-
PDF
- Split View
-
Views
-
Cite
Cite
Matteo Bassetti, Maddalena Peghin, Jean-Francois Timsit, The current treatment landscape: candidiasis, Journal of Antimicrobial Chemotherapy, Volume 71, Issue suppl_2, November 2016, Pages ii13–ii22, https://doi.org/10.1093/jac/dkw392
Close - Share Icon Share
Abstract
The epidemiology of Candida species infection has changed over recent decades, influenced by local hospital-related factors, patient predisposing conditions and type of antifungal agents administered. A shift from Candida albicans as the predominant pathogen towards an increasing prevalence of the species Candida glabrata and Candida parapsilosis amongst critically ill patients has been documented. Changes in Candida species distribution may impact treatment recommendations due to differences in susceptibility to antifungal agents among species. Previous exposure to antifungal agents has likely contributed to this shift in species distribution. Another evolving epidemiological factor to consider is the global increase in antifungal resistance to certain antifungal drug types, which has been contributed to by the inappropriate use of these agents. Proposed management strategies to optimize treatment of patients with Candida infection include starting prompt ‘early’ antifungal therapy, early cessation of inappropriate therapy, using an adequate dose and duration of therapy and de-escalating treatment whenever possible. The implementation of institutional antifungal stewardship programmes has the potential to promote appropriate utilization of antifungal agents and to significantly improve the care of patients with Candida infection. However, a cultural change among healthcare providers and authorities is currently needed to improve antifungal use worldwide.
Introduction
Invasive candidiasis (IC) comprises both bloodstream and other deep-seated invasive infections caused by Candida species, and represents a significant cause of morbidity and mortality. IC may affect many different patient populations cared for by a large number of physicians in varying types of hospital units. In recent years, there have been substantial changes in IC management and in the availability of antifungal agents, with greater complexity in prescribing suitable drugs and increasing interest in the development of stewardship programmes. The aim of this review is to provide an overview of the changing epidemiology of Candida species infection and to outline the current diagnostic and treatment options, with a specific focus on the role of stewardship programmes in optimizing management strategies.
The changing epidemiology of Candida infections
Over recent decades, the incidence of IC has shown stable or increasing rates in most regions,1 probably due to the growing complexity of surgical procedures, the existence of patient populations at higher risk of infection and the changes in patient demographic characteristics.2,3 In recent epidemiological studies, candidaemia has been described as the fourth to the tenth most common cause of bloodstream infection worldwide4 and its incidence has showed important geographical variation, ranging from 0.02 to 1.73 cases per 1000 hospital admissions.5–7
The epidemiology of candidaemia has been studied extensively in large series focusing on specific high-risk patient populations, including neonates, patients with cancer and surgical and critically ill patients.8,9 In recent years, however, candidaemia has emerged as a rising problem in patients hospitalized in other settings, such as internal medicine wards. This setting can represent a significant reservoir for Candida infections, showing prevalence ranging from 24% to 57% and higher mortality rates compared with other wards.10
During recent years, changes in species distribution have also occurred according to different geographical areas, local hospital-related factors, patient predisposing conditions and the types of antifungal agents received.11Candida albicans used to represent the predominant pathogen among the Candida species; however, today this species accounts for only half the isolates detected. A shift towards an increasing prevalence of Candida glabrata and Candida parapsilosis species has been recently documented, especially among critically ill patients, probably due to an increase in oncology–haematological patients previously exposed to antifungal therapy.11–13 Changes in species distribution may impact treatment recommendations because of differences in susceptibility to azoles and echinocandins among these species. Previous exposure to antifungals is clearly associated with a shift in species distribution and MICs of available antifungal agents.14,15 In addition, inappropriate antifungal use has contributed to the global increase in antifungal resistance to both triazole and echinocandin antifungal drugs, and merits continued vigilance.14
Current treatment options for Candida infections
During the past few years, new insights have substantially changed therapeutic strategies. The classes of antifungal agents that are mainly used in invasive Candida species infections include azoles, polyenes and echinocandins.
Azole antifungal agents target the 14-α-demethylase enzyme which mediates the conversion of lanosterol to ergosterol in the fungus wall. This class is metabolized by P450 cytochromes, which can result in drug–drug interactions. Fluconazole is used in the treatment of candidaemia as a de-escalation therapy, and for the treatment of non-critically ill patients without previous exposure to azoles and without evidence of colonization with a strain that has reduced susceptibility to azoles.15
Amphotericin B is a polyene antifungal agent that binds to the ergosterol in the fungal membrane. Owing to its toxicity, amphotericin B deoxycholate has now been replaced by better-tolerated polyenes. Three lipid formulations of amphotericin B are currently approved for clinical use: liposomal amphotericin B (L-AmB), amphotericin B lipid complex (ABLC) and amphotericin B colloidal dispersion (ABCD). L-AmB is widely used and has favourable pharmacokinetics along with high intracellular penetration in the cerebral spinal fluid and in the eye. Both L-AmB and ABLC achieve therapeutically effective concentrations in the epithelial lining fluid of critically ill patients.16 L-AmB is used as a first-line therapy for disseminated forms of Candida species infection, and as a second-line therapy for IC.17
Echinocandins target the fungal cell wall and act by inhibiting 1,3-β-d-glucan (BDG) synthesis, showing fungicidal activity against Candida species. As BDG is not part of human cells, echinocandins display strong tolerability and safety profiles. Other characteristics of these compounds include broad and strong fungicidal action with activity against biofilms, and few drug–drug interactions; the development of resistance is also rare. Currently, three echinocandins (anidulafungin, caspofungin and micafungin) are used for intravenous treatment and prevention of IC, although most data come from randomized controlled trials for candidaemia; very few data on other forms of IC are available. In addition, it should be taken into account that these drugs do not achieve therapeutically effective concentrations in some tissues (e.g. eyes, CNS, urine, endocardium and peritoneum) and their pharmacokinetic/pharmacodynamic properties are poorly known for critically ill patients.17 Moreover, CLSI clinical breakpoints have not yet been established for caspofungin due to significant inter-laboratory variation in MIC ranges. In general, isolates that are susceptible to anidulafungin as well as micafungin should be considered susceptible to caspofungin.18 Echinocandins are recommended as first-line therapy in the treatment of IC in critically ill patients15 and are preferred in non-critically ill patients with previous exposure to azoles and/or evidence of colonization with a Candida strain with reduced susceptibility to azoles.
Flucytosine (5-fluorocytosine) is an antifungal agent that interferes with both DNA and protein synthesis. Owing to the high incidence of primary and/or acquired resistance, use of flucytosine as monotherapy is significantly restricted. Nevertheless, it should be kept in mind that flucytosine has very good tissue distribution and should be used in combination with other antifungals in cases of endophthalmitis, endocarditis or CNS infections.19
A few new classes of antifungal agents are currently in the pipeline. These include agents with mechanisms of action similar to clinically available classes, and novel mechanisms of action that are either fungus-specific or more selective for fungal targets than mammalian cells.20 Many of these agents have demonstrated potent in vitro and in vivo activity against a broad range of fungi, with some also maintaining activity against isolates known to be resistant to azoles and echinocandins.21
Optimization of management of invasive candidiasis
The management of IC has changed markedly in the past decade. To minimize the negative impact of this infection, several management strategies have been proposed: prophylaxis, empirical therapy, pre-emptive therapy, and presumptive and culture-based targeted treatment.20 However, in this review, for practical purposes the term ‘early’ antifungal treatment refers to pre-emptive, presumptive or empirical therapy.20 The most important principles for the treatment of invasive Candida infections are summarized in Table 1. Each recommendation is discussed in turn below.
| Action . |
|---|
| ▪ Restrict antifungal prophylaxis |
| ▪ Start prompt ‘early’ antifungal treatment based on risk factors |
| ▪ Select an antifungal agent that the isolate is susceptible to |
| ▪ Achieve adequate source control |
| ▪ Use an adequate dose: low dose is associated with resistance |
| ▪ Stop ‘early’ inappropriate therapy |
| ▪ De-escalate whenever possible |
| ▪ Check duration of therapy |
| Action . |
|---|
| ▪ Restrict antifungal prophylaxis |
| ▪ Start prompt ‘early’ antifungal treatment based on risk factors |
| ▪ Select an antifungal agent that the isolate is susceptible to |
| ▪ Achieve adequate source control |
| ▪ Use an adequate dose: low dose is associated with resistance |
| ▪ Stop ‘early’ inappropriate therapy |
| ▪ De-escalate whenever possible |
| ▪ Check duration of therapy |
| Action . |
|---|
| ▪ Restrict antifungal prophylaxis |
| ▪ Start prompt ‘early’ antifungal treatment based on risk factors |
| ▪ Select an antifungal agent that the isolate is susceptible to |
| ▪ Achieve adequate source control |
| ▪ Use an adequate dose: low dose is associated with resistance |
| ▪ Stop ‘early’ inappropriate therapy |
| ▪ De-escalate whenever possible |
| ▪ Check duration of therapy |
| Action . |
|---|
| ▪ Restrict antifungal prophylaxis |
| ▪ Start prompt ‘early’ antifungal treatment based on risk factors |
| ▪ Select an antifungal agent that the isolate is susceptible to |
| ▪ Achieve adequate source control |
| ▪ Use an adequate dose: low dose is associated with resistance |
| ▪ Stop ‘early’ inappropriate therapy |
| ▪ De-escalate whenever possible |
| ▪ Check duration of therapy |
Restrict antifungal prophylaxis to high-risk patients
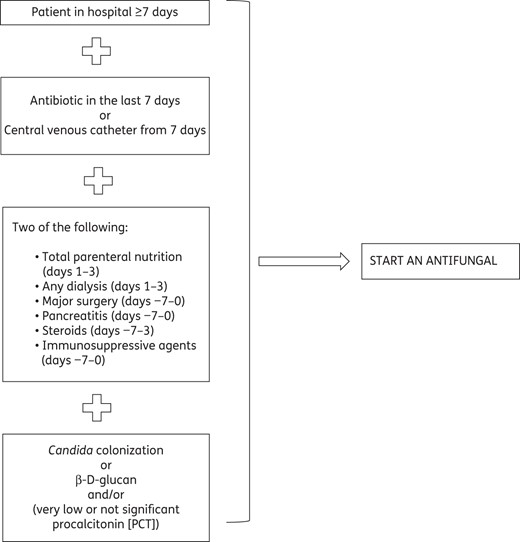
Although the benefits of antifungal prophylaxis are well established in neutropenic patients (e.g. haematological patients), its utility in non-immunocompromised, critically ill patients with sepsis and no confirmed fungal infection is still controversial.22,23 Fluconazole prophylaxis in patients admitted to an ICU can reduce the incidence of IC;24 however, the patient population should be carefully considered before the use of prophylactic fluconazole is recommended. For example, in critically ill patients fluconazole can promote the emergence of non-albicans Candida species, some of which are less susceptible or resistant to fluconazole and its use prophylactically.25 We suggest restricting antifungal prophylaxis to surgical patients presenting with anastomotic leakage after abdominal surgery or re-operation of the digestive tract during the same hospitalization in order to prevent the development of IC (Figure 1).26 The optimal duration of prophylactic treatment is not known.
Start prompt ‘early’ antifungal treatment
IC has considerable clinical and economic consequences.27 The high morbidity and mortality associated with IC are, in part, related to the risk factors of the critically ill patient population, but challenges with diagnosis of the disease due to a lack of accurate diagnostic tools may also be a causative factor. Conventional mycological blood cultures are currently the gold standard for the diagnosis of candidaemia, but data suggest that these tools may fail to diagnose IC in up to 25%–50% of cases.28 It is therefore vital to investigate methods to improve the diagnostic accuracy of these tests. For example, in order to maximize the sensitivity of blood cultures, guidelines recommend filling the culture bottles with 10 mL of blood. The use of bottles containing adsorbing resins and bottles containing non-resin selective milieu also increases the positivity rate, especially in patients who have received antifungal therapy prior to blood culture.15,29 Furthermore, the time required for test results must also be considered. For example, time to positivity of blood cultures in patients with candidaemia can be 2–5 days from the time the blood culture is drawn, depending on the Candida species.30 This delay in diagnosis may cause a delay in the initiation of appropriate antifungal therapy if treatment is not initiated until a diagnosis of candidaemia is confirmed.31,32 Numerous studies have demonstrated that a delay in the initiation of antifungal therapy (e.g. >12 h after the first positive blood culture) is associated with significant increases in in-hospital mortality and cost of care for patients with candidaemia.33,34 Due to the challenges faced in rapidly diagnosing a fungal infection, the majority of treatment courses (∼75%) are currently used as part of a pre-emptive, presumptive or empirical strategy.13,27,35
It is therefore vital to consider the value of predictive models. Predictive models of IC using the Candida Colonization Index (CCI) and corrected CCI (cCCI), or one of the available predictive scores (including the Candida score), are useful to stratify non-neutropenic critically ill patients at high risk; however, these are currently often indiscriminately applied.35,36–38 Recent studies comparing different clinical scores have demonstrated that the CCI has greater predictive ability for the diagnosis of intra-abdominal candidiasis in a highly selected group, whereas the Candida score is higher than the latter in non-selected (medical/surgical) patient populations with candidaemia.37 Although these prediction rules display high negative predictive values, insufficient positive predictive values (<15%) limit their use. The risks of toxicity, selection of resistance and treatment costs should also be factored in when considering the clinical application of these predictive scores.35,36,39
In recent years, other non-culture-based tests for diagnosis and susceptibility have been advocated to offset the diagnostic challenges and improve patient management, but their availability is currently limited to reference mycology laboratories and is associated with high costs.28,30,37 BDG testing has shown high negative predictive value and can be useful to rule out IC;28 nevertheless, it is not specific for IC as it is considered a pan-fungal diagnostic method. False-positive results are frequently observed in the most severe critically ill patients undergoing renal replacement therapies or extracorporeal membrane oxygenation, and in patients who have received immunoglobulin therapy.28 Interestingly, in a recent prospective cohort study, BDG showed superiority to culture, Candida score, CCI and cCCI in predicting the diagnosis of blood culture negative intra-abdominal candidiasis in a highly selected population of patients with gastrointestinal post-operative leakage or necrotizing pancreatitis, and correlated with severity of illness and outcome.39
Different commercially available Candida tests measuring mannan/antimannan antibodies in serum are specific diagnostic markers of Candida species, showing high negative predictive values in patients with IC and possibly being useful to detect early infections (∼6 days prior to blood culture).40 PCR detection of Candida DNA is a potentially powerful diagnostic tool to promptly identify Candida species but its diagnostic performance is still questioned because of the heterogeneity of the available results, the lack of reliable reference standards and differences in techniques used.15
Antibody detection kits, such as the Candida albicans germ tube antibody test (CAGTA) are under evaluation, but there are limited data about their clinical accuracy.41 Procalcitonin (PCT) for detection of Candida species has a high negative predictive value by either blood culture or PCR and may be a useful tool to rule out the presence of candidaemia and guide antifungal treatment regimens in critically ill patients.42 Overall, a combination of non-culture-based techniques may be needed to optimize the diagnosis of Candida infection and reduce the incidence of undiagnosed patients.
Understanding the relationship between microbiological findings, clinical symptoms and serological and molecular biomarkers may help clinicians in the stratification of patients and to more accurately select the candidates suitable for prompt ‘early’ antifungal treatment.
The criteria to start prophylaxis and early antifungal therapy based on the authors' experience are listed in Figure 1.26 Treatment should be based on expert opinion with a careful assessment of: (i) individual risk factors (immune suppression, surgery, invasive procedures, previous use of broad-spectrum antibiotics, pancreatitis, total parenteral nutrition, haemodialysis, steroids and length of hospital stay); (ii) severity of infection (sepsis or organ failure); (iii) fungal history (multiple colonizations, previous candidaemia or proven IC); and (iv) non-culture tests (positivity for a BDG level >250 pg/mL or Candida PCR, very low or non-significant PCT).
Start adequate antifungal treatment and determine source control
The selection and optimization of an antifungal regimen in treating Candida bloodstream infection are based on multiple factors, including the knowledge of local epidemiological data to guide empirical therapy as well as both species identification and antifungal susceptibility testing.
In patients with IC the synergistic effect of optimal treatment and source control within 24−48 h has been advantageous34,43,44 and has marked a paradigm shift in the treatment of IC. Current guidelines from the ESCMID and the IDSA recommend the empirical use of echinocandins in critically ill patients, whereas azoles and lipid formulations of amphotericin B are considered to be alternative or second-line options15,20,45–47 (Table 2). Amphotericin B should also be reserved for end-organ infections such as meningitis, endocarditis or osteomyelitis, or for patients where co-infection with other fungal pathogens is documented or suspected. There is currently no evidence of significant benefit in combining antifungals for the treatment of IC.37
Recommended drugs for empirical treatment of Candida species infections, according to different guidelines
Recommended drugs for empirical treatment of Candida species infections, according to different guidelines
New therapeutic practices may result in ecological shifts and the emergence of resistance, which could influence future recommendations for the management of patients suspected of having IC; this merits vigilance. A prospective multicentre study showed that both fluconazole and caspofungin, the two major drugs recommended for the first-line treatment of candidaemia and for early treatment in high-risk patients, were associated with changes in the epidemiology of Candida infection and with an overall decrease in susceptibility of the isolates to these drugs.48 In addition, the emergence of co-resistance to both azoles and echinocandins in clinical isolates of C. glabrata, a species that appears to be unique in its ability to sequentially acquire and express resistance mutations, has been documented over time.49
Source control, which encompasses all measures to control invasive infection and restore optimal function of the affected area, has been shown to be an important determinant of outcome for patients with candidiasis.50 Although catheter removal in patients with candidaemia remains a controversial issue,51 based on data reported in expert recommendations and previous studies, central venous catheter and device withdrawal should be attempted, any identified collection should be drained and adequate surgical source control should be performed.
Use an adequate dose of antifungal therapy
Although in the past lower doses of antifungal therapy were considered useful to avoid side effects, adequate doses of treatment are now considered paramount to avoid resistance development. Fluconazole should be used at a 12 mg/kg loading dose followed by 6 mg/kg daily. Three lipid formulations of amphotericin B are currently approved: L-AmB, ABLC and ABCD, which are generally used at the following doses: 3 mg/kg daily, 5 mg/kg daily and 3–4 mg/kg daily, respectively. Three echinocandin agents are available: caspofungin (dose of 70 mg on day 1 followed by 50 mg daily), micafungin (dose of 100 mg daily) and anidulafungin (dose of 200 mg on day 1 followed by 100 mg daily thereafter).
Stop inappropriate therapy early
Early treatment is frequently used, although not proven to be effective, and its discontinuation should be carefully evaluated to stop ‘inappropriate’ therapy as soon as possible. Antifungals are usually maintained for an extended time (∼10 days in patients without IC) with the associated unnecessary risk of toxicity and additional costs.43 A recent study suggested that stopping empirical treatment early, within 5 days in the absence of documented candidiasis, did not modify the prognosis of ICU patients.52 The low sensitivity of blood cultures complicates the decision to stop antifungal treatment safely. It should be emphasized that blood cultures should be appropriately taken before any treatment decision is made, in order to improve their accuracy and allow successful early discontinuation. Importantly, the added use of combinations of different biomarkers (BDG, mannan/antimannan and the CAGTA test) could help as a decision-making tool for discontinuing unnecessary empirical therapy in patients with suspected candidaemia.53
De-escalate antifungal therapy if possible
In cases of proven candidiasis or candidaemia, treatment should be given immediately; subsequently, early step-down therapy with a triazole should be considered. Antifungal susceptibility testing is not routinely performed at all institutions but can identify patients with resistant Candida species who are receiving an inappropriate agent and patients who would be candidates for de-escalation. De-escalation from an echinocandin to intravenous or oral fluconazole should be encouraged when the patient is clinically stable and the isolated strain is susceptible. Nevertheless, usually in daily practice a low proportion (around 20%–40%) of de-escalation from an echinocandin to fluconazole therapy in patients with fluconazole-susceptible isolates is performed.52,54 The exact timing for shifting to fluconazole is unknown and may vary from patient to patient, depending on patient- and pathogen-related factors. For IC, the ESCMID and IDSA guidelines recommend a de-escalation strategy (3 days in stabilized patients, as per IDSA, and 10 days overall, as per ESCMID) to limit the emergence of resistant strains and to reduce treatment costs.15,55 The safety of de-escalation (within 5 days) in the case of proven or probable IC has been recently suggested for non-neutropenic patients treated in the ICU, since it was not associated with increased mortality and led to a subsequent and significant decrease in antifungal use.52
Determine an adequate duration of antifungal therapy
The duration of treatment depends on the extent of organ involvement. For uncomplicated candidaemia, duration of treatment is generally considered to be 14 days from the first negative blood culture, requiring daily blood cultures to be performed until negativity. It is important to highlight that this duration applies only to patients in whom disseminated disease, abscesses, or end-organ disease have been excluded. Therefore, it is recommended that all patients with candidaemia undergo diagnostic procedures to detect organ involvement, including transoesophageal echocardiography, fundoscopy and searching for a thrombus. Treatment duration might be much longer in deep-seated infections and endocarditis.15
The role of Candida-specific stewardship programmes
The appropriate use of antifungal agents can improve efficacy, limit the potential for emergence of antifungal resistance and reduce treatment-related costs. The importance of care bundles has been described for the prevention and management of many bacterial infections. However, there is limited experience with antifungal stewardship (AFS) programmes targeting antifungal treatment bundles. We have analysed the most significant examples of AFS programmes that have been implemented to date, aiming to target antifungals active against invasive Candida infections. Table 3 includes the most recent publications.
| Reference . | Duration/type of study/fungal infection explored . | Antifungal stewardship programme bundles . | AFS programme team . | Number of episodes reviewed . | Hospital ward . | ‘Early’ antifungal therapy . | Empirical antifungal therapy adequacy . | Source control . | Antifungal therapy target . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de-escalation . | duration . | use reduction . | cost reduction . | |||||||||
| Swoboda et al. 200956 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. and Aspergillus spp. | Local guidelines | pharmacy | — | ICU | — | — | — | — | — | yes | yes |
| Apisarnthanarak et al. 201057 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. | Prescription review, dose adjustment tool, antifungal prescription forms, non-compulsory recommendations, educational programmes, prescription control strategies | ID, microbiology, pharmacy | 1106 | all | — | — | — | — | yes | yes | yes |
| Standiford et al. 201258 | 7 years, uncontrolled before, during, after intervention on fungi and bacteria | Preauthorization protocol, prescription review, non-compulsory recommendations, local guidelines, computer decision-support assistance | ID, pharmacy | — | all | — | — | — | — | — | — | yes |
| Lopez-Medrano et al. 201359 | 1 year, non-randomized uncontrolled before–after intervention on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | ID, microbiology, pharmacy, | 662 | all | — | — | — | yes | yes | no | yes |
| Guarascio et al. 201360 | 6 months, matched-control analysis on Candida spp. | Prescription review (echinocandins), non-compulsory recommendations, daily 5-point questionnaire assessment | pharmacy | 108 | ICU | — | — | — | yes | yes | yes | yes |
| Alfandari et al. 201363 | 9 years, retrospective study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, local guidelines | ID | 192 | haematology | — | — | — | — | — | yes | — |
| Antworth et al. 201361 | 2 years, quasi-experimental study on Candida spp. | Candida blood culture review, non-compulsory recommendations, educational programmes, local guidelines | ID, microbiology, pharmacy | 78 | all | no | no | no | no | yes | — | — |
| Mondain et al. 201362 | 6 years, prospective observational study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, educational programmes, improvement of diagnostic tools, local guidelines | ID, microbiology, pharmacy, haematologist | 636 | all | yes | yes | no | no | no | yes | yes |
| Micallef et al. 201464 | 1 year, observational prospective on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | microbiology, pharmacy | 294 | all | — | — | — | yes | yes | — | yes |
| Reference . | Duration/type of study/fungal infection explored . | Antifungal stewardship programme bundles . | AFS programme team . | Number of episodes reviewed . | Hospital ward . | ‘Early’ antifungal therapy . | Empirical antifungal therapy adequacy . | Source control . | Antifungal therapy target . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de-escalation . | duration . | use reduction . | cost reduction . | |||||||||
| Swoboda et al. 200956 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. and Aspergillus spp. | Local guidelines | pharmacy | — | ICU | — | — | — | — | — | yes | yes |
| Apisarnthanarak et al. 201057 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. | Prescription review, dose adjustment tool, antifungal prescription forms, non-compulsory recommendations, educational programmes, prescription control strategies | ID, microbiology, pharmacy | 1106 | all | — | — | — | — | yes | yes | yes |
| Standiford et al. 201258 | 7 years, uncontrolled before, during, after intervention on fungi and bacteria | Preauthorization protocol, prescription review, non-compulsory recommendations, local guidelines, computer decision-support assistance | ID, pharmacy | — | all | — | — | — | — | — | — | yes |
| Lopez-Medrano et al. 201359 | 1 year, non-randomized uncontrolled before–after intervention on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | ID, microbiology, pharmacy, | 662 | all | — | — | — | yes | yes | no | yes |
| Guarascio et al. 201360 | 6 months, matched-control analysis on Candida spp. | Prescription review (echinocandins), non-compulsory recommendations, daily 5-point questionnaire assessment | pharmacy | 108 | ICU | — | — | — | yes | yes | yes | yes |
| Alfandari et al. 201363 | 9 years, retrospective study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, local guidelines | ID | 192 | haematology | — | — | — | — | — | yes | — |
| Antworth et al. 201361 | 2 years, quasi-experimental study on Candida spp. | Candida blood culture review, non-compulsory recommendations, educational programmes, local guidelines | ID, microbiology, pharmacy | 78 | all | no | no | no | no | yes | — | — |
| Mondain et al. 201362 | 6 years, prospective observational study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, educational programmes, improvement of diagnostic tools, local guidelines | ID, microbiology, pharmacy, haematologist | 636 | all | yes | yes | no | no | no | yes | yes |
| Micallef et al. 201464 | 1 year, observational prospective on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | microbiology, pharmacy | 294 | all | — | — | — | yes | yes | — | yes |
| Reference . | Duration/type of study/fungal infection explored . | Antifungal stewardship programme bundles . | AFS programme team . | Number of episodes reviewed . | Hospital ward . | ‘Early’ antifungal therapy . | Empirical antifungal therapy adequacy . | Source control . | Antifungal therapy target . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de-escalation . | duration . | use reduction . | cost reduction . | |||||||||
| Swoboda et al. 200956 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. and Aspergillus spp. | Local guidelines | pharmacy | — | ICU | — | — | — | — | — | yes | yes |
| Apisarnthanarak et al. 201057 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. | Prescription review, dose adjustment tool, antifungal prescription forms, non-compulsory recommendations, educational programmes, prescription control strategies | ID, microbiology, pharmacy | 1106 | all | — | — | — | — | yes | yes | yes |
| Standiford et al. 201258 | 7 years, uncontrolled before, during, after intervention on fungi and bacteria | Preauthorization protocol, prescription review, non-compulsory recommendations, local guidelines, computer decision-support assistance | ID, pharmacy | — | all | — | — | — | — | — | — | yes |
| Lopez-Medrano et al. 201359 | 1 year, non-randomized uncontrolled before–after intervention on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | ID, microbiology, pharmacy, | 662 | all | — | — | — | yes | yes | no | yes |
| Guarascio et al. 201360 | 6 months, matched-control analysis on Candida spp. | Prescription review (echinocandins), non-compulsory recommendations, daily 5-point questionnaire assessment | pharmacy | 108 | ICU | — | — | — | yes | yes | yes | yes |
| Alfandari et al. 201363 | 9 years, retrospective study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, local guidelines | ID | 192 | haematology | — | — | — | — | — | yes | — |
| Antworth et al. 201361 | 2 years, quasi-experimental study on Candida spp. | Candida blood culture review, non-compulsory recommendations, educational programmes, local guidelines | ID, microbiology, pharmacy | 78 | all | no | no | no | no | yes | — | — |
| Mondain et al. 201362 | 6 years, prospective observational study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, educational programmes, improvement of diagnostic tools, local guidelines | ID, microbiology, pharmacy, haematologist | 636 | all | yes | yes | no | no | no | yes | yes |
| Micallef et al. 201464 | 1 year, observational prospective on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | microbiology, pharmacy | 294 | all | — | — | — | yes | yes | — | yes |
| Reference . | Duration/type of study/fungal infection explored . | Antifungal stewardship programme bundles . | AFS programme team . | Number of episodes reviewed . | Hospital ward . | ‘Early’ antifungal therapy . | Empirical antifungal therapy adequacy . | Source control . | Antifungal therapy target . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| de-escalation . | duration . | use reduction . | cost reduction . | |||||||||
| Swoboda et al. 200956 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. and Aspergillus spp. | Local guidelines | pharmacy | — | ICU | — | — | — | — | — | yes | yes |
| Apisarnthanarak et al. 201057 | 3 years (18 months before and after implementation), quasi-experimental study on Candida spp. | Prescription review, dose adjustment tool, antifungal prescription forms, non-compulsory recommendations, educational programmes, prescription control strategies | ID, microbiology, pharmacy | 1106 | all | — | — | — | — | yes | yes | yes |
| Standiford et al. 201258 | 7 years, uncontrolled before, during, after intervention on fungi and bacteria | Preauthorization protocol, prescription review, non-compulsory recommendations, local guidelines, computer decision-support assistance | ID, pharmacy | — | all | — | — | — | — | — | — | yes |
| Lopez-Medrano et al. 201359 | 1 year, non-randomized uncontrolled before–after intervention on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | ID, microbiology, pharmacy, | 662 | all | — | — | — | yes | yes | no | yes |
| Guarascio et al. 201360 | 6 months, matched-control analysis on Candida spp. | Prescription review (echinocandins), non-compulsory recommendations, daily 5-point questionnaire assessment | pharmacy | 108 | ICU | — | — | — | yes | yes | yes | yes |
| Alfandari et al. 201363 | 9 years, retrospective study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, local guidelines | ID | 192 | haematology | — | — | — | — | — | yes | — |
| Antworth et al. 201361 | 2 years, quasi-experimental study on Candida spp. | Candida blood culture review, non-compulsory recommendations, educational programmes, local guidelines | ID, microbiology, pharmacy | 78 | all | no | no | no | no | yes | — | — |
| Mondain et al. 201362 | 6 years, prospective observational study on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations, educational programmes, improvement of diagnostic tools, local guidelines | ID, microbiology, pharmacy, haematologist | 636 | all | yes | yes | no | no | no | yes | yes |
| Micallef et al. 201464 | 1 year, observational prospective on Candida spp. and Aspergillus spp. | Prescription review, non-compulsory recommendations | microbiology, pharmacy | 294 | all | — | — | — | yes | yes | — | yes |
Swoboda et al.56 demonstrated that the implementation of standardized practice guidelines for antifungal therapy in ICUs decreased the use of selected antifungal treatment bundles, resulting in a 50% reduction in costs. Nevertheless, quality indicators, including length of stay, readmission and mortality, did not change.
Apisarnthanarak et al.57 conducted a quasi-experimental study 18 months before and after the implementation of an AFS programme for IC. Measures included specific prescriptions, meetings with prescribers and the supervising team, and a systematic clinical assessment at three timepoints: initial (<48 h), between day 3 and day 7, and at the end of treatment. A 59% reduction in antifungal prescriptions (from 194 to 80 prescriptions per 1000 hospitalizations, P < 0.001) was reported after the intervention. The authors concluded that there was also a reduction in the inappropriate use of antifungals against Candida species from 71% to 24% (P < 0.001) and that fluconazole use decreased from 242 to 117 DDDs per 1000 patient-days (P < 0.0001). Notably, these measures had a positive impact on the species distribution, since the incidence C. glabrata and Candida krusei decreased significantly, whereas the rate of C. albicans infections tended to increase. Total cost savings were ∼US$ 30 000.57
Standiford et al.58 described the implementation of a 7 year AFS programme in a large tertiary hospital. The duties of the team were to provide an active computer-assisted real-time review of antimicrobial orders to provide active intervention when necessary and the development of guidelines and policies. Before implementation, annual antifungal expenditure exceeded US$ 3.7 million, declining to US$ 1.3 million by the end of the programme. This study also looked at outcome measures such as hospital stay, readmissions and mortality, finding no significant changes before, during and after programme termination.58
Lopez-Medrano et al.59 conducted an intervention study with a non-randomized uncontrolled ‘before and after’ methodology at a teaching hospital for 12 months. An infectious diseases (ID) specialist managed the supervision and was present in the hospital for 3 h per day, to provide advice for prescribers, and suggested rather than ordered therapies. A total of 662 antifungal therapies (51.7% of which targeted Candida species) were reviewed and a recommendation to change treatment was made in 29% of the cases, including a change from intravenous to oral treatment (15%), cessation of antifungal treatment (8%) and a change to fluconazole (6%). Expenditure on antifungals was reduced by US$ 370 000 (11.8% reduction), but use of antifungal therapy in DDDs was not affected by the intervention. The programme was not related to significant increases in the incidence of candidaemia, percentage of persistent or relapsing candidaemia cases and percentage of fluconazole-resistant Candida species.59
A study by Guarascio et al.60 evaluated a care bundle on the use of antifungals aimed at decreasing the prescription of echinocandins in the ICU setting in a matched control analysis (36 patients after implementation of the AFS programme were compared with 72 paired controls). Clinicians documented five points of a daily protocol (based on initial diagnosis or indication, planned duration of therapy and the potential for de-escalation or discontinuation of therapy) and the renewal of the treatment depended on the answers. The authors found a significant reduction in median days of caspofungin therapy (4.00 versus 2.00, P = 0.001) in the bundle group. Nevertheless, most of this reduction in use was realized in the medical ICU (P = 0.002) as opposed to the surgical ICU (P = 0.188), probably because of the higher compliance with the care bundle recommendations (79% versus 58%; not statistically significant). The average cost of treatment was decreased by approximately US$ 1000 per patient, compared with the previous period.60
In their quasi-experimental investigation, Antworth et al.61 developed an AFS team that advocated prospective recommendations in accordance with care bundles, which included utilization of appropriate antifungal treatment with appropriate duration of use, removal of intravenous catheters, repeat blood cultures, monitoring of time until clearance of candidaemia, and performance of ophthalmological examinations. In this study, a significant increase in all candidaemia care bundle elements in the AFS programme group versus the control group was demonstrated (78.0% versus 40.5%, P = 0.0016). Of note, the implementation of the AFS programme significantly improved the proportion of patients receiving an ophthalmological consultation (97.6% versus 75.7%, P = 0.0108), the selection of appropriate definitive antifungals (100% versus 86.5%, P = 0.0488) and an appropriate duration of therapy (97.6% versus 67.7%, P = 0.0012). Numerical increases were also seen for the other bundle components, such as removal of intravenous catheters and performance of repeat blood cultures, although they were not significantly different. Nevertheless, length of hospitalization, time until clearance of candidaemia, rate of persistent candidaemia and rate of recurrent candidaemia were similar in the AFS programme group versus the control group.61
Mondain et al.62 performed a prospective observational study describing a multifaceted AFS programme in a French teaching tertiary-care hospital from 2005 to 2010, inclusive. Activities included the systematic evaluation of all costly antifungal prescriptions, education of staff, creation of a multidisciplinary antifungal management team, improvement of diagnostic tools, presence of systematic real-time ID advice for candidaemia, and creation of local guidelines on antifungal prophylaxis and the diagnosis and treatment of candidaemia. Advice was given for 54% of cases reviewed, with a compliance rate of 88%. Regarding candidaemia, optimal standards of care were achieved for the timing of antifungal treatment (P = 0.0025), recommended first-line therapy (P = 0.0025), duration of therapy (P = 0.46) and the removal of central venous catheters (P = 0.27), compared with pre-AFS programme findings, even if statistical significance was not met for all process of care measures. Improvements were also noted in the performance of echocardiography and follow-up blood cultures, without reaching statistical significance. Total antifungal prescriptions in DDDs and their cost were contained between 2003 and 2010, corresponding to a 38% decrease in total antifungal use.62
Alfandari et al.63 found that a permanent collaboration between haematologists and an ID physician could improve antifungal prescribing, since antifungal consumption decreased by 40% (from ∼1000 to 620 DDDs per 1000 hospitalization days), but invasive fungal infection incidence remained stable, during the study period (2003–12).
Micallef et al.64 performed an observational prospective 12 month study conducted on an AFS programme targeting the use of high-cost antifungals (specifically L-AmB, voriconazole and echinocandins) in a tertiary referral hospital. Clinical and financial interventions were performed. The authors found that nearly half of all treatments reviewed were stopped or changed (except for voriconazole) and that there was a crude cost saving of £180 000 in antifungal drugs over the year.64
The future role of stewardship programmes in the management of Candida infection
The authors encourage further exploration in the use of care bundles in AFS programmes as part of a multifaceted approach to promote appropriate antifungal utilization and to optimize the management of patients with IC. These programmes cannot succeed without continuous exchange between clinicians managing the patients and a multidisciplinary team, including an ID specialist team, supported by a microbiologist and a pharmacist trained in ID quality information. Although long-term ecological benefits cannot yet be measured, data suggest that AFS programmes may decrease the rate of Candida strains resistant to fluconazole, without affecting the quality of care provided.57 As previous use of antimicrobial agents represents one of the major risk factors of IC, antifungals should be included in a global anti-infective stewardship programme. The integration of this programme with control of nosocomial infections and cross-transmission of multiresistant organisms is also a key point that must be considered.
Implementing, sustaining and assessing stewardship programmes require resources. Health professionals are now also tasked with the implementation and subsequent cost justification of new economic challenges. A report focusing on the cost analysis of such a programme demonstrated that the establishment of an AFS programme can be very cost-effective.58 Finally, it is important to emphasize that this stewardship work should be offered on an ongoing basis, as it has also been shown that the baseline prescribing culture returns shortly after an interruption.58 Further studies are needed and should explore the possibility of developing multicentre AFS programmes for extended periods of time.
Conclusions
There are several opportunities to optimize management strategies for Candida infections. The implementation of institutional AFS programmes has the potential to provide significant improvements in care for patients with Candida infections. However, a cultural change among healthcare providers and authorities will be needed to improve antifungal use worldwide.
Transparency declarations
M. B. serves on scientific advisory boards for AstraZeneca, Bayer, Gilead, Merck, Pfizer Inc., Paratek, Astellas Pharma Inc and Tetraphase and has received funding for travel or speaker honoraria from AstraZeneca, Gilead, Pfizer Inc., Merck, Novartis, Gilead Sciences, Angelini, Astellas Pharma Inc. and Vifor Pharma. J. F. T. serves on a scientific advisory board for Merck, has received research grants and/or speaker honoraria from Merck, Astellas, Pfizer and Gilead. M. P. has none to declare.
This article forms part of a Supplement sponsored and funded by Gilead Sciences Europe Ltd; editorial assistance was provided by Synergy Medical. The content of this Supplement is based on the sessions presented at the CARE VIII meeting, held in Madrid in November 2015.



