-
PDF
- Split View
-
Views
-
Cite
Cite
I. Viciana, C. M. González-Domenech, R. Palacios, M. Delgado, A. Del Arco, F. Tellez, F. Jarilla, S. Fernández, E. Clavijo, J. Santos, Clinical, virological and phylogenetic characterization of a multiresistant HIV-1 strain outbreak in naive patients in southern Spain, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 2, February 2016, Pages 357–361, https://doi.org/10.1093/jac/dkv332
Close - Share Icon Share
Abstract
We describe the characteristics of an HIV-1 strain with six viral reverse transcriptase mutations (D67N, T69N/D, V118I, V179D, T215S and K219Q), which we have called the Malaga strain. This strain was detected in treatment-naive patients from southern Spain.
The study was undertaken at the Virgen de la Victoria Hospital, Malaga, a reference centre for the study of HIV-1 genotype resistance in Andalusia (the ‘Costa del Sol’), Spain. Genotypic resistance testing was done in an automated sequencer. Phylogenetic analysis was performed using a 630 bp region of the reverse transcriptase with the mutations mentioned.
Between 2007 and 2014, we detected the Malaga strain in 30 treatment-naive patients. All were MSM, seen at five hospitals on the Costa del Sol. In all cases, the HIV-1 was subtype B with viral tropism R5. Phylogenetic analysis based on the reverse transcriptase sequence showed consistent grouping (with a bootstrap value of the common node of 100%) of the isolates that shared the mutation pattern mentioned. This strain has not been detected elsewhere or in previously treated patients. All of the patients treated with first-line combination ART responded.
We report a cluster of an HIV-1 strain with multiple resistance mutations that was transmitted over a period of >8 years, affecting 30 naive patients from the same geographical area. The strain was susceptible to first-line combination ART.
Introduction
The study of primary resistance in new diagnoses of HIV infection has resulted in a large number of series providing very diverse data about its prevalence, incidence and rates.1–4 Although the worldwide prevalence of primary resistance has fallen, an important percentage of patients still have strains with resistance mutations at the time of diagnosis.5,6
The possibility of sequencing the reverse transcriptase (RT) and protease (PR) in treatment-naive patients has enabled molecular epidemiological studies about HIV infection, as these regions in the viral genome cannot only be used to estimate the viral subtype, they can also be used for phylogenetic studies.7 Phylogenetic studies provide information about epidemiological dynamics and numerous studies have demonstrated the presence of groupings, particularly among recently infected persons. This has been useful to show the high rate of transmission that can occur during acute infection.8–10 Viruses in recently infected patients tend to group more than in patients with chronic infection.11,12 In addition, patients who are infected with resistant strains group together in clusters of several persons, over a variable time period, though usually several years, and almost always involving MSM.12–16
In this study, we describe the characteristics of an HIV strain with a pattern of six mutations in the viral RT (D67N, T69N/D, V118I, V179D, T215S and K219Q in the amino acid sequence) that confer resistance to RT inhibitors, both NRTIs and NNRTIs, and which we have called the Malaga strain. This strain has been transmitted as an outbreak among 30 MSM in southern Spain between 2007 and 2014.
Methods
The study was undertaken at the Virgen de la Victoria Hospital, Malaga, a reference centre for the study of HIV-1 genotype resistance in the region of Andalusia, southern Spain. All patients diagnosed with HIV infection routinely undergo genotype resistance testing at the time of diagnosis and before starting combination ART (cART) if >6 months has passed since diagnosis. The tests are done by nucleic acid extraction using the MagNApure system (Roche) and a fragment of the pol gene, which codes for RT and PR, is sequenced in both senses using RT–PCR and Sanger sequencing with commercial reagents (Trugene HIV Genotyping Kit, Siemens) in accordance with the manufacturer's instructions. Genotypic resistance testing was conducted in an automated sequencer (Long Read Tower, Visible Genetics). The sequences were aligned and compared with HIV-1 reference strain LAV-1 (GenBank accession number K02013) using OpenGene DNA Sequencing System software. Viral tropism was determined with an ‘in-house’ sequencing technique of the V3 region and interpreted with geno2 pheno FPR 10%. Clinically relevant drug resistance to antiretrovirals was evaluated using the Stanford database algorithm.17 Alternatively, transmitted drug resistance (TDR)-associated mutations were evaluated following the WHO surveillance drug resistance mutation list updated in 2009 by Bennett et al.18 Multiresistance was defined as genotypic resistance to two or three families of antiretrovirals. Ultradeep RT and PR sequencing with a GS Junior 454 (Roche), seeking minority populations, and sequencing of the proviral DNA in 500 μL of whole blood were performed in some patients carrying the Malaga strain.
All genotypes of the Malaga strain underwent phylogenetic analysis, using a partial region (630 bp) of the RT, with the mutations mentioned. For comparative purposes, we randomly included another 30 sequences from naive patients diagnosed in the same geographical area, belonging to both subtype B and other subtypes. The sequences were aligned using Clustal X19 and the phylogenetic reconstruction was done with the neighbour-joining method using the corresponding programs included in the Phylip software package (dnadist, neighbor and consense).20 Evolutionary distances were calculated from the two-parameter Kimura model.21 The reliability of each grouping on the resulting tree was assessed from its bootstrap resampling value (seqboot program of the Phylip package), from which it was statistically and randomly verified whether the order in which the sequences had been introduced influenced the clusters obtained; the bootstrap value was based on 1000 resamplings.
Epidemiological, clinical, virological and therapy-related data were collected from all patients with the Malaga strain.
Nucleotide sequence accession numbers
The sequences used in this study were registered in GenBank under accession numbers KP081410–KP081469.
Results
From January 2007 to December 2014, a total of 1856 resistance studies were performed for naive patients. Of these, 30 (1.6%) had the Malaga strain. All were MSM, 28 were Spanish and 1 was from Colombia and 1 from Argentina. The patients were seen at five hospitals on the Costa del Sol. The clinical and epidemiological characteristics of the patients are shown in Table 1. The median CD4 cell count was 525 cells/mm3 (range: 214–1170). The median plasma viral load (VL) was 27 855 copies/mL (range: 1189 to >10 000 000). No patient had AIDS and only two had a CD4 cell count <350 cells/mm3. In all cases, the HIV-1 was subtype B with viral tropism R5. Eleven patients had a prior negative HIV serology with a mean time to seroconversion of 23.1 months.
| Case . | Age at diagnosis of HIV-1 (years) . | Year of diagnosis of HIV . | Year of detection of the Malaga strain . | CD4 cell count at diagnosis (cells/mm3) . | Baseline VL (copies/mL) . | cART . | Therapeutic success . | GenBank . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 2006 | 2007 | 417 | 4799 | no | — | KP081423 |
| 2 | 46 | 2007 | 2007 | 510 | 62 570 | Atripla® | yes | KP081422 |
| 3 | 36 | 2008 | 2008 | 272 | 868 | TDF/FTC + DRV/RTV | yes | KP081425 |
| 4 | 37 | 1997 | 2008 | 264 | 313 549 | TDF/FTC + LPV/RTV | yes | KP081441 |
| 5 | 27 | 2008 | 2008 | 576 | 13 118 | Atripla® | yes | KP081428 |
| 6 | 36 | 2009 | 2009 | 480 | 2415 | no | — | KP081426 |
| 7 | 37 | 2009 | 2009 | 552 | 10 642 | Stribild® | yes | KP081434 |
| 8 | 27 | 2010 | 2010 | 342 | 83 728 | Atripla® | yes | KP081427 |
| 9 | 36 | 2011 | 2011 | 538 | 68 413 | Atripla® | yes | KP081432 |
| 10 | 37 | 2011 | 2011 | 639 | 10 502 | ABC/3TC + DRV/RTV | yes | KP081435 |
| 11 | 37 | 2011 | 2011 | 876 | 52 530 | Eviplera® | yes | KP081442 |
| 12 | 31 | 2011 | 2011 | 369 | 1382 | TDF/FTC + DRV/RTV | yes | KP081429 |
| 13 | 48 | 2011 | 2011 | 343 | 131 000 | Atripla® | yes | KP081443 |
| 14 | 41 | 2011 | 2011 | 214 | 303 300 | Eviplera® | yes | KP081430 |
| 15 | 52 | 1993 | 2011 | 406 | 19 160 | Atripla® | yes | KP081436 |
| 16 | 49 | 2012 | 2012 | 531 | 64 969 | ABC/3TC + ETV | yes | KP081449 |
| 17 | 29 | 2011 | 2012 | 886 | 96 912 | TDF/FTC + DRV/RTV | yes | KP081439 |
| 18 | 47 | 2012 | 2012 | 675 | 30 141 | Atripla® | yes | KP081448 |
| 19 | 38 | 2012 | 2012 | 769 | 1874 | no | — | KP081424 |
| 20 | 35 | 2012 | 2012 | 632 | 130 000 | ABC/3TC + RAL | yes | KP081451 |
| 21 | 72 | 2012 | 2012 | 361 | 3906 | ABC/3TC + ETV | yes | KP081450 |
| 22 | 48 | 2012 | 2012 | 539 | 10 000 000 | TDF/FTC + RAL | yes | KP081437 |
| 23 | 42 | 2012 | 2013 | 363 | 49 140 | TDF/FTC + ATV/RTV | yes | KP081444 |
| 24 | 45 | 2013 | 2014 | 541 | 25 569 | Eviplera® | KP081440 | |
| 25 | 24 | 2013 | 2014 | 403 | 88 730 | Atripla® | yes | KP081431 |
| 26 | 40 | 2014 | 2014 | 1170 | 14 340 | not known | yes | KP081445 |
| 27 | 33 | 2014 | 2014 | 608 | 12 210 | Atripla® | yes | KP081433 |
| 28 | 38 | 2014 | 2014 | 602 | 14 950 | Atripla® | yes | KP081446 |
| 29 | 25 | 2014 | 2014 | 790 | 1189 | no | — | KP081447 |
| 30 | 39 | 2014 | 2014 | 402 | 209 364 | Stribild® | yes | KP081438 |
| Case . | Age at diagnosis of HIV-1 (years) . | Year of diagnosis of HIV . | Year of detection of the Malaga strain . | CD4 cell count at diagnosis (cells/mm3) . | Baseline VL (copies/mL) . | cART . | Therapeutic success . | GenBank . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 2006 | 2007 | 417 | 4799 | no | — | KP081423 |
| 2 | 46 | 2007 | 2007 | 510 | 62 570 | Atripla® | yes | KP081422 |
| 3 | 36 | 2008 | 2008 | 272 | 868 | TDF/FTC + DRV/RTV | yes | KP081425 |
| 4 | 37 | 1997 | 2008 | 264 | 313 549 | TDF/FTC + LPV/RTV | yes | KP081441 |
| 5 | 27 | 2008 | 2008 | 576 | 13 118 | Atripla® | yes | KP081428 |
| 6 | 36 | 2009 | 2009 | 480 | 2415 | no | — | KP081426 |
| 7 | 37 | 2009 | 2009 | 552 | 10 642 | Stribild® | yes | KP081434 |
| 8 | 27 | 2010 | 2010 | 342 | 83 728 | Atripla® | yes | KP081427 |
| 9 | 36 | 2011 | 2011 | 538 | 68 413 | Atripla® | yes | KP081432 |
| 10 | 37 | 2011 | 2011 | 639 | 10 502 | ABC/3TC + DRV/RTV | yes | KP081435 |
| 11 | 37 | 2011 | 2011 | 876 | 52 530 | Eviplera® | yes | KP081442 |
| 12 | 31 | 2011 | 2011 | 369 | 1382 | TDF/FTC + DRV/RTV | yes | KP081429 |
| 13 | 48 | 2011 | 2011 | 343 | 131 000 | Atripla® | yes | KP081443 |
| 14 | 41 | 2011 | 2011 | 214 | 303 300 | Eviplera® | yes | KP081430 |
| 15 | 52 | 1993 | 2011 | 406 | 19 160 | Atripla® | yes | KP081436 |
| 16 | 49 | 2012 | 2012 | 531 | 64 969 | ABC/3TC + ETV | yes | KP081449 |
| 17 | 29 | 2011 | 2012 | 886 | 96 912 | TDF/FTC + DRV/RTV | yes | KP081439 |
| 18 | 47 | 2012 | 2012 | 675 | 30 141 | Atripla® | yes | KP081448 |
| 19 | 38 | 2012 | 2012 | 769 | 1874 | no | — | KP081424 |
| 20 | 35 | 2012 | 2012 | 632 | 130 000 | ABC/3TC + RAL | yes | KP081451 |
| 21 | 72 | 2012 | 2012 | 361 | 3906 | ABC/3TC + ETV | yes | KP081450 |
| 22 | 48 | 2012 | 2012 | 539 | 10 000 000 | TDF/FTC + RAL | yes | KP081437 |
| 23 | 42 | 2012 | 2013 | 363 | 49 140 | TDF/FTC + ATV/RTV | yes | KP081444 |
| 24 | 45 | 2013 | 2014 | 541 | 25 569 | Eviplera® | KP081440 | |
| 25 | 24 | 2013 | 2014 | 403 | 88 730 | Atripla® | yes | KP081431 |
| 26 | 40 | 2014 | 2014 | 1170 | 14 340 | not known | yes | KP081445 |
| 27 | 33 | 2014 | 2014 | 608 | 12 210 | Atripla® | yes | KP081433 |
| 28 | 38 | 2014 | 2014 | 602 | 14 950 | Atripla® | yes | KP081446 |
| 29 | 25 | 2014 | 2014 | 790 | 1189 | no | — | KP081447 |
| 30 | 39 | 2014 | 2014 | 402 | 209 364 | Stribild® | yes | KP081438 |
TDF/FTC, tenofovir/emtricitabine; DRV/RTV, darunavir/ritonavir; LPV/RTV, lopinavir/ritonavir; ABC/3TC, abacavir/lamivudine; ETV, etravirine; RAL, raltegravir; ATV/RTV, atazanavir/ritonavir.
| Case . | Age at diagnosis of HIV-1 (years) . | Year of diagnosis of HIV . | Year of detection of the Malaga strain . | CD4 cell count at diagnosis (cells/mm3) . | Baseline VL (copies/mL) . | cART . | Therapeutic success . | GenBank . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 2006 | 2007 | 417 | 4799 | no | — | KP081423 |
| 2 | 46 | 2007 | 2007 | 510 | 62 570 | Atripla® | yes | KP081422 |
| 3 | 36 | 2008 | 2008 | 272 | 868 | TDF/FTC + DRV/RTV | yes | KP081425 |
| 4 | 37 | 1997 | 2008 | 264 | 313 549 | TDF/FTC + LPV/RTV | yes | KP081441 |
| 5 | 27 | 2008 | 2008 | 576 | 13 118 | Atripla® | yes | KP081428 |
| 6 | 36 | 2009 | 2009 | 480 | 2415 | no | — | KP081426 |
| 7 | 37 | 2009 | 2009 | 552 | 10 642 | Stribild® | yes | KP081434 |
| 8 | 27 | 2010 | 2010 | 342 | 83 728 | Atripla® | yes | KP081427 |
| 9 | 36 | 2011 | 2011 | 538 | 68 413 | Atripla® | yes | KP081432 |
| 10 | 37 | 2011 | 2011 | 639 | 10 502 | ABC/3TC + DRV/RTV | yes | KP081435 |
| 11 | 37 | 2011 | 2011 | 876 | 52 530 | Eviplera® | yes | KP081442 |
| 12 | 31 | 2011 | 2011 | 369 | 1382 | TDF/FTC + DRV/RTV | yes | KP081429 |
| 13 | 48 | 2011 | 2011 | 343 | 131 000 | Atripla® | yes | KP081443 |
| 14 | 41 | 2011 | 2011 | 214 | 303 300 | Eviplera® | yes | KP081430 |
| 15 | 52 | 1993 | 2011 | 406 | 19 160 | Atripla® | yes | KP081436 |
| 16 | 49 | 2012 | 2012 | 531 | 64 969 | ABC/3TC + ETV | yes | KP081449 |
| 17 | 29 | 2011 | 2012 | 886 | 96 912 | TDF/FTC + DRV/RTV | yes | KP081439 |
| 18 | 47 | 2012 | 2012 | 675 | 30 141 | Atripla® | yes | KP081448 |
| 19 | 38 | 2012 | 2012 | 769 | 1874 | no | — | KP081424 |
| 20 | 35 | 2012 | 2012 | 632 | 130 000 | ABC/3TC + RAL | yes | KP081451 |
| 21 | 72 | 2012 | 2012 | 361 | 3906 | ABC/3TC + ETV | yes | KP081450 |
| 22 | 48 | 2012 | 2012 | 539 | 10 000 000 | TDF/FTC + RAL | yes | KP081437 |
| 23 | 42 | 2012 | 2013 | 363 | 49 140 | TDF/FTC + ATV/RTV | yes | KP081444 |
| 24 | 45 | 2013 | 2014 | 541 | 25 569 | Eviplera® | KP081440 | |
| 25 | 24 | 2013 | 2014 | 403 | 88 730 | Atripla® | yes | KP081431 |
| 26 | 40 | 2014 | 2014 | 1170 | 14 340 | not known | yes | KP081445 |
| 27 | 33 | 2014 | 2014 | 608 | 12 210 | Atripla® | yes | KP081433 |
| 28 | 38 | 2014 | 2014 | 602 | 14 950 | Atripla® | yes | KP081446 |
| 29 | 25 | 2014 | 2014 | 790 | 1189 | no | — | KP081447 |
| 30 | 39 | 2014 | 2014 | 402 | 209 364 | Stribild® | yes | KP081438 |
| Case . | Age at diagnosis of HIV-1 (years) . | Year of diagnosis of HIV . | Year of detection of the Malaga strain . | CD4 cell count at diagnosis (cells/mm3) . | Baseline VL (copies/mL) . | cART . | Therapeutic success . | GenBank . |
|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 2006 | 2007 | 417 | 4799 | no | — | KP081423 |
| 2 | 46 | 2007 | 2007 | 510 | 62 570 | Atripla® | yes | KP081422 |
| 3 | 36 | 2008 | 2008 | 272 | 868 | TDF/FTC + DRV/RTV | yes | KP081425 |
| 4 | 37 | 1997 | 2008 | 264 | 313 549 | TDF/FTC + LPV/RTV | yes | KP081441 |
| 5 | 27 | 2008 | 2008 | 576 | 13 118 | Atripla® | yes | KP081428 |
| 6 | 36 | 2009 | 2009 | 480 | 2415 | no | — | KP081426 |
| 7 | 37 | 2009 | 2009 | 552 | 10 642 | Stribild® | yes | KP081434 |
| 8 | 27 | 2010 | 2010 | 342 | 83 728 | Atripla® | yes | KP081427 |
| 9 | 36 | 2011 | 2011 | 538 | 68 413 | Atripla® | yes | KP081432 |
| 10 | 37 | 2011 | 2011 | 639 | 10 502 | ABC/3TC + DRV/RTV | yes | KP081435 |
| 11 | 37 | 2011 | 2011 | 876 | 52 530 | Eviplera® | yes | KP081442 |
| 12 | 31 | 2011 | 2011 | 369 | 1382 | TDF/FTC + DRV/RTV | yes | KP081429 |
| 13 | 48 | 2011 | 2011 | 343 | 131 000 | Atripla® | yes | KP081443 |
| 14 | 41 | 2011 | 2011 | 214 | 303 300 | Eviplera® | yes | KP081430 |
| 15 | 52 | 1993 | 2011 | 406 | 19 160 | Atripla® | yes | KP081436 |
| 16 | 49 | 2012 | 2012 | 531 | 64 969 | ABC/3TC + ETV | yes | KP081449 |
| 17 | 29 | 2011 | 2012 | 886 | 96 912 | TDF/FTC + DRV/RTV | yes | KP081439 |
| 18 | 47 | 2012 | 2012 | 675 | 30 141 | Atripla® | yes | KP081448 |
| 19 | 38 | 2012 | 2012 | 769 | 1874 | no | — | KP081424 |
| 20 | 35 | 2012 | 2012 | 632 | 130 000 | ABC/3TC + RAL | yes | KP081451 |
| 21 | 72 | 2012 | 2012 | 361 | 3906 | ABC/3TC + ETV | yes | KP081450 |
| 22 | 48 | 2012 | 2012 | 539 | 10 000 000 | TDF/FTC + RAL | yes | KP081437 |
| 23 | 42 | 2012 | 2013 | 363 | 49 140 | TDF/FTC + ATV/RTV | yes | KP081444 |
| 24 | 45 | 2013 | 2014 | 541 | 25 569 | Eviplera® | KP081440 | |
| 25 | 24 | 2013 | 2014 | 403 | 88 730 | Atripla® | yes | KP081431 |
| 26 | 40 | 2014 | 2014 | 1170 | 14 340 | not known | yes | KP081445 |
| 27 | 33 | 2014 | 2014 | 608 | 12 210 | Atripla® | yes | KP081433 |
| 28 | 38 | 2014 | 2014 | 602 | 14 950 | Atripla® | yes | KP081446 |
| 29 | 25 | 2014 | 2014 | 790 | 1189 | no | — | KP081447 |
| 30 | 39 | 2014 | 2014 | 402 | 209 364 | Stribild® | yes | KP081438 |
TDF/FTC, tenofovir/emtricitabine; DRV/RTV, darunavir/ritonavir; LPV/RTV, lopinavir/ritonavir; ABC/3TC, abacavir/lamivudine; ETV, etravirine; RAL, raltegravir; ATV/RTV, atazanavir/ritonavir.
Two patients (Cases 4 and 15) were diagnosed in 1997 and 1993; Case 4 in the Canary Islands and Case 15 in Gijon (northern Spain). Case 4 moved to Malaga in 2005 and started cART in 2008. Genotyping prior to starting ART showed the Malaga strain, but no genotype data are available for the time of diagnosis. Case 15, who moved to Malaga in 2003, was considered an elite controller as he had an undetectable VL until May 2008, at which time he had persistent viraemia. Genotyping in 2011 prior to starting cART detected the Malaga strain. Given that this Malaga strain has not been detected in the places of origin of these two patients, and despite the lack of genotype studies at the time of their diagnosis, it is reasonable to suppose that in both cases reinfection with the Malaga strain occurred during their time in Malaga. In all other cases, the Malaga strain was detected at the time of diagnosis, all in 2007–14. We studied the in vivo evolution of the viral population in the four patients who did not initiate cART (follow-up 24–48 months) and the resistance pattern persisted in all. In the two patients who underwent proviral DNA sequencing, the same sequence was obtained in plasma. In the patients who underwent ultradeep sequencing of RT and PR by GS Junior 454 (Roche), no minority populations with resistance mutations different from the Malaga strain were found. To date, 26 patients have started cART (Table 1), all with a good response (undetectable VL), independent of the cART used.
Phylogenetic analysis showed consistent grouping of the isolates sharing the relevant mutation pattern (Figure 1). Phylogenetic grouping by subtypes was maintained, showing a clear separation between the transmission cluster of the Malaga strain and the non-B subtype sequences, and slightly less though still highly significant, the other 16 subtype B sequences chosen randomly from among the naive patients diagnosed at our hospital. Finally, the mean genetic distance between the 30 sequences of the Malaga strain was very small, about (or very slightly above) 0.01 (variation of one nucleotide for every 100 positions in the nucleotide sequences). When the sequences were compared with others in the GenBank database, even in cases showing a high percentage of sequence identity, the exact mutation pattern described could not be found.
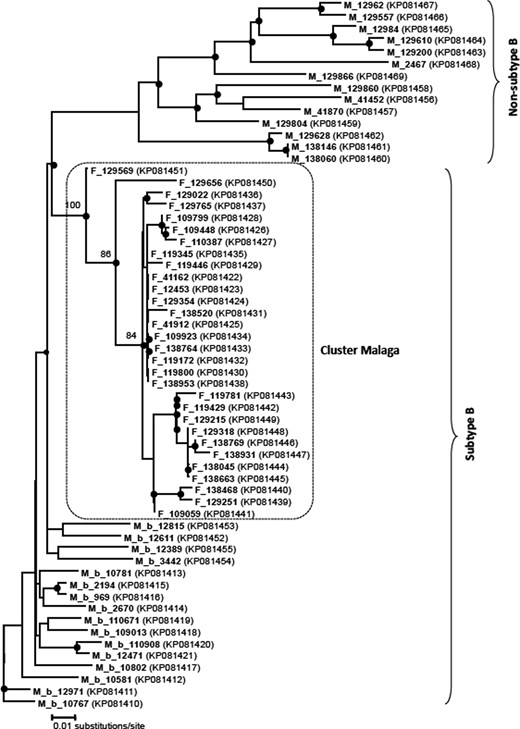
Phylogenetic tree obtained by the neighbour-joining method and based on the partial RT region of the HIV genome. The box shows the position of the Malaga transmission cluster compared with the same region in other therapy-naive patients. Shaded circles at the nodes indicate bootstrap values >50%, showing the main groupings of the Malaga cluster in more detail. Each patient is represented by their sample ID. GenBank accession numbers are given in parentheses.
Discussion
This study reports an outbreak of HIV-1 due to a strain with a characteristic mutation pattern (D67N, T69N/D, V118I, V179D, T215S and K219Q) that has been circulating in our area for ≥8 years. As far as we are aware, this is the outbreak with the highest number of reported cases.
The Malaga strain was first detected in 2007 in a patient diagnosed in 2006 and most recently in a patient diagnosed in 2014. The centre of this outbreak was the province of Malaga, where all the patients live. To date, this strain has not been detected in either non-naive patients or in patients outside Malaga.
Mutations in codons 67, 215 and 219 are thymidine analogue mutations (TAMs) and the mutation at position 69 is also often seen in patients exposed to thymidine analogues. T215S is a revertant of the resistance mutation 215Y/F that has been involved in many outbreaks.8,16,22,23 V118I occurs in <2% of untreated patients, though its prevalence increases in persons who have received multiple analogues and it facilitates the reduction in TAM sensitivity. V179D/E occurs in <1% of persons who have not received an NNRTI and reduces by 2-fold the susceptibility to nevirapine and efavirenz.17,18 In summary, the Malaga strain has low-level resistance to tenofovir and abacavir, intermediate resistance to zidovudine, stavudine and didanosine and potential low-level resistance to all NNRTIs. Despite this resistance pattern, the viraemia can be well controlled with first-line cART.
Other characteristics of the strain are its persistence over time in the same person and its ability to be transmitted. Although we have not determined its replicative capacity, our isolate has been being transmitted for a long time, suggesting high viral fitness. Likewise, the fact that a strain with various resistance mutations can be transmitted to many persons suggests relatively high infectivity, in contrast to the loss of replicative capacity that occurs with resistant variants in naive patients and which tend to revert to the WT strain.24 The mutations in this cluster, though, do not appear to have much impact on the replicative capacity, given the absence of reversion to the WT strain seen in four of our patients. In addition, the CD4 cell count and VL suggest that this strain is not highly virulent, though nevertheless it is easily transmissible given the number of persons infected. However, it is susceptible to first-line cART.
Phylogenetic analysis showed that 30 sequences of this Malaga strain were grouped together, forming a robust cluster (Figure 1). The existence of this strong grouping is supported by both the very high bootstrap values and a very low mean genetic distance. Many phylogenetic studies have found that transmission of resistant strains is via clusters. A phylogenetic study of sequences of the pol gene in 637 patients newly diagnosed with HIV infection in Geneva showed that over half of the recent infections were linked to transmission clusters and clusters were more frequent in individuals with TDR than in those with susceptible strains.15 A study undertaken in Greece involving 369 naive patients found the prevalence of TDR to be 12.5%, while phylogenetic analysis revealed three statistically robust transmission clusters involving drug-resistant strains, including one cluster of 12 patients.23 Another study in the Basque Country (northern Spain) undertaken in 2004–07 found that 119 of 261 (45%) newly diagnosed patients were involved in transmission clusters. These sequences were grouped into 43 clusters, each comprising between 2 and 19 persons. Additionally, 11% had at least one resistance-related mutation in RT or PR.8 Another study, in Granada (south-eastern Spain), of 693 sequences detected 77 clusters, of which 15 had TDR, though they were all small.25 Phylogenetic analysis is, therefore, very useful for the epidemiological analysis of different HIV-1 transmission chains and the identification of clusters and source subjects to prevent the expansion of resistance mutations. In our case, we do not know the source subject of the Malaga strain. Probably, the strain arose from a patient treated with thymidine analogues, though this is yet to be confirmed.
In summary, we report a cluster of a strain with six mutations in RT, transmitted over a period of >8 years and that affected 30 naive patients from the same geographical area. This Malaga strain persists over time, is easily transmitted and is susceptible to first-line cART. Phylogenetic analysis showed a strong grouping of the carrier strains of this pattern of mutations, suggesting they are all part of the same transmission cluster.
Funding
Partially funded by the RD12/0017/0017 project (Plan Nacional R+D+I) and cofinanced by Instituto de Salud Carlos III-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional.
Transparency declarations
None to declare.
Acknowledgements
We thank Natalia Chueca and Federico García, for their comments about the study prior to submission, and Josefa Ruiz, Enrique Nuño and Manuel Márquez, for their assistance to some patients. We also thank Ian Johnstone for help with English language.



