-
PDF
- Split View
-
Views
-
Cite
Cite
Katy Jeannot, Marion Danassie, Pauline Triponney, Maxime Bour, Thomas Gueudet, Racha Beyrouthy, Richard Bonnet, Patrick Plésiat, A novel IncQ plasmid carrying gene blaCTX-M-3 in Pseudomonas aeruginosa, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 3, March 2019, Pages 823–825, https://doi.org/10.1093/jac/dky454
Close - Share Icon Share
Sir,
Several types of ESBLs belonging to Ambler classes A (TEM, PER, SHV, GES, PME, CTX-M, VEB, BEL) and D (OXA enzyme derivatives) have been described in Pseudomonas aeruginosa.1 While the CTX-M enzymes have rapidly spread among enterobacterial species worldwide, they remain relatively uncommon in this opportunistic pathogen.2,3 CTX-M-1, -2, -3, -14, -15 and -43 have sporadically been detected in strains recovered from Bolivia, Brazil, China and the Netherlands.3–6 However, except the description of an ISCR1-blaCTX-M2 structure, no information on the genetic environment of the blaCTX-M genes found in P. aeruginosa has been reported so far.7 Here, we describe an IncQ plasmid carrying the blaCTX-M-3 gene in a strain isolated in France.
A P. aeruginosa strain, named 15.2986, was isolated from two urine samples of an outpatient previously admitted to a Russian hospital for prosthetic surgery. According to the EUCAST breakpoints, this ST244 strain was resistant to antipseudomonal β-lactams ticarcillin (MIC >128 mg/L), piperacillin/tazobactam (128/4 mg/L), ceftazidime (>32 mg/L), cefepime, (>64 mg/L) and ceftolozane/tazobactam (32/4 mg/L), as well as to tobramycin (>128 mg/L) and ciprofloxacin (64 mg/L). On the other hand, the isolate remained susceptible to ceftazidime/avibactam (4/4 mg/L) and colistin (1 mg/L). Suggesting involvement of a class A ESBL in this resistance profile, a synergy between ceftazidime (disc load of 10 μg) and amoxicillin/clavulanate (20/10 μg) was observed with the disc diffusion method. Drug susceptibility testing of strain 15.2986 in the presence of class C β-lactamase inhibitor cloxacillin at 2000 mg/L in contrast failed to demonstrate any involvement of intrinsic cephalosporinase AmpC in the resistance pattern of that isolate to β-lactams. PCR-sequencing experiments with specific primers confirmed the presence of ESBL gene blaCTX-M-3 in strain 15.2986. Though blaCTX-M determinants are often borne by self-conjugative plasmids in Enterobacteriaceae,8 all our attempts to horizontally transfer ticarcillin resistance to rifampicin-resistant P. aeruginosa PU21 remained unsuccessful. To determine the genetic environment of blaCTX-M-3, the whole DNA content of the strain was sequenced by hybrid de novo assembly of 2 × 150 paired-end reads using MiSeq Illumina technology. Alignment of resultant reads with the reference strain PAO1 genome (accession number NC_002516.2) allowed the selection of unmapped sequences (3 743 640 reads), which when assembled using CLC Genomics Workbench 10.0.1 software (Qiagen Aahrus A/S) led to the identification of a small plasmid of 9910 bp in length, which was named pPSTRAS1. As previously reported for blaCTX-M-3 genes harboured by Enterobacteriaceae, the blaCTX-M-3 gene in strain 15.2986 is flanked by a truncated transposition unit ΔISEcp1-blaCTX-M-3-Δorf477, itself embedded in a strB gene (Figure 1).8 A 1534 bp region upstream of this transposition unit shares 100% sequence identity with a DNA region consisting of the end of gene strB, gene aphA6 and the IRR of transposon Tn5393, a chromosomally located element carried by a carbapenemase IMP-1-producing P. aeruginosa, Pae-32183cz, from the Czech Republic.9 A truncated IS belonging to the IS91 family is also present in this region, likely as a remnant of previous rearrangement events.
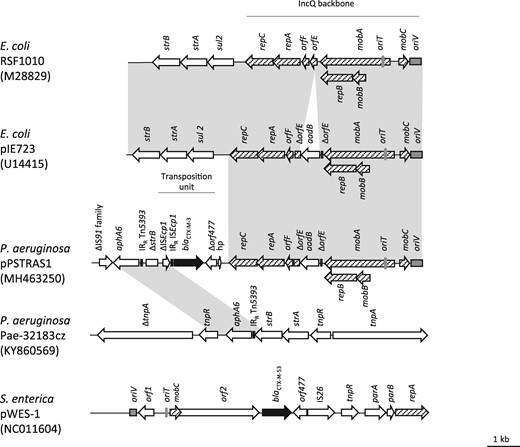
Linear schematic representation of IncQ plasmids RSF1010, pIE723, pPSTRAS1, Pae-32183cz and pWES-1. The IncQ plasmid backbone is composed of genes involved in the mobilization of IncQ plasmids (mobC and mobB) and genes required for replication (repB, orfE, orfF, repA and repC) (hatched arrows). The oriV (replication) and oriT (mobilization) regions are represented by grey boxes. Accessory genes are represented by white arrows, except for blaCTX-M genes that are represented by black arrows. IRR of Tn5393 and IRR of ISEcp1 are indicated by vertical bars. Grey areas correspond to 100% sequence identity.
Downstream of the transposition unit, a sequence of 5768 bp is clearly related to the backbone of IncQ plasmids.10 Indeed, examination of this region showed the presence of replicase genes (repA, repB and repC), origin of replication oriV and mobilization genes (mobA, mobB and mobC), including the primase gene repB fused to relaxase gene mobA, as in other IncQ plasmids.10 Sequence analysis of Rep proteins and the oriV-like iteron strongly suggests that pPSTRAS1 belongs to the IncQ1 subgroup of plasmids10 whose archetype is RSF1010.11 Plasmid pPSTRAS1 shares 74.3% sequence identity with pIE723, which itself differs from RSF1010 by the presence of an aadB gene cassette inserted in gene orfE (Figure 1).12 The expression of the cassette is under the control of promoter P4, which also regulates the orfE-orfF-repA-repC operon.13 The pIE723 plasmid was identified in Escherichia coli strain IE1003 from Bulgaria in 1984.14
IncQ plasmids have been frequently associated with β-lactam resistance determinants, e.g. blaCMY-4 in E. coli, blaVEB-18 in Vibrio spp., blaGES-1 in E. coli and Klebsiella pneumoniae, blaGES-5 in E. coli and blaBKC in K. pneumoniae.15–20 However, to our knowledge, this work is the first description of a plasmid-borne blaCTX-M gene in P. aeruginosa. Interestingly, an IncQ-like plasmid, named pWES-1, was found to encode another CTX-M-type ESBL (CTX-M-53) in strains of Salmonella enterica serotypes Westhampton and Stenftenberg recovered from cockles in France.21 However, pWES-1 does not show any significant sequence homology with pSTRAS1 (Figure 1).
While IncQ plasmids are known to be mobilized at high frequency to different hosts by a variety of conjugative plasmids (RP4, RK2, R68 and R751), we could not obtain transconjugants with helper plasmid pRK2013, suggesting that pPSTRAS1 has lost its capacity to be co-transmitted, at least under the conditions used.10 Whether disruption of orfF by gene cassette aadB accounts for this apparent defect is not clear. Deletion of orfE and orfF in a RSF1010 derivative was associated with a significant decrease in electro-transformation efficiency.13 Similarly, in our case very few transformants of E. coli DH5α could be obtained with pPSTRAS1 by electroporation. Compared with the parent strain E. coli DH5α, these transformants were more resistant to cefotaxime (>32 versus 0.016 mg/L), ceftazidime (4 versus 0.06 mg/L), aztreonam (16 versus 0.016 mg/L), ceftolozane/tazobactam (1/4 versus 0.25/4 mg/L), gentamicin (16 versus 0.25 mg/L), tobramycin (8 versus 0.5 mg/L) and amikacin (4 versus 0.25 mg/L), due to acquisition together with CTX-M-3 of enzymes APH-3′-VIb and ANT-2″ encoded by genes aphA6b and aadB, respectively. Because of the strong inhibitory activity of avibactam on CTX-M enzymes, resistance to ceftazidime/avibactam increased only 2-fold upon pPSTRAS1 transfer (0.125 versus 0.064 mg/L).
In conclusion, to the best of our knowledge, pPSTRAS1 is the first example of an IncQ1 plasmid found in P. aeruginosa that encodes a CTX-M β-lactamase. This finding further illustrates the potential of IncQ replicons to collect and propagate resistance genes in phylogenetically distant hosts. Evidence accumulates on the role of these broad-host-range vectors in global antibiotic resistance development.
Nucleotide sequence accession number
The whole nucleotide sequence of plasmid pPSTRAS1 was deposited in the GenBank nucleotide database under accession number MH463250.1.
Funding
The French National Reference Centre for Antibiotic Resistance is funded by the French Ministry of Health through the Santé publique France agency.
Transparency declarations
None to declare.



