-
PDF
- Split View
-
Views
-
Cite
Cite
Patricia Muñoz, Emilio Bouza, on behalf of the COMIC (Collaboration Group on Mycosis) study group, The current treatment landscape: the need for antifungal stewardship programmes, Journal of Antimicrobial Chemotherapy, Volume 71, Issue suppl_2, November 2016, Pages ii5–ii12, https://doi.org/10.1093/jac/dkw391
Close - Share Icon Share
Abstract
There is increasing evidence supporting the need for antifungal stewardship (AFS) programmes in order to promote appropriate antifungal use, improve diagnosis and quality of care, and decrease the costs of antifungal treatment. AFS programmes delivered by experienced teams can be efficacious and cost effective. However, there are a variety of challenges often faced during the implementation of AFS programmes which can present barriers to their success. These can include lack of dedicated personnel, lack of investment in new diagnostic and prescription tools, and misperception by other physicians.
Introduction
The use of antifungal drugs poses a daily challenge for professionals of many different specialties in modern hospitals.1 Clinical manifestations of invasive fungal infection (IFI) may be non-specific, diagnostic tests are far from perfect and patients requiring antifungal agents frequently have significant comorbidities that increase the risk of toxicity and severe drug–drug interactions.2
In the 1970s and 1980s, conventional amphotericin B was largely the only therapeutic option, which, due to its high toxicity and infusion-related adverse events, was mainly prescribed by highly trained physicians. With the advent of fluconazole, which is considered easy to administer and is generally well-tolerated, the use of antifungal agents widened significantly; this has continued with the use of candins, new azoles and lipid formulations. It is also important to consider where patients with IFIs are located within the hospital. In a recent survey of 100 patients receiving systemic antifungal agents at the Hospital General Universitario Gregorio Marañón, 43% of the prescriptions came from medical departments, 25% from haematology/oncology departments, 17% from ICUs and 12% from surgical departments.3 These findings therefore support having an effective antifungal stewardship (AFS) programme throughout the entire hospital, if feasible.
Since the introduction of newer antifungal agents as alternative options to conventional amphotericin B, many changes have occurred in the field of IFI, some positive and some not as positive. The prognosis of critical care and oncology patients has improved significantly, partially due to effective antifungal prophylaxis strategies. However, antifungal resistance is now a reality, due to a shift from susceptible species towards more resistant ones. Some of these changes may be related to the overuse of antifungals.
Empirical/pre-emptive antifungal therapy is especially troublesome, particularly in ICUs. In the Hospital General Universitario Gregorio Marañón Centre, the majority of antifungal drugs are used for empirical therapy.3 In a study from Houston, Texas, USA, 64% of all micafungin treatment courses and 62% of fluconazole courses were for empirical treatment.4 This practice is frequently based on risk scores with extremely low positive predictive values.5,6 For example, the risk of having a fungal infection was evaluated in thousands of patients from different ICUs using the Candida score. According to the score, 180 patients (17%) received empirical antifungal treatment. However, only 5% of those 180 patients actually developed a proven fungaemia.7 This therefore demonstrated that the Candida score had a very poor positive predictive value that led to unnecessary antifungal therapy in a large number of patients.
Overuse of antifungal agents is also partially due to the use of other strategies, such as antifungal prophylaxis or pre-emptive therapy. These have proven to be useful in highly immunosuppressed patients but less so in ICUs.8 The overuse of antifungal agents results in exposure to unnecessary medication and increased costs.9
Quality of antifungal use in general hospitals and the consequences of their misuse
It is not easy to establish the quality of use of antifungal agents, given the difficulty in establishing a proven diagnosis in many instances. However, different studies have shown that lack of compliance with national or international guidelines has a negative impact on patient outcomes. In one study performed in France, only 65% of the antifungal agents used in ICUs, oncology and haematology departments were prescribed according to labelling or international guidelines.10 The indication and dosage were found to be appropriate in 65% and 62% of cases, inappropriate in 22% and 21%, and equivocal in 13% and 17%, respectively. Interestingly, the overall survival rate at 12 weeks was significantly higher in patients receiving appropriate therapy. In another study, performed in Thailand, the rate of inappropriate antifungal use reached 70%.11 The most common reason was unnecessary treatment of candiduria, in the absence of an infectious disease (ID) consultation, which could have helped prevent inappropriate use.
Adherence to recommendations on antifungal drug use was also found to be extremely poor among solid organ transplant physicians, in both the USA12 and Spain.13,14 In these studies, antifungal prophylaxis was frequently administered to patients with no indication, combination therapy was provided without strong supporting evidence and, in general, dose and duration of treatment differed widely between centres.
When the AFS programme was initiated at the Hospital General Universitario Gregorio Marañón, two baseline surveys were performed in order to identify gaps in knowledge relating to IFI and clinical practice.3,15 To evaluate the quality of antifungal use with an easily measurable index, we created a composite point score ranging from 0 to 10 (with each criterion scoring 0–2 points). We assigned the highest impact (2 points) to severe mistakes (prescription of an unnecessary antifungal agent) or to aspects that were clear intervention targets (lack of adjustment following receipt of microbiological information or excessive duration of treatment). Less severe mistakes (incorrect dosage or lack of switching to an oral form of the drug) were given a smaller impact (0 or 1 point) in the global score. In the case of drug selection, a non-efficacious drug was a major mistake (0 points), an effective but less optimal selection was a minor mistake (1 point) and a perfect selection was awarded 2 points.3
According to our evaluation, 16% of antifungal prescriptions were considered unnecessary and a degree of inappropriateness was found in 57% of the prescriptions. The mean point score for antifungal use in the study was 7.7 ± 2.6; scores were lower for empirical (6.6) than for tailored (9.5) or prophylactic (9.1) therapies. The most common problems found were lack of adjustment to microbiological results (35%), inappropriate drug selection (31%), length of therapy (27%), no switch from intravenous to oral administration when indicated (20%) and inappropriate dosing (16%). Inappropriate empirical therapy accounted for 78.6% of total potential cost savings.3
Interestingly, the rate of inappropriate prescriptions was higher in patients for whom an ID consultation was not requested (74.1% versus 33.3%, P < 0.001). An ID consultation was requested in 42% of patients (54.8% from medical wards, 28.6% from ICUs, 14.3% from surgical wards and 2.4% from oncology and haematology wards).3
Antimicrobial stewardship programmes
According to the aforementioned studies, most centres agree with the belief that an antimicrobial stewardship (AMS) programme is necessary. However, it is important to consider which initiatives actually deserve this title.
An AMS programme can be defined as a coordinated intervention designed to improve and measure the appropriate use of antimicrobials by promoting the selection of the optimal antimicrobial drug regimen, dose, duration of therapy and route of administration.16 The ultimate goal of all AMS programmes is not cost containment, but improvement of the quality of care and a better outcome for treated patients.
In our opinion it is debatable whether the application of a standardized protocol of antifungal treatment in a single department should be called a stewardship programme. There are very successful examples of AFS programmes dedicated to just one type of patient.17,18 However, by definition, AMS programmes need to have goals, measurable indicators that are prospectively recorded and a multidisciplinary team that designs the policies. As recently shown in a cross-sectional survey in Europe, most centres in Europe have in place some type of AMS programme, although not many of them extend their scope to the use of antifungals.19
The development of AFS programmes
There are some key steps that need to be considered before an AFS programme can be initiated (Figure 1).20 One of the first steps is to organize a collaborative group that receives official support. An AFS intervention will usually require monitoring of the prescriptions of other physicians, so empowerment by and full support from the hospital authorities are essential. As an example, the group at the Hospital General Universitario Gregorio Marañón reports to the Hospital Committee for Antimicrobial Policy and Nosocomial Infections.
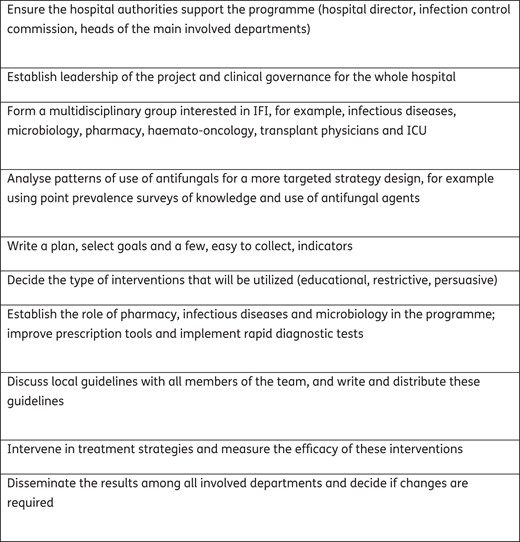
The key steps in initiation of an antifungal stewardship programme.
A multidisciplinary team is absolutely essential;21,22 the role of the team is discussed in detail in the article entitled ‘The role of the multidisciplinary team in antifungal stewardship’ later in this Supplement.23
It is important to also select a respected leader for the AFS programme who can assure clinical governance. The specialty of the leader may differ in each hospital but it is essential that they have a deep understanding of the diagnosis and management of fungal infections and the capacity to coordinate a multidisciplinary group. In the European survey it was shown that in AMS programmes the main medical input was provided by ID physicians.19
Another factor to consider in the development of an AFS programme is the selection of goals and indicators. The multidisciplinary team has to choose a suitable number of objectives, as well as designing a written annual plan and selecting the metrics that will be used to check whether the programme is working. It is also necessary to plan for data collection and graphic representation of the results, and to establish periodic meetings to discuss the data with all professionals involved. The indicators must be simple to obtain, as the team members should generally be dedicated to intervention and treatment, not to data collection. Some examples of frequently used indicators include days of therapy (DOT), daily defined doses (DDDs), episodes of candidaemia and rates of resistance or cost of antifungal agents. It is also possible to measure the percentage of patients treated with antifungal agents who are included in the AFS programme, the percentage of acceptance of the recommendations, the number of people who are exposed to the educational activities, the satisfaction with the AFS programme, and the evolution of the knowledge score of prescribing physicians, for example. Point prevalence studies can be performed to analyse the evolution of the appropriateness of antifungal agent use in depth, as has been done at the Hospital General Universitario Gregorio Marañón Centre.
The next step is to select interventions. Interventions can be restrictive, e.g. necessitating a limited formulary and requiring pre-approval for antifungal use by nominated people or automatic stop orders. Alternatively, they can be persuasive, e.g. encouraging education, developing guidelines, improving access to experts to discuss cases or post-prescription bedside review for dose optimization and sequential treatment. Persuasive measures require significantly more time and effort and require high expertise but are believed to have improved long-term acceptance compared with restrictive measures.24,25
It must be noted that the new IDSA guidelines on AMS programmes state that passive educational activities, such as lectures or information pamphlets, should be used only to complement other stewardship activities.26 In these guidelines, the authors encourage the use of strategies (e.g. antibiotic time-outs and stop orders) that encourage prescribers to perform routine reviews of antibiotic regimens in order to improve antibiotic prescribing. In our opinion this practice is more difficult to implement with fungal infections, since for some clinical presentations the diagnostic criteria are not as clear as for bacterial infections. At the Hospital General Universitario Gregorio Marañón we do not have personal experience with automatic stop orders for antifungal drugs, although this could be considered in some cases, e.g. excessive durations of prophylaxis and some cases of empirical therapy. However, a safety mechanism should be paired with stop orders to avoid unintended interruptions and to prevent the alienation of prescribers against antibiotic stewardship interventions.25 The safety and effectiveness of these types of measures have to be evaluated specifically for antifungal use before they are implemented.
In the European survey, 81% of centres restricted use of some antibiotics (mostly carbapenems and quinolones), 64% carried out post-prescription review of restricted antimicrobials and only 20% had electronic prescribing for all patients.19 In a study performed in the USA,27 all systemic antifungal agents were classified as restricted drugs, with the exception of intravenous fluconazole, which was classified as a controlled drug. A pharmacist, with input from the ID practitioner, placed recommendations to change or stop the drug in the instructions section of the patient's chart. If after 24 h there was no response, the pharmacist would then enforce the recommended changes. Total antifungal agent use decreased by 28% and mortality rates were not significantly altered by the programme. This type of intervention would not be easy to implement at all institutions, although in this study the acceptance of the programme was excellent (>90%).27
Some AFS programmes may contain multiple simultaneous activities (bundled interventions), including educational initiatives, dose adjustment tools, antifungal prescription forms and prescription-control strategies. In a hospital in Thailand with a high rate of antifungal misuse, a programme focused on the treatment of candidosis significantly reduced antifungal use and was associated with a decrease in fluconazole resistance.28 Improved quality of care and improved outcomes have also been reported by other authors.29,30
At the Hospital General Universitario Gregorio Marañón, selected persuasive interventions have been chosen based on advice given in the prescription electronic tool and on a bedside intervention for post-prescription evaluation performed by an experienced ID physician.20,25 The roles of different specialists are summarized in Figure 2. The electronic prescription tool requires the selection of an approved indication, or a justified explanation, for every antifungal agent. It also provides a link to local guidelines and alerts on possible drug–drug interactions and toxicities. Pharmacists also monitor the cost of antifungal agents (overall cost and unit-level number of DDDs or prescribed DDDs per 1000 patient-days).
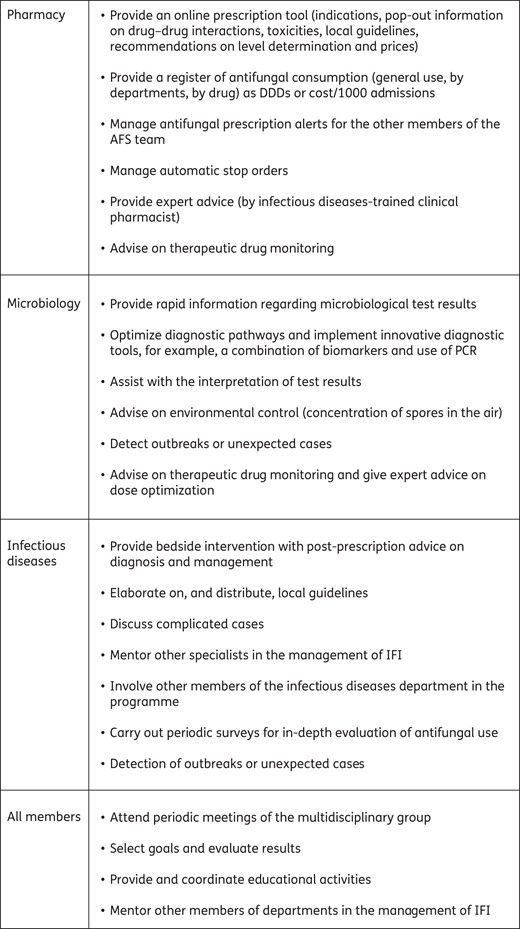
Potential activities that each member of the antifungal stewardship (AFS) team may perform.
Development of local guidelines is very worthwhile and highly recommended. Many physicians feel that their type of patient is not fully represented in official guidelines, so discussion of local epidemiological and demographic characteristics is likely to help compliance with AFS programmes. The programme at the Hospital General Universitario Gregorio Marañón includes diagnostic criteria, recommendations for prophylaxis and treatment, indications for dose adjustments and contact phone numbers of members of the AFS group. The guidelines should be agreed upon within the multidisciplinary group and updated at least every 2 years. Pocket-sized leaflets containing the local guidelines are routinely offered to all physicians contacted by the AFS programme.
A great deal of effort is given to bedside interventions at the Hospital General Universitario Gregorio Marañón. An experienced ID specialist receives a daily alert on the prescription of candins, liposomal amphotericin B, voriconazole and posaconazole. The patient and prescribing physician are visited, and, if necessary, diagnostic tests and therapeutic changes are suggested. De-escalation to fluconazole is recommended when an azole-susceptible species is isolated, organ dysfunctions are resolved and drug–drug interactions seem unlikely. During the first year, 453 patients were visited in haematology (35%), medical (23%) and ICU (20%) departments. Antifungal drugs were prescribed for targeted therapy (36%), prophylaxis (32%) and empirical/pre-emptive treatment (22%). At the initial visit, extra diagnostic procedures were suggested in 40.4% of the patients and some change in therapy in 32%, including de-escalating the antifungal drug (17.4%) or stopping it (7.1%). A fungal infection was finally proven in 28.9% of patients and in 45% of patients the treatment intervention was found to be sub-optimal or inappropriate. The most common problems found were inadequate duration of therapy (28.5%), inadequate selection of antifungal drug (24.1%) and lack of adjustment in light of microbiology results (18.5%). Compared with the 12 months prior to programme implementation, expenditure on antifungals was reduced by $915 808 (26.2%), mainly due to a reduction in the consumption of candins and voriconazole.25
Challenges of AFS programmes
Challenges are varied and may differ according to each hospital and healthcare model. In the recent European survey19 the top barriers selected by the participants were lack of personnel or funding (29%), lack of technological support (23%) and opposition from prescribers (17%). Rejection of the AFS programme may appear to be due to perception of loss of autonomy.31,32 This attitude may be present in prescribers, but also in other members of the ID team not directly involved in the AFS programme. It is thus desirable to maintain a constructive attitude and to include as many members of the team as possible. The AFS coordinator remains accountable for the outcome of the programme, and it is important to disseminate information, to communicate the successes and to give credit to the whole team.
Another issue to consider is that the knowledge of prescribers may not be as good as their personal perception. A knowledge survey on different aspects of diagnosis and management of candidosis and aspergillosis was performed both in the Hospital General Universitario Gregorio Marañón Centre15 and in Europe.33 Significant gaps in the knowledge of everyday prescribers were identified that may underlie the causes of antifungal misuse. Misconceptions were mainly related to the confusion between colonization and real infection, overestimation of the real rate of resistance, and lack of knowledge on available diagnostic methods, prophylaxis indications and first-line therapy. This questionnaire is repeated at the Hospital General Universitario Gregorio Marañón before and after yearly educational activities, with very encouraging results.
Lack of funding for implementation of new diagnostic methods is also a common complaint. The Hospital General Universitario Gregorio Marañón has started using a combination of Candida biomarkers and has found that their high negative predictive value may help to stop empirical antifungal use.34–36 Antifungal levels are also measured,37 molecular analysis of strains causing fungaemia is performed in order to detect horizontal transmission,38 resistance in clinical isolates is followed up39–43 and environmental surveillance of spore concentrations in the air is performed.44–46
Several authors have demonstrated that the cost of microbiology techniques is extremely low in comparison with other components of the budget required to treat patients with IFIs.47,48 However, new and promising diagnostic methods will need to demonstrate cost effectiveness in current resource- and cost-constrained environments.49–52
Another issue that must be considered is the lack of adjustment of initial therapy according to microbiology results (streamlining). We have already mentioned that only a very small proportion of patients receiving empirical antifungal treatment will go on to have a diagnosis of fungal infection. These patients offer a very good opportunity for effective AFS and management bundles.18,29,53 Unfortunately, many studies have demonstrated that physicians frequently do not adapt therapy according to the laboratory results. In a number of studies of candidaemia, most patients were not treated until Candida was detected in blood cultures, and, surprisingly, after microbiological information was available the majority of echinocandin-treated patients with fluconazole-susceptible isolates were not de-escalated to fluconazole.54,55 This practice has been proven safe, and in stable patients with no risk of major drug–drug interactions, early step-down when clinically feasible should be a clear objective of the AFS programme.56
It is also challenging to maintain a registry of all IFIs. Microbiology departments can maintain records of isolates reflecting proven infections, such as episodes of candidaemia per 1000 admissions. However, in order to establish the real meaning of infection with filamentous fungi, a very experienced physician must review the case. In the Hospital General Universitario Gregorio Marañón each case of potential nosocomial mould infection is discussed in the multidisciplinary group and the host, clinical and microbiological criteria are reviewed before it is admitted to the registry.
Finally, it can also be challenging to keep AFS programmes going, due to fatigue of the team. AFS requires a high degree of personal enthusiasm and effort which may not always be appreciated by colleagues and frequently results in no obvious personal benefit. When an AFS programme begins, it is likely to rapidly show a significant impact with reduction in inappropriate prescribing and direct expenditure, but this may then decrease after 2–3 years. It is important to consider ways to prevent this fatigue, e.g. by involving young staff in the team, publishing the team's achievements and designing new interventions.
It is vital to consider approaches to overcome the challenges highlighted above, as stewardship programmes have a vast potential to improve the quality of care and the safety of patients. In the future, AFS programmes are likely to make use of electronic decision trees and automatic instructions; however, the opinion of the physician will always be key to ensuring a high standard of medical practice, taking into consideration the individual characteristics and presentation of each patient. AMS programmes have already demonstrated positive results in a variety of settings, and although AFS programmes face distinct key challenges, lessons from AMS can be considered in order to improve outcomes. AFS programmes have the potential to optimize antifungal agent use and improve patient diagnosis and quality of care. Collaboration between pharmacy, microbiology, infectious diseases, haematology, intensive care, anaesthesiology, internal medicine and surgery healthcare professionals is essential to meet the challenges faced during the implementation of AFS programmes, and to optimize care for people with invasive fungal diseases.
Funding
The Antifungal Stewardship Project at the Hospital General Universitario Gregorio Marañón was financed by the PROgrama MULtidisciplinar para la Gestión de Antifúngicos y la Reducción de Candidiasis Invasora (PROMULGA) II Project, Instituto de Salud Carlos III, Madrid, Spain, and by the European Regional Development Fund (FEDER) ‘A way of making Europe’ (grant number PI13/01148). Antonio Vena is supported by a Rio Hortega from the Instituto de Salud Carlos III, Madrid, Spain, partially financed by the European Regional Development Fund (FEDER) ‘A way of making Europe’ (grant number CM15/00181).
Transparency declarations
E. B. has participated in meetings and advisory boards for Pfizer, Novartis, Janssen, Baxter, McDonalds, Astellas, Wyeth Lederle, Optimer, several scientific societies and non-profit foundations (Fundación de Ciencias de la Salud); received research funds from Pfizer, Astra-Zeneca, Novartis, Schering-Plough, Covidien, FIS, CIBER Enf Respiratorias, REIPI, Mutua Madrileña, European Community funds, Fundación del Pino; and received payment for conferences from Pfizer, Novartis, Astellas, Wyeth Lederle and other private and public sources. P. M. has none to declare.
This article forms part of a Supplement sponsored and funded by Gilead Sciences Europe Ltd; editorial assistance was provided by Synergy Medical. The content of this Supplement is based on the sessions presented at the CARE VIII meeting, held in Madrid in November 2015.
Acknowledgements
Members of the COMIC study group (Collaboration group on Mycosis)
Roberto Alonso, Fernando Anaya, Rafael Bañares, Emilio Bouza, Amaya Bustinza, Betsabé Cáliz, Ana Fernández-Cruz, Pilar Escribano, Lorenzo Fernández-Quero, Isabel Frias, Jorge Gayoso, Paloma Gijón, Jesús Guinea, Javier Hortal, Maria del Carmen Martínez, Iván Márquez, Patricia Muñoz, Belén Padilla, Teresa Peláez, José Peral, Blanca Pinilla, Carmen Guadalupe Rodríguez, Magdalena Salcedo, Carlos Sánchez, Mar Sánchez-Somolinos, Maria Sanjurjo, David Serrano, Maricela Valerio, Antonio Vena, Eduardo Verde, Encarnación Vilalta and Elena Zamora.
References
Author notes
Members are listed in the Acknowledgements.



