-
PDF
- Split View
-
Views
-
Cite
Cite
Silvia Nozza, Andrea Malagoli, Lilian Maia, Andrea Calcagno, Emanuele Focà, Giuseppe De Socio, Stefania Piconi, Giancarlo Orofino, Anna Maria Cattelan, Benedetto Maurizio Celesia, Elena Gervasi, Giovanni Guaraldi, GEPPO Study Group, Members of the GEPPO Study Group , Antiretroviral therapy in geriatric HIV patients: the GEPPO cohort study, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 10, October 2017, Pages 2879–2886, https://doi.org/10.1093/jac/dkx169
Close - Share Icon Share
Abstract
GEPPO is a prospective observational multi-centric cohort including HIV-infected geriatric patients. We hypothesized that the GEPPO cohort may help characterize antiretroviral (ARV) prescribing criteria used in real life by Italian infectious disease (ID) physicians.
This was a cross-sectional study describing the current ARV regimen in a geriatric HIV population (≥65 years). Antiretroviral strategies were categorized as follows: (i) multidrug regimens (MDRs), which comprised triple or mega ART combinations; (ii) less drug regimens (LDRs), which comprised fewer than three ART compounds. Multi-morbidity (MM) was defined as the presence of three or more non-communicable diseases, and polypharmacy (PP) as the use of five or more medications in chronic use. Four alternative combinations (MM+PP+, MM+PP−, MM−PP+, MM−PP−) were used in logistic regression analyses.
A total of 1222 HIV-positive patients were included (median age 70 years). Females composed 16% of the cohort. Median duration of HIV infection was 17 years; 335 population members had been infected for >20 years. MM was present in 64% and PP in 37% of the patients. Treatment consisted of triple therapy in 66.4%, dual therapy in 25.3%, monotherapy in 6.5% and ‘mega-ART’ with more than three drugs in 1.64% of the patients. In multivariate logistic regression MM and PP were predictive for mono-dual, NRTI-sparing and tenofovir disoproxil fumarate (TDF)-sparing combinations. Female gender and age were predictors of unboosted ARV regimens.
High prevalence of non-conventional ARV regimens in elderly HIV patients suggests that clinicians try to tailor ARV regimens according to age, HIV duration, MM and PP.
Introduction
Antiretroviral therapy has been one of the most impressive achievements of contemporary medicine, credited as it is with enabling people to grow old with HIV.
Standard ART consists of the combination of a minimum of three different antiretroviral (ARV) drugs. These underlie the so-called multidrug regimens (MDRs), preferably containing drugs from at least two different classes, to maximally suppress HIV replication and stop the progression of HIV disease. Currently, there are six classes of antiretroviral agents available: NRTIs, NNRTIs, PIs, fusion inhibitors (FIs), entry inhibitors (EIs) and integrase strand transfer inhibitors (INSTIs).
Based on evidence from clinical trials and expert opinion, current international treatment guidelines have established preferred recommended regimens that include two NRTIs plus one INSTI, two NRTIs plus one NNRTI or two NRTIs plus one PI.1–3 However, all agree that ART must be tailored according to the clinical condition and preferences of the patient.
The pillars of the choice of ART in both naive and experienced patients are ARV potency and resistance, ARV impact on comorbidity, the risk of drug–drug interactions, costs, tolerability and convenience in fixed-dose combination [single-tablet regimens (STRs) in particular].
The tailored approach to ART has produced an increasing number of ‘non-conventional’ ARV regimens either in dual regimens (one NRTI plus one PI or one INSTI plus one PI) or monotherapy [one PI boosted with ritonavir (PI/r), either lopinavir/r or darunavir/r]—the so-called less drug regimens (LDRs)—as an alternative option.
In recent years, some guidelines have suggested the tailoring principle for the management of the elderly HIV-infected population. What all agree on is the need for an intensive screening for co-morbidities owing to the association of these clinical conditions with advanced age. However, only a few guidelines (CDC and Italian Society of Infectious and Tropical Diseases)3 attempt preferred options, but they identify areas of uncertainty in the use of ARV in elderly HIV patients.
In particular, older HIV-infected patients may suffer from age-related co-morbidities, in particular kidney, bone and heart disease, that challenge ARV toxicities. From this perspective, an increasing number of LDRs have been used, albeit supported by limited data from randomized clinical studies, in order to build regimens sparing tenofovir, abacavir, NRTIs or boosted combinations.
Comorbidities frequently aggregate in complex multi-morbidity pictures, which implies the need for polypharmacy with potential high risk of drug–drug interaction (DDI).4,5 From this perspective, ARV classes with less potential for DDI are increasingly used, INSTIs in particular, parallel to the reduction of boosted regimens, PI/r in particular. Ritonavir and cobicistat are ‘boosters’ known to inhibit the CYP3A4 and 2D66,7 cytochrome pathway, metabolizing nearly 70% of all medications undergoing CYP450 metabolism.8,9 PIs and NNRTIs can also decrease the activity of P-glycoprotein, a ubiquitous transport protein10 that plays a significant role in drug absorption and disposition.11
Finally, ARV prescribers should also consider age-associated physiological changes altering pharmacokinetics (for example, decreased gastrointestinal transit, increased fat-to-lean body ratio, reduced hepatic metabolism and renal elimination12) and pharmacodynamics (physiological and biochemical effects of drugs on the body), resulting in increased sensitivity to medications and higher risk of adverse side effects.
GEriatric Patients living with HIV/AIDS: a Prospective Multidimensional cOhort (GEPPO), is a prospective observational multi-centric cohort including consecutive HIV-infected geriatric patients in care in 10 HIV clinics in Italy compared with HIV-negative individuals. It aims to describe health status and transition over time in HIV-infected patients >65 years old.
We hypothesized that the HIV-positive GEPPO cohort may help to characterize the ARV prescribing criteria used in real life by Italian infectious disease (ID) physicians.
The present analysis of the GEPPO cohort aims to describe the current use of ART in a well-characterized HIV geriatric population.
Patients and methods
Study design
This is a cross-sectional study describing the current ART regimen in a geriatric HIV-infected population aged ≥65 years at the time of cohort entry. We chose this age according to the geriatric literature. The initial visit was performed between June 2015 and May 2016.
The inclusion criteria were as follows: age ≥65 years; HIV antibody positive; being on HAART for at least 6 months; and signed informed consent. The patients were recruited in 10 HIV clinics in Italy.
Demographic and clinical characteristics, such as current and nadir CD4 cell counts, CD4/CD8 ratio, plasma HIV RNA, duration of HIV infection, presence of coinfection with a hepatotropic virus, current ART regimen and concomitant therapeutic drugs were recorded.
Duration of HIV infection was calculated as the time between HIV diagnosis and the last visit. This variable was stratified into <10, 10–20 and >20 years. The choice of these time periods not only paralleled the tertile distribution of the last variable, but also identified the subset of individuals ageing with HIV since pre-HAART, early and late HAART periods.
Comorbidity diagnoses were based on criteria previously used in our studies.13 The category of cardiovascular disease (CVD) included the following diagnoses: myocardial infarction, coronary artery disease, peripheral vascular disease, stroke, angina pectoris, coronary artery bypass grafting, and angioplasty. Hypertension was defined as blood pressure >140/90 mmHg over two consecutive measurements, type 2 diabetes mellitus (T2DM) as fasting serum glucose levels >126 mg/dL, and chronic kidney disease (CKD) as estimated glomerular filtration rate (eGFR) <60 mL/min using the Modification of Diet in Renal Disease (MDRD) estimating equation. Hypertension and T2DM diagnoses were also identified through current use of antihypertensive and antidiabetic drugs, respectively. Dyslipidaemia was diagnosed in patients with fasting total cholesterol >200 mg/dL or triglycerides >150 mg/dL, or current use of statins. COPD was defined with pulmonary function testing [spirometry, diffusion capacity of carbon monoxide (DLCO)], demonstrating forced expiratory volume (FEV1)/forced vital capacity (FVC) ratios <70%. Multi-morbidity (MM) was defined as the presence of three or more non-communicable diseases.
Polypharmacy (PP) was defined as five or more medications in chronic use, excluding ARV medications. To distinguish acute exposure to a drug from chronic use of medication, the latter was classified as use of the drug for at least 4 months consecutively.
Antiretroviral strategies were categorized as follows: (i) MDRs, triple or mega combinations of ARV; and (ii) LDRs, fewer than three ART compounds administered as either monotherapy or dual combination therapy.
Statistical analysis
Comparisons between the groups (MDR and LDR) were performed using the χ2 test for categorical variables and the t-test or Mann–Whitney U-test for normally and non-normally distributed continuous variables, respectively.
Results were expressed as mean (SD) or median (IQR) for normal and non-normal continuously distributed variables, or frequency (%) for categorical variables. Separate multivariate logistic regression analyses were used to identify predictors of ‘non-conventional’ ARV strategies, including tenofovir-sparing, unboosted, NRTI-sparing and mono/dual therapies.
Logistic regression was used as the following clinically meaningful variables co-vary: age (per 1 year increment); gender (female as reference); HIV duration (<10 years as reference); MM; and PP. By virtue of the potential overlap between MM and PP, we built a joint dummy variable, using MM-negative PP-negative (MM−PP−) as reference and three alternative combinations, i.e. MM+PP+, MM+PP − and MM−PP+.
Statistical analyses were performed using R software, version 3.2.
Ethics
Institutional review board (IRB) approval was obtained from the Research Ethics Board of each individual centre belonging to the GEPPO cohort (protocol number 1710, reference 39/16, Servizio Sanitario Regionale Emilia Romagna, Azienda Ospedaliero Universitaria di Modena). All participants provided written consent at their initial in-clinic visit.
Results
A total of 1222 HIV-positive patients were included. Table 1 describes the demographic and clinical characteristic of the HIV-infected population in the GEPPO cohort, comparing the ART regimen groups (MDR and LDR).
| Variable . | Total (n = 1222) . | LDR (n = 390, 31.91%) . | MDR (n = 832, 68.09%) . | P value . |
|---|---|---|---|---|
| Female | 205 (16.3) | 59 (15.13) | 138 (16.59) | 0.57 |
| Age, years, mean (SD) | 70 (68–74)a | 71.28 (4.22) | 71.12 (4.01) | 0.78 |
| HBV coinfection | 103 (9.83) | 33 (10.58) | 68 (9.58) | 0.7 |
| HCV coinfection | 141 (12.57) | 45 (13.35) | 91 (11.99) | 0.66 |
| Age at HIV diagnosis, years, mean (SD) | 54.03 (8.83) | 52.7 (9.02) | 54.51 (8.6) | <0.01 |
| HIV duration, years, mean (SD) | 17.17 (7.65) | 18.55 (7.83) | 16.62 (7.45) | <0.01 |
| <10 years | 263 (21.23) | 71 (18.39) | 182 (22.11) | <0.01 |
| 10–20 years | 561 (45.28) | 154 (39.9) | 247 (30.01) | |
| >20 years | 415 (33.49) | 161 (41.71) | 247 (30.01) | |
| CD4 counta, cells/mm3 | ||||
| nadir | 197.5 (84–310) | 214 (101–308.5) | 190 (78–307) | 0.12 |
| current (SD) | 644.58 (289.04) | 655.59 (290.82) | 638.06 (287.59) | 0.45 |
| CD4/CD8 (SD) | 0.97 (1.45) | 1.09 (2.46) | 0.92 (0.55) | 0.75 |
| Viral load | ||||
| ≤40 copies/mL | 1044 (94.31) | 332 (95.13) | 692 (94.54) | 0.79 |
| undetectable | 925 (86.53) | 264 (84.62) | 647 (88.03) | 0.16 |
| Dyslipidaemia | 618 (71.12) | 205 (76.49) | 404 (69.06) | 0.03 |
| Type 2 diabetes mellitus | 241 (28.45) | 91 (34.47) | 144 (25.4) | 0.01 |
| Hypertension | 551 (63.55) | 186 (69.14) | 353 (60.86) | 0.02 |
| Cardiovascular disease | 164 (19.83) | 72 (28.24) | 89 (15.98) | <0.01 |
| Chronic kidney disease | 171 (19.21) | 76 (27.24) | 92 (15.51) | <0.01 |
| COPD | 60 (7.37) | 29 (11.69) | 31 (5.63) | <0.01 |
| MM | 510 (64.31) | 124 (61.08) | 212 (43.8) | <0.01 |
| PP | 242 (37.29) | 97 (42.73) | 138 (33.91) | 0.03 |
| MM−PP− | 138 (30.32) | 32 (20.78) | 106 (35.22) | <0.01 |
| MM+PP− | 138 (30.32) | 49 (31.82) | 89 (29.57) | |
| MM−PP+ | 28 (6.1) | 8 (5.19) | 20 (6.64) | |
| MM+PP+ | 151 (33.18) | 65 (42.21) | 86 (28.57) | |
| NRTI-sparing | 702 (57.4) | 291 (74.62) | 410 (49.28) | <0.01 |
| TDF-sparing | 842 (66.9) | 369 (94.6) | 437 (52.5) | <0.01 |
| INSTI use | 357 (28.3) | 162 (41.5) | 195 (23.4) | <0.01 |
| Unboosted PI | 623 (54.5) | 107 (34.5) | 516 (62) | <0.01 |
| Variable . | Total (n = 1222) . | LDR (n = 390, 31.91%) . | MDR (n = 832, 68.09%) . | P value . |
|---|---|---|---|---|
| Female | 205 (16.3) | 59 (15.13) | 138 (16.59) | 0.57 |
| Age, years, mean (SD) | 70 (68–74)a | 71.28 (4.22) | 71.12 (4.01) | 0.78 |
| HBV coinfection | 103 (9.83) | 33 (10.58) | 68 (9.58) | 0.7 |
| HCV coinfection | 141 (12.57) | 45 (13.35) | 91 (11.99) | 0.66 |
| Age at HIV diagnosis, years, mean (SD) | 54.03 (8.83) | 52.7 (9.02) | 54.51 (8.6) | <0.01 |
| HIV duration, years, mean (SD) | 17.17 (7.65) | 18.55 (7.83) | 16.62 (7.45) | <0.01 |
| <10 years | 263 (21.23) | 71 (18.39) | 182 (22.11) | <0.01 |
| 10–20 years | 561 (45.28) | 154 (39.9) | 247 (30.01) | |
| >20 years | 415 (33.49) | 161 (41.71) | 247 (30.01) | |
| CD4 counta, cells/mm3 | ||||
| nadir | 197.5 (84–310) | 214 (101–308.5) | 190 (78–307) | 0.12 |
| current (SD) | 644.58 (289.04) | 655.59 (290.82) | 638.06 (287.59) | 0.45 |
| CD4/CD8 (SD) | 0.97 (1.45) | 1.09 (2.46) | 0.92 (0.55) | 0.75 |
| Viral load | ||||
| ≤40 copies/mL | 1044 (94.31) | 332 (95.13) | 692 (94.54) | 0.79 |
| undetectable | 925 (86.53) | 264 (84.62) | 647 (88.03) | 0.16 |
| Dyslipidaemia | 618 (71.12) | 205 (76.49) | 404 (69.06) | 0.03 |
| Type 2 diabetes mellitus | 241 (28.45) | 91 (34.47) | 144 (25.4) | 0.01 |
| Hypertension | 551 (63.55) | 186 (69.14) | 353 (60.86) | 0.02 |
| Cardiovascular disease | 164 (19.83) | 72 (28.24) | 89 (15.98) | <0.01 |
| Chronic kidney disease | 171 (19.21) | 76 (27.24) | 92 (15.51) | <0.01 |
| COPD | 60 (7.37) | 29 (11.69) | 31 (5.63) | <0.01 |
| MM | 510 (64.31) | 124 (61.08) | 212 (43.8) | <0.01 |
| PP | 242 (37.29) | 97 (42.73) | 138 (33.91) | 0.03 |
| MM−PP− | 138 (30.32) | 32 (20.78) | 106 (35.22) | <0.01 |
| MM+PP− | 138 (30.32) | 49 (31.82) | 89 (29.57) | |
| MM−PP+ | 28 (6.1) | 8 (5.19) | 20 (6.64) | |
| MM+PP+ | 151 (33.18) | 65 (42.21) | 86 (28.57) | |
| NRTI-sparing | 702 (57.4) | 291 (74.62) | 410 (49.28) | <0.01 |
| TDF-sparing | 842 (66.9) | 369 (94.6) | 437 (52.5) | <0.01 |
| INSTI use | 357 (28.3) | 162 (41.5) | 195 (23.4) | <0.01 |
| Unboosted PI | 623 (54.5) | 107 (34.5) | 516 (62) | <0.01 |
Values shown are n (%) unless otherwise indicated.
Values are median (IQR).
| Variable . | Total (n = 1222) . | LDR (n = 390, 31.91%) . | MDR (n = 832, 68.09%) . | P value . |
|---|---|---|---|---|
| Female | 205 (16.3) | 59 (15.13) | 138 (16.59) | 0.57 |
| Age, years, mean (SD) | 70 (68–74)a | 71.28 (4.22) | 71.12 (4.01) | 0.78 |
| HBV coinfection | 103 (9.83) | 33 (10.58) | 68 (9.58) | 0.7 |
| HCV coinfection | 141 (12.57) | 45 (13.35) | 91 (11.99) | 0.66 |
| Age at HIV diagnosis, years, mean (SD) | 54.03 (8.83) | 52.7 (9.02) | 54.51 (8.6) | <0.01 |
| HIV duration, years, mean (SD) | 17.17 (7.65) | 18.55 (7.83) | 16.62 (7.45) | <0.01 |
| <10 years | 263 (21.23) | 71 (18.39) | 182 (22.11) | <0.01 |
| 10–20 years | 561 (45.28) | 154 (39.9) | 247 (30.01) | |
| >20 years | 415 (33.49) | 161 (41.71) | 247 (30.01) | |
| CD4 counta, cells/mm3 | ||||
| nadir | 197.5 (84–310) | 214 (101–308.5) | 190 (78–307) | 0.12 |
| current (SD) | 644.58 (289.04) | 655.59 (290.82) | 638.06 (287.59) | 0.45 |
| CD4/CD8 (SD) | 0.97 (1.45) | 1.09 (2.46) | 0.92 (0.55) | 0.75 |
| Viral load | ||||
| ≤40 copies/mL | 1044 (94.31) | 332 (95.13) | 692 (94.54) | 0.79 |
| undetectable | 925 (86.53) | 264 (84.62) | 647 (88.03) | 0.16 |
| Dyslipidaemia | 618 (71.12) | 205 (76.49) | 404 (69.06) | 0.03 |
| Type 2 diabetes mellitus | 241 (28.45) | 91 (34.47) | 144 (25.4) | 0.01 |
| Hypertension | 551 (63.55) | 186 (69.14) | 353 (60.86) | 0.02 |
| Cardiovascular disease | 164 (19.83) | 72 (28.24) | 89 (15.98) | <0.01 |
| Chronic kidney disease | 171 (19.21) | 76 (27.24) | 92 (15.51) | <0.01 |
| COPD | 60 (7.37) | 29 (11.69) | 31 (5.63) | <0.01 |
| MM | 510 (64.31) | 124 (61.08) | 212 (43.8) | <0.01 |
| PP | 242 (37.29) | 97 (42.73) | 138 (33.91) | 0.03 |
| MM−PP− | 138 (30.32) | 32 (20.78) | 106 (35.22) | <0.01 |
| MM+PP− | 138 (30.32) | 49 (31.82) | 89 (29.57) | |
| MM−PP+ | 28 (6.1) | 8 (5.19) | 20 (6.64) | |
| MM+PP+ | 151 (33.18) | 65 (42.21) | 86 (28.57) | |
| NRTI-sparing | 702 (57.4) | 291 (74.62) | 410 (49.28) | <0.01 |
| TDF-sparing | 842 (66.9) | 369 (94.6) | 437 (52.5) | <0.01 |
| INSTI use | 357 (28.3) | 162 (41.5) | 195 (23.4) | <0.01 |
| Unboosted PI | 623 (54.5) | 107 (34.5) | 516 (62) | <0.01 |
| Variable . | Total (n = 1222) . | LDR (n = 390, 31.91%) . | MDR (n = 832, 68.09%) . | P value . |
|---|---|---|---|---|
| Female | 205 (16.3) | 59 (15.13) | 138 (16.59) | 0.57 |
| Age, years, mean (SD) | 70 (68–74)a | 71.28 (4.22) | 71.12 (4.01) | 0.78 |
| HBV coinfection | 103 (9.83) | 33 (10.58) | 68 (9.58) | 0.7 |
| HCV coinfection | 141 (12.57) | 45 (13.35) | 91 (11.99) | 0.66 |
| Age at HIV diagnosis, years, mean (SD) | 54.03 (8.83) | 52.7 (9.02) | 54.51 (8.6) | <0.01 |
| HIV duration, years, mean (SD) | 17.17 (7.65) | 18.55 (7.83) | 16.62 (7.45) | <0.01 |
| <10 years | 263 (21.23) | 71 (18.39) | 182 (22.11) | <0.01 |
| 10–20 years | 561 (45.28) | 154 (39.9) | 247 (30.01) | |
| >20 years | 415 (33.49) | 161 (41.71) | 247 (30.01) | |
| CD4 counta, cells/mm3 | ||||
| nadir | 197.5 (84–310) | 214 (101–308.5) | 190 (78–307) | 0.12 |
| current (SD) | 644.58 (289.04) | 655.59 (290.82) | 638.06 (287.59) | 0.45 |
| CD4/CD8 (SD) | 0.97 (1.45) | 1.09 (2.46) | 0.92 (0.55) | 0.75 |
| Viral load | ||||
| ≤40 copies/mL | 1044 (94.31) | 332 (95.13) | 692 (94.54) | 0.79 |
| undetectable | 925 (86.53) | 264 (84.62) | 647 (88.03) | 0.16 |
| Dyslipidaemia | 618 (71.12) | 205 (76.49) | 404 (69.06) | 0.03 |
| Type 2 diabetes mellitus | 241 (28.45) | 91 (34.47) | 144 (25.4) | 0.01 |
| Hypertension | 551 (63.55) | 186 (69.14) | 353 (60.86) | 0.02 |
| Cardiovascular disease | 164 (19.83) | 72 (28.24) | 89 (15.98) | <0.01 |
| Chronic kidney disease | 171 (19.21) | 76 (27.24) | 92 (15.51) | <0.01 |
| COPD | 60 (7.37) | 29 (11.69) | 31 (5.63) | <0.01 |
| MM | 510 (64.31) | 124 (61.08) | 212 (43.8) | <0.01 |
| PP | 242 (37.29) | 97 (42.73) | 138 (33.91) | 0.03 |
| MM−PP− | 138 (30.32) | 32 (20.78) | 106 (35.22) | <0.01 |
| MM+PP− | 138 (30.32) | 49 (31.82) | 89 (29.57) | |
| MM−PP+ | 28 (6.1) | 8 (5.19) | 20 (6.64) | |
| MM+PP+ | 151 (33.18) | 65 (42.21) | 86 (28.57) | |
| NRTI-sparing | 702 (57.4) | 291 (74.62) | 410 (49.28) | <0.01 |
| TDF-sparing | 842 (66.9) | 369 (94.6) | 437 (52.5) | <0.01 |
| INSTI use | 357 (28.3) | 162 (41.5) | 195 (23.4) | <0.01 |
| Unboosted PI | 623 (54.5) | 107 (34.5) | 516 (62) | <0.01 |
Values shown are n (%) unless otherwise indicated.
Values are median (IQR).
HIV patients undergoing LDR appear to have acquired HIV infection at an earlier age, and they have been living with HIV for a longer period of time. The comorbidity burden is higher in this patient group, which displays a higher prevalence of MM and PP. MM+PP+ correlates with LDR prescription.
The ARV prescriptions of GEPPO participants were triple therapy in 66.4%, dual therapy in 25.3%, monotherapy in 6.5% and ‘mega-ART’ with more than three drugs in 1.64% of the patients (Figure 1).
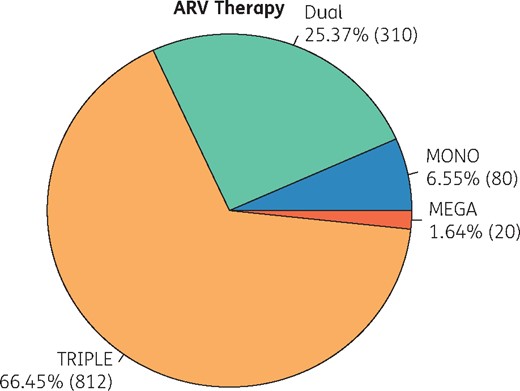
Figure 2 shows mono/dual or triple/mega strategies according to HIV duration, stratified into <10, 10–20 and >20 years of HIV exposure (Figure 2a), and by the four possible combinations of MM and PP (MM−PP−, MM+PP−, MM−PP + and MM+PP+) (Figure 2b).
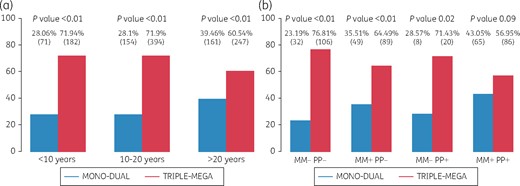
ARV prescription strategies. (a) According to duration of HIV infection (categorized in three intervals: <10, 10–20 and >20 years). (b) According to combinations of MM and PP.
Our univariate analysis indicates that mono/dual therapy, but not triple/mega therapy, is driven by HIV duration.
Figure 3 shows the top 10 prescribed ARV combinations and drug classes in MDR and LDR regimens. Both MDR and LDR regimens show widely varying numbers of ARV combinations. In mono/dual therapy for 384 patients, there were 68 different ARV regimens, while in the triple/mega group 113 ARV regimens were recorded for 839 patients. The most commonly prescribed third agent in MDR was an NNRTI (44.82%). The LDR regimen was an INSTI dual regimen in 40.62% of the cases.
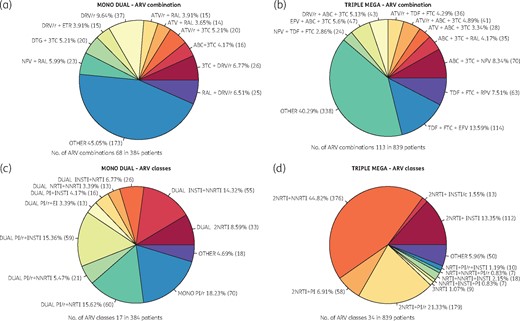
Top 10 prescribed ARV combinations and drug classes in MDRs and LDRs. ATV, atazanavir; RAL, raltegravir; /r, ritonavir boosted; /c, cobicistat boosted; 3TC, lamivudine; ABC, abacavir; NFV, nelfinavir; DTG, dolutegravir; ETR, etravirine; TDF, tenofovir disoproxil fumarate; RPV, rilpivirine; EFV, efavirenz; FTC, emtricitabine.
Figure 4 shows the multivariate logistic regression for the use of non-conventional ARV strategies. MM and PP were predictive of mono/dual, NRTI-sparing and tenofovir disoproxil fumarate (TDF)-sparing combinations. Female gender and age were predictors of boosted-free ARV regimens.
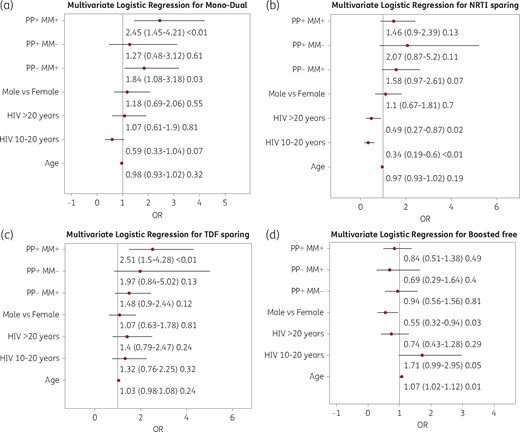
Multivariate logistic regression for the use of non-conventional ARV strategies. (a) Mono and dual combination of therapy. (b) NRTI-sparing therapy. (c) TDF-sparing regimen. (d) Booster-free therapy.
Discussion
The GEPPO cohort is one of the largest existing geriatric HIV cohorts. It is a well-characterized population of people ageing with HIV, with a median duration of HIV of 17 years and a homogeneous exposure to decades of HIV infection: <10, 10–20 and >20 years. Virological control is similar in patients treated with MDR or LDR, highlighting that in this population, tailored antiretroviral therapy is efficacious.
By reason of female predominance in the gender distribution of the general geriatric population, the GEPPO cohort is over-representative of male patients in relation to the HIV epidemic in Italy, mainly represented by MSM in this cohort (data not shown). This population is doing remarkably well with regard to immunovirological control of HIV. The WHO objective of 90% of HIV-infected people with undetectable HIV viral load is fully reached (94%). Current median CD4 is >600 cells/mm3 and CD4/CD8 is >0.9. Prevalence of HBV coinfection (10%) is higher than in the general population and even higher than in the HIV population of a younger age. This may be correlated with the fact that HBV vaccination was introduced in Italy as a public health policy in 1991; by that time, people belonging to this cohort were above the age of 40, and presumably most of them were already HBV infected. As expected, this population suffers from many comorbidities, most of the time aggregating in a complex picture called ‘multi-morbidity’. The 64% prevalence of MM turns this condition into the norm in this cohort, and it drives the high prevalence of PP (37%). In this study, we chose a restrictive definition of PP (without taking note of the burden of ARV in the count of the chemical products prescribed in the same individual at the same time) to avoid saturation of the prevalence of this condition in the study sample.
The choice to divide the study population into MDR and LDR ARV prescription strategies was driven by the observation that one-third of the cohort (32%) was exposed to unconventional LDR regimens, either mono (7%) or dual (25%) regimens. These regimens were more frequently NRTI-sparing (57%) and TDF-sparing (67%), but less likely to be free from boosting (34%).
We hypothesized that the GEPPO cohort may help characterize ARV prescribing criteria. However, it must be acknowledged that many of the driving forces in ARV prescription in real life cannot be reconstructed retrospectively. The major limitation of this study is inherent in its multicentre observational nature, meaning we were unable to check for resistance patterns, tolerability, cost, patient convenience or even calendar year. As a matter of fact, when a new ARV agent is introduced, this is not always immediately available to all centres across Italy. Nevertheless, we focused on two major ARV prescription drivers that are of paramount importance in geriatric cohorts, MM and PP.
In the GEPPO cohort, MM was a combination of highly prevalent comorbidity conditions, including dyslipidaemia (71%), hypertension (63%), CVD (20%) and CKD (19%). Dual-energy X-ray absorptiometry (DEXA) data were available only in a subset of the cohort (157 participants). In these patients, low bone mass (lumbar t-score < −2) was present in 26% of the cases (data not shown). These comorbidities may impact on the use of boosted regimens frequently associated with dyslipidaemia,14,15 abacavir being presumably associated with an increased risk of CVD13,16 and tenofovir definitively associated with kidney impairment and fracture risk.17–19
PP is a well-recognized public health concern but has been poorly studied in HIV infection.
PP in the older population might raise several concerns related to an increased risk of drug–drug and drug–disease interactions, poor adherence to treatment and increased risk of adverse drug reactions.20,21
A boosted regimen does increase the risk of DDI. Therefore, several guidelines recommend avoiding boosted ARV regimens in cases of PP.22,23
In addition, medications often used to treat chronic and acute diseases are rarely tested in the older population.24 In the ‘oldest old’ population, this is further complicated by the high prevalence of geriatric conditions (cognitive impairment, functional deficits and geriatric syndromes). These can impact on treatment adherence and limit life expectancy, which can further reduce the beneficial effect of prescribed medications.25–27
The strong association between PP and MM represents a methodological challenge to separately evaluating the associations between PP and clinical harm. As expected, in the GEPPO cohort comorbidities implied the need for specific pharmacological interventions for treatment or prevention (33% were MM+PP+). Interestingly, nonetheless, there was a similar proportion of patients with MM who did not have PP (30% were MM+PP−). A quite worrying subset of people with PP did not have MM (6% were MM−PP+) and merit further clinical attention.
The classification into LDRs and MDRs very clearly stratified the cohort into two different populations. The former is significantly exposed to HIV infection for a longer time, acquired HIV at a younger age, and has a higher prevalence of MM and PP. Apparently, clinicians’ choice of LDRs in this population bears witness to the recognition of the higher vulnerability of this subgroup of people.
One of the most surprising findings in the GEPPO cohort is the impressive number of different ARV drugs and drug classes: 72 and 17, respectively, in the 390 patients undergoing LDRs, and 110 and 34 for the 839 patients undergoing MDRs. In the latter group, the following ‘third agent’ classes may be recognized: NNRTI 49%, PI/r 26% and INSTI 25%.
In the LDR group, NNRTI is present in 23%, PI/r in 58% and INSTI in 42%. The latter are mainly associated with a PI/r (15%), an NNRTI (14%) or lamivudine (7%).
Unfortunately, there is very little high-quality evidence to guide ARV prescriptions for the elderly HIV population, particularly those with MM,4,5 because these patients are generally excluded from clinical trials.24,28–31
In the multivariate logistic regression, the presence of MM and PP more than doubled the likelihood of a mono-dual regimen (OR 2.45, 95% CI 1.45–4.21) and a TDF-sparing regimen (OR 2.51, 95% CI 1.5–4.28). These two groups of people almost overlap and TDF appears to be the main driver for these dual regimens.
People exposed to HIV for >10 years had a higher probability of NRTI-sparing regimens. In this subgroup, the duration of HIV exposure may be a proxy for comorbidities, but these individuals are more likely to have an issue related to NRTI resistance, mainly generated in the pre- and early HAART era.
Age was the only identified independent risk factor for unboosted regimens. Clinicians may in fact be worried about the pharmacy–dynamic behaviour of drugs in ageing metabolism.
Our data lead us to argue strongly for a tailored approach to drug treatment in HIV-positive patients with MM. Studies addressing ARV efficacy in elderly people with end-stage organ function represent innovative drug ‘stress tests’; in fact, these are the most informative studies at the bedside on the switch from TDF to tenofovir alafenamide (TAF), a recommendation for all geriatric patients due to a lower impact on bone or kidney toxicity.27 TAF also has the potential to reduce both LDR and MDR regimens, decreasing the burden of ARV variability with combinations not supported by randomized clinical trials. In spite of this, LDRs may well continue to exist. Indeed, recent research into the superior efficacy of PI/r plus lamivudine as opposed to standard PI/r plus two NRTIs32 and the ongoing trials on dolutegravir plus lamivudine (Gemini 1 and PADDLE trials) may definitively leave room for LDR maintenance strategies. The SWORD trial demonstrated viral suppression with a two-drug regimen combining an INSTI (dolutegravir) and an NNRTI (rilpivirine) in patients with HIV who have already achieved viral suppression with a three-drug regimen;33 on the other hand, more adverse events were reported and led to withdrawal from the study in the dolutegravir and rilpivirine arm compared with the current antiretroviral therapy arm. This strategy could be studied in an aged population, who usually need a drug with a high genetic barrier to resistance in order to achieve continuing virological suppression.
ARV tailoring may also consider PP interventions. Although ‘de-prescribing’ is relatively new to HIV medicine, the use of tools such as the Beers criteria,34 the IPET (Improving Prescribing in the Elderly Tool)35 and the STOPP-START criteria36,37 to tailor therapy and reduce harmful PP are well established in gerontology practice, and should be extended to the HIV field.38
Italian data on dual therapies based on boosted PI are available in some clinical settings and these strategies are used in 6.7%–12.8% of patients.39
Conclusions
We have described ARV use in a large well-characterized geriatric cohort. The present scenario outlines the clinicians’ effort to tailor ARV regimens according to age, duration of HIV and, in particular, MM and PP. The advent of TAF and unboosted regimens, mainly in the INSTI class, holds the potential to change this scenario rapidly, while at the same time calling for randomized clinical trials specifically addressing geriatric HIV patients.
Acknowledgements
Members of the GEPPO Study Group
Department of Infectious Diseases, San Raffaele Scientific Institute, Milano: Silvia Nozza, Antonella Castagna, Andrea Poli, Nadia Galizzi. University of Modena and Reggio Emilia, Department of Mother, Child and Adult Medicine and Surgical Science, Infectious Disease Clinic, Modena: Giovanni Guaraldi, Federica Carli, Andrea Malagoli. Unit of Infectious Diseases, Department of Medical Sciences, University of Torino, Torino: Andrea Calcagno, Giovanni Di Perri, Stefano Bonora, Chiara Montrucchio. Department of Infectious and Tropical Diseases, University of Brescia: Emanuele Focà, Francesco Castelli, Paola Magro, Eugenia Quiros Roldan. Department of Infectious Diseases, Azienda Ospedaliero-Universitaria di Perugia, Perugia: Giuseppe Vittorio De Socio, Serena Marinello. 1st Division of Infectious Diseases Unit, University of Milano, Ospedale L. Sacco, Milano: Stefania Piconi. Unit of Infectious Diseases, ‘Divisione A’, Ospedale Amedeo di Savoia, ASLTO2, Torino: Giancarlo Orofino, Mariana Farenga. Unit of Infectious Diseases, Department of Internal Medicine, Azienda Ospedaliero-Universitaria di Padova, Padova: Anna Maria Cattela, Serena Marinello. Department of Clinical and Molecular Biomedicine, Division of Infectious Diseases, University of Catania, ARNAS Garibaldi, Catania: Benedetto Maurizio Celesia, Andrea Marino, Bruno Cacopardo. 3rd Division of Infectious Diseases, University of Milano, Ospedale L. Sacco, Milano: Massimo Galli, Agostino Riva, Valeria Morena, Elena Gervasi.
Funding
The GEPPO cohort has been designed in the ‘Ageing & Frailty Working Group’ sponsored by ViiV Healthcare Italy.
Transparency declarations
S. N. received travel grants and speaker’s honoraria from Gilead, Viiv, Janssen-Cilag and MSD. A. C. received grants, travel grants and speaker’s honoraria from Abbvie, BMS, Gilead, Viiv, Janssen-Cilag and MSD. E. F. received travel grants or speaker's honoraria from BMS, Gilead, Janssen-Cilag, MSD, Viiv Healthcare and consultancy fees from Gilead, Janssen-Cilag, Viiv Healthcare and BMS. G. D. S. received travel grants from Abbvie, BMS, Gilead, Viiv, Janssen-Cilag and MSD. A. M. C. received grants and speaker's honoraria from Abbvie, BMS, Gilead, Viiv, Janssen-Cilag and MSD. B. M. C. received grants and speaker's honoraria from Abbvie, BMS, Gilead, Viiv, Janssen-Cilag and MSD. G. G. received travel grants and speaker’s honoraria from Abbvie, BMS, Gilead, Viiv, Janssen-Cilag and MSD. G. O. received grants and speaker honoraria from GSK, MSD and Viiv Healthcare. A. M., L. M., S. P. and E. G. have none to declare.
References
Author notes
Members are listed in the Acknowledgements section.



