-
PDF
- Split View
-
Views
-
Cite
Cite
Adrian Curran, Jhon Rojas, Alfonso Cabello, Jesús Troya, Arkaitz Imaz, Pere Domingo, Esteban Martinez, Pablo Ryan, Miguel Górgolas, Daniel Podzamczer, Hernando Knobel, Félix Gutiérrez, Esteban Ribera, Effectiveness and safety of an abacavir/lamivudine + rilpivirine regimen for the treatment of HIV-1 infection in naive patients, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 12, 1 December 2016, Pages 3510–3514, https://doi.org/10.1093/jac/dkw347
Close - Share Icon Share
To describe the effectiveness and safety of an abacavir/lamivudine + rilpivirine regimen in naive HIV-1-infected patients, as there is a lack of data with this combination.
This was an observational, retrospective, multicentre study in eight Spanish hospitals. All antiretroviral-naive patients ≥18 years old and starting abacavir/lamivudine + rilpivirine were included. Effectiveness (ITT and on-treatment) and safety (adverse events and laboratory parameters) were assessed during follow-up. Values are expressed as n (%) or median (IQR). The Wilcoxon signed-rank test was used to compare baseline and 6 and 12 month values.
Eighty-four patients were included [93% males, age = 36 (30–45) years]. Time since HIV diagnosis was 12 (4–35) months. Fifty-one per cent of patients had comorbidities. Baseline CD4+ was 425 (340–519) cells/mm3 and baseline HIV-RNA was 19 000 (9500–42 000) copies/mL. Median follow-up was 18 (9–22) months; 100% and 68% patients with at least 6 and 12 months, respectively. At 6 and 12 months effectiveness was 94% and 86% by ITT analysis and 96% and 97% by on-treatment analysis. At 12 months, there were significant increases in CD4+ (+262 cell/mm3) and HDL cholesterol (+4 mg/dL) and a significant decrease in the total cholesterol/HDL cholesterol ratio (−0.2). There were two (2.4%) virological failures (HIV-RNA 50–100 copies/mL); one patient later achieving virological suppression without changing the treatment. Six patients (7.1%) changed treatment due to reasons other than virological failure or side effects. One patient discontinued treatment due to gastrointestinal complaints attributed to abacavir/lamivudine.
Abacavir/lamivudine + rilpivirine was an effective and safe option in a selected group of HIV-1-infected treatment-naive patients.
Introduction
Current ART against HIV is highly effective and physicians have to take into consideration other characteristics when selecting the ART regimen, such as convenience, toxicity or cost.
Abacavir/lamivudine offers some advantages compared with tenofovir/emtricitabine, mainly avoiding potential renal and bone toxicity.1,2 It is also a fixed-dose combination, allowing once-daily regimens with low pill burden, and a reduced cost compared with tenofovir/emtricitabine.
Rilpivirine is a second-generation NNRTI approved for the treatment of HIV-infected patients with HIV-RNA <100 000 copies/mL in combination with other ART.3
Efficacy and safety of rilpivirine in naive patients has been demonstrated in randomized clinical trials, combined with two NRTIs.4–6 Most of these patients were receiving tenofovir/emtricitabine as the backbone.4 Only 35 patients (10%) in the THRIVE study received abacavir/lamivudine.5
However, abacavir/lamivudine + rilpivirine is not considered a preferred option in naive patients in guidelines,6–9 probably due to scarce data from clinical trials.
The aim of this study is to describe in the clinical practice the effectiveness and safety of abacavir/lamivudine + rilpivirine in naive HIV-infected patients.
Methods
This was an observational, retrospective, multicentre study carried out in eight Spanish university hospitals (KiRilNa study).
Subjects and design
All consecutive HIV-1-infected adult patients having started a combination of abacavir/lamivudine (600/300 mg fixed-dose combination once daily) plus rilpivirine (25 mg once daily) as their first ART before the 30 June 2015 were included, irrespective of being currently on this regimen or not. All patients tested negative for HLA_B*5701 and hepatitis B surface antigen and had baseline resistance testing without mutations before initiating ART.9
Eligible patients were selected from each hospital-specific HIV database. Study-defined data were obtained from patient clinical charts and electronic medical records. The follow-up was censored for analyses on the 31 December 2015.
Demographic data, HIV-related data, comorbidities and other relevant medical conditions were recorded. The main reason for initiating the abacavir/lamivudine + rilpivirine regimen was also recorded. During follow-up, decided by the physician in charge of the patient as routine clinical care, information regarding CD4+, HIV-RNA, laboratory parameters (blood count, renal, liver and fasting lipids), adverse events and reasons for discontinuing abacavir/lamivudine + rilpivirine if any were recorded. If ART was stopped due to virological failure (VF), the results of resistance testing when performed, the subsequent prescribed therapy and outcomes were analysed.
VF was defined as not achieving HIV-RNA <50 copies/mL, confirmed HIV-RNA >50 copies/mL after achieving undetectable viral load or last determination >50 copies/mL if treatment was changed or patient was lost to follow-up. Treatment failure included VF, ART change due to any reason, death or loss irrespective of follow-up.
The primary objective was to analyse in real life the effectiveness of abacavir/lamivudine + rilpivirine in naive patients, using ITT (missing/discontinuation/VF equals failure) and on-treatment (censoring any cause of treatment failure other than VF) analyses at 6 and 12 months (±1 month).
Ethics
The protocol was approved by the Clinical Research Ethics Committees of the participating centres in accordance with the 2008 Declaration of Helsinki.
Statistical analysis
For qualitative variables, number of patients and percentages were given. For quantitative variables, median and IQR were used.
Comparisons between baseline and follow-up variables were performed with the Wilcoxon signed-rank test.
All statistical tests were two-tailed and performed at a level of statistical significance of 0.05 (SPSS 20.0; IBM Corp., Armonk, NY, USA).
Results
Eighty-four patients were included. Baseline characteristics are described in Table 1. Median follow-up with the study regimen was 18 (9–22) months, with 100% and 68% patients reaching 6 and 12 months, respectively.
| Male | 78 (93) |
| Age (years) | 36 (30–45) |
| HIV infection diagnosis (months) | 12 (4–35) |
| HIV transmission route | |
| IVDU | 3 (4) |
| MSM | 64 (76) |
| heterosexual | 15 (18) |
| others | 2 (2) |
| Baseline CD4+ (cells/mm3) | 425 (340–519) |
| <200 | 3 (4) |
| 200–350 | 21 (25) |
| >350–500 | 36 (43) |
| >500 | 24 (28) |
| Baseline HIV-RNA (copies/mL) | 19 000 (9500–42 000) |
| <10 000 | 21 (25) |
| 10 000–50 000 | 47 (56) |
| >50 000 | 16 (19) |
| AIDS-defining illness | 0 |
| Positive for hepatitis C virus | 5 (6) |
| Comorbidities | 43 (51) |
| Creatinine (mg/dL) | 0.90 (0.79–0.98) |
| Total cholesterol (mg/dL) | 158 (138–190) |
| HDL cholesterol (mg/dL) | 40 (35–47) |
| LDL cholesterol (mg/dL) | 95 (77–122) |
| Triglycerides (mg/dL) | 89 (66–126) |
| Total cholesterol/HDL cholesterol ratio | 4.02 (3.36–4.82) |
| Male | 78 (93) |
| Age (years) | 36 (30–45) |
| HIV infection diagnosis (months) | 12 (4–35) |
| HIV transmission route | |
| IVDU | 3 (4) |
| MSM | 64 (76) |
| heterosexual | 15 (18) |
| others | 2 (2) |
| Baseline CD4+ (cells/mm3) | 425 (340–519) |
| <200 | 3 (4) |
| 200–350 | 21 (25) |
| >350–500 | 36 (43) |
| >500 | 24 (28) |
| Baseline HIV-RNA (copies/mL) | 19 000 (9500–42 000) |
| <10 000 | 21 (25) |
| 10 000–50 000 | 47 (56) |
| >50 000 | 16 (19) |
| AIDS-defining illness | 0 |
| Positive for hepatitis C virus | 5 (6) |
| Comorbidities | 43 (51) |
| Creatinine (mg/dL) | 0.90 (0.79–0.98) |
| Total cholesterol (mg/dL) | 158 (138–190) |
| HDL cholesterol (mg/dL) | 40 (35–47) |
| LDL cholesterol (mg/dL) | 95 (77–122) |
| Triglycerides (mg/dL) | 89 (66–126) |
| Total cholesterol/HDL cholesterol ratio | 4.02 (3.36–4.82) |
Results are expressed as n (%) or median (IQR).
| Male | 78 (93) |
| Age (years) | 36 (30–45) |
| HIV infection diagnosis (months) | 12 (4–35) |
| HIV transmission route | |
| IVDU | 3 (4) |
| MSM | 64 (76) |
| heterosexual | 15 (18) |
| others | 2 (2) |
| Baseline CD4+ (cells/mm3) | 425 (340–519) |
| <200 | 3 (4) |
| 200–350 | 21 (25) |
| >350–500 | 36 (43) |
| >500 | 24 (28) |
| Baseline HIV-RNA (copies/mL) | 19 000 (9500–42 000) |
| <10 000 | 21 (25) |
| 10 000–50 000 | 47 (56) |
| >50 000 | 16 (19) |
| AIDS-defining illness | 0 |
| Positive for hepatitis C virus | 5 (6) |
| Comorbidities | 43 (51) |
| Creatinine (mg/dL) | 0.90 (0.79–0.98) |
| Total cholesterol (mg/dL) | 158 (138–190) |
| HDL cholesterol (mg/dL) | 40 (35–47) |
| LDL cholesterol (mg/dL) | 95 (77–122) |
| Triglycerides (mg/dL) | 89 (66–126) |
| Total cholesterol/HDL cholesterol ratio | 4.02 (3.36–4.82) |
| Male | 78 (93) |
| Age (years) | 36 (30–45) |
| HIV infection diagnosis (months) | 12 (4–35) |
| HIV transmission route | |
| IVDU | 3 (4) |
| MSM | 64 (76) |
| heterosexual | 15 (18) |
| others | 2 (2) |
| Baseline CD4+ (cells/mm3) | 425 (340–519) |
| <200 | 3 (4) |
| 200–350 | 21 (25) |
| >350–500 | 36 (43) |
| >500 | 24 (28) |
| Baseline HIV-RNA (copies/mL) | 19 000 (9500–42 000) |
| <10 000 | 21 (25) |
| 10 000–50 000 | 47 (56) |
| >50 000 | 16 (19) |
| AIDS-defining illness | 0 |
| Positive for hepatitis C virus | 5 (6) |
| Comorbidities | 43 (51) |
| Creatinine (mg/dL) | 0.90 (0.79–0.98) |
| Total cholesterol (mg/dL) | 158 (138–190) |
| HDL cholesterol (mg/dL) | 40 (35–47) |
| LDL cholesterol (mg/dL) | 95 (77–122) |
| Triglycerides (mg/dL) | 89 (66–126) |
| Total cholesterol/HDL cholesterol ratio | 4.02 (3.36–4.82) |
Results are expressed as n (%) or median (IQR).
Forty-three patients (51%) had comorbidities [five (6%) patients had more than one comorbidity]. Fourteen patients had bone disease (osteopenia/osteoporosis), seven chronic liver disease, seven depression, five diabetes, five hypertension, four dyslipidaemia, two cardiovascular disease and four other diseases.
The main reasons for starting the abacavir/lamivudine + rilpivirine regimen were due to pre-existing comorbidities or to prevent toxicities in 50 (60%) patients and other reasons (cost, trust in the regimen) in 34 (40%) patients.
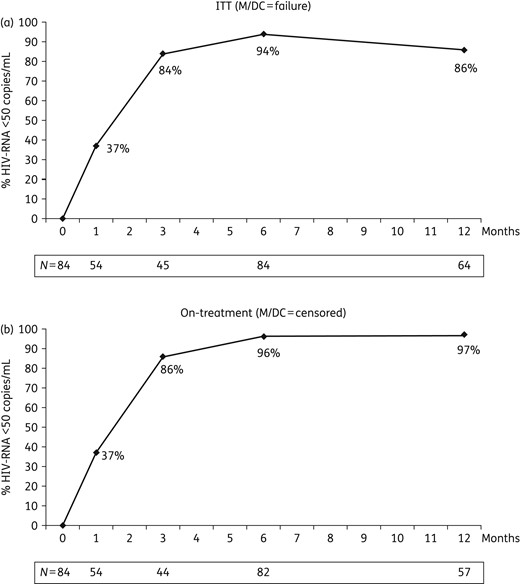
(a) Effectiveness by ITT (missing/discontinuation equals failure) analysis. (b) Effectiveness by on-treatment (missing or discontinuation censored) analysis. M, missing; DC, discontinuation.
At 12 months there were two VF. One patient had HIV-RNA of 96 and 94 copies/mL and ART was switched to abacavir/lamivudine + raltegravir, achieving undetectable HIV-RNA. The other patient had HIV-RNA 90 and 66 copies/mL and virological suppression was achieved later without changing the treatment. Resistance testing could not be performed in these patients due to low HIV-RNA.
At 12 months seven patients had stopped abacavir/lamivudine + rilpivirine due to reasons other than VF: two patients were lost to follow-up, three switched to abacavir/lamivudine/dolutegravir (two due to omeprazole use and one for simplification), one switched to zidovudine/lamivudine + nevirapine due to pregnancy and one switched to tenofovir/emtricitabine/rilpivirine due to adverse events attributed to abacavir (gastrointestinal complaints). Five patients reported mild gastrointestinal disturbances without discontinuing ART. One patient started atorvastatin.
After 12 months there were significant increases in serum creatinine [0.07 (−0.03 to 0.14) mg/dL, P = 0.015] and HDL cholesterol [+4 (−1 to 10) mg/dL, P < 0.001] and significant decreases in the total cholesterol/HDL cholesterol ratio [−0.20 (−0.74 to 0.10), P = 0.006]. One patient presented a grade 3 increase in liver enzymes at month 6 with spontaneous recovery without modifying ART.
Discussion
We have observed that, in clinical practice, an abacavir/lamivudine + rilpivirine regimen is effective and safe in this selected group of naive HIV-1-infected patients.
There are scarce data in the literature using this combination. In naive patients, in the THRIVE study only 10% (n = 35) of the included patients received abacavir/lamivudine + rilpivirine. The overall efficacy was 86%, without apparent differences depending on the NRTI backbone.5 Recently, a study evaluating this combination has been presented following 33 patients for 12 months. Only one VF was seen in a patient with intermittent adherence (with NRTI and NNRTI resistance) and seven patients discontinued treatment (for toxicity/intolerance in four).10
In patients with undetectable HIV-RNA, this regimen has been used as a switch strategy. Imaz et al.11 presented a series of 80 patients with effectiveness at 6 months of 85% (ITT) and 92% (on-treatment), improvement in lipid profile and two discontinuations due to adverse events. In the SIMKE study, 85 patients were switched to abacavir/lamivudine + rilpivirine. After 12 months the effectiveness was 96%; there was one VF and four discontinuations due to adverse events.12 In the SIMRIKI study, the effectiveness after 12 months in 205 patients was 91% (ITT) and 97% (on-treatment), with five VF and four discontinuations due to toxicity.13
There are several arguments in favour of using this combination in selected patients. One is avoiding the potential renal and bone side effects of tenofovir. Even when tenofovir alafenamide (a pro-drug with less renal and bone toxicity than tenofovir disoproxil fumarate)14 becomes available, it will be more expensive than abacavir. Another reason for using abacavir/lamivudine + rilpivirine is the good tolerability of the regimen, as previously described.15 Furthermore, we observed a good metabolic profile, significantly increasing HDL cholesterol without changes in total cholesterol or LDL cholesterol, resulting in a small but significant decrease of the total cholesterol/HDL cholesterol ratio. These HDL cholesterol increases have also been seen with abacavir/lamivudine in larger randomized trials, but without significant decreases in total cholesterol/HDL cholesterol ratios.16,17 Rilpivirine is a weak inhibitor of OCT2 transporters in the proximal tubular cell and can slightly increase serum creatinine due to inhibition of the renal active tubular secretion, as seen in our patients, without affecting the actual glomerular filtration rate.18
It is also a convenient regimen, containing only two pills (rilpivirine being the smallest ART tablet available) taken once daily. The potential for drug–drug interactions with rilpivirine is low.15 Last but not least, it is a relatively cheap regimen.
Of course, some precautions have to be taken, all inherent to the drugs that form the combination. Rilpivirine is only approved for patients with HIV-RNA <100 000 copies/mL3 and probably not recommended in patients with CD4+ cell counts <200 cells/mm3. It has to be taken with food and the patient cannot take proton pump inhibitors,3 but these precautions are applicable irrespective of the drugs that accompany rilpivirine. Regarding abacavir use in patients with high cardiovascular risk, evidence in the literature is inconsistent, with an association found in some cohorts19 but not in others nor in a meta-analysis performed by the FDA.20 However, some guidelines recommend caution when using abacavir in these patients.5,8 Furthermore, patients must have a negative HLA_B*5701 before starting abacavir.
The retrospective, observational nature of this study is a clear limitation that can entail a selection bias and does not allow controlling for factors such as adherence or adverse events that might have not been reported. Furthermore, not all patients have the same follow-up. Longer evaluation of these patients will be very useful to determine the real effectiveness and safety of this regimen in the clinical setting.
In conclusion, abacavir/lamivudine + rilpivirine was an effective and safe option in a selected group of HIV-1-infected treatment-naive patients.
Funding
Study supported by Fondo de Investigaciones Sanitarias (FIS PS09/02123), RD12/0017/0003 project as part of the Plan Nacional R+D+I and cofinanced by ISCIII-Subdirección General de Evaluación y el Fondo Europeo de Desarrollo Regional (FEDER).
Transparency declarations
A. Cu. has received honoraria, speakers' fees and/or funds for research from Bristol-Myers Squibb, Abbvie, Gilead Sciences, Janssen-Cilag, MSD and ViiV. A. Ca. has received honoraria and speakers' fees from Gilead Sciences, Janssen-Cilag, MSD and ViiV. J. T. has received honoraria for educational presentations from ViiV Healthcare, MSD and Bristol-Myers Squibb. A. I. has received honoraria for lectures, consultancies and/or research grants from Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD and ViiV. P. D. has received honoraria, speakers' fees and/or funds for research from Bristol-Myers Squibb, Abbott, Boehringer Ingelheim, Gilead, Janssen-Cilag, MSD, ViiV, Roche Farma and GlaxoSmithKline. E. M. has received honoraria, speakers' fees, consultant fees and/or funds for research from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, GSK, MSD, Theratechnologies, Tibotec and ViiV Healthcare. M. G. has received speakers' fees from ViiV. D. P. has received research grants and/or honoraria for advisories and/or lectures from Boehringer Ingelheim, GSK, Viiv, Pfizer, BMS, Abbott, Gilead, Janssen and Merck. H. K. has received honoraria, speakers' fees and/or funds for research from Bristol-Myers Squibb, Abbvie, Gilead Sciences, Janssen-Cilag, MSD and ViiV. F. G. has received honoraria, speakers' fees and/or funds for research from Bristol-Myers Squibb, Gilead Sciences, Janssen-Cilag, MSD and ViiV Healthcare. E. R. has received honoraria, speakers' fees and/or funds for research from Bristol-Myers Squibb, Abbott, Boehringer Ingelheim, Gilead, Janssen-Cilag, MSD, ViiV, Roche Farma and GlaxoSmithKline. J. R. and P. R.: none to declare.
Author contributions
A. Cu. and E. R. conceived the study, participated in its design, coordination and data analysis, and drafted the manuscript. J. R., A. Ca., J. T., A. I., P. D., E. M., P. R, M. G., D. P., H. K. and F. G. recruited patients, carried out the study protocol and supervised data integrity and analysis. All the authors contributed to the final version of the manuscript.
References



