-
PDF
- Split View
-
Views
-
Cite
Cite
Cathia Soulie, Maxime Grudé, Diane Descamps, Corinne Amiel, Laurence Morand-Joubert, Stéphanie Raymond, Coralie Pallier, Pantxika Bellecave, Sandrine Reigadas, Mary-Anne Trabaud, Constance Delaugerre, Brigitte Montes, Francis Barin, Virginie Ferré, Hélène Jeulin, Chakib Alloui, Sabine Yerly, Anne Signori-Schmuck, Aurélie Guigon, Samira Fafi-Kremer, Stéphanie Haïm-Boukobza, Audrey Mirand, Anne Maillard, Sophie Vallet, Catherine Roussel, Lambert Assoumou, Vincent Calvez, Philippe Flandre, Anne-Geneviève Marcelin, on behalf of the ANRS AC11 Resistance Study Group, Antiretroviral-treated HIV-1 patients can harbour resistant viruses in CSF despite an undetectable viral load in plasma, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 8, August 2017, Pages 2351–2354, https://doi.org/10.1093/jac/dkx128
Close - Share Icon Share
Abstract
Background: HIV therapy reduces the CSF HIV RNA viral load (VL) and prevents disorders related to HIV encephalitis. However, these brain disorders may persist in some cases. A large population of antiretroviral-treated patients who had a VL > 1.7 log10 copies/mL in CSF with detectable or undetectable VL in plasma associated with cognitive impairment was studied, in order to characterize discriminatory factors of these two patient populations.
Methods: Blood and CSF samples were collected at the time of neurological disorders for 227 patients in 22 centres in France and 1 centre in Switzerland. Genotypic HIV resistance tests were performed on CSF. The genotypic susceptibility score was calculated according to the last Agence Nationale de Recherche sur le Sida et les hépatites virales Action Coordonnée 11 (ANRS AC11) genotype interpretation algorithm.
Results: Among the 227 studied patients with VL > 1.7 log10 copies/mL in CSF, 195 had VL detectable in plasma [median (IQR) HIV RNA was 3.7 (2.7–4.7) log10 copies/mL] and 32 had discordant VL in plasma (VL < 1.7 log10 copies/mL). The CSF VL was lower (median 2.8 versus 4.0 log10 copies/mL; P < 0.001) and the CD4 cell count was higher (median 476 versus 214 cells/mm3; P < 0.001) in the group of patients with VL < 1.7 log10 copies/mL in plasma compared with patients with plasma VL > 1.7 log10 copies/mL. Resistance to antiretrovirals was observed in CSF for the two groups of patients.
Conclusions: Fourteen percent of this population of patients with cognitive impairment and detectable VL in CSF had well controlled VL in plasma. Thus, it is important to explore CSF HIV (VL and genotype) even if the HIV VL is controlled in plasma because HIV resistance may be observed.
Introduction
HIV disease can be associated with pathological changes in the brain resulting in HIV-associated neurocognitive disorders. Several studies have shown that cognitive impairment and brain injury may persist in chronically HIV-infected patients on ART.1,2 HIV-1 may enter the CNS rapidly after infection and continue to replicate due to failure of drug pressure in this compartment and progressive immune deficiency.3,4
In some HIV-1-infected patients, HIV-1 RNA viral load (VL) is higher in CSF than in plasma.5–7 The reason for this discordance remains to be determined. Furthermore, it was shown several years ago that small groups of HIV-infected patients experienced ‘CSF viral escape’. These patients had undetectable VL in plasma, but HIV was detected in CSF.5–7
This retrospective multicentre study supported by the Agence Nationale de Recherche sur le Sida et les hépatites virales (ANRS) aimed to evaluate a large population of antiretroviral-treated patients who had an HIV RNA VL >1.7 log10 copies/mL in CSF associated with cognitive impairment. In the analysis, two groups were considered in order to investigate their discriminatory factors: patients with detectable VL in plasma and patients with VL <1.7 log10 copies/mL in plasma.
Materials and methods
Patients
We studied all CSF/plasma pairs with detectable VL (>1.7 log10 copies/mL) in CSF between 2000 and 2013 in 22 centres in France and 1 centre in Switzerland. The indications for lumbar puncture are reserved for people affected by severe cognitive impairment without aetiological orientation (disabling cognitive impairment accompanied by motor dysfunction, speech problems and/or behavioural change). Socio-demographic and clinical data as well as treatment regimen were collected for all studied patients. Participating laboratories belong to ANRS and participate in the ANRS quality control assessment of HIV-1 drug resistance sequencing.8 The study was approved by the scientific committee of Action Coordonnée 11 of ANRS.
Genotyping resistance testing
The reverse transcriptase (RT) and protease resistance mutations were determined in each laboratory using the ANRS consensus technique (http://www.hivfrenchresistance.org), a Bayer TrueGene Kit, an Abbott ViroSeq Kit or an in-house method. The RT and protease mutations were identified from the latest IAS-USA resistance testing panel (http://www.iasusa.org) and were interpreted with the ANRS genotypic algorithm (http://www.hivfrenchresistance.org). According to the ANRS algorithm, the genotypic susceptibility score (GSS) of treatment was calculated on antiretrovirals currently available (n = 18) using a value of 1 for a susceptible drug and 0 for a resistant or possibly resistant drug. Antiretroviral penetration was estimated using the revised CNS penetration effectiveness score.9
Statistical methods
Quantitative variables are summarized as median and IQR and qualitative variables as percentages. Comparisons between independent groups were performed using the Kruskal–Wallis non-parametric test. No correction for multiple testing was made and the analysis was done with SAS (version 9.4).
Results
Overall, 227 patients (65% male, median age 45 years) were studied. Current and nadir CD4 cell counts were 230 (110–452) and 67 (24–165) cells/mm3, respectively. Overall, the median CSF HIV RNA was 3.8 (3.1–4.6) log10 copies/mL. Among the 227 patients, 195 had HIV RNA detectable in plasma [median HIV RNA was 3.7 (2.7–4.7) log10 copies/mL] and 32 had discordant VL (<1.7 log10 copies/mL in plasma) (Table 1). The clinical and virological factors were then compared between these two groups of patients.
| Characteristic . | Total population (n = 227) . | Patients with VL < 50 copies/mL in plasma (n = 32) . | Patients with VL > 50 copies/mL in plasma (n = 195) . | P value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 45 (39–52) | 47 (42–52) | 45 (39–52) | 0.229 |
| Male, % | 65.3 | 59.4 | 66.3 | 0.547 |
| B subtype in CSF, % | 54.3 | 59.4 | 53.3 | 0.570 |
| B subtype in plasma, % | 53.9 | 59.1 | 53.4 | 0.650 |
| CSF HIV-1 RNA (log10 copies/mL), median (IQR) | 3.84 (3.13–4.57) | 2.77 (2.05–3.34) | 3.99 (3.29–4.69) | 0.001 |
| Plasma HIV-1 RNA (log10 copies/mL), median (IQR) | 3.34 (2.32–4.48) | 1.6 (1.30–1.60) | 3.70 (2.73–4.69) | 0.001 |
| Nadir CD4 cell count (cells/mm3), median (IQR) | 67 (24–165) | 92 (53–175) | 63 (19–162) | 0.064 |
| CD4 cell count (cells/mm3), median (IQR) | 230 (110–452) | 476 (169–658) | 214 (96–407) | 0.001 |
| Current treatment, % | ||||
| NRTIs | 90.8 | 87.5 | 91.3 | 0.603 |
| NNRTIs | 12.8 | 15.6 | 12.3 | 0.603 |
| PIs | 86.8 | 78.1 | 88.2 | 0.499 |
| integrase inhibitors | 12.3 | 15.6 | 11.8 | 0.542 |
| other | 12.8 | 15.6 | 11.3 | 0.612 |
| GSS | 2 | 2 | 2 | 0.332 |
| Charter score | 7.5 | 8.5 | 7.5 | 0.347 |
| Characteristic . | Total population (n = 227) . | Patients with VL < 50 copies/mL in plasma (n = 32) . | Patients with VL > 50 copies/mL in plasma (n = 195) . | P value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 45 (39–52) | 47 (42–52) | 45 (39–52) | 0.229 |
| Male, % | 65.3 | 59.4 | 66.3 | 0.547 |
| B subtype in CSF, % | 54.3 | 59.4 | 53.3 | 0.570 |
| B subtype in plasma, % | 53.9 | 59.1 | 53.4 | 0.650 |
| CSF HIV-1 RNA (log10 copies/mL), median (IQR) | 3.84 (3.13–4.57) | 2.77 (2.05–3.34) | 3.99 (3.29–4.69) | 0.001 |
| Plasma HIV-1 RNA (log10 copies/mL), median (IQR) | 3.34 (2.32–4.48) | 1.6 (1.30–1.60) | 3.70 (2.73–4.69) | 0.001 |
| Nadir CD4 cell count (cells/mm3), median (IQR) | 67 (24–165) | 92 (53–175) | 63 (19–162) | 0.064 |
| CD4 cell count (cells/mm3), median (IQR) | 230 (110–452) | 476 (169–658) | 214 (96–407) | 0.001 |
| Current treatment, % | ||||
| NRTIs | 90.8 | 87.5 | 91.3 | 0.603 |
| NNRTIs | 12.8 | 15.6 | 12.3 | 0.603 |
| PIs | 86.8 | 78.1 | 88.2 | 0.499 |
| integrase inhibitors | 12.3 | 15.6 | 11.8 | 0.542 |
| other | 12.8 | 15.6 | 11.3 | 0.612 |
| GSS | 2 | 2 | 2 | 0.332 |
| Charter score | 7.5 | 8.5 | 7.5 | 0.347 |
| Characteristic . | Total population (n = 227) . | Patients with VL < 50 copies/mL in plasma (n = 32) . | Patients with VL > 50 copies/mL in plasma (n = 195) . | P value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 45 (39–52) | 47 (42–52) | 45 (39–52) | 0.229 |
| Male, % | 65.3 | 59.4 | 66.3 | 0.547 |
| B subtype in CSF, % | 54.3 | 59.4 | 53.3 | 0.570 |
| B subtype in plasma, % | 53.9 | 59.1 | 53.4 | 0.650 |
| CSF HIV-1 RNA (log10 copies/mL), median (IQR) | 3.84 (3.13–4.57) | 2.77 (2.05–3.34) | 3.99 (3.29–4.69) | 0.001 |
| Plasma HIV-1 RNA (log10 copies/mL), median (IQR) | 3.34 (2.32–4.48) | 1.6 (1.30–1.60) | 3.70 (2.73–4.69) | 0.001 |
| Nadir CD4 cell count (cells/mm3), median (IQR) | 67 (24–165) | 92 (53–175) | 63 (19–162) | 0.064 |
| CD4 cell count (cells/mm3), median (IQR) | 230 (110–452) | 476 (169–658) | 214 (96–407) | 0.001 |
| Current treatment, % | ||||
| NRTIs | 90.8 | 87.5 | 91.3 | 0.603 |
| NNRTIs | 12.8 | 15.6 | 12.3 | 0.603 |
| PIs | 86.8 | 78.1 | 88.2 | 0.499 |
| integrase inhibitors | 12.3 | 15.6 | 11.8 | 0.542 |
| other | 12.8 | 15.6 | 11.3 | 0.612 |
| GSS | 2 | 2 | 2 | 0.332 |
| Charter score | 7.5 | 8.5 | 7.5 | 0.347 |
| Characteristic . | Total population (n = 227) . | Patients with VL < 50 copies/mL in plasma (n = 32) . | Patients with VL > 50 copies/mL in plasma (n = 195) . | P value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 45 (39–52) | 47 (42–52) | 45 (39–52) | 0.229 |
| Male, % | 65.3 | 59.4 | 66.3 | 0.547 |
| B subtype in CSF, % | 54.3 | 59.4 | 53.3 | 0.570 |
| B subtype in plasma, % | 53.9 | 59.1 | 53.4 | 0.650 |
| CSF HIV-1 RNA (log10 copies/mL), median (IQR) | 3.84 (3.13–4.57) | 2.77 (2.05–3.34) | 3.99 (3.29–4.69) | 0.001 |
| Plasma HIV-1 RNA (log10 copies/mL), median (IQR) | 3.34 (2.32–4.48) | 1.6 (1.30–1.60) | 3.70 (2.73–4.69) | 0.001 |
| Nadir CD4 cell count (cells/mm3), median (IQR) | 67 (24–165) | 92 (53–175) | 63 (19–162) | 0.064 |
| CD4 cell count (cells/mm3), median (IQR) | 230 (110–452) | 476 (169–658) | 214 (96–407) | 0.001 |
| Current treatment, % | ||||
| NRTIs | 90.8 | 87.5 | 91.3 | 0.603 |
| NNRTIs | 12.8 | 15.6 | 12.3 | 0.603 |
| PIs | 86.8 | 78.1 | 88.2 | 0.499 |
| integrase inhibitors | 12.3 | 15.6 | 11.8 | 0.542 |
| other | 12.8 | 15.6 | 11.3 | 0.612 |
| GSS | 2 | 2 | 2 | 0.332 |
| Charter score | 7.5 | 8.5 | 7.5 | 0.347 |
The CSF VL was lower (2.8 versus 4.0 log10 copies/mL; P < 0.001) and the CD4 cell count was higher (476 versus 214 cells/mm3; P < 0.001) in the group of patients with VL <1.7 log10 copies/mL in plasma compared with patients with plasma VL >1.7 log10 copies/mL (Table 1). The nadir CD4 tended to be higher (92 versus 63 cells/mm3; P = 0.064) in the group of patients with undetectable VL in plasma. No difference was observed between patients with plasma HIV VL <1.7 and >1.7 log10 copies/mL for GSS and Charter score (Table 1). The presence of HIV CSF resistance for at least one antiretroviral drug was detected for 75% (24/32) and 78% (152/195) of patients with plasma HIV VL <1.7 and >1.7 log10 copies/mL. The details of HIV resistance are described for the two groups of patients in Figure 1.
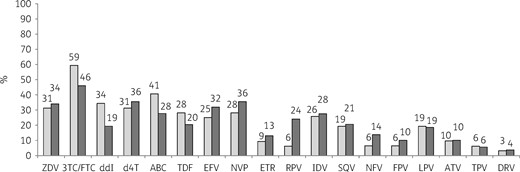
HIV-1 antiretroviral resistance in CSF for patients with HIV RNA VL < 1.7 and >1.7 log10 copies/mL in plasma. Light grey bars, patients with VL < 1.7 log10 copies/mL in plasma (n = 32); dark grey bars, patients with VL > 1.7 log10 copies/mL in plasma (n = 195). ZDV, zidovudine; 3TC, lamivudine; FTC, emtricitabine; ddI, didanosine; d4T, stavudine; ABC, abacavir; TDF, tenofovir; EFV, efavirenz; NVP, nevirapine; ETR, etravirine; RPV, rilpivirine; IDV, indinavir, SQV, saquinavir; NFV, nelfinavir; FPV, fosamprenavir; LPV, lopinavir; ATV, atazanavir; TPV, tipranavir; DRV, darunavir.
Discussion
In this multicentre study, among 227 HIV-1 patients with neurological disorders and HIV-1 RNA VL >1.7 log10 copies/mL in CSF, 32 (14%) patients presented virological discordance with an undetectable VL in plasma. This rate of discordance is similar to other reports from clinical settings.5,6
The VL observed in CSF in the group of patients with HIV VL <1.7 log10 copies/mL in plasma was lower than that in patients with detectable VL in plasma, probably due to HIV local replication (sanctuary site). For the patients with detectable VL in both compartments, the CSF VL probably reflected HIV crossing the blood–brain barrier from circulating blood. The CSF/plasma VL discordance could also be associated with an increased expression of host inflammatory mediators in the CSF of discordant patients in comparison with non-discordant patients.10 It could be interesting to study the microglial activation that is a hallmark of neuro-inflammation and to evaluate a potential role in HIV neurological disorders.11
The CD4 cell count at the time of lumbar puncture was higher for patients with discordant VL than in patients with detectable VL in both compartments. This result was in accordance with the fact that patients with discordant VL had a plasma VL <1.7 log10 copies/mL. Indeed, the ART was effective and allowed CD4 recovery.
The genotypic resistance testing in CSF for all included patients in this study revealed that patients with detectable or undetectable VL in plasma could have HIV antiretroviral resistance in CSF whatever their VL in plasma. It is important to note that despite an undetectable VL in plasma, the virus in CSF may continue to evolve and develop some resistance to antiretrovirals and become less susceptible to treatment.12,13 An implication for clinical practice is the need to perform an HIV genotypic resistance test.14 There was evidence for a correspondence between the drug’s penetration properties and its CNS efficacy during ART, but ART should not be based only on the Charter score but also on HIV genotypic resistance testing.9,15,16 Indeed, changing ART based on the CSF resistance genotype has led to clinical improvement.7,17 Furthermore, it would be interesting to keep studying this type of cohort to evaluate the impact of the most recent antiretrovirals.
Higher CNS penetration effectiveness scores correlate with lower CSF viraemia and a CNS penetration effectiveness score >6 is thus recommended to achieve control of HIV replication in CNS.18 In our study, the patients of the two studied groups had a similar CNS penetration effectiveness score, >6. Thus, the replication in the CNS was not explained by a Charter score that was too low.
In conclusion, 14% of patients with cognitive impairment and HIV RNA >1.7 log10 copies/mL in CSF had well controlled VL in plasma. Thus, it is important to explore HIV CSF (VL and genotype) even if the HIV VL is controlled in plasma because HIV resistance may be observed. Optimization of ART could be necessary, using fully active drugs with improved CNS penetration.
Acknowledgements
This work was presented at the Twenty-fourth Conference on Retroviruses and Opportunistic Infections, Seattle, WA, USA, 2017 (Poster 349).
ANRS AC11 Resistance Study Group
Aix en Provence (Dr E. Lagier); Amiens (Dr C. Roussel); Angers (Dr H. Le Guillou); Avicenne (Dr C. Alloui); Besançon (Dr D. Bettinger); Bicêtre (Dr C. Pallier); Bordeaux (Pr H. Fleury, Dr S. Reigadas, Dr P. Bellecave, Dr P. Recordon-Pinson); Brest (Pr C. Payan, Dr S. Vallet); Caen (Pr A. Vabret); Clermont-Ferrand (Dr C. Henquell, Dr A. Mirand); Créteil (Dr M. Bouvier-Alias); Dijon (Dr A. de Rougemont); Fort de France (Dr G. Dos Santos); Grenoble (Pr P. Morand, Dr A. Signori-Schmuck); Lille (Dr L. Bocket); Limoges (Pr S. Rogez); Lyon (Dr P. Andre, Dr J. C. Tardy, Dr M. A. Trabaud); Marseille (Dr C. Tamalet); Metz-Thionville (Dr C. Delamare); Montpellier (Dr B. Montes); Nancy (Pr E. Schvoerer); Nantes (Pr V. Ferré, Dr E. André-Garnier); Nice (Dr J. Cottalorda); Orléans (Dr J. Guinard, Dr A. Guiguon); Paris-Bichat Claude Bernard (Pr D. Descamps, Pr F. Brun-Vézinet, Dr C. Charpentier, Dr B. Visseaux, Dr G. Peytavin); Paris-Cochin (Dr A. Krivine); Paris-HEGP (Dr A. Si-Mohamed); Paris-Necker (Dr V. Avettand-Fenoel); Paris-Pitié-Salpêtrière (Pr A. G. Marcelin, Pr V. Calvez, Dr S. Lambert-Niclot, Dr C. Soulié, Dr M. Wirden); Paris Saint-Antoine (Dr L. Morand-Joubert); Paris-Saint Louis (Pr C. Delaugerre, Dr M. L. Chaix); Paris-Tenon (Dr C. Amiel, Dr V. Schneider); Poitiers (Dr G. Giraudeau); Reims (Dr V. Brodard); Rennes (Dr A. Maillard); Rouen (Pr J. C. Plantier); Saint Denis (Dr C. Chaplain); Saint Etienne (Dr T. Bourlet); Strasbourg (Pr S. Fafi-Kremer, Pr F. Stoll-Keller, Dr M. P. Schmitt, Dr H. Barth); Suisse (Dr S. Yerly); Toulon (Dr C. Poggi); Toulouse (Pr J. Izopet, Dr S. Raymond); Tours (Pr F. Barin, Dr A. Chaillon); Versailles (Dr S. Marque-Juillet); Villejuif (Pr A. M. Roque-Afonso, Dr S. Haïm-Boukobza); INSERM UMR-S1136 (Dr P. Flandre, M. Grudé, Dr L. Assoumou, Dr D. Costagliola).
ANRS Clinical Centres
Aix en Provence (Dr T. Allegre); Amiens (Pr J. L. Schmit); Angers (Dr J. M. Chennebault); Avicenne (Pr O. Bouchaud); Besançon (Pr N. Magy-Bertrand); Bicêtre (Pr J. F. Delfraissy); Bordeaux (Pr M. Dupon, Pr P. Morlat, Pr D. Neau); Brest (Pr S. Ansart, Dr S. Jaffuel); Caen (Pr R. Verdon); Clermont-Ferrand (Dr C. Jacomet); Créteil (Pr Y. Lévy, Dr S. Dominguez); Dijon (Pr P. Chavanet; Pr L. Piroth); Fort de France (Dr A. Cabié); Grenoble (Dr P. Leclercq); Lille-Tourcoing (Dr F. Ajana, Dr A. Cheret); Limoges (Pr P. Weinbreck); Lyon (Dr L. Cotte); Marseille (Dr I. Poizot-Martin, Dr I. Ravaud); Metz-Thionville (Dr B. Christian, Dr F. Truchetet, Dr M. Grandidier); Montpellier (Pr J. Reynes); Nancy (Pr T. May, Dr F. Goehringer); Nantes (Pr F. Raffi); Nice (Pr P. Dellamonica); Orléans (Dr T. Prazuck, Dr L. Hocqueloux); Paris-Bichat Claude Bernard (Dr R. Landman, Pr Yazdanpanah); Paris-Cochin (Dr O. Launay); Paris-HEGP (Pr L. Weiss); Paris-Necker (Dr J. P. Viard); Paris-Pitié-Salpêtrière (Pr C. Katlama, Dr A. Simon); Paris-Saint Antoine (Pr P. M. Girard, Dr J. L. Meynard); Paris-Saint Louis (Pr J. M. Molina); Paris Tenon (Pr G. Pialoux); Pointe-à-Pitre (Pr B. Hoen, Dr M.T. Goeger-Sow, Dr I. Lamaury, Dr G. Beaucaire); Reims (Pr R. Jaussaud, Dr C. Rouger); Rennes (Pr C. Michelet); Rouen (Dr F. Borsa-Lebas, Pr F. Caron); Saint Denis (Dr M. A. Khuong); Saint Etienne (Pr F. Lucht); Strasbourg (Dr D. Rey); Suisse (Dr A. Calmy); Toulon (Dr A. Lafeuillade); Toulouse (Pr B. Marchou); Tours (Dr G. Gras), Versailles (Dr A. Greder-Belan); Villejuif (Pr D. Vittecoq, Dr E. Teicher).
Funding
The work was supported by the Agence Nationale de Recherche sur le Sida et les hépatites virales.
Transparency declarations
None to declare.
References
Author notes
Members are listed in the Acknowledgements section.



