-
PDF
- Split View
-
Views
-
Cite
Cite
Xiaoliang Ba, Lajos Kalmar, Nazreen F Hadjirin, Heidrun Kerschner, Petra Apfalter, Fiona J Morgan, Gavin K Paterson, Samantha L Girvan, Rui Zhou, Ewan M Harrison, Mark A Holmes, Truncation of GdpP mediates β-lactam resistance in clinical isolates of Staphylococcus aureus, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 5, May 2019, Pages 1182–1191, https://doi.org/10.1093/jac/dkz013
Close - Share Icon Share
Abstract
High-level β-lactam resistance in MRSA is mediated in the majority of strains by a mecA or mecC gene. In this study, we identified 10 mec gene-negative MRSA human isolates from Austria and 11 bovine isolates from the UK showing high levels of β-lactam resistance and sought to understand the molecular basis of the resistance observed.
Different antimicrobial resistance testing methods (disc diffusion, Etest and VITEK® 2) were used to establish the β-lactam resistance profiles for the isolates and the isolates were further investigated by WGS.
A number of mutations (including novel ones) in PBPs, AcrB, YjbH and the pbp4 promoter were identified in the resistant isolates, but not in closely related susceptible isolates. Importantly, a truncation in the cyclic diadenosine monophosphate phosphodiesterase enzyme, GdpP, was identified in 7 of the 10 Austrian isolates and 10 of the 11 UK isolates. Complementation of four representative isolates with an intact copy of the gdpP gene restored susceptibility to penicillins and abolished the growth defects caused by the truncation.
This study reports naturally occurring inactivation of GdpP protein in Staphylococcus aureus of both human origin and animal origin, and demonstrates clinical relevance to a previously reported association between this truncation and increased β-lactam resistance and impaired bacterial growth in laboratory-generated mutants. It also highlights possible limitations of genomic determination of antibiotic susceptibility based on single gene presence or absence when choosing the appropriate antimicrobial treatment for patients.
Introduction
Resistance to all classes of β-lactams in Staphylococcus aureus has been reported, initially to penicillin in the 1940s,1 subsequently to methicillin in the 1960s2 and most recently to fifth-generation cephalosporins such as ceftaroline.3 In addition to β-lactamase (encoded by blaZ), which confers resistance to penicillins, and the acquisition of an alternative PBP (encoded by mecA/C or the recently reported mecB4), which confers resistance to all β-lactams5 (although in the case of fifth-generation cephalosporins this requires further mutations in PBP2a3), other mechanisms or factors that contribute to β-lactam resistance have also been reported. For example, hyperproduction of β-lactamase in borderline oxacillin-resistant (BORSA) strains,6 mutations in native pbp genes,7–13 the pbp4 promoter14–16 and other chromosomal genes such as acrB (cation multidrug efflux transporter),13,yjbH (disulphide stress effector)17 and gdpP (GGDEF domain protein containing phosphodiesterase)13,18,19 have been linked to β-lactam resistance in S. aureus.
GdpP functions to hydrolyse the secondary messenger cyclic diadenosine monophosphate (c-di-AMP), a signalling nucleotide recently found to be produced in S. aureus.19 It has been shown that insertional inactivation or deletion of the gdpP gene leads to an increased level of c-di-AMP, which produces a reduced susceptibility to β-lactams and other cell wall-targeting antimicrobials in S. aureus laboratory mutants.18,19 However, to our knowledge, naturally occurring mutations and inactivation of GdpP do not appear to be common, with only one report of a truncated GdpP protein in clinical human isolates.7 Here, in this study, we initially investigated 10 isolates of mec gene-negative S. aureus that were phenotypically methicillin resistant (mgn-MRSA) from human patients in Austria and found that 7 of them contained a naturally truncated gdpP gene. A further investigation into a collection of mgn-MRSA from bovine milk in the UK revealed another 10 isolates also containing the natural gdpP truncation. We then investigated the effect of the truncation on β-lactam resistance and bacterial growth in these clinical isolates.
Materials and methods
Media and culture conditions
Clinical S. aureus isolates used in this study are described in Table 1. For routine culture, S. aureus was grown on horse blood agar (Oxoid, UK) and in tryptone soya broth (TSB) (Oxoid) at 37°C. Escherichia coli was grown in LB or on LB agar (Oxoid) at 37°C. Media were supplemented with 10 mg/L chloramphenicol, as appropriate.
Phenotypic and genotypic characteristics of S. aureus isolates from Austria and the UK
| Country . | Isolate . | Isolated from . | Year isolated . | MLST ST . | Oxacillin . | Cefoxitin . | Penicillin . | β- Lactamase . | blaZ gene . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | |||||||
| Austria | 5116 | wound | 2011 | 1 | 6 | 16 | R | 28 | 6 | R | 8 | 1 | R | + | + |
| 3277 | nose | 2007 | 7 | 6 | 12 | R | 27 | 6 | R | 6 | 24 | R | + | + | |
| 1556 | unknown | 2005 | 15 | 6 | 32 | S | 26 | 6 | S | 6 | 6 | R | + | − | |
| 2725 | unknown | 2007 | 22 | 6 | 6 | R | 32 | 6 | R | 16 | 0.19 | R | − | − | |
| 5441 | nose | 2012 | 22 | 6 | 12 | R | 28 | 6 | R | 10 | 1.5 | R | + | + | |
| 3258 | nose | 2007 | 22 | 6 | 8 | R | 30 | 6 | R | 17 | 0.19 | R | − | − | |
| 3555 | nose | 2009 | 22 | 6 | 8 | S | 28 | 4 | S | 11 | 1 | R | + | + | |
| 4613 | unknown | 2010 | 25 | 6 | 6 | S | 30 | 3 | S | 6 | 2 | R | + | + | |
| 3322 | unknown | 2008 | 45 | 6 | 6 | S | 30 | 3 | S | 17 | 0.38 | R | + | + | |
| 3397 | unknown | 2007 | 225 | 6 | 16 | R | 26 | 6 | R | 18 | 0.19 | R | − | − | |
| 1724 | wound | 2005 | 1560 | 23 | 1 | S | 29 | 3 | S | 29 | 0.064 | S | − | − | |
| 5278 | unknown | 2011 | 5 | 16 | 0.75 | S | 28 | 3 | S | 11 | 1 | R | + | + | |
| UK | m-30-76 | milk | 2012 | 8 | 6 | 12 | R | 32 | 4 | R | 12 | 0.5 | R | + | + |
| m-36-28 | milk | 2012 | 8 | 6 | 6 | R | 34 | 3 | R | 6 | 2 | R | + | + | |
| m-35-38 | milk | 2012 | 97 | 6 | 4 | S | 34 | 4 | S | 12 | 0.38 | R | + | + | |
| m-MR-11G | milk | 2012 | 97 | 6 | 4 | S | 30 | 4 | S | 30 | 0.064 | S | − | − | |
| m-MR-11Z | milk | 2012 | 97 | 6 | 6 | S | 29 | 6 | S | 10 | 2 | R | + | + | |
| m-MR-089A | milk | 2012 | 97 | 6 | 8 | R | 30 | 4 | R | 10 | 1.5 | R | + | + | |
| m-30-4 | milk | 2012 | 133 | 6 | 4 | R | 30 | 4 | R | 20 | 0.19 | R | − | − | |
| m-30-64 | milk | 2012 | 133 | 6 | 3 | R | 32 | 4 | R | 18 | 0.19 | R | − | − | |
| m-36-35 | milk | 2012 | 133 | 6 | 8 | R | 32 | 4 | R | 28 | 0.064 | S | − | − | |
| m-29-22 | milk | 2012 | 425 | 6 | 4 | S | 28 | 6 | S | 20 | 0.19 | R | − | − | |
| m-30-43 | milk | 2012 | 425 | 6 | 3 | R | 30 | 4 | R | 19 | 0.19 | R | − | − | |
| GKP136-23 | milk | 2012 | 151 | 20 | 0.38 | S | 31 | 3 | S | 36 | 0.016 | S | − | − | |
| Country . | Isolate . | Isolated from . | Year isolated . | MLST ST . | Oxacillin . | Cefoxitin . | Penicillin . | β- Lactamase . | blaZ gene . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | |||||||
| Austria | 5116 | wound | 2011 | 1 | 6 | 16 | R | 28 | 6 | R | 8 | 1 | R | + | + |
| 3277 | nose | 2007 | 7 | 6 | 12 | R | 27 | 6 | R | 6 | 24 | R | + | + | |
| 1556 | unknown | 2005 | 15 | 6 | 32 | S | 26 | 6 | S | 6 | 6 | R | + | − | |
| 2725 | unknown | 2007 | 22 | 6 | 6 | R | 32 | 6 | R | 16 | 0.19 | R | − | − | |
| 5441 | nose | 2012 | 22 | 6 | 12 | R | 28 | 6 | R | 10 | 1.5 | R | + | + | |
| 3258 | nose | 2007 | 22 | 6 | 8 | R | 30 | 6 | R | 17 | 0.19 | R | − | − | |
| 3555 | nose | 2009 | 22 | 6 | 8 | S | 28 | 4 | S | 11 | 1 | R | + | + | |
| 4613 | unknown | 2010 | 25 | 6 | 6 | S | 30 | 3 | S | 6 | 2 | R | + | + | |
| 3322 | unknown | 2008 | 45 | 6 | 6 | S | 30 | 3 | S | 17 | 0.38 | R | + | + | |
| 3397 | unknown | 2007 | 225 | 6 | 16 | R | 26 | 6 | R | 18 | 0.19 | R | − | − | |
| 1724 | wound | 2005 | 1560 | 23 | 1 | S | 29 | 3 | S | 29 | 0.064 | S | − | − | |
| 5278 | unknown | 2011 | 5 | 16 | 0.75 | S | 28 | 3 | S | 11 | 1 | R | + | + | |
| UK | m-30-76 | milk | 2012 | 8 | 6 | 12 | R | 32 | 4 | R | 12 | 0.5 | R | + | + |
| m-36-28 | milk | 2012 | 8 | 6 | 6 | R | 34 | 3 | R | 6 | 2 | R | + | + | |
| m-35-38 | milk | 2012 | 97 | 6 | 4 | S | 34 | 4 | S | 12 | 0.38 | R | + | + | |
| m-MR-11G | milk | 2012 | 97 | 6 | 4 | S | 30 | 4 | S | 30 | 0.064 | S | − | − | |
| m-MR-11Z | milk | 2012 | 97 | 6 | 6 | S | 29 | 6 | S | 10 | 2 | R | + | + | |
| m-MR-089A | milk | 2012 | 97 | 6 | 8 | R | 30 | 4 | R | 10 | 1.5 | R | + | + | |
| m-30-4 | milk | 2012 | 133 | 6 | 4 | R | 30 | 4 | R | 20 | 0.19 | R | − | − | |
| m-30-64 | milk | 2012 | 133 | 6 | 3 | R | 32 | 4 | R | 18 | 0.19 | R | − | − | |
| m-36-35 | milk | 2012 | 133 | 6 | 8 | R | 32 | 4 | R | 28 | 0.064 | S | − | − | |
| m-29-22 | milk | 2012 | 425 | 6 | 4 | S | 28 | 6 | S | 20 | 0.19 | R | − | − | |
| m-30-43 | milk | 2012 | 425 | 6 | 3 | R | 30 | 4 | R | 19 | 0.19 | R | − | − | |
| GKP136-23 | milk | 2012 | 151 | 20 | 0.38 | S | 31 | 3 | S | 36 | 0.016 | S | − | − | |
IZD, inhibition zone diameter; R, resistant; S, susceptible.
6 mm in disc diffusion results indicates no inhibition zone.
For oxacillin, the resistance breakpoint for disc diffusion is a diameter of ≤14 mm (BSAC) and for MIC it is >2 mg/L.
For cefoxitin, the resistance breakpoint for disc diffusion is a diameter of <22 mm and for MIC it is >4 mg/L.
For penicillin, the resistance breakpoint for disc diffusion is a diameter of <26 mm and for MIC it is >0.125 mg/L.
Phenotypic and genotypic characteristics of S. aureus isolates from Austria and the UK
| Country . | Isolate . | Isolated from . | Year isolated . | MLST ST . | Oxacillin . | Cefoxitin . | Penicillin . | β- Lactamase . | blaZ gene . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | |||||||
| Austria | 5116 | wound | 2011 | 1 | 6 | 16 | R | 28 | 6 | R | 8 | 1 | R | + | + |
| 3277 | nose | 2007 | 7 | 6 | 12 | R | 27 | 6 | R | 6 | 24 | R | + | + | |
| 1556 | unknown | 2005 | 15 | 6 | 32 | S | 26 | 6 | S | 6 | 6 | R | + | − | |
| 2725 | unknown | 2007 | 22 | 6 | 6 | R | 32 | 6 | R | 16 | 0.19 | R | − | − | |
| 5441 | nose | 2012 | 22 | 6 | 12 | R | 28 | 6 | R | 10 | 1.5 | R | + | + | |
| 3258 | nose | 2007 | 22 | 6 | 8 | R | 30 | 6 | R | 17 | 0.19 | R | − | − | |
| 3555 | nose | 2009 | 22 | 6 | 8 | S | 28 | 4 | S | 11 | 1 | R | + | + | |
| 4613 | unknown | 2010 | 25 | 6 | 6 | S | 30 | 3 | S | 6 | 2 | R | + | + | |
| 3322 | unknown | 2008 | 45 | 6 | 6 | S | 30 | 3 | S | 17 | 0.38 | R | + | + | |
| 3397 | unknown | 2007 | 225 | 6 | 16 | R | 26 | 6 | R | 18 | 0.19 | R | − | − | |
| 1724 | wound | 2005 | 1560 | 23 | 1 | S | 29 | 3 | S | 29 | 0.064 | S | − | − | |
| 5278 | unknown | 2011 | 5 | 16 | 0.75 | S | 28 | 3 | S | 11 | 1 | R | + | + | |
| UK | m-30-76 | milk | 2012 | 8 | 6 | 12 | R | 32 | 4 | R | 12 | 0.5 | R | + | + |
| m-36-28 | milk | 2012 | 8 | 6 | 6 | R | 34 | 3 | R | 6 | 2 | R | + | + | |
| m-35-38 | milk | 2012 | 97 | 6 | 4 | S | 34 | 4 | S | 12 | 0.38 | R | + | + | |
| m-MR-11G | milk | 2012 | 97 | 6 | 4 | S | 30 | 4 | S | 30 | 0.064 | S | − | − | |
| m-MR-11Z | milk | 2012 | 97 | 6 | 6 | S | 29 | 6 | S | 10 | 2 | R | + | + | |
| m-MR-089A | milk | 2012 | 97 | 6 | 8 | R | 30 | 4 | R | 10 | 1.5 | R | + | + | |
| m-30-4 | milk | 2012 | 133 | 6 | 4 | R | 30 | 4 | R | 20 | 0.19 | R | − | − | |
| m-30-64 | milk | 2012 | 133 | 6 | 3 | R | 32 | 4 | R | 18 | 0.19 | R | − | − | |
| m-36-35 | milk | 2012 | 133 | 6 | 8 | R | 32 | 4 | R | 28 | 0.064 | S | − | − | |
| m-29-22 | milk | 2012 | 425 | 6 | 4 | S | 28 | 6 | S | 20 | 0.19 | R | − | − | |
| m-30-43 | milk | 2012 | 425 | 6 | 3 | R | 30 | 4 | R | 19 | 0.19 | R | − | − | |
| GKP136-23 | milk | 2012 | 151 | 20 | 0.38 | S | 31 | 3 | S | 36 | 0.016 | S | − | − | |
| Country . | Isolate . | Isolated from . | Year isolated . | MLST ST . | Oxacillin . | Cefoxitin . | Penicillin . | β- Lactamase . | blaZ gene . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | IZD (mm) . | Etest (mg/L) . | VITEK® 2 . | |||||||
| Austria | 5116 | wound | 2011 | 1 | 6 | 16 | R | 28 | 6 | R | 8 | 1 | R | + | + |
| 3277 | nose | 2007 | 7 | 6 | 12 | R | 27 | 6 | R | 6 | 24 | R | + | + | |
| 1556 | unknown | 2005 | 15 | 6 | 32 | S | 26 | 6 | S | 6 | 6 | R | + | − | |
| 2725 | unknown | 2007 | 22 | 6 | 6 | R | 32 | 6 | R | 16 | 0.19 | R | − | − | |
| 5441 | nose | 2012 | 22 | 6 | 12 | R | 28 | 6 | R | 10 | 1.5 | R | + | + | |
| 3258 | nose | 2007 | 22 | 6 | 8 | R | 30 | 6 | R | 17 | 0.19 | R | − | − | |
| 3555 | nose | 2009 | 22 | 6 | 8 | S | 28 | 4 | S | 11 | 1 | R | + | + | |
| 4613 | unknown | 2010 | 25 | 6 | 6 | S | 30 | 3 | S | 6 | 2 | R | + | + | |
| 3322 | unknown | 2008 | 45 | 6 | 6 | S | 30 | 3 | S | 17 | 0.38 | R | + | + | |
| 3397 | unknown | 2007 | 225 | 6 | 16 | R | 26 | 6 | R | 18 | 0.19 | R | − | − | |
| 1724 | wound | 2005 | 1560 | 23 | 1 | S | 29 | 3 | S | 29 | 0.064 | S | − | − | |
| 5278 | unknown | 2011 | 5 | 16 | 0.75 | S | 28 | 3 | S | 11 | 1 | R | + | + | |
| UK | m-30-76 | milk | 2012 | 8 | 6 | 12 | R | 32 | 4 | R | 12 | 0.5 | R | + | + |
| m-36-28 | milk | 2012 | 8 | 6 | 6 | R | 34 | 3 | R | 6 | 2 | R | + | + | |
| m-35-38 | milk | 2012 | 97 | 6 | 4 | S | 34 | 4 | S | 12 | 0.38 | R | + | + | |
| m-MR-11G | milk | 2012 | 97 | 6 | 4 | S | 30 | 4 | S | 30 | 0.064 | S | − | − | |
| m-MR-11Z | milk | 2012 | 97 | 6 | 6 | S | 29 | 6 | S | 10 | 2 | R | + | + | |
| m-MR-089A | milk | 2012 | 97 | 6 | 8 | R | 30 | 4 | R | 10 | 1.5 | R | + | + | |
| m-30-4 | milk | 2012 | 133 | 6 | 4 | R | 30 | 4 | R | 20 | 0.19 | R | − | − | |
| m-30-64 | milk | 2012 | 133 | 6 | 3 | R | 32 | 4 | R | 18 | 0.19 | R | − | − | |
| m-36-35 | milk | 2012 | 133 | 6 | 8 | R | 32 | 4 | R | 28 | 0.064 | S | − | − | |
| m-29-22 | milk | 2012 | 425 | 6 | 4 | S | 28 | 6 | S | 20 | 0.19 | R | − | − | |
| m-30-43 | milk | 2012 | 425 | 6 | 3 | R | 30 | 4 | R | 19 | 0.19 | R | − | − | |
| GKP136-23 | milk | 2012 | 151 | 20 | 0.38 | S | 31 | 3 | S | 36 | 0.016 | S | − | − | |
IZD, inhibition zone diameter; R, resistant; S, susceptible.
6 mm in disc diffusion results indicates no inhibition zone.
For oxacillin, the resistance breakpoint for disc diffusion is a diameter of ≤14 mm (BSAC) and for MIC it is >2 mg/L.
For cefoxitin, the resistance breakpoint for disc diffusion is a diameter of <22 mm and for MIC it is >4 mg/L.
For penicillin, the resistance breakpoint for disc diffusion is a diameter of <26 mm and for MIC it is >0.125 mg/L.
Antimicrobial susceptibility testing
Both disc (Oxoid) diffusion and Etest (bioMérieux) methods for antimicrobial susceptibility testing were carried out according to EUCAST (Version 7.1, March 2017). Briefly, an inoculum with a turbidity equivalent to that of a 0.5 McFarland standard was prepared using a direct colony suspension method in saline. The inoculum was then inoculated on Mueller–Hinton agar (MHA) by swabbing in three directions. After applying antibiotic discs or Etest strips, all agar plates were incubated at 35°C for 20 h before interpretation of the results. As EUCAST doesn’t define a disc diffusion breakpoint for oxacillin, we also performed oxacillin disc diffusion according to BSAC (Version 14, January 2015). For BSAC oxacillin disc diffusion, a 1:10 dilution of an inoculum with a turbidity equivalent to that of a 0.5 McFarland standard was inoculated on MHA with 2% sodium chloride, and agar plates were incubated at 30°C for 24 h. The VITEK® 2 system was used to validate some antimicrobial susceptibility testing results with an AST-P635 card, in accordance with the manufacturer’s instructions.
WGS and bioinformatic analysis
Genomic DNA of S. aureus was extracted from overnight cultures grown in TSB at 37°C using the MasterPure Gram-Positive DNA Purification Kit (Cambio, UK). Illumina library preparation and HiSeq sequencing were carried out as previously described.20 NCBI BLAST21 was used to identify orthologous DNA and protein sequences and multiple sequence alignment was performed using Unipro UGENE software.22 Mutations in the native pbp genes, the pbp4 promoter, arcB, yjbH and gdpP were identified by comparing the DNA or translated protein sequences extracted from oxacillin-resistant isolates with those from a panel of MSSA isolates of various STs.
GdpP complementation
For complementation of gdpP, the gene was cloned into expression plasmid pXB01, a derivative of the tetracycline-inducible expression vector pRMC2 with the bla gene deleted.23 The gene, including its ribosome-binding site, was amplified from RN422024 genomic DNA with primers gdpP-KpnI (ATCGGGTACCCTAAAAAGTGAATAGAGGTG) and gdpP-SacI (CGATGAGCTCTACTTTCATGCATCTTCACT). The PCR product was digested with KpnI and SacI and ligated with pXB01 cleaved with the same enzymes. The resulting plasmid pXB01gdpP was passed through E. coli DC10B before being electroporated into four isolates with a truncated GdpP. Expression of gdpP was induced with 200 ng/mL anhydrotetracycline (Atc) (Sigma–Aldrich, UK). The empty plasmid pXB01 was also electroporated into the same four isolates to generate control strains with no GdpP expression.
Growth analysis
To assess the effect of the truncated GdpP on the growth of S. aureus strains in liquid culture, a Bioscreen C optical growth analyser (Labsystems, Finland) was used to monitor the growth rate of the strains with plasmid pXB01gdpP or pXB01. Briefly, overnight cultures were diluted 1:1000 into minimal medium SSM9PR25 (1× M9 salts, 2 mM MgSO4, 0.1 mM CaCl2, 1% glucose, 1% casamino acids, 1 mM thiamine-HCl and 0.05 mM nicotinamide) or SSM9PR supplemented with 200 ng/mL Atc to induce the expression of the plasmid-borne gdpP gene. For each strain, 300 μL of inoculated medium was added into wells of the microplate in triplicate. Fresh medium with Atc was also added to three wells acting as blank controls. Cultures were incubated at 37°C with continuous shaking for 24 h and OD600 was measured every 30 min.
A spot growth assay was also developed to evaluate bacterial growth on agar medium for the complementary strains. Briefly, S. aureus strains were cultured overnight on horse blood agar or tryptone soya agar (Oxoid) supplemented with 10 mg/L chloramphenicol, as appropriate. An inoculum with a turbidity equivalent to that of a 0.5 McFarland standard was then prepared as described above. For each strain, 20 μL of the inoculum was spotted onto MHA supplemented with 200 ng/mL Atc and the plate was incubated at 37°C for 20 h. For analysis, the growth of each strain was quantified as ‘mean area intensity’ using ImageJ software and the difference was compared between WT, empty plasmid-complemented and gdpP-complemented strains.
Results
β-Lactam resistance found in mgn-MRSA
Twelve human clinical isolates from Austria that were isolated on chromID® MRSA (bioMérieux), but were subsequently found to be lacking a mec gene by PCR, were tested for penicillin, oxacillin and cefoxitin resistance using both disc diffusion and Etest. Both tests showed that 11 isolates were resistant to penicillin (MICs 0.19–24 mg/L) and 10 isolates to oxacillin (MICs 6–32 mg/L) (Table 1). Although no isolate was classified as resistant to cefoxitin by disc diffusion, seven of them had an MIC of 6 mg/L by Etest, which indicated resistance (Table 1). Further antimicrobial testing using the VITEK® 2 system reported that 11 isolates were penicillin resistant and 6 were resistant both to cefoxitin and oxacillin (Table 1). The VITEK® 2 also showed that isolate 5116 was resistant to clindamycin, erythromycin and tetracycline and 3397 was resistant to ciprofloxacin and erythromycin. Interestingly, isolate 1556 was susceptible to both oxacillin and cefoxitin by VITEK® 2, although it was highly oxacillin resistant (MIC 32 mg/L) by Etest (Table 1).
Resistance not mediated by a β-lactamase
Previously, borderline oxacillin resistance in BORSA isolates has been reported to be due to overexpression of the staphylococcal β-lactamase.6 Therefore, we first investigated whether β-lactamase played a role in the resistance observed in these isolates. WGS showed that 4 out of the 10 oxacillin-resistant isolates were negative for the blaZ gene despite all being resistant to penicillin (Table 1). To rule out the possibility of a lost blaZ-carrying plasmid during the WGS process, a colony PCR for blaZ amplification using primer pair 487–37326 was performed on these blaZ-negative isolates. The PCR results confirmed the absence of the blaZ gene in these isolates (data not shown). Interestingly, isolate 1556, which was highly penicillin resistant (MIC 6 mg/L) and generated a positive result using β-lactamase (Nitrocefin) Identification Sticks (Oxoid), appeared not to possess a blaZ gene (Table 1). A cloverleaf assay,27 performed to confirm the β-lactamase phenotype, showed that isolate 1556 was not a β-lactamase producer, indicating that the β-lactamase Identification Sticks had given a false-positive result. Overall, this demonstrated that blaZ was not responsible for the penicillin and oxacillin resistance observed in the isolates lacking the gene. However, to demonstrate whether overexpression of blaZ was responsible for the resistance in isolates possessing the gene, antibiotic discs (penicillin and oxacillin) containing 0, 1, 2, 5 and 10 μg of clavulanic acid (a β-lactamase inhibitor) were used to repeat the disc diffusion assays.8 The results showed an obvious increase in inhibition zone diameters (but not to susceptible level) for the discs in combination with clavulanic acid. This could be explained by the neutralization of β-lactamase by clavulanic acid in these isolates. However, considering that these isolates remained resistant to penicillin and oxacillin in the presence of clavulanic acid, we suspected that mechanism(s) other than β-lactamase also contributed to the resistance in these blaZ-positive isolates.
Mutations found in resistant isolates
As β-lactamase hyperproduction was not responsible for the resistance observed and WGS confirmed the absence of a mec gene, further analyses were performed to investigate other possible resistance mechanisms. Mutations in the pbp4 promoter, PBPs, AcrB, YjbH and GdpP have been previously associated with β-lactam resistance. We therefore started with identifying possible mutations by comparing DNA and translated protein sequences from the oxacillin-resistant isolates (ST1, ST7, ST15, ST22, ST25, ST45 and ST225; Table 2) with sequences from a panel of MSSA isolates of different STs [ST1 (n = 5), ST97 (n = 5), ST425 (n = 5), ST133 (n = 4), ST8 (n = 2) and ST15 (n = 2)].
Mutations and amino acid substitutions identified in oxacillin-resistant isolates from Austria (n = 10) and the UK (n = 11)
| Country . | Isolate . | MLST ST . | ERA accession . | PBP1 . | PBP2 . | PBP3 . | pbp4 promoter . | PBP4 . | AcrB . | YjbH . | GdpP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 5116 | 1 | ERR599830 | − | − | − | − | R200L | − | − | R397* |
| 3277 | 7 | ERR599821 | − | Q629P | − | − | C12F, T101R | − | − | Q572* | |
| 1556 | 15 | ERR599824 | M361T | S569A | – | G>A at −39 bp | − | − | M262I | − | |
| 2725 | 22 | ERR599822 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 5441 | 22 | ERR599823 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | S207* | |
| 3298 | 22 | ERR599826 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 3955 | 22 | ERR599825 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | E334* | |
| 4613 | 25 | ERR599829 | D149E | R117H, Q629P | K6N | − | − | K35N, F581L, V700L | S267L | − | |
| 3322 | 45 | ERR599831 | − | A470T | − | G>T at −62 bp | − | V138I | − | S50* | |
| 3397 | 225 | ERR599832 | − | S707L | − | − | − | − | − | V609F | |
| UK | m-30-76 | 8 | ERR211695 | − | − | − | − | − | − | − | Q642* |
| m-36-28 | 8 | ERR234780 | P100T, P476S | − | − | A>C at 117 bp | − | − | − | K411* | |
| m-35-38 | 97 | ERR234716 | − | − | G374D,A596T | − | − | − | − | Q480* | |
| m-MR-11G | 97 | ERR175884 | − | − | − | − | − | − | − | Q101* | |
| m-MR-11Z | 97 | ERR175932 | − | − | − | − | − | − | − | A639* | |
| m-MR-089A | 97 | ERR175934 | − | − | − | − | − | − | − | T104K, E423* | |
| m-30-4 | 133 | ERR211688 | − | − | − | − | − | − | − | 542–546 deletion | |
| m-30-64 | 133 | ERR211693 | − | − | − | − | A22V | − | A242S | E267* | |
| m-36-35 | 133 | ERR234788 | − | V233I | − | − | − | − | − | R382* | |
| m-29-22 | 425 | ERR211684 | − | − | − | − | − | − | − | I233* | |
| m-30-43 | 425 | ERR211692 | − | − | − | − | − | − | − | R540* |
| Country . | Isolate . | MLST ST . | ERA accession . | PBP1 . | PBP2 . | PBP3 . | pbp4 promoter . | PBP4 . | AcrB . | YjbH . | GdpP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 5116 | 1 | ERR599830 | − | − | − | − | R200L | − | − | R397* |
| 3277 | 7 | ERR599821 | − | Q629P | − | − | C12F, T101R | − | − | Q572* | |
| 1556 | 15 | ERR599824 | M361T | S569A | – | G>A at −39 bp | − | − | M262I | − | |
| 2725 | 22 | ERR599822 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 5441 | 22 | ERR599823 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | S207* | |
| 3298 | 22 | ERR599826 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 3955 | 22 | ERR599825 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | E334* | |
| 4613 | 25 | ERR599829 | D149E | R117H, Q629P | K6N | − | − | K35N, F581L, V700L | S267L | − | |
| 3322 | 45 | ERR599831 | − | A470T | − | G>T at −62 bp | − | V138I | − | S50* | |
| 3397 | 225 | ERR599832 | − | S707L | − | − | − | − | − | V609F | |
| UK | m-30-76 | 8 | ERR211695 | − | − | − | − | − | − | − | Q642* |
| m-36-28 | 8 | ERR234780 | P100T, P476S | − | − | A>C at 117 bp | − | − | − | K411* | |
| m-35-38 | 97 | ERR234716 | − | − | G374D,A596T | − | − | − | − | Q480* | |
| m-MR-11G | 97 | ERR175884 | − | − | − | − | − | − | − | Q101* | |
| m-MR-11Z | 97 | ERR175932 | − | − | − | − | − | − | − | A639* | |
| m-MR-089A | 97 | ERR175934 | − | − | − | − | − | − | − | T104K, E423* | |
| m-30-4 | 133 | ERR211688 | − | − | − | − | − | − | − | 542–546 deletion | |
| m-30-64 | 133 | ERR211693 | − | − | − | − | A22V | − | A242S | E267* | |
| m-36-35 | 133 | ERR234788 | − | V233I | − | − | − | − | − | R382* | |
| m-29-22 | 425 | ERR211684 | − | − | − | − | − | − | − | I233* | |
| m-30-43 | 425 | ERR211692 | − | − | − | − | − | − | − | R540* |
, Stop codon; −, absence of mutations or amino acid substitutions.
Novel amino acid substitutions are in bold and amino acid substitutions in transpeptidase domains of PBPs are in italics.
Mutations and amino acid substitutions listed in the table were not present in the MSSA isolates used as controls. MSSAs were five ST1 isolates (ERR237623, ERR246646, ERR246644, ERR246656 and ERR234851), five ST97 isolates (ERR234740, ERR175922, ERR175911, ERR175906 and ERR175903), five ST425 isolates (ERR211673, ERR211668, ERR246593, ERR084608 and ERR211685), four ST133 isolates (ERR211694, ERR246594, ERR246606 and ERR237612), two ST8 isolates (ERR294352 and ERR144823) and two ST15 isolates (ERR234888 and ERR234842).
Mutations and amino acid substitutions identified in oxacillin-resistant isolates from Austria (n = 10) and the UK (n = 11)
| Country . | Isolate . | MLST ST . | ERA accession . | PBP1 . | PBP2 . | PBP3 . | pbp4 promoter . | PBP4 . | AcrB . | YjbH . | GdpP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 5116 | 1 | ERR599830 | − | − | − | − | R200L | − | − | R397* |
| 3277 | 7 | ERR599821 | − | Q629P | − | − | C12F, T101R | − | − | Q572* | |
| 1556 | 15 | ERR599824 | M361T | S569A | – | G>A at −39 bp | − | − | M262I | − | |
| 2725 | 22 | ERR599822 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 5441 | 22 | ERR599823 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | S207* | |
| 3298 | 22 | ERR599826 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 3955 | 22 | ERR599825 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | E334* | |
| 4613 | 25 | ERR599829 | D149E | R117H, Q629P | K6N | − | − | K35N, F581L, V700L | S267L | − | |
| 3322 | 45 | ERR599831 | − | A470T | − | G>T at −62 bp | − | V138I | − | S50* | |
| 3397 | 225 | ERR599832 | − | S707L | − | − | − | − | − | V609F | |
| UK | m-30-76 | 8 | ERR211695 | − | − | − | − | − | − | − | Q642* |
| m-36-28 | 8 | ERR234780 | P100T, P476S | − | − | A>C at 117 bp | − | − | − | K411* | |
| m-35-38 | 97 | ERR234716 | − | − | G374D,A596T | − | − | − | − | Q480* | |
| m-MR-11G | 97 | ERR175884 | − | − | − | − | − | − | − | Q101* | |
| m-MR-11Z | 97 | ERR175932 | − | − | − | − | − | − | − | A639* | |
| m-MR-089A | 97 | ERR175934 | − | − | − | − | − | − | − | T104K, E423* | |
| m-30-4 | 133 | ERR211688 | − | − | − | − | − | − | − | 542–546 deletion | |
| m-30-64 | 133 | ERR211693 | − | − | − | − | A22V | − | A242S | E267* | |
| m-36-35 | 133 | ERR234788 | − | V233I | − | − | − | − | − | R382* | |
| m-29-22 | 425 | ERR211684 | − | − | − | − | − | − | − | I233* | |
| m-30-43 | 425 | ERR211692 | − | − | − | − | − | − | − | R540* |
| Country . | Isolate . | MLST ST . | ERA accession . | PBP1 . | PBP2 . | PBP3 . | pbp4 promoter . | PBP4 . | AcrB . | YjbH . | GdpP . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 5116 | 1 | ERR599830 | − | − | − | − | R200L | − | − | R397* |
| 3277 | 7 | ERR599821 | − | Q629P | − | − | C12F, T101R | − | − | Q572* | |
| 1556 | 15 | ERR599824 | M361T | S569A | – | G>A at −39 bp | − | − | M262I | − | |
| 2725 | 22 | ERR599822 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 5441 | 22 | ERR599823 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | S207* | |
| 3298 | 22 | ERR599826 | E606K, S629T, S664T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | V417* | |
| 3955 | 22 | ERR599825 | S629T, S644T | − | R504K, K584N | G>T at −62 bp, C>T at −298 bp, C>T at 407 bp | D98E | A284S, M291V, K846E | − | E334* | |
| 4613 | 25 | ERR599829 | D149E | R117H, Q629P | K6N | − | − | K35N, F581L, V700L | S267L | − | |
| 3322 | 45 | ERR599831 | − | A470T | − | G>T at −62 bp | − | V138I | − | S50* | |
| 3397 | 225 | ERR599832 | − | S707L | − | − | − | − | − | V609F | |
| UK | m-30-76 | 8 | ERR211695 | − | − | − | − | − | − | − | Q642* |
| m-36-28 | 8 | ERR234780 | P100T, P476S | − | − | A>C at 117 bp | − | − | − | K411* | |
| m-35-38 | 97 | ERR234716 | − | − | G374D,A596T | − | − | − | − | Q480* | |
| m-MR-11G | 97 | ERR175884 | − | − | − | − | − | − | − | Q101* | |
| m-MR-11Z | 97 | ERR175932 | − | − | − | − | − | − | − | A639* | |
| m-MR-089A | 97 | ERR175934 | − | − | − | − | − | − | − | T104K, E423* | |
| m-30-4 | 133 | ERR211688 | − | − | − | − | − | − | − | 542–546 deletion | |
| m-30-64 | 133 | ERR211693 | − | − | − | − | A22V | − | A242S | E267* | |
| m-36-35 | 133 | ERR234788 | − | V233I | − | − | − | − | − | R382* | |
| m-29-22 | 425 | ERR211684 | − | − | − | − | − | − | − | I233* | |
| m-30-43 | 425 | ERR211692 | − | − | − | − | − | − | − | R540* |
, Stop codon; −, absence of mutations or amino acid substitutions.
Novel amino acid substitutions are in bold and amino acid substitutions in transpeptidase domains of PBPs are in italics.
Mutations and amino acid substitutions listed in the table were not present in the MSSA isolates used as controls. MSSAs were five ST1 isolates (ERR237623, ERR246646, ERR246644, ERR246656 and ERR234851), five ST97 isolates (ERR234740, ERR175922, ERR175911, ERR175906 and ERR175903), five ST425 isolates (ERR211673, ERR211668, ERR246593, ERR084608 and ERR211685), four ST133 isolates (ERR211694, ERR246594, ERR246606 and ERR237612), two ST8 isolates (ERR294352 and ERR144823) and two ST15 isolates (ERR234888 and ERR234842).
A number of mutations, either previously reported or novel, were found in the resistant isolates but not in the susceptible controls (Table 2). Identical mutations were found in PBP1, PBP3, the pbp4 promoter, PBP4 and AcrB in all four of the ST22 isolates. These mutations have been reported in two clonal complex (CC) 22 strains described in a recent study.7 Further bioinformatic analysis on more ST22 strains revealed that the same mutations were present in all MSSA strains reported previously28 as well as in all mecA-MRSA strains (n = 10) investigated in this study (data not shown). This suggests that the mutations found in the ST22 strains are likely to be clonally specific to the ST22 lineage and unlikely to mediate resistance alone, although this needs to be validated.
Apart from the mutations found in ST22 isolates, a few novel amino acid substitutions were also identified in the transpeptidase domain of the PBPs (in italics, Table 2) and in proteins AcrB and YjiH. In the pbp4 promoter, a novel mutation (a G to A change at 39 bp upstream of the pbp4 start codon) was identified in isolate 1556. Most interestingly, a naturally truncated GdpP was identified in 7 out of 10 oxacillin-resistant isolates of four different STs (Table 2). Protein domain prediction using InterPro (https://www.ebi.ac.uk/interpro/) revealed three domains in GdpP, namely the GGDEF domain, the DDH domain and the DHHA1 domain. The mutations resulting in GdpP truncation were different and located throughout the three domains (Table 3). In comparison with other mutations identified in a small number of isolates, the GdpP truncation showed a very strong association with the resistance as it was found in the majority of resistant isolates.
| Isolate . | . | GGDEF domain (135–299) . | . | DDH domain (337–494) . | DHHA1 domain (516–645) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 . | 101 . | 104 . | 207 . | 233 . | 267 . | 334 . | 382 . | 397 . | 411 . | 417 . | 423 . | 480 . | 540 . | 542– 546 . | 572 . | 609 . | 639 . | 642 . | |
| S . | Q . | T . | S . | I . | E . | E . | R . | R . | K . | V . | E . | Q . | R . | ELIRT . | Q . | V . | A . | Q . | |
| 5116 | * | ||||||||||||||||||
| 3277 | * | ||||||||||||||||||
| 2725 | * | ||||||||||||||||||
| 5441 | * | ||||||||||||||||||
| 3298 | * | ||||||||||||||||||
| 3955 | * | ||||||||||||||||||
| 3322 | * | ||||||||||||||||||
| 3397 | F | ||||||||||||||||||
| m-30-76 | * | ||||||||||||||||||
| m-36-28 | * | ||||||||||||||||||
| m-35-38 | * | ||||||||||||||||||
| m-MR-11G | * | ||||||||||||||||||
| m-MR-11Z | * | ||||||||||||||||||
| m-MR-089A | K | * | |||||||||||||||||
| m-30-4 | deletion | ||||||||||||||||||
| m-30-64 | * | ||||||||||||||||||
| m-36-35 | * | ||||||||||||||||||
| m-29-22 | * | ||||||||||||||||||
| m-30-43 | * | ||||||||||||||||||
| Isolate . | . | GGDEF domain (135–299) . | . | DDH domain (337–494) . | DHHA1 domain (516–645) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 . | 101 . | 104 . | 207 . | 233 . | 267 . | 334 . | 382 . | 397 . | 411 . | 417 . | 423 . | 480 . | 540 . | 542– 546 . | 572 . | 609 . | 639 . | 642 . | |
| S . | Q . | T . | S . | I . | E . | E . | R . | R . | K . | V . | E . | Q . | R . | ELIRT . | Q . | V . | A . | Q . | |
| 5116 | * | ||||||||||||||||||
| 3277 | * | ||||||||||||||||||
| 2725 | * | ||||||||||||||||||
| 5441 | * | ||||||||||||||||||
| 3298 | * | ||||||||||||||||||
| 3955 | * | ||||||||||||||||||
| 3322 | * | ||||||||||||||||||
| 3397 | F | ||||||||||||||||||
| m-30-76 | * | ||||||||||||||||||
| m-36-28 | * | ||||||||||||||||||
| m-35-38 | * | ||||||||||||||||||
| m-MR-11G | * | ||||||||||||||||||
| m-MR-11Z | * | ||||||||||||||||||
| m-MR-089A | K | * | |||||||||||||||||
| m-30-4 | deletion | ||||||||||||||||||
| m-30-64 | * | ||||||||||||||||||
| m-36-35 | * | ||||||||||||||||||
| m-29-22 | * | ||||||||||||||||||
| m-30-43 | * | ||||||||||||||||||
, Stop codon.
| Isolate . | . | GGDEF domain (135–299) . | . | DDH domain (337–494) . | DHHA1 domain (516–645) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 . | 101 . | 104 . | 207 . | 233 . | 267 . | 334 . | 382 . | 397 . | 411 . | 417 . | 423 . | 480 . | 540 . | 542– 546 . | 572 . | 609 . | 639 . | 642 . | |
| S . | Q . | T . | S . | I . | E . | E . | R . | R . | K . | V . | E . | Q . | R . | ELIRT . | Q . | V . | A . | Q . | |
| 5116 | * | ||||||||||||||||||
| 3277 | * | ||||||||||||||||||
| 2725 | * | ||||||||||||||||||
| 5441 | * | ||||||||||||||||||
| 3298 | * | ||||||||||||||||||
| 3955 | * | ||||||||||||||||||
| 3322 | * | ||||||||||||||||||
| 3397 | F | ||||||||||||||||||
| m-30-76 | * | ||||||||||||||||||
| m-36-28 | * | ||||||||||||||||||
| m-35-38 | * | ||||||||||||||||||
| m-MR-11G | * | ||||||||||||||||||
| m-MR-11Z | * | ||||||||||||||||||
| m-MR-089A | K | * | |||||||||||||||||
| m-30-4 | deletion | ||||||||||||||||||
| m-30-64 | * | ||||||||||||||||||
| m-36-35 | * | ||||||||||||||||||
| m-29-22 | * | ||||||||||||||||||
| m-30-43 | * | ||||||||||||||||||
| Isolate . | . | GGDEF domain (135–299) . | . | DDH domain (337–494) . | DHHA1 domain (516–645) . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 . | 101 . | 104 . | 207 . | 233 . | 267 . | 334 . | 382 . | 397 . | 411 . | 417 . | 423 . | 480 . | 540 . | 542– 546 . | 572 . | 609 . | 639 . | 642 . | |
| S . | Q . | T . | S . | I . | E . | E . | R . | R . | K . | V . | E . | Q . | R . | ELIRT . | Q . | V . | A . | Q . | |
| 5116 | * | ||||||||||||||||||
| 3277 | * | ||||||||||||||||||
| 2725 | * | ||||||||||||||||||
| 5441 | * | ||||||||||||||||||
| 3298 | * | ||||||||||||||||||
| 3955 | * | ||||||||||||||||||
| 3322 | * | ||||||||||||||||||
| 3397 | F | ||||||||||||||||||
| m-30-76 | * | ||||||||||||||||||
| m-36-28 | * | ||||||||||||||||||
| m-35-38 | * | ||||||||||||||||||
| m-MR-11G | * | ||||||||||||||||||
| m-MR-11Z | * | ||||||||||||||||||
| m-MR-089A | K | * | |||||||||||||||||
| m-30-4 | deletion | ||||||||||||||||||
| m-30-64 | * | ||||||||||||||||||
| m-36-35 | * | ||||||||||||||||||
| m-29-22 | * | ||||||||||||||||||
| m-30-43 | * | ||||||||||||||||||
, Stop codon.
Investigation of truncated GdpP in bovine mec gene-negative S. aureus in the UK
Previously, when screening bovine milk for the presence of MRSA,29,30 we identified a panel of mec-negative isolates (n = 108) that were potentially methicillin resistant as demonstrated by growth on Brilliance MRSA agar (Oxoid). WGS data revealed that mec genes were absent in all isolates. Further analysis revealed that a truncated GdpP protein was present in a total of 10 isolates belonging to four STs (ST8, ST97, ST133 and ST425) and an eleventh isolate, m-30-4 (ST133), had an amino acid deletion in position 542–546 of GdpP (Table 2).
Antimicrobial susceptibility testing showed that 11 isolates were resistant to oxacillin and 9 to penicillin (Table 1). None of the isolates was classified as resistant to cefoxitin by disc diffusion, but two isolates (m-29-22 and m-MR-11Z) were classified as resistant by Etest (6 mg/L; Table 1). The VITEK® 2 system reported that nine isolates were penicillin resistant and seven were resistant both to cefoxitin and oxacillin (Table 1). WGS data and β-lactamase production assay using β-lactamase Identification Sticks revealed that 7 of the 11 oxacillin-resistant isolates lacked the blaZ gene or β-lactamase activity (Table 1), indicating that the resistance observed was not caused by an overproduction of β-lactamase.
Apart from the truncated GdpP protein, a small number of novel amino acid substitutions were also identified in the native PBPs and YjbH protein of these isolates (Table 2). For instance, amino acid substitution P476S in the transpeptidase domain of PBP1 was found in isolate m-36-28 and a V233I substitution in the transglycosylase domain of PBP2 was found in isolate m-36-35. In the transpeptidase domain of PBP3 of isolate m-35-28, a G374D and an A596T substitution were identified. An A22V substitution in PBP4 and an A242S substitution in YjbH were present in isolate m-30-64. In the promoter region of the pbp4 gene, an A to C change was identified at 117 bp upstream of the pbp4 start codon.
Truncated GdpP causes increased β-lactam resistance
Altogether, 7 human and 10 bovine mgn-MRSA isolates were found to contain a truncated GdpP. In order to confirm the resistance was indeed caused by the truncated GdpP in these isolates, as previously suggested,18,19 a plasmid-borne copy of the intact gdpP gene (pXB01gdpP) was introduced into four resistant clinical isolates from three different STs (ST1, ST7 and ST22; Figure 1). Oxacillin, penicillin and cefoxitin disc diffusion susceptibility testing was then carried out on these pXB01gdpP-containing strains as well as the control strains with the empty plasmid. Results showed that all four gdpP-complemented strains became completely susceptible to oxacillin when induced with 200 ng/mL Atc while strains with the empty plasmid remained resistant (Figure 1a). Full susceptibility to penicillin was only restored in strain 3298 (pXB01gdpP), which was blaZ negative, but not in the other strains possessing blaZ (Figure 1b). However, the blaZ-positive strains became fully susceptible to penicillin when 5 μg of clavulanic acid was added to the penicillin discs (Figure 1d), indicating that GdpP loss of function also mediates penicillin resistance in these isolates. The resistance to cefoxitin in these GdpP-complemented strains seemed to be not noticeably affected (Figure 1c).
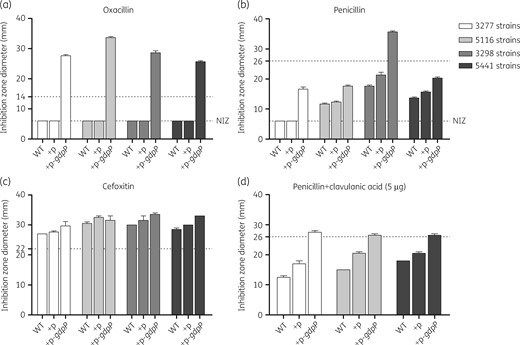
Effect of gdpP complementation on oxacillin and penicillin resistance in GdpP-truncated strains. Four isolates with GdpP truncation were complemented with plasmid pXB01gdpP or the empty plasmid pXB01. Resistance of the strains was determined using the disc diffusion method recommended by BSAC (for oxacillin) and EUCAST (for penicillin and cefoxitin) as detailed in the Materials and methods section. Media were supplemented with 200 ng/mL Atc to induce the expression of the plasmid-borne gdpP gene. For oxacillin, the resistance breakpoint for disc diffusion is a diameter of ≤14 mm (BSAC). For penicillin, the resistance breakpoint for disc diffusion is a diameter of <26 mm (EUCAST). For cefoxitin, the resistance breakpoint for disc diffusion is a diameter of <22 mm (EUCAST). NIZ, no inhibition zone (disc diameter was 6 mm); +p, complementation with the empty vector; +p-gdpP, complementation with pXB01gdpP.
GdpP contributes to S. aureus growth in vitro
It has been shown that disruption of gdpP compensates for a lack of lipoteichoic acid (LTA), which is crucial for normal bacterial growth and cell division, allowing LTA-deficient S. aureus to grow.19 We therefore performed genomic analysis to investigate the integrity of the LTA synthase gene ltaS in the isolates that lacked a functional GdpP. The results showed that all these isolates had an intact copy of the ltaS gene.
We next sought to understand the effect of truncated GdpP on the growth of S. aureus in minimal medium SSM9PR. As shown in Figure 2, an isolate complemented with plasmid pXB01gdpP had its growth noticeably improved in comparison with the same isolate complemented with the empty plasmid in the presence of 200 ng/mL Atc. Even without the Atc induction, isolates complemented with pXB01gdpP had an obvious growth advantage over the ones with the empty pXB01 (Figure 2). This could be explained by incomplete repression by the TetR repressor over the Pxyl/tetO promoter in the expression plasmid used,31 resulting in a low level of GdpP expression. A spot growth assay also showed that pXB01gdpP-complemented strains had better growth compared with the WT and the empty vector-complemented strains on MHA supplemented with 200 ng/mL Atc (Figure 3). The results suggest that a functional GdpP protein contributes to better bacterial growth, but is not essential for the growth.
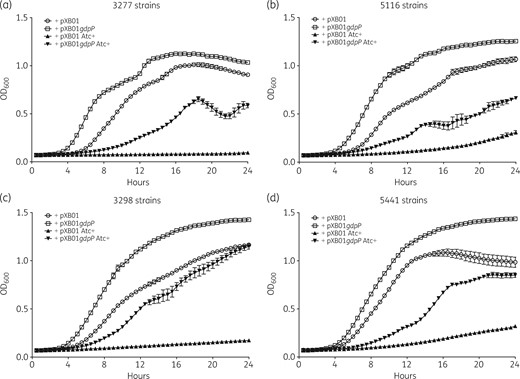
Growth analysis for gdpP-complemented strains in minimal medium SSM9PR. Four isolates with GdpP truncation were complemented with plasmid pXB01gdpP or the empty plasmid pXB01. For the growth curve analysis, overnight cultures were diluted 1:1000 into fresh SSM9PR or SSM9PR supplemented with 200 ng/mL Atc to induce the expression of the plasmid-borne gdpP gene. For each strain, 300 μL of inoculated medium was added into wells of the microplate in triplicate. Fresh medium with Atc was also added to three wells acting as blank controls. Cultures were incubated at 37°C with continuous shaking for 24 h and OD600 was measured every 30 min.
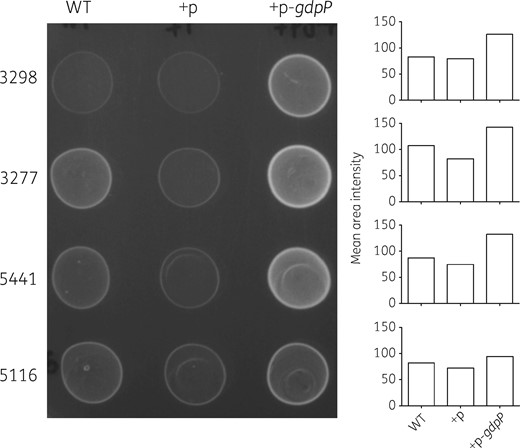
Spot growth assay for gdpP-complemented strains on MHA with 200 ng/mL Atc. For each strain, an inoculum with a turbidity equivalent to that of a 0.5 McFarland standard was prepared and 20 μL of the inoculum was spotted onto MHA supplemented with 200 ng/mL Atc. The plate was then incubated at 37°C for 20 h. Growth was quantified as ‘mean area intensity’ using ImageJ and is represented as a bar graph. +p, complementation with the empty vector; +p-gdpP, complementation with pXB01gdpP.
Discussion
In this work, we have reported 10 mgn-MRSA of various STs isolated from human clinical samples in Austria and 11 from bovine milk in the UK (Table 1). These isolates are different from BORSA6 strains as they have a higher oxacillin MIC and a proportion of them are also resistant to cefoxitin. Also, the resistance observed is not mediated by hyperproduction of β-lactamase, as demonstrated by the clavulanic acid assay, and the lack of the blaZ gene itself in 11 of the 21 isolates. To understand the resistance mechanism in these isolates, sequence analysis was carried out on proteins and the pbp4 promoter region that had previously been reported to be associated with β-lactam resistance in S. aureus. We identified a number of novel amino acid substitutions in PBPs, AcrB and YjbH, as well as some previously reported substitutions, and found a truncated GdpP protein in the majority of the resistant isolates (Table 2).
Amino acid substitution S569A in the transpeptidase domain of PBP2 had been previously reported to reduce affinity to penicillin and resulted in high-level penicillin resistance (MIC 8 mg/L) in strain BB255R.11 This mutation may explain the high-level penicillin resistance observed in isolate 1556, which lacked a gdpP mutation. It has been suggested that mutations in the pbp4 promoter cause overexpression of PBP4, resulting in low-level methicillin resistance.14,15 However, in this study, only a few pbp4 promoter mutations were found and most of these were found in all CC22 strains investigated, both MRSA and MSSA, suggesting that these are ancestral lineage-specific mutations (Table 2). Interestingly, a previously reported mutation (G to T at 62 bp upstream of the pbp4 start codon) in isolates resistant to cefoxitin and penicillin (but not to oxacillin)7 was found in oxacillin-resistant isolate 3322 (Table 2) and in β-lactam susceptible isolates 1724 and GKP136-23, indicating this mutation may not be sufficient to mediate resistance alone. Unfortunately, the small number of isolates made it unlikely that we would be able to identify the contribution of other genes involved in resistance. Further experimental validation is required to understand the mechanism(s) of resistance in these isolates.
This study focused on the truncated GdpP, which was found in the majority of the resistant isolates across various STs. Mutations in GdpP have been associated with increased resistance to cell wall-targeting antimicrobials in different Gram-positive bacteria, such as Bacillus subtilis,32,33,Listeria monocytogenes34 and S. aureus.18,19,35 It has been suggested that gdpP deletion in S. aureus results in an increased amount of cross-linked peptidoglycan, which could be responsible for increased resistance to antimicrobials that target cell-wall synthesis.19 In this work, the expression of a plasmid-borne copy of the gdpP gene restored susceptibility to penicillins in the complemented strains (Figure 1), suggesting that the truncation was responsible for the increased resistance, as previously suggested. Also, importantly, only mutation Q642* has been reported previously7 and it seems that there is not a truncation ‘hot spot’ in the GdpP protein as the premature stop codons appear at multiple locations throughout the sequence (Table 3), suggesting that loss of function is the determinant of resistance.
It has been suggested that a high level of cellular c-di-AMP impairs bacterial growth in B. subtilis32 and S. aureus.36 Given that GdpP functions to hydrolyse c-di-AMP and the inactivation of the gdpP gene leads to high and relatively constant levels of c-di-AMP,36 we investigated the growth rate of the strains complemented with and without the gdpP gene. The growth curves and spot growth assay showed that strains with a plasmid-borne copy of the intact gdpP gene grew noticeably faster than the ones with the empty plasmid, suggesting that GdpP truncation in these isolates contributes to a decreased growth rate.
This work has provided experimental evidence that naturally occurring GdpP truncations in mgn-MRSA isolates mediate resistance to oxacillin and penicillin. However, it seems that the truncation does not mediate resistance to cefoxitin, although some of the isolates are resistant to cefoxitin as determined by Etest and VITEK® 2 (Table 1). This suggests that other mechanism(s) may be involved in the determination of cefoxitin resistance in these isolates. Since cefoxitin disc diffusion testing failed to detect the resistance in the isolates reported in this study, it is important to raise the concern that MRSA screening based only on cefoxitin disc diffusion may report these highly oxacillin-resistant isolates as MSSA. It is important to use both cefoxitin and oxacillin disc diffusion testing for routine β-lactam resistance screening in S. aureus.
The finding of GdpP truncations in a range of STs of both human origin and animal origin demonstrates that this mutation is not restricted to a single S. aureus lineage. It is likely that GdpP loss-of-function mutants arise as a result of the selective pressure of exposure to β-lactam antibiotics, but as resistance comes with a fitness cost in the form of reduced growth this may explain why GdpP mutants are relatively rare. Nonetheless, these findings raise further concerns about β-lactam antibiotic resistance in S. aureus that could be missed by conventional molecular diagnostic methods targeting mec genes (including WGS). Patients who have received prolonged or multiple courses of β-lactam antibiotics may also be at risk of treatment failure when these gdpP truncations occur.
Acknowledgements
We thank the core sequencing and informatics team at the Wellcome Sanger Institute for sequencing of the isolates described in this study.
Funding
This work was funded by the UK Medical Research Council grants MR/P007201/1, MR/N002660/1 and G1001787/1. R. Z. is supported by a National Natural Science Foundation of China (NSFC) grant: 81661138003. E. M. H. is supported by a UK Research and Innovation (UKRI) Fellowship: MR/S00291X/1.
Transparency declarations
None to declare.
References
Author notes
E. M. H. and M. A. H. are joint senior authors.



