-
PDF
- Split View
-
Views
-
Cite
Cite
M. Doumith, J. Findlay, H. Hirani, K. L. Hopkins, D. M. Livermore, A. Dodgson, N. Woodford, Major role of pKpQIL-like plasmids in the early dissemination of KPC-type carbapenemases in the UK, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 8, August 2017, Pages 2241–2248, https://doi.org/10.1093/jac/dkx141
Close - Share Icon Share
Abstract
Objectives:Klebsiella pneumoniae carbapenemase (KPC)-producing Enterobacteriaceae were first seen in the UK in 2003 and have been increasingly reported since 2010, largely owing to an ongoing outbreak in North-West England. We examined the role of clonal spread and plasmid transmission in their emergence.
Methods: Isolates comprised KPC-positive K. pneumoniae (n = 33), Escherichia coli (n = 7) and Enterobacter spp. (n = 4) referred to the national reference laboratory between 2008 and 2010 from 17 UK centres, including three in North-West England. Isolates were typed by MLST. Plasmids were transferred by electroporation and characterized by PCR or sequencing. PCR screening assays were developed to distinguish plasmid pKpQIL variants.
Results: The K. pneumoniae isolates included 10 STs, of which three belonged to clonal group (CG) 258. CG258 (n = 19) isolates were detected in 13 centres but accounted for only 7/19 (36.8%) of those from North-West England. Most KPC-producers (37/44, 84.1%), including 16/19 CG258 K. pneumoniae, carried blaKPC on IncFIIK2 plasmids. Sequencing of a subset of these plasmids (n = 11) revealed similarities with published pKpQIL. One variant, pKpQIL-UK [identified in K. pneumoniae CG258 (n = 5) and ST468 (n = 1) isolates from distinct centres] had only a few nucleotide changes from classical pKpQIL, whereas pKpQIL-D1 (n = 1) and pKpQIL-D2 (n = 4), from isolates of various species in the North-West, harboured large variations, reflecting replacement of the partitioning and replication functions and potentially thereby facilitating spread. PCR revealed that 36/37 (97.3%) IncFIIK2-type plasmids in KPC-positive isolates had pKpQIL markers.
Conclusions: pKpQIL-like plasmids played a major role in the early dissemination of KPC enzymes in the UK.
Introduction
Klebsiella pneumoniae carbapenemase (KPC) enzymes are geographically widespread and increasingly prevalent.1,2 The family includes 23 variants (KPC-2 to KPC-24), with KPC-2 and KPC-3 being globally predominant. They are mainly associated with K. pneumoniae, and in particular with the ST258 lineage, although production by other Enterobacteriaceae, Pseudomonas and Acinetobacter spp. is increasingly reported.3–8,K. pneumoniae with KPC enzymes have been endemic in the United States since the late 1990s with later, dramatic, spread in, for example, Israel, Greece and Italy.9–13 The first KPC enzyme identified in the UK was KPC-4, found in 2003 in an Enterobacter cloacae complex isolate from Scotland.14 Since then, KPC-positive organisms have been occasionally reported in various part of the country, with the first KPC-carrying ST258 K. pneumoniae isolate from Scotland in 2007.15 Numbers of KPC-positive isolates rose substantially from 2010, largely due to an outbreak centred on the Greater Manchester area in North-West England.16 This outbreak remains ongoing 6 years later. In contrast to most international KPC problems, which are largely associated with the ST258 K. pneumoniae clone, the North-West England outbreak is unusual in being polyclonal: its KPC-positive K. pneumoniae isolates have diverse PFGE profiles and belong to multiple MLST types, and the ‘outbreak’ also includes KPC-positive isolates belonging to other Enterobacteriaceae species, principally Enterobacter spp. and Escherichia coli.1,K. pneumoniae clonal group (CG) 258 is dominant among KPC-positive isolates from elsewhere in the UK.17
The first fully sequenced plasmid encoding a KPC enzyme was pKpQIL, a self-conjugative IncFIIK2 replicon-type element from a K. pneumoniae ST258 isolate collected in Israel.18 Later studies have suggested a major role for pKpQIL-like plasmids in the dissemination of KPC enzymes in Israel, Italy, Greece and the United States.19–21
In this study, 44 KPC-positive isolates referred to PHE’s Antimicrobial Resistance and Healthcare Associated Infections (AMRHAI) Reference Unit in the early spread (2008–10) of KPC enzymes in the UK were investigated, to examine the role of pKpQIL-like plasmids in their emergence.
Materials and methods
Clinical isolates and transformants
Isolates (n = 44) comprised KPC-positive K. pneumoniae (n = 33), E. coli (n = 7) and Enterobacter spp. (n = 4) referred to PHE’s AMRHAI Reference Unit between 2008 and 2010 from 17 centres in the UK, including three in the Greater Manchester area (Table 1). They comprised 19 of 27 geographically scattered isolates examined by the Unit between 2008 and 2010 and 25 representatives (out of the 214 referred) from the start of the North-West England outbreak, all collected in 2010. Plasmids were extracted by an alkaline lysis method and were transferred by electroporation into E. coli α-Select Strain (Bioline, London, UK) using a GenePulser electroporator (Bio-Rad, Hemel Hempstead, UK). Transformants were selected on Luria-Bertani agar supplemented with 1 mg/L ertapenem.
| . | . | . | . | . | . | . | . | pKpQIL PCR . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate . | Species . | Year . | ST . | Centre . | KPC variant . | Tn4401 isoform . | Replicon type . | parB-O . | KPC . | rplQ . | traK . | traI . | parB-D2 . | hyp . | interpretation . |
| T1 | K. pneumoniae | 2008 | 512 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T2 | K. pneumoniae | 2008 | 258 (CG258) | B | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T3 | K. pneumoniae | 2008 | 258 (CG258) | C | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T4 | K. pneumoniae | 2008 | 258 (CG258) | D | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T5 | K. pneumoniae | 2009 | 258 (CG258) | E | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T6 | K. pneumoniae | 2009 | 258 (CG258) | F | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L16 | K. pneumoniae | 2009 | 258 (CG258) | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T8 | K. pneumoniae | 2009 | 258 (CG258) | G | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T9 | Enterobacter spp. | 2009 | ND | H | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T10 | K. pneumoniae | 2009 | 258 (CG258) | I | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T11 | K. pneumoniae | 2009 | 258 (CG258) | NW-C3 | 2 | a | IncFIIK5 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T12 | K. pneumoniae | 2009 | 258 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T13 | K. pneumoniae | 2009 | 11 (CG258) | J | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T14 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 3 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T15 | K. pneumoniae | 2010 | 321 | E | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T16 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T17 | K. pneumoniae | 2010 | 248 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T18 | K. pneumoniae | 2010 | 321 | K | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T19 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T20 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T21 | E. coli | 2010 | ND | NW-C1 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T22 | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | unknown | |||||
| T23 | Enterobacter spp. | 2010 | ND | L | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T24 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T25 | K. pneumoniae | 2010 | 27 | NW-C3 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T26 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T27 | K. pneumoniae | 2010 | 258 (CG258) | M | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T28 | K. pneumoniae | 2010 | 258 (CG258) | C | 3 | a | IncFIIK1 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T29 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T30 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T31 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T32 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T33 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L19 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| L22 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L23 | K. pneumoniae | 2010 | 25 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L27 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L33 | K. pneumoniae | 2010 | 468 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L37 | K. pneumoniae | 2010 | 491 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L38 | K. pneumoniae | 2010 | 490 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L39 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| LENT | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| LESC | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| I2 | E. coli | 2010 | ND | N | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| . | . | . | . | . | . | . | . | pKpQIL PCR . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate . | Species . | Year . | ST . | Centre . | KPC variant . | Tn4401 isoform . | Replicon type . | parB-O . | KPC . | rplQ . | traK . | traI . | parB-D2 . | hyp . | interpretation . |
| T1 | K. pneumoniae | 2008 | 512 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T2 | K. pneumoniae | 2008 | 258 (CG258) | B | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T3 | K. pneumoniae | 2008 | 258 (CG258) | C | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T4 | K. pneumoniae | 2008 | 258 (CG258) | D | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T5 | K. pneumoniae | 2009 | 258 (CG258) | E | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T6 | K. pneumoniae | 2009 | 258 (CG258) | F | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L16 | K. pneumoniae | 2009 | 258 (CG258) | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T8 | K. pneumoniae | 2009 | 258 (CG258) | G | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T9 | Enterobacter spp. | 2009 | ND | H | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T10 | K. pneumoniae | 2009 | 258 (CG258) | I | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T11 | K. pneumoniae | 2009 | 258 (CG258) | NW-C3 | 2 | a | IncFIIK5 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T12 | K. pneumoniae | 2009 | 258 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T13 | K. pneumoniae | 2009 | 11 (CG258) | J | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T14 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 3 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T15 | K. pneumoniae | 2010 | 321 | E | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T16 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T17 | K. pneumoniae | 2010 | 248 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T18 | K. pneumoniae | 2010 | 321 | K | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T19 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T20 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T21 | E. coli | 2010 | ND | NW-C1 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T22 | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | unknown | |||||
| T23 | Enterobacter spp. | 2010 | ND | L | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T24 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T25 | K. pneumoniae | 2010 | 27 | NW-C3 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T26 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T27 | K. pneumoniae | 2010 | 258 (CG258) | M | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T28 | K. pneumoniae | 2010 | 258 (CG258) | C | 3 | a | IncFIIK1 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T29 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T30 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T31 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T32 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T33 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L19 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| L22 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L23 | K. pneumoniae | 2010 | 25 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L27 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L33 | K. pneumoniae | 2010 | 468 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L37 | K. pneumoniae | 2010 | 491 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L38 | K. pneumoniae | 2010 | 490 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L39 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| LENT | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| LESC | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| I2 | E. coli | 2010 | ND | N | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
Isolates with sequenced KPC-encoding plasmids are shown in bold. An asterisk indicates plasmids failing to amplify any parB fragments. ND, not determined; NT, not tested. Centres in North-West England have codes starting NW-C; the other centres (A–N) were elsewhere in the UK.
| . | . | . | . | . | . | . | . | pKpQIL PCR . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate . | Species . | Year . | ST . | Centre . | KPC variant . | Tn4401 isoform . | Replicon type . | parB-O . | KPC . | rplQ . | traK . | traI . | parB-D2 . | hyp . | interpretation . |
| T1 | K. pneumoniae | 2008 | 512 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T2 | K. pneumoniae | 2008 | 258 (CG258) | B | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T3 | K. pneumoniae | 2008 | 258 (CG258) | C | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T4 | K. pneumoniae | 2008 | 258 (CG258) | D | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T5 | K. pneumoniae | 2009 | 258 (CG258) | E | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T6 | K. pneumoniae | 2009 | 258 (CG258) | F | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L16 | K. pneumoniae | 2009 | 258 (CG258) | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T8 | K. pneumoniae | 2009 | 258 (CG258) | G | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T9 | Enterobacter spp. | 2009 | ND | H | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T10 | K. pneumoniae | 2009 | 258 (CG258) | I | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T11 | K. pneumoniae | 2009 | 258 (CG258) | NW-C3 | 2 | a | IncFIIK5 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T12 | K. pneumoniae | 2009 | 258 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T13 | K. pneumoniae | 2009 | 11 (CG258) | J | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T14 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 3 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T15 | K. pneumoniae | 2010 | 321 | E | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T16 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T17 | K. pneumoniae | 2010 | 248 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T18 | K. pneumoniae | 2010 | 321 | K | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T19 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T20 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T21 | E. coli | 2010 | ND | NW-C1 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T22 | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | unknown | |||||
| T23 | Enterobacter spp. | 2010 | ND | L | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T24 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T25 | K. pneumoniae | 2010 | 27 | NW-C3 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T26 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T27 | K. pneumoniae | 2010 | 258 (CG258) | M | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T28 | K. pneumoniae | 2010 | 258 (CG258) | C | 3 | a | IncFIIK1 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T29 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T30 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T31 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T32 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T33 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L19 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| L22 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L23 | K. pneumoniae | 2010 | 25 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L27 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L33 | K. pneumoniae | 2010 | 468 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L37 | K. pneumoniae | 2010 | 491 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L38 | K. pneumoniae | 2010 | 490 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L39 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| LENT | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| LESC | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| I2 | E. coli | 2010 | ND | N | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| . | . | . | . | . | . | . | . | pKpQIL PCR . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isolate . | Species . | Year . | ST . | Centre . | KPC variant . | Tn4401 isoform . | Replicon type . | parB-O . | KPC . | rplQ . | traK . | traI . | parB-D2 . | hyp . | interpretation . |
| T1 | K. pneumoniae | 2008 | 512 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T2 | K. pneumoniae | 2008 | 258 (CG258) | B | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T3 | K. pneumoniae | 2008 | 258 (CG258) | C | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T4 | K. pneumoniae | 2008 | 258 (CG258) | D | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T5 | K. pneumoniae | 2009 | 258 (CG258) | E | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T6 | K. pneumoniae | 2009 | 258 (CG258) | F | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L16 | K. pneumoniae | 2009 | 258 (CG258) | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T8 | K. pneumoniae | 2009 | 258 (CG258) | G | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T9 | Enterobacter spp. | 2009 | ND | H | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T10 | K. pneumoniae | 2009 | 258 (CG258) | I | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T11 | K. pneumoniae | 2009 | 258 (CG258) | NW-C3 | 2 | a | IncFIIK5 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T12 | K. pneumoniae | 2009 | 258 (CG258) | A | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T13 | K. pneumoniae | 2009 | 11 (CG258) | J | 3 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T14 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 3 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T15 | K. pneumoniae | 2010 | 321 | E | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T16 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T17 | K. pneumoniae | 2010 | 248 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T18 | K. pneumoniae | 2010 | 321 | K | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T19 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T20 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T21 | E. coli | 2010 | ND | NW-C1 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T22 | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | unknown | |||||
| T23 | Enterobacter spp. | 2010 | ND | L | 4 | b | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T24 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T25 | K. pneumoniae | 2010 | 27 | NW-C3 | 2 | a | ND | NT | NT | NT | NT | NT | NT | NT | unknown |
| T26 | K. pneumoniae | 2010 | 321 | NW-C3 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T27 | K. pneumoniae | 2010 | 258 (CG258) | M | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T28 | K. pneumoniae | 2010 | 258 (CG258) | C | 3 | a | IncFIIK1 | NT | NT | NT | NT | NT | NT | NT | unknown |
| T29 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T30 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| T31 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| T32 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| T33 | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L19 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| L22 | K. pneumoniae | 2010 | 11 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L23 | K. pneumoniae | 2010 | 25 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L27 | K. pneumoniae | 2010 | 321 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L33 | K. pneumoniae | 2010 | 468 | NW-C2 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L37 | K. pneumoniae | 2010 | 491 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
| L38 | K. pneumoniae | 2010 | 490 | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| L39 | K. pneumoniae | 2010 | 258 (CG258) | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | pKpQIL-like* | ||
| LENT | Enterobacter spp. | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| LESC | E. coli | 2010 | ND | NW-C1 | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like (D2) | |
| I2 | E. coli | 2010 | ND | N | 2 | a | IncFIIK2 | + | + | + | + | + | + | pKpQIL-like | |
Isolates with sequenced KPC-encoding plasmids are shown in bold. An asterisk indicates plasmids failing to amplify any parB fragments. ND, not determined; NT, not tested. Centres in North-West England have codes starting NW-C; the other centres (A–N) were elsewhere in the UK.
Antimicrobial susceptibility testing and molecular characterization of KPC-producing isolates
MICs were determined by BSAC agar dilution22 and with results interpreted according to EUCAST guidelines (http://www.eucast.org/clinical_breakpoints). Conventional MLST was performed as detailed on the K. pneumoniae MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html). The blaKPC gene and its direct environment were amplified as previously described23 and sequenced using an ABI Genetic Analyser capillary platform 3130XL (Applied Biosystems, CA, USA). Plasmids were classified using PCR-based replicon typing (PBRT);24 IncFII replicons were subgrouped by replicon sequence typing (RST).25
Plasmid sequencing and bioinformatics analyses
The complete DNA sequences of 11 KPC-encoding plasmids from isolates randomly selected to proportionally represent the distribution of isolates from the outbreak (6/25, 24%) and other UK regions (5/19, 26%), were obtained using a 454-Genome Sequencer FLX (Roche, Branford, CT, USA) on libraries generated using plasmid DNA purified from E. coli α-select transformants according to the standard protocol for whole-genome shotgun sequencing, producing 250 bp reads. A draft assembly was produced de novo with Newbler 2.6 (Roche, Branford, CT, USA); plasmid sequences were further cleaned from residual genomic contigs by in silico subtraction of the host E. coli published genome sequences (E. coli str. K12 substr. DH10B, Genbank CP000948).
Based on BLAST homologies, the published sequence of the archetypal pKpQIL plasmid (NC_014016) was used as a reference to identify nucleotide variations with Newbler 2.6. Single nucleotide polymorphisms and indels were inspected manually and those located in regions of homopolymers consisting of more than six units were excluded from the analysis. The MAUVE program (http://darlinglab.org/mauve/mauve.html) was used to reorder assembled contigs according to the pKpQIL sequence. Remaining gaps were closed by standard PCR and sequencing using manually designed primers. Coding sequences were identified using Glimmer 2.13 (http://www.cs.jhu.edu/∼genomics/Glimmer/) and gene functions were inferred based on homology searches with BLAST. Sequence homologies with published plasmids were checked by BLAST using the Blast Ring Image Generator (BRIG) software.26 The reference plasmids included: (i) pKpQIL (GenBank NC_014016), pGR-1504 (KF874496), pIT-01C03 (HG969995), pKp41 (CP012000), pKpQIL-10 (KJ146687), pKpQIL-531 (CP008833), pKpQIL-6e6 (CP014650), pUHKPC07 (CP011986), pUHKPC33 (CP011991), pG12-KPC2 (KU665642), pIT-01C22 (HG969997), pKpQIL-IT (JN233705), pGR-1870 (KF874498), pKPN207_p2(LT216438) and pIT-11C07 (HG969998), all of which were previously reported from CG258 K. pneumoniae isolates; (ii) pGR-3913 (KF874499), pKpQIL-9b8 (CP014765), pGR-1780 (KF874497) and pKpQIL-234 (KJ146689), which were variously reported from K. pneumoniae isolates belonging to ST35, ST37, ST147 and ST234, respectively; and (iii) pBK33689 (KU295133), pKpQIL-571 (CP014669) and pKpQIL-Ec (KJ146688), all of which were reported from E. coli.
The complete nucleotide sequences of plasmids pKpQIL-UK, pKpQIL-D1 and pKpQIL-D2 generated in this study were submitted to GenBank under the accession numbers KY798507, KY798505 and KY798506, respectively.
Detection of pKpQIL-like IncFIIK2 plasmids by PCR
PCR primers were designed to amplify size-distinguishable fragments from six markers on pKpQIL-like backbones; these markers were selected based on the comparison of available plasmid sequences (Table 2). They covered four distinct regions of the pKpQIL-backbone, comprising: (i) both the traI and traK genes encoding the transfer-conjugation functions; (ii) the blaKPC carbapenemase gene; and (iii) a gene encoding a conserved hypothetical protein (Table 1). Primers targeting parB of pKpQIL and its homologue in pKpQIL-D2 were added to differentiate between these two plasmid variants. The 50S ribosomal protein gene rplQ was targeted as an internal PCR control from a chromosomal region conserved among Enterobacteriaceae (Table 2).
| Target . | Primer . | Sequence . | Size (bp) . |
|---|---|---|---|
| hyp | hyp-for | GGTCAGAAAATCACGTCTGAA | 412 |
| hyp-rev | CTCACCGTGAATGTCATAGC | ||
| parB-D2 | parBD2-for | GTAAGACCTTCGTAAACCAGGA | 315 |
| parBD2-rev | AAGAGCGATCAATCTCAGGC | ||
| traI | traI-for | TCGTTGCTCTCGTGTTTTTC | 247 |
| traI-Rev | GGTGAAACCAGAATGACCAC | ||
| traK | traK-for | CAGGCAAATATTGCCGTGAG | 203 |
| traK-rev | GCACGAATGGAGAAGTTCAG | ||
| rplQ | IC-for | ATGCGCCATCGTAAGAGTGGT | 170 |
| IC-rev | GTCTTGGCAAGAGTAATCAGCGG | ||
| blaKPC | KPC-for | GCTTGCTGGACACACCCAT | 127 |
| KPC-rev | ATCACTGTATTGCACGGCG | ||
| parB-O | parB-for | ACCTATGAATTTGCCCGTCT | 91 |
| parB-rev | TTTCGAAGGACTGCATGTTG |
| Target . | Primer . | Sequence . | Size (bp) . |
|---|---|---|---|
| hyp | hyp-for | GGTCAGAAAATCACGTCTGAA | 412 |
| hyp-rev | CTCACCGTGAATGTCATAGC | ||
| parB-D2 | parBD2-for | GTAAGACCTTCGTAAACCAGGA | 315 |
| parBD2-rev | AAGAGCGATCAATCTCAGGC | ||
| traI | traI-for | TCGTTGCTCTCGTGTTTTTC | 247 |
| traI-Rev | GGTGAAACCAGAATGACCAC | ||
| traK | traK-for | CAGGCAAATATTGCCGTGAG | 203 |
| traK-rev | GCACGAATGGAGAAGTTCAG | ||
| rplQ | IC-for | ATGCGCCATCGTAAGAGTGGT | 170 |
| IC-rev | GTCTTGGCAAGAGTAATCAGCGG | ||
| blaKPC | KPC-for | GCTTGCTGGACACACCCAT | 127 |
| KPC-rev | ATCACTGTATTGCACGGCG | ||
| parB-O | parB-for | ACCTATGAATTTGCCCGTCT | 91 |
| parB-rev | TTTCGAAGGACTGCATGTTG |
| Target . | Primer . | Sequence . | Size (bp) . |
|---|---|---|---|
| hyp | hyp-for | GGTCAGAAAATCACGTCTGAA | 412 |
| hyp-rev | CTCACCGTGAATGTCATAGC | ||
| parB-D2 | parBD2-for | GTAAGACCTTCGTAAACCAGGA | 315 |
| parBD2-rev | AAGAGCGATCAATCTCAGGC | ||
| traI | traI-for | TCGTTGCTCTCGTGTTTTTC | 247 |
| traI-Rev | GGTGAAACCAGAATGACCAC | ||
| traK | traK-for | CAGGCAAATATTGCCGTGAG | 203 |
| traK-rev | GCACGAATGGAGAAGTTCAG | ||
| rplQ | IC-for | ATGCGCCATCGTAAGAGTGGT | 170 |
| IC-rev | GTCTTGGCAAGAGTAATCAGCGG | ||
| blaKPC | KPC-for | GCTTGCTGGACACACCCAT | 127 |
| KPC-rev | ATCACTGTATTGCACGGCG | ||
| parB-O | parB-for | ACCTATGAATTTGCCCGTCT | 91 |
| parB-rev | TTTCGAAGGACTGCATGTTG |
| Target . | Primer . | Sequence . | Size (bp) . |
|---|---|---|---|
| hyp | hyp-for | GGTCAGAAAATCACGTCTGAA | 412 |
| hyp-rev | CTCACCGTGAATGTCATAGC | ||
| parB-D2 | parBD2-for | GTAAGACCTTCGTAAACCAGGA | 315 |
| parBD2-rev | AAGAGCGATCAATCTCAGGC | ||
| traI | traI-for | TCGTTGCTCTCGTGTTTTTC | 247 |
| traI-Rev | GGTGAAACCAGAATGACCAC | ||
| traK | traK-for | CAGGCAAATATTGCCGTGAG | 203 |
| traK-rev | GCACGAATGGAGAAGTTCAG | ||
| rplQ | IC-for | ATGCGCCATCGTAAGAGTGGT | 170 |
| IC-rev | GTCTTGGCAAGAGTAATCAGCGG | ||
| blaKPC | KPC-for | GCTTGCTGGACACACCCAT | 127 |
| KPC-rev | ATCACTGTATTGCACGGCG | ||
| parB-O | parB-for | ACCTATGAATTTGCCCGTCT | 91 |
| parB-rev | TTTCGAAGGACTGCATGTTG |
Amplification mixtures contained each of the primers described in Table 2 at a final concentration of 0.2 μM and were performed with the following cycling conditions: 95°C for 5 min, 30 cycles of 95°C for 30 s, 55°C for 30 s and 72°C for 30 s, and one final cycle at 72°C for 5 min.
Results
Characteristics of the KPC producers
Thirty-three of the 44 KPC producers, including 24 K. pneumoniae, 7 E. coli and 2 Enterobacter spp., harboured blaKPC-2, whereas 9 K. pneumoniae had blaKPC-3 and 2 Enterobacter spp. carried blaKPC-4. Almost all (41/44, 93.2%) of the KPC-encoding genes were embedded in Tn4401 isoform ‘a’ transposons, predominantly on IncFIIK2 replicon-type plasmids (n = 37), although some were carried on IncFIIK1 (n = 1), IncFIIK5 (n = 1) or non-typeable (n = 2) plasmids. Of the remainder, genes encoding KPC-3 (n = 1) or KPC-4 (n = 2) enzymes were located within Tn4401 isoform ‘b’ transposons on non-typeable plasmids. Among 27 isolates from North-West England, only 4 (14.8%) had their blaKPC gene on non-IncFIIK2 plasmids; these comprised 3 K. pneumoniae and 1 E. coli carrying the gene on IncFIIK5 or non-typeable plasmids.
The 33 K. pneumoniae isolates included 10 STs, with 19 isolates belonging to CG258 and comprising ST258 (n = 15), ST11 (n = 3) and ST512 (n = 1) (Table 1). Non-CG258 STs (n = 14) comprised ST321, with eight representatives mainly (6/8, 75%) from North-West England, as well as ST25, ST27, ST248, ST468, ST490 and ST491 each represented by a single isolate (Table 1). CG258 was widespread, being identified in 13 different centres across the UK. It dominated among K. pneumoniae isolates (12/14, 85.7%) recovered outside North-West England. By contrast, K. pneumoniae isolates (n = 19) from North-West England were diverse and belonged to nine different STs, with CG258 (7/19, 36.8%) and ST321 (6/19, 31.6%) the most represented (Table 1).
Antibiotic susceptibility testing showed that the majority of the KPC producers were resistant to all β-lactams (88.6%) with the exception of five isolates showing susceptibility to meropenem, alone (n = 4) or to both meropenem and imipenem (n = 1). In contrast, isolates remained mostly susceptible to colistin (88.6%) and variably susceptible to amikacin (70.5%), gentamicin (68.2%), ciprofloxacin (38.6%) and tigecycline (65.9%), with no marked regional differences in susceptibility frequencies. Colistin resistance was detected in only five K. pneumoniae isolates, including three from North-West England (Table 3).
| . | . | . | Number of isolates with MIC (mg/L) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic . | Species . | Region . | ≤0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | ≥32 . | %Sa . | |
| Ertapenem | K. pneumoniae | North-West England | 19 | 0 | 0 | ||||||
| other centres | 14 | 0 | |||||||||
| other spp. | North-West England | 1 | 1 | 2 | 1 | 3 | 0 | ||||
| other centres | 2b | 1 | 0 | ||||||||
| Imipenem | K. pneumoniae | North-West England | 2 | 8 | 9 | 0 | 2.3 | ||||
| other centres | 1 | 2 | 11 | 0 | |||||||
| other spp. | North-West England | 2 | 4 | 2 | 0 | ||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| Meropenem | K. pneumoniae | North-West England | 3 | 7 | 9 | 0 | 11.4 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 2 | 2 | 1 | 2 | 37.5 | ||||
| other centres | 1 | 1 | 1 | 66.7 | |||||||
| Cefotaxime | K. pneumoniae | North-West England | 5 | 6 | 8 | 0 | 0 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 1 | 2 | 4 | 0 | |||||
| other centres | 3 | 0 | |||||||||
| Ceftazidime | K. pneumoniae | North-West England | 1 | 2 | 6 | 10 | 0 | 0 | |||
| other centres | 3 | 11 | 0 | ||||||||
| other spp. | North-West England | 2 | 1 | 2 | 3 | 0 | |||||
| other centres | 1 | 2 | 0 | ||||||||
| Amikacin | K. pneumoniae | North-West England | 5 | 6 | 1 | 2 | 5 | 73.7 | 70.5 | ||
| other centres | 1 | 1 | 1 | 1 | 3 | 1 | 6 | 50 | |||
| other spp. | North-West England | 1 | 3 | 2 | 2 | 100 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Gentamicin | K. pneumoniae | North-West England | 8 | 4 | 2 | 2 | 3 | 73.7 | 68.2 | ||
| other centres | 2 | 5 | 2 | 2 | 2 | 1 | 64.3 | ||||
| other spp. | North-West England | 2 | 3 | 2 | 1 | 62.5 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Ciprofloxacin | K. pneumoniae | North-West England | 8 | 1 | 1 | 1 | 1 | 7c | 42.1 | 38.6 | |
| other centres | 2 | 12c | 14.3 | ||||||||
| other spp. | North-West England | 7 | 1c | 87.5 | |||||||
| other centres | 3c | 0 | |||||||||
| Colistin | K. pneumoniae | North-West England | 12 | 4 | 1 | 2 | 84.2 | 88.6 | |||
| other centres | 11 | 1 | 1 | 1 | 85.7 | ||||||
| other spp. | North-West England | 8 | 100 | ||||||||
| other centres | 3 | 100 | |||||||||
| Tigecycline | K. pneumoniae | North-West England | 6 | 8 | 5 | 73.7 | 65.9 | ||||
| other centres | 1 | 5 | 7 | 1 | 42.9 | ||||||
| other spp. | North-West England | 5 | 3 | 100 | |||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| . | . | . | Number of isolates with MIC (mg/L) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic . | Species . | Region . | ≤0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | ≥32 . | %Sa . | |
| Ertapenem | K. pneumoniae | North-West England | 19 | 0 | 0 | ||||||
| other centres | 14 | 0 | |||||||||
| other spp. | North-West England | 1 | 1 | 2 | 1 | 3 | 0 | ||||
| other centres | 2b | 1 | 0 | ||||||||
| Imipenem | K. pneumoniae | North-West England | 2 | 8 | 9 | 0 | 2.3 | ||||
| other centres | 1 | 2 | 11 | 0 | |||||||
| other spp. | North-West England | 2 | 4 | 2 | 0 | ||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| Meropenem | K. pneumoniae | North-West England | 3 | 7 | 9 | 0 | 11.4 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 2 | 2 | 1 | 2 | 37.5 | ||||
| other centres | 1 | 1 | 1 | 66.7 | |||||||
| Cefotaxime | K. pneumoniae | North-West England | 5 | 6 | 8 | 0 | 0 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 1 | 2 | 4 | 0 | |||||
| other centres | 3 | 0 | |||||||||
| Ceftazidime | K. pneumoniae | North-West England | 1 | 2 | 6 | 10 | 0 | 0 | |||
| other centres | 3 | 11 | 0 | ||||||||
| other spp. | North-West England | 2 | 1 | 2 | 3 | 0 | |||||
| other centres | 1 | 2 | 0 | ||||||||
| Amikacin | K. pneumoniae | North-West England | 5 | 6 | 1 | 2 | 5 | 73.7 | 70.5 | ||
| other centres | 1 | 1 | 1 | 1 | 3 | 1 | 6 | 50 | |||
| other spp. | North-West England | 1 | 3 | 2 | 2 | 100 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Gentamicin | K. pneumoniae | North-West England | 8 | 4 | 2 | 2 | 3 | 73.7 | 68.2 | ||
| other centres | 2 | 5 | 2 | 2 | 2 | 1 | 64.3 | ||||
| other spp. | North-West England | 2 | 3 | 2 | 1 | 62.5 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Ciprofloxacin | K. pneumoniae | North-West England | 8 | 1 | 1 | 1 | 1 | 7c | 42.1 | 38.6 | |
| other centres | 2 | 12c | 14.3 | ||||||||
| other spp. | North-West England | 7 | 1c | 87.5 | |||||||
| other centres | 3c | 0 | |||||||||
| Colistin | K. pneumoniae | North-West England | 12 | 4 | 1 | 2 | 84.2 | 88.6 | |||
| other centres | 11 | 1 | 1 | 1 | 85.7 | ||||||
| other spp. | North-West England | 8 | 100 | ||||||||
| other centres | 3 | 100 | |||||||||
| Tigecycline | K. pneumoniae | North-West England | 6 | 8 | 5 | 73.7 | 65.9 | ||||
| other centres | 1 | 5 | 7 | 1 | 42.9 | ||||||
| other spp. | North-West England | 5 | 3 | 100 | |||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
S, susceptible; cell shading shows the number of isolates susceptible (white), intermediate (light grey) and resistant (dark grey) to each tested antibiotic according to EUCAST breakpoints.
The number in the second column is the percentage of susceptible isolates per antibiotic, irrespective of species or centre.
Indicates the ertapenem MIC of the E. cloacae isolate showing susceptibility to imipenem and meropenem.
Shows numbers with MIC greater than or equal to the indicated value, which represents the maximum concentration tested for the corresponding antibiotic.
| . | . | . | Number of isolates with MIC (mg/L) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic . | Species . | Region . | ≤0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | ≥32 . | %Sa . | |
| Ertapenem | K. pneumoniae | North-West England | 19 | 0 | 0 | ||||||
| other centres | 14 | 0 | |||||||||
| other spp. | North-West England | 1 | 1 | 2 | 1 | 3 | 0 | ||||
| other centres | 2b | 1 | 0 | ||||||||
| Imipenem | K. pneumoniae | North-West England | 2 | 8 | 9 | 0 | 2.3 | ||||
| other centres | 1 | 2 | 11 | 0 | |||||||
| other spp. | North-West England | 2 | 4 | 2 | 0 | ||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| Meropenem | K. pneumoniae | North-West England | 3 | 7 | 9 | 0 | 11.4 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 2 | 2 | 1 | 2 | 37.5 | ||||
| other centres | 1 | 1 | 1 | 66.7 | |||||||
| Cefotaxime | K. pneumoniae | North-West England | 5 | 6 | 8 | 0 | 0 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 1 | 2 | 4 | 0 | |||||
| other centres | 3 | 0 | |||||||||
| Ceftazidime | K. pneumoniae | North-West England | 1 | 2 | 6 | 10 | 0 | 0 | |||
| other centres | 3 | 11 | 0 | ||||||||
| other spp. | North-West England | 2 | 1 | 2 | 3 | 0 | |||||
| other centres | 1 | 2 | 0 | ||||||||
| Amikacin | K. pneumoniae | North-West England | 5 | 6 | 1 | 2 | 5 | 73.7 | 70.5 | ||
| other centres | 1 | 1 | 1 | 1 | 3 | 1 | 6 | 50 | |||
| other spp. | North-West England | 1 | 3 | 2 | 2 | 100 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Gentamicin | K. pneumoniae | North-West England | 8 | 4 | 2 | 2 | 3 | 73.7 | 68.2 | ||
| other centres | 2 | 5 | 2 | 2 | 2 | 1 | 64.3 | ||||
| other spp. | North-West England | 2 | 3 | 2 | 1 | 62.5 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Ciprofloxacin | K. pneumoniae | North-West England | 8 | 1 | 1 | 1 | 1 | 7c | 42.1 | 38.6 | |
| other centres | 2 | 12c | 14.3 | ||||||||
| other spp. | North-West England | 7 | 1c | 87.5 | |||||||
| other centres | 3c | 0 | |||||||||
| Colistin | K. pneumoniae | North-West England | 12 | 4 | 1 | 2 | 84.2 | 88.6 | |||
| other centres | 11 | 1 | 1 | 1 | 85.7 | ||||||
| other spp. | North-West England | 8 | 100 | ||||||||
| other centres | 3 | 100 | |||||||||
| Tigecycline | K. pneumoniae | North-West England | 6 | 8 | 5 | 73.7 | 65.9 | ||||
| other centres | 1 | 5 | 7 | 1 | 42.9 | ||||||
| other spp. | North-West England | 5 | 3 | 100 | |||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| . | . | . | Number of isolates with MIC (mg/L) . | . | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic . | Species . | Region . | ≤0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | ≥32 . | %Sa . | |
| Ertapenem | K. pneumoniae | North-West England | 19 | 0 | 0 | ||||||
| other centres | 14 | 0 | |||||||||
| other spp. | North-West England | 1 | 1 | 2 | 1 | 3 | 0 | ||||
| other centres | 2b | 1 | 0 | ||||||||
| Imipenem | K. pneumoniae | North-West England | 2 | 8 | 9 | 0 | 2.3 | ||||
| other centres | 1 | 2 | 11 | 0 | |||||||
| other spp. | North-West England | 2 | 4 | 2 | 0 | ||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
| Meropenem | K. pneumoniae | North-West England | 3 | 7 | 9 | 0 | 11.4 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 2 | 2 | 1 | 2 | 37.5 | ||||
| other centres | 1 | 1 | 1 | 66.7 | |||||||
| Cefotaxime | K. pneumoniae | North-West England | 5 | 6 | 8 | 0 | 0 | ||||
| other centres | 1 | 13 | 0 | ||||||||
| other spp. | North-West England | 1 | 1 | 2 | 4 | 0 | |||||
| other centres | 3 | 0 | |||||||||
| Ceftazidime | K. pneumoniae | North-West England | 1 | 2 | 6 | 10 | 0 | 0 | |||
| other centres | 3 | 11 | 0 | ||||||||
| other spp. | North-West England | 2 | 1 | 2 | 3 | 0 | |||||
| other centres | 1 | 2 | 0 | ||||||||
| Amikacin | K. pneumoniae | North-West England | 5 | 6 | 1 | 2 | 5 | 73.7 | 70.5 | ||
| other centres | 1 | 1 | 1 | 1 | 3 | 1 | 6 | 50 | |||
| other spp. | North-West England | 1 | 3 | 2 | 2 | 100 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Gentamicin | K. pneumoniae | North-West England | 8 | 4 | 2 | 2 | 3 | 73.7 | 68.2 | ||
| other centres | 2 | 5 | 2 | 2 | 2 | 1 | 64.3 | ||||
| other spp. | North-West England | 2 | 3 | 2 | 1 | 62.5 | |||||
| other centres | 2 | 1 | 66.7 | ||||||||
| Ciprofloxacin | K. pneumoniae | North-West England | 8 | 1 | 1 | 1 | 1 | 7c | 42.1 | 38.6 | |
| other centres | 2 | 12c | 14.3 | ||||||||
| other spp. | North-West England | 7 | 1c | 87.5 | |||||||
| other centres | 3c | 0 | |||||||||
| Colistin | K. pneumoniae | North-West England | 12 | 4 | 1 | 2 | 84.2 | 88.6 | |||
| other centres | 11 | 1 | 1 | 1 | 85.7 | ||||||
| other spp. | North-West England | 8 | 100 | ||||||||
| other centres | 3 | 100 | |||||||||
| Tigecycline | K. pneumoniae | North-West England | 6 | 8 | 5 | 73.7 | 65.9 | ||||
| other centres | 1 | 5 | 7 | 1 | 42.9 | ||||||
| other spp. | North-West England | 5 | 3 | 100 | |||||||
| other centres | 1 | 1 | 1 | 33.3 | |||||||
S, susceptible; cell shading shows the number of isolates susceptible (white), intermediate (light grey) and resistant (dark grey) to each tested antibiotic according to EUCAST breakpoints.
The number in the second column is the percentage of susceptible isolates per antibiotic, irrespective of species or centre.
Indicates the ertapenem MIC of the E. cloacae isolate showing susceptibility to imipenem and meropenem.
Shows numbers with MIC greater than or equal to the indicated value, which represents the maximum concentration tested for the corresponding antibiotic.
Structure of KPC-encoding IncFIIK2 plasmids
The presence of IncFIIK2 plasmids among diverse STs of KPC-positive K. pneumoniae, as well as E. coli and Enterobacter spp., suggested that these were playing a major role in the early dissemination of KPC enzymes in the UK. Therefore, 11 IncFIIK2 plasmids originating from K. pneumoniae (n = 9), Escherichia coli (n = 1) and Enterobacter spp. (n = 1) from the North-West outbreak and five other UK centres were fully sequenced (Table 1).
Plasmids, designated pKpQIL-UK, from K. pneumoniae ST486 isolate L33 (North-West England) and CG258 isolates T4, T6, T8, T13 and T27 (from centres outside North West England), were nearly identical to the archetypal IncFIIK2 KPC plasmid pKpQIL. At most, these plasmids were distinguished by 32 nucleotide variations (Figure 1a) and, of these, 22 were confined in a single non-coding region of 100 bp (positions 5270–5370 of pKpQIL) and were observed in plasmid T8 only. Based on the published annotation of pKpQIL, eight other variations found in the present plasmids were located in non-coding regions or were synonymous, having no effect on the amino acid sequences of the inferred gene products. A more significant variation was that all the plasmids from CG258 variants had a 1 bp deletion, creating a translational shift in a region predicted to encode a 130 amino acid hypothetical protein (positions 86 406–86 795 pKpQIL); the plasmids from CG258 isolates T4, T8 and L33 also had a substitution in the coding region of blaKPC, changing KPC-3 to a KPC-2 enzyme.
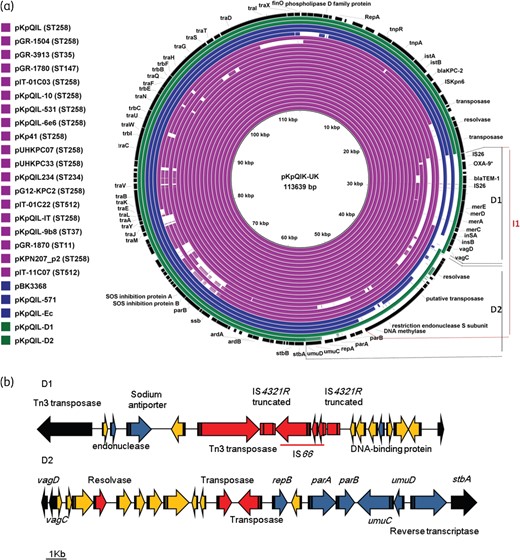
(a) Sequence homologies amongst published pKpQIL-like plasmids. Coloured rings show published plasmids from K. pneumoniae (purple), E. coli (blue) and those generated in this study (green). D1 and D2 indicate the fragments of pKpQIL plasmid that were substituted in pKpQIL-D1 and pKpQIL-D2, respectively, while the pKpQIL fragment indicated I1 is inversely oriented in plasmid pKpQIL-234. (b) Gene contents of the substituted DNA fragments of pKpQIL-D1 and pKpQIL-D2. Colours in the substituted DNA fragments show genes encoding known functions (blue), hypothetical proteins (yellow) and mobile elements (red). Irrespective of their encoding functions, genes of pKpQIL present at the flanking regions of the two substitutions are coloured in black. This figure appears in colour in the online version of JAC and in black and white in the printed version of JAC.
The remaining five IncFIIK2 plasmids, from isolates L27, L38, T19, LESC and LENT, all from the North-West England outbreak, harboured large DNA fragment substitutions. Nevertheless, the portions of these plasmids located outside the substitutions, representing 86.8% of the total length of plasmid T19 and 84.2% of the four other derived plasmids, remained almost identical to pKpQIL with at most 25 nucleotide variations to distinguish them. Of these variations, eight, including mutations in the KPC-encoding gene, were identical to those observed in the pKpQIL-UK variant, suggesting that plasmids pKpQIL-UK, -D1 and -D2 have evolved from the same ancestor.
The T19 plasmid, named pKpQIL-D1 (117 903 bp), which originated from an ST11 K. pneumoniae isolate, had the 11 260 bp fragment (positions 25 539–36 799 of pKpQIL) located between the insertion sequence IS26 and the resolvase and carrying blaTEM-1, truncated blaOXA-9, mercuric resistance genes and the vagCD addiction system genes replaced by a 15 524 bp fragment encoding 14 ORFs comprising IS66, truncated IS4321R, Tn3 transposase, resolvase and nine hypothetical proteins of which three showed low homologies to endonuclease, Na+/H+ antiporter and DNA binding proteins (Figure 1).
The plasmids from K. pneumoniae isolates L27 and L38, from E. coli LESC and Enterobacter LENT, were similar to each other and were designated pKpQIL-D2 (111 742 bp). This plasmid shared the pKpQIL regions from 1 to 36 830 bp and from 56 350 to 113 637 bp, but had the 19 520 bp fragment located between the addiction operon vagCD and the plasmid stability stbA gene replaced by a 17 625 bp fragment harbouring 19 genes (Figure 1). Although markedly different at the sequence level, some of the replacement genes encoded proteins homologous to those encoded by the deleted pKpQIL fragment, such as the UmuC and UmuD SOS mutagenesis and repair proteins, the ParA and ParB plasmid-partitioning proteins, and an origin of replication RepB (Figure 1b). Interestingly, the ‘new’ portion, encoding the plasmid-partitioning genes, has been described in other plasmid sequences deposited in the GenBank database and originating from various Enterobacteriaceae genera including Klebsiella (KP008371), Salmonella (CP006054), Escherichia (KT845955), Providencia (JQ824049), Enterobacter (CP011587) and Proteus (CP015347). This degree of dissemination suggests that ParA and ParB, as encoded by pKpQIL-D2, may favour stable plasmid maintenance in diverse host species.
Comparison of pKpQIL-like IncFIIK2 plasmids
Sequences of the present pKpQIL-UK, -D1 and -D2 plasmids were compared with similar (≥75% identity) pKpQIL-like plasmids previously reported from K. pneumoniae (n = 19) and E. coli (n = 3) in Greece, Italy, Norway and the United States (Figure 1a).
The majority (15/19) of the published plasmids from K. pneumoniae were from CG258 isolates with the four exceptions being from ST35, ST37, ST147 and ST234 organisms (Figure 1a). In 11/19 plasmids, described from CG258 (n = 8), ST35 and ST147 isolates of various origins, differences were limited to a few nucleotide variations from the archetypal pKpQIL, and were similar to those identified in this study. The remaining eight plasmids, of which six were from CG258 isolates, had various genetic rearrangements including insertions or deletions of insertion sequences resulting in the acquisition of genes encoding mainly resistance genes, as well as inversions or substitutions in pKpQIL portions carrying genes encoding the partitioning, transfer and conjugal activities, similar to those identified here in pKpQIL-D2 (Figure 1a). The detection of plasmids almost identical to pKpQIL in non-CG258 isolates, (i) as in the case of published plasmids pGR-3913 (ST35)21 and pGR-1780 (ST147)21 and (ii) as with pKpQIL-UK (ST486) here, indicates that these plasmids have the potential to spread among distinct K. pneumoniae lineages.
In contrast to the dominance of near-classical pKpQIL in K. pneumoniae, all the published pKpQIL-like plasmids from E. coli, and those sequenced here, had major modifications in their backbones. Like the pKpQIL-D1 present, both pKpQIL-Ec and pKPQIL-571 plasmids (originally described from E. coli isolates in the United States)20 had deletions in the pKpQIL region located between the IS26 element and genes encoding the restriction endonuclease units (Figure 1a). On the other hand, plasmid pBK33689 (KU295133), also described in United States, had the 5.5 kb region located upstream of the repA gene substituted by a fragment carrying, among others, a gene encoding an additional replication protein RepB (Figure 1a).
Distribution of pKpQIL-like plasmids in the UK
The screening assay for pKpQIL-like plasmids was validated on the 11 isolates harbouring the fully sequenced KPC plasmids. As expected, all isolates amplified the pKpQIL markers traI, traK, hyp and blaKPC whilst the banding pattern of parB varied according to whether pKpQIL or pKPQIL-D2 was present (Table 1). The assay was then applied to the remaining (n = 26) KPC-positive clinical isolates that had been shown to carry IncFIIK2 plasmids by PCR-based replicon typing but where sequencing had not been performed. Nearly all (25/26, 96%) yielded the traI, traK, hyp and the blaKPC fragments. Of these, 21 amplified also the partitioning parB fragments of either pKpQIL (n = 15), or pKpQIL-D2 (n = 6) while 4 had neither (Table 1). Only one isolate failed to amplify any pKpQIL-marker and yielded the fragments of only blaKPC and the rplQ internal control, suggesting that the KPC gene is harboured on an IncFIIK2 plasmid distinct from pKpQIL. Overall, the assay inferred the presence of pKpQIL-like plasmids in seven K. pneumoniae STs and pKpQIL-D2 in three STs; only the most represented CG258 and ST321 lineages were associated with both variants.
This screening supports the view that pKpQIL plasmids were disseminating in the UK in the 2008–10 study period and suggests considerable plasticity in the region carrying their partitioning functions. The assay showed that only two of the five E. coli isolates carried the parB of pKpQIL-D2 and all four isolates failing to amplify any parB fragments belonged to K. pneumoniae. Although no clear association was found between species and amplification of the plasmid-partitioning gene types carried, the presence of other types of modifications that could potentially affect the replication or segregation of these plasmids cannot be excluded, particularly in those plasmids profiled only by PCR and not by sequencing.
Discussion
Our analysis revealed that IncFIIK2 pKpQIL-like elements played a major role in the early (2008–10) spread of KPC carbapenemases among diverse Enterobacteriaceae species in the UK. Plasmids related to archetypal IncFIIK2 pKpQIL were identified in 36/37 isolates carrying an IncFIIK2 replicon type and in 81.8% (36/44) of all KPC-bearing isolates included in this study.
Sequencing identified plasmids (designated pKpQIL-UK) that were nearly identical to each other and to published pKpQIL from K. pneumoniae isolates belonging to CG258 and ST468 from distinct UK centres, including in North-West England. Screening for pKpQIL markers inferred the presence of these classical forms of pKpQIL in K. pneumoniae isolates belonging to four other STs (e.g. ST25, 248, 321 and 491) among those identified in this study. Published plasmid sequences from non-CG258 isolates,20,21 and from those reported in this study, clearly suggest that highly conserved pKpQIL plasmids, although mainly associated with CG258, are able to spread among other lineages of K. pneumoniae.
Sequenced plasmids with large DNA fragment replacements in their pKpQIL-backbone were identified from the North-West England outbreak only. Critically though, they were found not only in K. pneumoniae isolates, but also in Enterobacter spp. and E. coli. One variant, pKpQIL-D2, had lost a 19.5 kb DNA fragment of pKpQIL that carries genes encoding the plasmid partitioning and replication functions and had this replaced with a 17.6 kb fragment partly encoding similar functions. In contrast to the original parAB genes of pKpQIL, which seem to be confined to K. pneumoniae, the variants present in pKpQIL-D2 have been described previously in plasmids from various Enterobacteriaceae species and might favour the stable inheritance of this variant plasmid across diverse species.
A further variant plasmid, termed pKpQIL-D1, was identified in a single K. pneumoniae isolate, from North-West England and had the 11.2 kb DNA fragment of pKpQIL harbouring the antimicrobial and mercury resistance genes and the plasmid maintenance system VagCD substituted. Interestingly, the fully sequenced plasmids pKpQIL-Ec (KJ146688) and pKpQIL-571 (CP014669) from E. coli and pKpQIL-98 b (CP014765) from K. pneumoniae, both recently described in the United States, harboured deletions located in the same region substituted in pKpQIL-D1. The system vagCD is thought to help plasmid maintenance by preventing cell division until plasmid replication is complete.27 The loss of the plasmid maintenance system in these pKpQIL-derivatives may have increased the chances of being acquired by hosts in which their replication may be less efficient.
Comparison of published pKpQIL-like sequences with those generated in this study showed that the pKpQIL-region between the IS26 element and the genes encoding UmuCD are the most affected by modifications. The identification of isolates failing to amplify any of the parB genes sought in the screening assay for pKpQIL markers developed in this study further supports the inference of high plasticity in this region.
Overall, these results showed that CG258 K. pneumoniae with conserved pKpQIL-like plasmids played a major role in the early spread of KPC enzymes in multiple regions of the UK. Their distribution among further K. pneumoniae STs shows that these relatively conserved pKpQIL plasmids can spread among lineages of this species. We postulate that in North-West England they evolved mainly by modifications of portions encoding plasmid partitioning and replication activities. We suggest that this, in turn, facilitated their spread into various Enterobacteriaceae species, again notably in North-West England. The evolution of unusually transmissible pKpQIL-like plasmids in the early years after the first appearance of KPC enzymes in the UK could also explain the polyclonal nature of K. pneumoniae isolates from the ongoing North-West England outbreak, as compared with the international experience, where the epidemiology of KPC K. pneumoniae is dominated by CG258 K. pneumoniae variants.
Funding
The work was funded by the National Infection Service, Public Health England.
Transparency declarations
M. D., J. F., K. L. H. and N. W. are part of PHE’s AMRHAI Reference Unit which has received financial support for conference attendance, lectures, research projects or contracted evaluations from numerous sources, including: Accelerate Diagnostics, Achaogen Inc., Allecra Therapeutics, Amplex, AstraZeneca UK Ltd, Basilea Pharmaceutica, Becton Dickinson Diagnostics, bioMérieux, Bio-Rad Laboratories, The BSAC, Cepheid, Check-Points BV, Cubist Pharmaceuticals, Department of Health, Enigma Diagnostics, Food Standards Agency, GlaxoSmithKline Services Ltd, Henry Stewart Talks, IHMA Ltd, Kalidex Pharmaceuticals, Melinta Therapeutics, Merck Sharpe & Dohme Corp., Meiji Seika Pharma Co., Mobidiag, Momentum Biosciences Ltd, Nordic Pharma Ltd, Norgine Pharmaceuticals, Rempex Pharmaceuticals Ltd, Roche, Rokitan Ltd, Smith & Nephew UK Ltd, Trius Therapeutics, VenatoRx Pharmaceuticals and Wockhardt Ltd. D. M. L. advisory boards or ad hoc consultancy: Accelerate, Achaogen, Adenium, Alere, Allecra, Altermune, Astellas, AstraZeneca, Auspherix, Basilea, Bayer, BioVersys, Cubist, Curetis, Cycle, Discuva, Forest, GSK, Meiji, Pfizer, Roche, Shionogi, Tetraphase, VenatoRx, Wockhardt; paid lectures: AOP Orphan, Astellas, AstraZeneca, Bruker, Curetis, Merck, Pfizer, Leo; shareholdings in: Dechra, GSK, Merck, Perkin Elmer, Pfizer amounting to <10% of portfolio value. A. D. advisory boards: MSD and Basilea; honoraria for talks: AstraZeneca, Astellas, Pfizer and Cepheid. The remaining authors have none to declare.
Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the UK Department of Health.



