in 214 immunocompetent female CBA mice (8 weeks old, weight 20–23 g) as previously described, with several modifications.11 Briefly, at day (D) 3 post-inoculation (PI) urine samples were collected to screen uninfected animals. D3-PI urine culture was performed to predict positive kidney culture at D4-PI in 28 mice, for which sensitivity and specificity were 86% and 79%, respectively. For each strain, mice with positive D3-PI urine culture were randomly assigned as follows: ≥7 were sacrificed at D4-PI (start of treatment), ≥7 were left untreated and sacrificed at D6-PI (controls), and ≥15 were treated for 24 h starting at D4-PI with temocillin (200 mg/kg q2h or q6h) or (only for Ec8) with imipenem (100 mg/kg q2h).
The pharmacokinetic/pharmacodynamic parameter the most predictive of efficacy for temocillin and imipenem is free-drug T>MIC (fT>MIC). Hence, fT>MIC values reproduced in mice were those obtained in humans (Table 1). Since pharmacokinetic parameters of temocillin differ between humans (Cmax = 147 mg/L, t½ = 4.3 h and protein binding = 76% after 2 g intravenously)12 and mice (Cmax=199 mg/L, t½ = 0.5 h and protein binding = 16% after 200 mg subcutaneously),6 therapeutic regimens were subsequently adapted in mice. Due to ethical concerns (no more than 12 injections per animal) the human regimens of 2 g q8h against Ec8 and Ec16 were not reproduced in mice. To avoid a carry-over effect, treated mice were sacrificed 24 h after the last dose of antibiotic. Kidneys were aseptically removed and homogenized in 1 mL of saline. Dilutions of the solution were spread onto LB agar plates without antibiotic or with temocillin (to detect mutants resistant to 4-fold MIC) and incubated for 72 h. The detection threshold was 1 log10 cfu/mL. Results are the median (IQR) for continuous variables. The statistical tests realized with R software (www.r-project.org/) were Mood’s test for MGR values, Mann–Whitney or Kruskal–Wallis tests for continuous variables and Fisher’s exact test for the proportion of sterile kidneys. A P value <0.05 was considered to be significant.
Bacterial count reduction and sterility rate in kidneys according to simulated human antibiotic regimen and strain MIC
| . | Antibiotic . | |||||
|---|---|---|---|---|---|---|
imipenem | temocillin | |||||
| Strain MIC (mg/L) | 0.125 | 8 | 16 | 32 | 64 | |
| Simulated human regimen (%fT>MIC) | 1 g q8h (100%) | 2 g q12h (80%) | 2 g q12h (45%) | 2 g q12h (10%) | 2 g q8h (36%) | 2 g q12h (0%) |
| Mouse regimen (%fT>MIC) | 100 mg/kg q2h (100%) | 200 mg/kg q2h (70%) | 200 mg/kg q2h (58%) | 200 mg/kg q6h (14%) | 200 mg/kg q2h (43%) | 200 mg/kg q2h (10%) |
| Bacterial count reduction versus controls | ||||||
| median | 3.35 | 3.32a | 2.92a | 0.01a | 1.55a | 0.55a |
| IQR | 0.04 | 0.04 | 1.31 | 2.23 | 2.10 | 2.76 |
| P | <0.001 | <0.001 | 0.002 | 0.981 | 0.197 | 0.523 |
| No. sterilized/total no. (%) | 14/15 (93%) | 14/15 (93%)a | 11/23 (48%)a | 3/18 (17%)a | 3/15 (20%)a | 4/16 (25%)a |
| . | Antibiotic . | |||||
|---|---|---|---|---|---|---|
imipenem | temocillin | |||||
| Strain MIC (mg/L) | 0.125 | 8 | 16 | 32 | 64 | |
| Simulated human regimen (%fT>MIC) | 1 g q8h (100%) | 2 g q12h (80%) | 2 g q12h (45%) | 2 g q12h (10%) | 2 g q8h (36%) | 2 g q12h (0%) |
| Mouse regimen (%fT>MIC) | 100 mg/kg q2h (100%) | 200 mg/kg q2h (70%) | 200 mg/kg q2h (58%) | 200 mg/kg q6h (14%) | 200 mg/kg q2h (43%) | 200 mg/kg q2h (10%) |
| Bacterial count reduction versus controls | ||||||
| median | 3.35 | 3.32a | 2.92a | 0.01a | 1.55a | 0.55a |
| IQR | 0.04 | 0.04 | 1.31 | 2.23 | 2.10 | 2.76 |
| P | <0.001 | <0.001 | 0.002 | 0.981 | 0.197 | 0.523 |
| No. sterilized/total no. (%) | 14/15 (93%) | 14/15 (93%)a | 11/23 (48%)a | 3/18 (17%)a | 3/15 (20%)a | 4/16 (25%)a |
P < 0.05 between strain with temocillin MIC of 8 mg/L treated with a regimen simulating 2 g q12h and other groups.
Bacterial count reduction and sterility rate in kidneys according to simulated human antibiotic regimen and strain MIC
| . | Antibiotic . | |||||
|---|---|---|---|---|---|---|
imipenem | temocillin | |||||
| Strain MIC (mg/L) | 0.125 | 8 | 16 | 32 | 64 | |
| Simulated human regimen (%fT>MIC) | 1 g q8h (100%) | 2 g q12h (80%) | 2 g q12h (45%) | 2 g q12h (10%) | 2 g q8h (36%) | 2 g q12h (0%) |
| Mouse regimen (%fT>MIC) | 100 mg/kg q2h (100%) | 200 mg/kg q2h (70%) | 200 mg/kg q2h (58%) | 200 mg/kg q6h (14%) | 200 mg/kg q2h (43%) | 200 mg/kg q2h (10%) |
| Bacterial count reduction versus controls | ||||||
| median | 3.35 | 3.32a | 2.92a | 0.01a | 1.55a | 0.55a |
| IQR | 0.04 | 0.04 | 1.31 | 2.23 | 2.10 | 2.76 |
| P | <0.001 | <0.001 | 0.002 | 0.981 | 0.197 | 0.523 |
| No. sterilized/total no. (%) | 14/15 (93%) | 14/15 (93%)a | 11/23 (48%)a | 3/18 (17%)a | 3/15 (20%)a | 4/16 (25%)a |
| . | Antibiotic . | |||||
|---|---|---|---|---|---|---|
imipenem | temocillin | |||||
| Strain MIC (mg/L) | 0.125 | 8 | 16 | 32 | 64 | |
| Simulated human regimen (%fT>MIC) | 1 g q8h (100%) | 2 g q12h (80%) | 2 g q12h (45%) | 2 g q12h (10%) | 2 g q8h (36%) | 2 g q12h (0%) |
| Mouse regimen (%fT>MIC) | 100 mg/kg q2h (100%) | 200 mg/kg q2h (70%) | 200 mg/kg q2h (58%) | 200 mg/kg q6h (14%) | 200 mg/kg q2h (43%) | 200 mg/kg q2h (10%) |
| Bacterial count reduction versus controls | ||||||
| median | 3.35 | 3.32a | 2.92a | 0.01a | 1.55a | 0.55a |
| IQR | 0.04 | 0.04 | 1.31 | 2.23 | 2.10 | 2.76 |
| P | <0.001 | <0.001 | 0.002 | 0.981 | 0.197 | 0.523 |
| No. sterilized/total no. (%) | 14/15 (93%) | 14/15 (93%)a | 11/23 (48%)a | 3/18 (17%)a | 3/15 (20%)a | 4/16 (25%)a |
P < 0.05 between strain with temocillin MIC of 8 mg/L treated with a regimen simulating 2 g q12h and other groups.
Results
The MIC of imipenem was 0.125 mg/L for the four studied isolates. The MGRs were 1.99, 1.58, 1.87 and 1.89 h−1 for Ec8, Ec16, Ec32 and Ec64, respectively. The MGR was lower for Ec16 than for Ec64 (P < 0.001). Median bacterial counts in kidneys in untreated animals were ∼5 log10 cfu/g and were stable from D4-PI to D6-PI whatever the strain used (data not shown). No important adverse event was noted during the experimental procedure.
Table 1 shows the bacterial count reduction and the sterilization rate in kidneys for each strain, according to the antibiotic regimen, the fT>MIC achieved in mice and the corresponding parameters in humans. Compared with controls, simulating temocillin at 2 g q12h in humans produced a bacterial reduction ≥2.9 log10 cfu/g after 24 h in kidneys of mice infected by Ec8 and Ec16, whereas no difference was observed in mice infected by Ec32 and Ec64 (Figure 1). Significant differences in the sterility rate of kidneys and in bacterial count reduction were noted between Ec8 and the three other isolates (P < 0.05), but not in other comparisons (P > 0.05) (Table 1). A regimen simulating temocillin at 2 g q8h in mice infected by Ec32 (Figure 1) resulted in a 1.55 log10 cfu/g bacterial reduction that did not differ significantly from controls (P = 0.197), similarly to a regimen simulating temocillin at 2 g q12h (P = 0.155). The kidney sterility rate after a regimen simulating temocillin at 2 g q8h against Ec32 was 20% (3/15), not significantly different from the 17% rate observed with a regimen simulating temocillin at 2 g q12h (P = 1) (Table 1). No temocillin-resistant mutant was detected in mice whatever the regimen used.
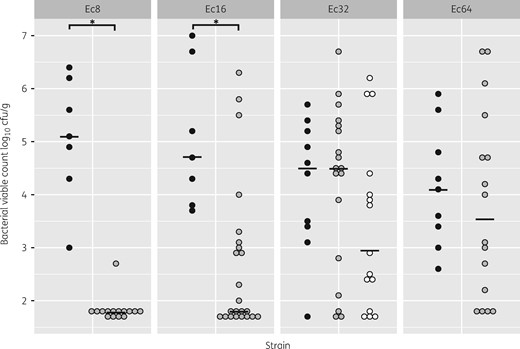
Bacterial counts at D6-PI in kidneys of mice infected by strains with different temocillin MICs (Ec8, 8 mg/L; Ec16, 16 mg/L; Ec32, 32 mg/L; Ec64, 64 mg/L) in control and treated groups. Each circle represents one mouse. Filled black circles, controls; grey circles, mice treated with a regimen simulating temocillin at 2 g q12h; white circles, mice treated with a regimen simulating temocillin at 2 g q8h. The black line represents the median. *P < 0.001 between groups.
Imipenem simulating 1 g q8h in humans for 24 h resulted in a significant bacterial count reduction compared with controls (Table 1), similar to that induced by temocillin at 2 g q12h against Ec8 (P > 0.10). The sterility rate of kidneys in mice infected by Ec8 was similar between imipenem (93%; 14/15) and temocillin at 2 g q12h (P > 0.05). In contrast, in mice infected by Ec16 the sterility rate of kidneys and bacterial count reduction were significantly lower for temocillin at 2 g q12h than for imipenem (Table 1).
Discussion
For now, there is no international consensus about temocillin clinical breakpoints and the optimal regimen. Our study, using clinical uropathogenic E. coli strains with similar fitness, demonstrates that temocillin at 2 g q12h displays excellent activity against a strain with an MIC of 8 mg/L, comparable to that of imipenem at 1 g q8h.
Regarding strains with a temocillin MIC of 16 mg/L, two previous experimental studies using isogenic strains in murine pyelonephritis or sepsis of intra-abdominal origin indicated the high efficacy of temocillin at 2 g q12h.6,7 In our experiments with Ec16, the mean bacterial reduction in kidneys was 2.9 log10 cfu/g and 48% of kidneys were sterilized after only 24 h of temocillin at 2 g q12h. However, as compared with imipenem and with a similar temocillin regimen against Ec8 the sterility rate of kidneys and the bacterial reduction in kidneys were significantly lower. Optimizing the fT>MIC using a 2 g q8h regimen might improve the efficacy of the treatment, but such a regimen against Ec16 was not evaluated for ethical reasons. Finally, our model also demonstrates a lack of efficacy of temocillin against strains with MICs of ≥32 mg/L, even with a 2 g q8h regimen. It could be argued that we reproduced in mice the demanding pharmacokinetics seen in patients hospitalized in ICUs, i.e. with high volumes of distribution.2,12 It cannot be excluded that better pharmacokinetic/pharmacodynamic parameters might be reached in patients with less severe infections. Furthermore, the 24 h washout period used in our model to ensure the absence of a carry-over effect might be responsible for regrowth of Ec32 and hence underestimation of the efficacy of treatments.
Our data are consistent with the only study in humans available to date.13 In a retrospective analysis of 19 patients with febrile UTI, the authors used temocillin at 2 g q12h to treat febrile UTIs caused by ESBL/AmpC-producing isolates with MICs up to 16 mg/L, with clinical and microbial cure rates of 100%. However, the authors did not determine the exact values of MICs; hence most, if not all, causative strains might have MICs <16 mg/L. This debate about whether the critical concentration is 8 or 16 mg/L is of particular importance in determining whether temocillin could be used as a first-line treatment in systemic UTIs. In fact, the MIC90 for Enterobacteriaceae isolated from community-acquired UTI was recently found to be 6 mg/L in an area of low ESBL prevalence14 and 16 mg/L in series including only MDR strains.1
In conclusion, our results confirm the in vivo efficacy of temocillin at 2 g q12h for the treatment of pyelonephritis due to E. coli strains with MICs of 8 mg/L and suggest that such efficacy might be conserved in non-severe infections for strains with MICs up to 16 mg/L. In contrast, even higher temocillin doses (2 g q8h) failed to treat UTI due to strains with temocillin MICs ≥32 mg/L. Prospective clinical studies regarding clinical efficacy according to MIC and temocillin regimen are now awaited to confirm our findings.
Funding
This study was supported by internal funding. Part of the animal purchase was supported by a grant from Eumedica SA (grant number CIC/010/2017/FR/GR).
Transparency declarations
K. A. and M. E. each received a travel grant from Eumedica. All other authors: none to declare.
References
Comité de l’Antibiogramme de la Société Française de Microbiologie. Tableaux des concentrations critiques pour l’interprétation des CMI et des diamètres des zones d’inhibition. In:



