-
PDF
- Split View
-
Views
-
Cite
Cite
Nannan Zhou, Zhou Han, Sandra Hartman-Neumann, Brenda DeGray, Joseph Ueland, Vincent Vellucci, Dennis Hernandez, Fiona McPhee, Characterization of NS5A polymorphisms and their impact on response rates in patients with HCV genotype 2 treated with daclatasvir-based regimens, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 12, 1 December 2016, Pages 3495–3505, https://doi.org/10.1093/jac/dkw336
Close - Share Icon Share
Daclatasvir (DCV) is a pan-genotypic non-structural protein 5A (NS5A) inhibitor that is approved for treatment of hepatitis C virus (HCV) genotype (GT)1 and GT3 in the USA and GT1, GT3 and GT4 in Europe. We set out to examine the impact of daclatasvir-based regimens on the sustained virologic response (SVR) in patients with GT2 infection with respect to GT2 subtype and NS5A polymorphisms at amino acid positions associated with daclatasvir resistance.
Analyses were performed on 283 GT2 NS5A sequences from five daclatasvir regimen-based clinical trials (ClinicalTrials.gov: NCT-01257204, NCT-01359644, NCT-02032875, NCT-02032888 and NCT-01616524) and 143 NS5A sequences from the Los Alamos HCV database. Susceptibility analyses of substitutions at amino acid positions associated with daclatasvir resistance and patient-derived NS5A sequences were performed using an in vitro HCV replication assay.
Of 13 GT2 subtypes identified from 426 NS5A sequences, the most prevalent were GT2a (32%), GT2b (48%) and GT2c (10%). The most prevalent NS5A polymorphism was L31M (GT2a = 88%; GT2b = 59%; GT2c = 10%). Substitutions identified in 96% of GT2 NS5A sequences exhibited daclatasvir EC50 values ranging from 0.005 to 20 nM when tested in vitro. A similar range in daclatasvir EC50 values was observed for 16 diverse GT2 patient-derived NS5A sequences (EC50 = 0.005–60 nM). Depending on the daclatasvir-based regimen studied (daclatasvir/interferon-based or daclatasvir/sofosbuvir-based), SVR rates ranged from 90% to 100% in GT2 patients with the most prevalent baseline NS5A-L31M polymorphism, compared with from 96% to 100% without this polymorphism.
High SVR rates were achieved in patients infected with GT2 treated with daclatasvir-based regimens irrespective of GT2 subtype or baseline NS5A polymorphisms.
Introduction
Hepatitis C virus (HCV) genotype 2 (GT2) is highly heterogeneous, with 11 confirmed subtypes and others awaiting assignment. The major GT2 subtypes are GT2a and GT2b.1 HCV GT2 can be found throughout the world and represents ∼9% of chronic HCV infections.2 Although GT2 is the predominant genotype in certain countries of West Africa and Central America, East Asia accounts for the greatest number (8.4 million) of GT2 cases. The prevalence of GT2 in North America and Western Europe is 12% and 10.8%, respectively.2
The introduction of direct-acting antiviral (DAA) agents for treatment of chronic HCV infection has markedly improved response rates.3–8 The HCV genotype is an important component of the information needed to guide DAA treatment strategies.9,10 Most studies have focused on GT1 due to it being the predominant genotype in North America, eastern Asia and most European countries. Recently, several DAA-based regimens have been evaluated in GT2 infection, including combinations of a DAA with peginterferon-alfa (pegIFNα) and ribavirin (RBV),11–13 and more recently, all-oral, interferon-free regimens.14–16 Consistent with results in patients with GT1 infection, these DAA-based regimens have demonstrated greater efficacy than pegIFNα/RBV treatment in patients with GT2 infection. There is extensive evidence that GT1 subtypes (1a or 1b) may influence the efficacy and resistance barrier of some DAA-based regimens.15,17,18 However, the potential influence of GT2 subtype on the efficacy and resistance profile of DAA-based regimens has not been reported.
Daclatasvir (DCV) is a pan-genotypic inhibitor of the HCV non-structural protein 5A (NS5A).19 It has been evaluated in multiple clinical studies and has demonstrated robust antiviral responses, as well as good safety and tolerability profiles in combination with a number of other agents;8,20–24 some of these daclatasvir-based studies have included patients with GT2 infection.
This analysis describes the resistance profile of daclatasvir in patients infected with GT2 from five Phase 2 and 3 clinical trials of daclatasvir-based regimens.13,15,21,22,25 Furthermore, the prevalence of GT2 subtypes and baseline NS5A polymorphisms at amino acid positions associated with daclatasvir resistance is described, as is the effect of these NS5A polymorphisms on daclatasvir susceptibility in vitro, and their impact on therapeutic outcome.
Materials and methods
Clinical samples
Baseline NS5A sequences derived from 283 patients with HCV GT2 infection were available from five daclatasvir-based clinical studies (ClinicalTrials.gov: NCT-01257204, NCT-01359644, NCT-02032875, NCT-02032888 and NCT-01616524).13,15,21,22,25 Information regarding these five clinical studies is summarized in Table S1 (available as Supplementary data at JAC Online).
Phylogenetic analysis
A phylogenetic analysis was performed on NS5A sequences (amino acid positions 1–166) obtained at baseline from 283 patients with HCV GT2 infection and 24 sequences with confirmed GT2 subtypes1 from the Los Alamos HCV Sequence Database (usHCVdb; Table S2).26 All of these sequences and a GT2a (JFH-1) reference strain27 were aligned using Mega v. 628 with the ClustalW algorithm. A neighbour-joining tree was then created. Subtyping of the GT2 NS5A sequences depended on the phylogenetic grouping of patient-derived sequences with confirmed sequence subtypes from the usHCVdb.
Genotypic and phenotypic analysis of clinical samples
Viral RNA purification, cDNA synthesis and NS5A amplification were performed as described previously.29 The NS5A coding region was amplified and sequenced by population-based sequencing (polymorphisms representing ≥20% of the sequence population reported) with genotype-specific primers (Table S3). NS5A sequences were compared with a GT2a (JFH-1) reference strain.27 Daclatasvir resistance-associated amino acid positions monitored in GT2 included F28, K30, L31, C92 and Y93.30 To determine GT2 subtype distribution and the prevalence of NS5A polymorphisms, all 283 patient-derived and 143 usHCVdb (Table S2) NS5A sequences were used.
For GT2 subtype phenotypic analyses, the GT2a (JFH-1) replicon31 was the reference strain except for GT2b. A patient-derived baseline sequence with 99% sequence homology to the consensus GT2b NS5A sequence (amino acids 1–213) was the reference, calculated from 27 baseline NS5A sequences from the US-based Study AI44403113 (Table S1) and 12 NS5A sequences from the usHCVdb (Table S2). A truncated NS5A region (amino acids 3–425) from the GT2b reference strain was introduced into the GT2a (JFH-1) replicon backbone, producing a hybrid replicon. To evaluate the susceptibility of GT2 NS5A variants to NS5A inhibitors, substitutions of interest were introduced into the JFH-1 or JFH/2b-NS5A hybrid replicon by site-directed mutagenesis and confirmed by sequence analysis. Patient-derived NS5A sequences were PCR-amplified using patient-specific primers. The PCR products were infused onto JFH-1 replicon cDNA to replace the coding region representing NS5A amino acids 3–425 using an In-Fusion Cloning kit (Clontech Laboratories, Mountain View, CA). The mean 50% effective concentration (EC50) was calculated from data generated using a previously described transient HCV replication luciferase assay.30
Results
HCV GT2 subtype distribution

Phylogenetic analysis of HCV GT2 NS5A sequences. 283 patient-derived baseline HCV GT2 NS5A sequences and 24 NS5A sequences from the usHCVdb were aligned. Open circles represent NS5A sequences from patients enrolled at sites in the Americas. Closed circles represent patients at European sites. Open triangles represent patients enrolled at sites in Asia and open squares represent patients at sites in Australia and New Zealand. Sequences P1–P16 represent the major clusters and were used for phenotypic testing (Table 5).
The prevalence of GT2 subtypes in 426 NS5A sequences (283 patient-derived and 143 from the usHCVdb) is shown in Table 1. The most common subtypes were GT2b (48%; 203/426) and GT2a (32%; 137/426), while 10% (42/426) were GT2c. Of the remaining 44 NS5A sequences, 7% (28/426) were distributed across 10 GT2 subtypes and 4% (16/426) had no assigned subtype. When examining the geographic distribution of GT2 subtypes, GT2b predominated (59%) followed by GT2a (23%) in the Americas. In Asia, GT2b (51%) and GT2a (47%) were equally prevalent. In Europe, GT2c was the most common subtype (48%) while GT2a and GT2b were less common (8% and 6%, respectively). Available NS5A sequences were too few in number to determine the prevalence of GT2 subtypes in Australia and New Zealand. Seven GT2 sequences had no country information.
| . | GT2 subtype (n) . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . | Total . |
| Americas, n (%) | 36 (23.4) | 91 (59.1) | 10 (6.5) | 1 (0.6) | 1 (0.6) | 3 (1.9) | 4 (2.6) | 1 (0.6) | 7 (4.5) | 154 | |||||
| USA | 16 | 59 | 1 | ||||||||||||
| Canada | 11 | 15 | 2 | 1 | 1 | 1 | 4 | 1 | 6 | ||||||
| Puerto Rico | 3 | ||||||||||||||
| Mexico | 3 | 13 | |||||||||||||
| Argentina | 6 | 1 | 8 | ||||||||||||
| Venezuela | 2 | ||||||||||||||
| Europe, n (%) | 5 (7.8) | 4 (6.3) | 31 (48.4) | 4 (6.3) | 2 (3.1) | 5 (7.8) | 2 (3.1) | 2 (3.1) | 9 (14.1) | 64 | |||||
| France | 1 | 1 | 5 | 4 | 2 | 3 | 2 | 9 | |||||||
| Denmark | 1 | 2 | |||||||||||||
| UK | 1 | 1 | 5 | 1 | |||||||||||
| Italy | 18 | ||||||||||||||
| Belgium | 1 | ||||||||||||||
| Spain | 2 | ||||||||||||||
| Moldova | 1 | ||||||||||||||
| Russia | 2 | 2 | |||||||||||||
| Asia, n (%) | 89 (47.3) | 96 (51.1) | 2 (1.1) | 1 (0.5) | 188 | ||||||||||
| China | 7 | 1 | 2 | ||||||||||||
| Japan | 26 | 86 | |||||||||||||
| Korea | 43 | 3 | |||||||||||||
| Taiwan | 13 | 6 | |||||||||||||
| Vietnam | 1 | ||||||||||||||
| Oceania, n (%) | 3 (23.1) | 10 (76.9) | 13 | ||||||||||||
| Australia | 2 | 10 | |||||||||||||
| New Zealand | 1 | ||||||||||||||
| Unknowna, n (%) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 | |||||||||||
| Total, n (%) | 137 (32.2) | 203 (47.7) | 42 (9.9) | 1 (0.2) | 1 (0.2) | 2 (0.5) | 5 (1.2) | 5 (1.2) | 5 (1.2) | 2 (0.5) | 4 (0.9) | 2 (0.5) | 1 (0.2) | 16 (3.8) | 426 |
| . | GT2 subtype (n) . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . | Total . |
| Americas, n (%) | 36 (23.4) | 91 (59.1) | 10 (6.5) | 1 (0.6) | 1 (0.6) | 3 (1.9) | 4 (2.6) | 1 (0.6) | 7 (4.5) | 154 | |||||
| USA | 16 | 59 | 1 | ||||||||||||
| Canada | 11 | 15 | 2 | 1 | 1 | 1 | 4 | 1 | 6 | ||||||
| Puerto Rico | 3 | ||||||||||||||
| Mexico | 3 | 13 | |||||||||||||
| Argentina | 6 | 1 | 8 | ||||||||||||
| Venezuela | 2 | ||||||||||||||
| Europe, n (%) | 5 (7.8) | 4 (6.3) | 31 (48.4) | 4 (6.3) | 2 (3.1) | 5 (7.8) | 2 (3.1) | 2 (3.1) | 9 (14.1) | 64 | |||||
| France | 1 | 1 | 5 | 4 | 2 | 3 | 2 | 9 | |||||||
| Denmark | 1 | 2 | |||||||||||||
| UK | 1 | 1 | 5 | 1 | |||||||||||
| Italy | 18 | ||||||||||||||
| Belgium | 1 | ||||||||||||||
| Spain | 2 | ||||||||||||||
| Moldova | 1 | ||||||||||||||
| Russia | 2 | 2 | |||||||||||||
| Asia, n (%) | 89 (47.3) | 96 (51.1) | 2 (1.1) | 1 (0.5) | 188 | ||||||||||
| China | 7 | 1 | 2 | ||||||||||||
| Japan | 26 | 86 | |||||||||||||
| Korea | 43 | 3 | |||||||||||||
| Taiwan | 13 | 6 | |||||||||||||
| Vietnam | 1 | ||||||||||||||
| Oceania, n (%) | 3 (23.1) | 10 (76.9) | 13 | ||||||||||||
| Australia | 2 | 10 | |||||||||||||
| New Zealand | 1 | ||||||||||||||
| Unknowna, n (%) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 | |||||||||||
| Total, n (%) | 137 (32.2) | 203 (47.7) | 42 (9.9) | 1 (0.2) | 1 (0.2) | 2 (0.5) | 5 (1.2) | 5 (1.2) | 5 (1.2) | 2 (0.5) | 4 (0.9) | 2 (0.5) | 1 (0.2) | 16 (3.8) | 426 |
NS, subtype unassigned.
aThe country was not stated for seven NS5A sequences from the usHCVdb.
| . | GT2 subtype (n) . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . | Total . |
| Americas, n (%) | 36 (23.4) | 91 (59.1) | 10 (6.5) | 1 (0.6) | 1 (0.6) | 3 (1.9) | 4 (2.6) | 1 (0.6) | 7 (4.5) | 154 | |||||
| USA | 16 | 59 | 1 | ||||||||||||
| Canada | 11 | 15 | 2 | 1 | 1 | 1 | 4 | 1 | 6 | ||||||
| Puerto Rico | 3 | ||||||||||||||
| Mexico | 3 | 13 | |||||||||||||
| Argentina | 6 | 1 | 8 | ||||||||||||
| Venezuela | 2 | ||||||||||||||
| Europe, n (%) | 5 (7.8) | 4 (6.3) | 31 (48.4) | 4 (6.3) | 2 (3.1) | 5 (7.8) | 2 (3.1) | 2 (3.1) | 9 (14.1) | 64 | |||||
| France | 1 | 1 | 5 | 4 | 2 | 3 | 2 | 9 | |||||||
| Denmark | 1 | 2 | |||||||||||||
| UK | 1 | 1 | 5 | 1 | |||||||||||
| Italy | 18 | ||||||||||||||
| Belgium | 1 | ||||||||||||||
| Spain | 2 | ||||||||||||||
| Moldova | 1 | ||||||||||||||
| Russia | 2 | 2 | |||||||||||||
| Asia, n (%) | 89 (47.3) | 96 (51.1) | 2 (1.1) | 1 (0.5) | 188 | ||||||||||
| China | 7 | 1 | 2 | ||||||||||||
| Japan | 26 | 86 | |||||||||||||
| Korea | 43 | 3 | |||||||||||||
| Taiwan | 13 | 6 | |||||||||||||
| Vietnam | 1 | ||||||||||||||
| Oceania, n (%) | 3 (23.1) | 10 (76.9) | 13 | ||||||||||||
| Australia | 2 | 10 | |||||||||||||
| New Zealand | 1 | ||||||||||||||
| Unknowna, n (%) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 | |||||||||||
| Total, n (%) | 137 (32.2) | 203 (47.7) | 42 (9.9) | 1 (0.2) | 1 (0.2) | 2 (0.5) | 5 (1.2) | 5 (1.2) | 5 (1.2) | 2 (0.5) | 4 (0.9) | 2 (0.5) | 1 (0.2) | 16 (3.8) | 426 |
| . | GT2 subtype (n) . | . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . | Total . |
| Americas, n (%) | 36 (23.4) | 91 (59.1) | 10 (6.5) | 1 (0.6) | 1 (0.6) | 3 (1.9) | 4 (2.6) | 1 (0.6) | 7 (4.5) | 154 | |||||
| USA | 16 | 59 | 1 | ||||||||||||
| Canada | 11 | 15 | 2 | 1 | 1 | 1 | 4 | 1 | 6 | ||||||
| Puerto Rico | 3 | ||||||||||||||
| Mexico | 3 | 13 | |||||||||||||
| Argentina | 6 | 1 | 8 | ||||||||||||
| Venezuela | 2 | ||||||||||||||
| Europe, n (%) | 5 (7.8) | 4 (6.3) | 31 (48.4) | 4 (6.3) | 2 (3.1) | 5 (7.8) | 2 (3.1) | 2 (3.1) | 9 (14.1) | 64 | |||||
| France | 1 | 1 | 5 | 4 | 2 | 3 | 2 | 9 | |||||||
| Denmark | 1 | 2 | |||||||||||||
| UK | 1 | 1 | 5 | 1 | |||||||||||
| Italy | 18 | ||||||||||||||
| Belgium | 1 | ||||||||||||||
| Spain | 2 | ||||||||||||||
| Moldova | 1 | ||||||||||||||
| Russia | 2 | 2 | |||||||||||||
| Asia, n (%) | 89 (47.3) | 96 (51.1) | 2 (1.1) | 1 (0.5) | 188 | ||||||||||
| China | 7 | 1 | 2 | ||||||||||||
| Japan | 26 | 86 | |||||||||||||
| Korea | 43 | 3 | |||||||||||||
| Taiwan | 13 | 6 | |||||||||||||
| Vietnam | 1 | ||||||||||||||
| Oceania, n (%) | 3 (23.1) | 10 (76.9) | 13 | ||||||||||||
| Australia | 2 | 10 | |||||||||||||
| New Zealand | 1 | ||||||||||||||
| Unknowna, n (%) | 4 (57.1) | 2 (28.6) | 1 (14.3) | 7 | |||||||||||
| Total, n (%) | 137 (32.2) | 203 (47.7) | 42 (9.9) | 1 (0.2) | 1 (0.2) | 2 (0.5) | 5 (1.2) | 5 (1.2) | 5 (1.2) | 2 (0.5) | 4 (0.9) | 2 (0.5) | 1 (0.2) | 16 (3.8) | 426 |
NS, subtype unassigned.
aThe country was not stated for seven NS5A sequences from the usHCVdb.
Baseline NS5A polymorphisms in patients infected with HCV GT2
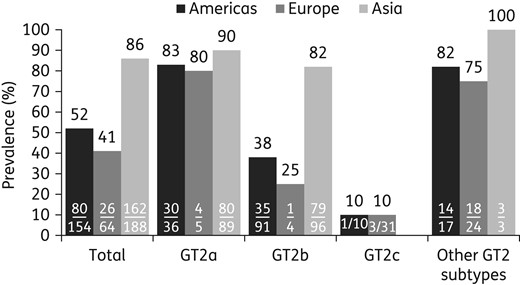
Geographic prevalence of NS5A-L31M across HCV GT2 subtypes. Bars indicate the prevalence (%) of L31M in the Americas (black), Europe (dark grey) and Asia (light grey). n/N represents the number of NS5A sequences harbouring L31M over the number of NS5A sequences for the noted GT2 subtype. The percentage prevalence (n/N) of L31M is shown above the bar. The country was not stated for seven NS5A sequences from the usHCVdb. Other subtypes include 2d, 2e, 2f, 2i, 2j, 2k, 2l, 2m, 2q, 2r and unassigned subtypes.
Polymorphisms were also frequently observed at NS5A-F28, an amino acid position associated with daclatasvir resistance in GT1a.19,30 NS5A-F28L was observed in 97% (196/203) of GT2b sequences and in none of the 137 GT2a and 42 GT2c sequences (Table S5). NS5A-F28C was observed in 29% (12/42) and 1% (2/137) of GT2c and GT2a sequences, respectively, and in none of the 203 GT2b sequences. NS5A-F28S and NS5A-F28I were each observed in only one database sequence. The prevalence of NS5A polymorphisms at amino acid positions K30 and C92 was low (3%) in GT2 sequences, while NS5A-Y93 polymorphisms were not observed.
The susceptibilities of GT2 NS5A polymorphisms to inhibition by daclatasvir are summarized in Table 2. Polymorphisms identified in 96% (408/426) of the GT2 NS5A sequences (including those without polymorphisms) exhibited daclatasvir EC50 values ranging from 0.005 to 20 nM, while EC90 values ranged from 0.03 to 27 nM [>8-fold lower than the mean daclatasvir trough concentration (221 nM) at the recommended dose].32 The most prevalent NS5A polymorphisms, NS5A-L31M (GT2a) and F28L-L31M (GT2b), conferred daclatasvir EC50 values of 11 and 13 nM, respectively. NS5A-F28L was dominant in GT2b, and when combined with L31M, it was shown to enhance susceptibility to daclatasvir by 5-fold [EC50 (GT2b NS5A-L31M) = 64 nM]. The anti-HCV activity of daclatasvir against single NS5A polymorphisms identified in this study ranged from 0.003 to 563 nM (F28L > K30R > F28I ∼ C92S > C92A > L31M > F28C > F28S) when examined in the GT2a (JFH-1) replicon (N. Zhou, unpublished data). Combinations of NS5A substitutions exhibited EC50 values ranging from 1.3 to >5000 nM (Table 2). Only 4% (18/426) of NS5A sequences harboured polymorphisms (GT2a F28C-L31M, L31M-C92S, F28S-L31M-C92S and GT2b L31M, F28L-L31M-C92A, F28L-L31M-C92S) that conferred EC90 values ranging from 99 to >5000 nM—values similar to or greater than the mean daclatasvir trough concentration.32 Eleven of the 18 NS5A sequences with these RAVs were from the usHCVdb with unknown patient treatment history, while 4 of 7 from clinical trials achieved a sustained virologic response (SVR).
| . | . | . | Polymorphism frequency, % (n) . | Number of patients with baseline NS5A polymorphisms by GT2 subtype . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS5A polymorphisma . | DCV EC50 (nM)b . | DCV EC90 (nM)b . | patients (N = 283) . | usHCVdb (N = 143) . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . |
| None | 0.02 | 0.1 | 9.5 (27) | 9.1 (13) | 16 | 1 | 21 | 2 | ||||||||||
| F28C | 8 | 20 | 2.8 (8) | 2.1 (3) | 11 | |||||||||||||
| F28I | 0.2 | 0.7 | 0.7 (1) | 1 | ||||||||||||||
| F28L | 0.005c | 0.03c | 24.7 (70) | 11.2 (16) | 81 | 1 | 1 | 3 | ||||||||||
| K30R | 0.05 | 0.1 | 1.8 (5) | 0.7 (1) | 6 | |||||||||||||
| L31M | 11 | 25 | 39.6 (112) | 21.7 (31) | 115 | 6d | 3 | 1 | 5 | 3 | 1 | 1 | 8 | |||||
| F28C-L31M | 200 | 392 | 1 (3) | 0.7 (1) | 2 | 1 | 1 | |||||||||||
| F28L-L31Ie | 1.3c | 2.7c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M | 13c | 20c | 18 (51) | 46.9 (67) | 109 | 2 | 4 | 1 | 2 | |||||||||
| F28L-C92S | 0.007 | 0.04 | 0.7 (2) | 1.4 (2) | 2 | 2 | ||||||||||||
| K30R-L31M | 2.2 | 5.3 | 0.7 (2) | 2 | ||||||||||||||
| L31M-C92S | 118 | 178 | 0.4 (1) | 0.7 (1) | 1 | 1 | ||||||||||||
| F28L-K30R-L31Mf | 2.5c | 6.3c | 0.4 (1) | 1 | ||||||||||||||
| F28L-K30R-L31Vf | 20c | 27c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M-C92A | 180c | 229c | 0.7 (1) | 1 | ||||||||||||||
| F28L-L31M-C92S | 186c | 206c | 0.4 (1) | 2.1 (3) | 2 | 2 | ||||||||||||
| F28S-L31M-C92S | >5000 | >5000 | 0.7 (1) | 1 | ||||||||||||||
| F28L-K30R-L31M-C92S | 20 | 24 | 1.4 (2) | 2 | ||||||||||||||
| Total with NS5A polymorphisms | NA | NA | 90.5 (256) | 90.9 (130) | 121 | 202 | 21 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 14 |
| Total NS5A sequences | NA | NA | 100 (283) | 100 (143) | 137 | 203 | 42 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 16 |
| . | . | . | Polymorphism frequency, % (n) . | Number of patients with baseline NS5A polymorphisms by GT2 subtype . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS5A polymorphisma . | DCV EC50 (nM)b . | DCV EC90 (nM)b . | patients (N = 283) . | usHCVdb (N = 143) . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . |
| None | 0.02 | 0.1 | 9.5 (27) | 9.1 (13) | 16 | 1 | 21 | 2 | ||||||||||
| F28C | 8 | 20 | 2.8 (8) | 2.1 (3) | 11 | |||||||||||||
| F28I | 0.2 | 0.7 | 0.7 (1) | 1 | ||||||||||||||
| F28L | 0.005c | 0.03c | 24.7 (70) | 11.2 (16) | 81 | 1 | 1 | 3 | ||||||||||
| K30R | 0.05 | 0.1 | 1.8 (5) | 0.7 (1) | 6 | |||||||||||||
| L31M | 11 | 25 | 39.6 (112) | 21.7 (31) | 115 | 6d | 3 | 1 | 5 | 3 | 1 | 1 | 8 | |||||
| F28C-L31M | 200 | 392 | 1 (3) | 0.7 (1) | 2 | 1 | 1 | |||||||||||
| F28L-L31Ie | 1.3c | 2.7c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M | 13c | 20c | 18 (51) | 46.9 (67) | 109 | 2 | 4 | 1 | 2 | |||||||||
| F28L-C92S | 0.007 | 0.04 | 0.7 (2) | 1.4 (2) | 2 | 2 | ||||||||||||
| K30R-L31M | 2.2 | 5.3 | 0.7 (2) | 2 | ||||||||||||||
| L31M-C92S | 118 | 178 | 0.4 (1) | 0.7 (1) | 1 | 1 | ||||||||||||
| F28L-K30R-L31Mf | 2.5c | 6.3c | 0.4 (1) | 1 | ||||||||||||||
| F28L-K30R-L31Vf | 20c | 27c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M-C92A | 180c | 229c | 0.7 (1) | 1 | ||||||||||||||
| F28L-L31M-C92S | 186c | 206c | 0.4 (1) | 2.1 (3) | 2 | 2 | ||||||||||||
| F28S-L31M-C92S | >5000 | >5000 | 0.7 (1) | 1 | ||||||||||||||
| F28L-K30R-L31M-C92S | 20 | 24 | 1.4 (2) | 2 | ||||||||||||||
| Total with NS5A polymorphisms | NA | NA | 90.5 (256) | 90.9 (130) | 121 | 202 | 21 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 14 |
| Total NS5A sequences | NA | NA | 100 (283) | 100 (143) | 137 | 203 | 42 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 16 |
DCV, daclatasvir; NA, not applicable; NS, subtype not assigned.
aNS5A polymorphisms at amino acid positions F28, K30, L31 or Y93 were examined as these positions have been associated with DCV resistance.
bDCV EC50 and EC90 were determined using the JFH-1 replicon harbouring the respective substitution. EC50 values are the average of three independent experiments.
cDCV EC50 and EC90 were determined using the JFH-2b NS5A hybrid replicon harbouring the respective substitution. EC50 values are the average of three independent experiments.
dFor GT2b NS5A-L31M, daclatasvir EC50 = 64 nM and EC90 = 99 nM.
eOne GT2b sequence had F28L and L31I/M.
fOne GT2b sequence had F28L, K30R and L31M/V.
| . | . | . | Polymorphism frequency, % (n) . | Number of patients with baseline NS5A polymorphisms by GT2 subtype . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS5A polymorphisma . | DCV EC50 (nM)b . | DCV EC90 (nM)b . | patients (N = 283) . | usHCVdb (N = 143) . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . |
| None | 0.02 | 0.1 | 9.5 (27) | 9.1 (13) | 16 | 1 | 21 | 2 | ||||||||||
| F28C | 8 | 20 | 2.8 (8) | 2.1 (3) | 11 | |||||||||||||
| F28I | 0.2 | 0.7 | 0.7 (1) | 1 | ||||||||||||||
| F28L | 0.005c | 0.03c | 24.7 (70) | 11.2 (16) | 81 | 1 | 1 | 3 | ||||||||||
| K30R | 0.05 | 0.1 | 1.8 (5) | 0.7 (1) | 6 | |||||||||||||
| L31M | 11 | 25 | 39.6 (112) | 21.7 (31) | 115 | 6d | 3 | 1 | 5 | 3 | 1 | 1 | 8 | |||||
| F28C-L31M | 200 | 392 | 1 (3) | 0.7 (1) | 2 | 1 | 1 | |||||||||||
| F28L-L31Ie | 1.3c | 2.7c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M | 13c | 20c | 18 (51) | 46.9 (67) | 109 | 2 | 4 | 1 | 2 | |||||||||
| F28L-C92S | 0.007 | 0.04 | 0.7 (2) | 1.4 (2) | 2 | 2 | ||||||||||||
| K30R-L31M | 2.2 | 5.3 | 0.7 (2) | 2 | ||||||||||||||
| L31M-C92S | 118 | 178 | 0.4 (1) | 0.7 (1) | 1 | 1 | ||||||||||||
| F28L-K30R-L31Mf | 2.5c | 6.3c | 0.4 (1) | 1 | ||||||||||||||
| F28L-K30R-L31Vf | 20c | 27c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M-C92A | 180c | 229c | 0.7 (1) | 1 | ||||||||||||||
| F28L-L31M-C92S | 186c | 206c | 0.4 (1) | 2.1 (3) | 2 | 2 | ||||||||||||
| F28S-L31M-C92S | >5000 | >5000 | 0.7 (1) | 1 | ||||||||||||||
| F28L-K30R-L31M-C92S | 20 | 24 | 1.4 (2) | 2 | ||||||||||||||
| Total with NS5A polymorphisms | NA | NA | 90.5 (256) | 90.9 (130) | 121 | 202 | 21 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 14 |
| Total NS5A sequences | NA | NA | 100 (283) | 100 (143) | 137 | 203 | 42 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 16 |
| . | . | . | Polymorphism frequency, % (n) . | Number of patients with baseline NS5A polymorphisms by GT2 subtype . | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NS5A polymorphisma . | DCV EC50 (nM)b . | DCV EC90 (nM)b . | patients (N = 283) . | usHCVdb (N = 143) . | a . | b . | c . | d . | e . | f . | i . | j . | k . | l . | m . | q . | r . | NS . |
| None | 0.02 | 0.1 | 9.5 (27) | 9.1 (13) | 16 | 1 | 21 | 2 | ||||||||||
| F28C | 8 | 20 | 2.8 (8) | 2.1 (3) | 11 | |||||||||||||
| F28I | 0.2 | 0.7 | 0.7 (1) | 1 | ||||||||||||||
| F28L | 0.005c | 0.03c | 24.7 (70) | 11.2 (16) | 81 | 1 | 1 | 3 | ||||||||||
| K30R | 0.05 | 0.1 | 1.8 (5) | 0.7 (1) | 6 | |||||||||||||
| L31M | 11 | 25 | 39.6 (112) | 21.7 (31) | 115 | 6d | 3 | 1 | 5 | 3 | 1 | 1 | 8 | |||||
| F28C-L31M | 200 | 392 | 1 (3) | 0.7 (1) | 2 | 1 | 1 | |||||||||||
| F28L-L31Ie | 1.3c | 2.7c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M | 13c | 20c | 18 (51) | 46.9 (67) | 109 | 2 | 4 | 1 | 2 | |||||||||
| F28L-C92S | 0.007 | 0.04 | 0.7 (2) | 1.4 (2) | 2 | 2 | ||||||||||||
| K30R-L31M | 2.2 | 5.3 | 0.7 (2) | 2 | ||||||||||||||
| L31M-C92S | 118 | 178 | 0.4 (1) | 0.7 (1) | 1 | 1 | ||||||||||||
| F28L-K30R-L31Mf | 2.5c | 6.3c | 0.4 (1) | 1 | ||||||||||||||
| F28L-K30R-L31Vf | 20c | 27c | 0.4 (1) | 1 | ||||||||||||||
| F28L-L31M-C92A | 180c | 229c | 0.7 (1) | 1 | ||||||||||||||
| F28L-L31M-C92S | 186c | 206c | 0.4 (1) | 2.1 (3) | 2 | 2 | ||||||||||||
| F28S-L31M-C92S | >5000 | >5000 | 0.7 (1) | 1 | ||||||||||||||
| F28L-K30R-L31M-C92S | 20 | 24 | 1.4 (2) | 2 | ||||||||||||||
| Total with NS5A polymorphisms | NA | NA | 90.5 (256) | 90.9 (130) | 121 | 202 | 21 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 14 |
| Total NS5A sequences | NA | NA | 100 (283) | 100 (143) | 137 | 203 | 42 | 1 | 1 | 2 | 5 | 5 | 5 | 2 | 4 | 2 | 1 | 16 |
DCV, daclatasvir; NA, not applicable; NS, subtype not assigned.
aNS5A polymorphisms at amino acid positions F28, K30, L31 or Y93 were examined as these positions have been associated with DCV resistance.
bDCV EC50 and EC90 were determined using the JFH-1 replicon harbouring the respective substitution. EC50 values are the average of three independent experiments.
cDCV EC50 and EC90 were determined using the JFH-2b NS5A hybrid replicon harbouring the respective substitution. EC50 values are the average of three independent experiments.
dFor GT2b NS5A-L31M, daclatasvir EC50 = 64 nM and EC90 = 99 nM.
eOne GT2b sequence had F28L and L31I/M.
fOne GT2b sequence had F28L, K30R and L31M/V.
Baseline NS5A polymorphisms and virologic response in patients infected with HCV GT2
In patients infected with GT2 and treated with different daclatasvir-based regimens, baseline NS5A RAVs had minimal impact on virologic outcome (Table 3).
| . | Percentage SVR (n/N) . | P valueb . | |
|---|---|---|---|
| Polymorphisma . | with polymorphism . | without polymorphism . | |
| DCV 60 mg + SOF ± RBV (12 or 24 week) | |||
| L31M | 94 (17/18) | 100 (18/18) | >0.25 |
| C92S | 100 (2/2) | 97 (33/34) | |
| 28, 30, 31, 92 or 93 | 95 (18/19) | 100 (17/17) | >0.25 |
| DCV 60 mg + pegIFNα + RBV (12 or 16 week) | |||
| F28C | 100 (3/3) | 100 (37/37) | |
| L31M | 100 (21/21) | 100 (19/19) | |
| 28, 30, 31, 92 or 93 | 100 (24/24) | 100 (16/16) | |
| DCV 60 mg + pegIFNλ + RBV (12 week) | |||
| F28C | 83 (5/6) | 93 (152/164) | >0.25 |
| K30 | 100 (6/6) | 92 (151/164) | |
| L31Mc | 90 (104/115) | 96 (53/55) | >0.15 |
| C92Sd | 0 (0/1) | 93 (157/169) | |
| 28, 30, 31, 92 or 93 | 91 (110/121) | 96 (47/49) | >0.25 |
| . | Percentage SVR (n/N) . | P valueb . | |
|---|---|---|---|
| Polymorphisma . | with polymorphism . | without polymorphism . | |
| DCV 60 mg + SOF ± RBV (12 or 24 week) | |||
| L31M | 94 (17/18) | 100 (18/18) | >0.25 |
| C92S | 100 (2/2) | 97 (33/34) | |
| 28, 30, 31, 92 or 93 | 95 (18/19) | 100 (17/17) | >0.25 |
| DCV 60 mg + pegIFNα + RBV (12 or 16 week) | |||
| F28C | 100 (3/3) | 100 (37/37) | |
| L31M | 100 (21/21) | 100 (19/19) | |
| 28, 30, 31, 92 or 93 | 100 (24/24) | 100 (16/16) | |
| DCV 60 mg + pegIFNλ + RBV (12 week) | |||
| F28C | 83 (5/6) | 93 (152/164) | >0.25 |
| K30 | 100 (6/6) | 92 (151/164) | |
| L31Mc | 90 (104/115) | 96 (53/55) | >0.15 |
| C92Sd | 0 (0/1) | 93 (157/169) | |
| 28, 30, 31, 92 or 93 | 91 (110/121) | 96 (47/49) | >0.25 |
DCV, daclatasvir; pegIFNα, peginterferon-alfa-2a; pegIFNλ, peginterferon-lambda-1a; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response.
aF28L was not included as resistance-associated polymorphism.
bA χ2 test was performed to examine significance of the SVR rate difference between the patients with and without the polymorphisms.
cMixed substitutions at position L31 were observed in two patients at baseline; one had L31I/M and one had L31M/V.
dχ2 test was not performed because only one patient had C92S.
| . | Percentage SVR (n/N) . | P valueb . | |
|---|---|---|---|
| Polymorphisma . | with polymorphism . | without polymorphism . | |
| DCV 60 mg + SOF ± RBV (12 or 24 week) | |||
| L31M | 94 (17/18) | 100 (18/18) | >0.25 |
| C92S | 100 (2/2) | 97 (33/34) | |
| 28, 30, 31, 92 or 93 | 95 (18/19) | 100 (17/17) | >0.25 |
| DCV 60 mg + pegIFNα + RBV (12 or 16 week) | |||
| F28C | 100 (3/3) | 100 (37/37) | |
| L31M | 100 (21/21) | 100 (19/19) | |
| 28, 30, 31, 92 or 93 | 100 (24/24) | 100 (16/16) | |
| DCV 60 mg + pegIFNλ + RBV (12 week) | |||
| F28C | 83 (5/6) | 93 (152/164) | >0.25 |
| K30 | 100 (6/6) | 92 (151/164) | |
| L31Mc | 90 (104/115) | 96 (53/55) | >0.15 |
| C92Sd | 0 (0/1) | 93 (157/169) | |
| 28, 30, 31, 92 or 93 | 91 (110/121) | 96 (47/49) | >0.25 |
| . | Percentage SVR (n/N) . | P valueb . | |
|---|---|---|---|
| Polymorphisma . | with polymorphism . | without polymorphism . | |
| DCV 60 mg + SOF ± RBV (12 or 24 week) | |||
| L31M | 94 (17/18) | 100 (18/18) | >0.25 |
| C92S | 100 (2/2) | 97 (33/34) | |
| 28, 30, 31, 92 or 93 | 95 (18/19) | 100 (17/17) | >0.25 |
| DCV 60 mg + pegIFNα + RBV (12 or 16 week) | |||
| F28C | 100 (3/3) | 100 (37/37) | |
| L31M | 100 (21/21) | 100 (19/19) | |
| 28, 30, 31, 92 or 93 | 100 (24/24) | 100 (16/16) | |
| DCV 60 mg + pegIFNλ + RBV (12 week) | |||
| F28C | 83 (5/6) | 93 (152/164) | >0.25 |
| K30 | 100 (6/6) | 92 (151/164) | |
| L31Mc | 90 (104/115) | 96 (53/55) | >0.15 |
| C92Sd | 0 (0/1) | 93 (157/169) | |
| 28, 30, 31, 92 or 93 | 91 (110/121) | 96 (47/49) | >0.25 |
DCV, daclatasvir; pegIFNα, peginterferon-alfa-2a; pegIFNλ, peginterferon-lambda-1a; RBV, ribavirin; SOF, sofosbuvir; SVR, sustained virologic response.
aF28L was not included as resistance-associated polymorphism.
bA χ2 test was performed to examine significance of the SVR rate difference between the patients with and without the polymorphisms.
cMixed substitutions at position L31 were observed in two patients at baseline; one had L31I/M and one had L31M/V.
dχ2 test was not performed because only one patient had C92S.
In 36 patients infected with GT2 (8 GT2a and 28 GT2b) treated with daclatasvir/sofobusvir (SOF), an HCV NS5B nucleoside inhibitor, with or without ribavirin (Table S1), 92% (33/36) had NS5A polymorphisms at daclatasvir-resistant positions including F28L (n = 14), L31M (n = 5), F28L-L31M (n = 12), F28L-C92S (n = 1) and L31M-C92S (n = 1). Only one GT2b patient (F1) who was treated with DCV/SOF/RBV relapsed 12 weeks after treatment (Table 4). The patient had NS5A-L31M at both baseline and relapse. The daclatasvir EC90 value in vitro for GT2b NS5A-L31M (EC90 = 99 nM) was ∼2-fold less than the mean trough daclatasvir exposure.32 The NS5B variants K77N/K, V309V/M and T340S/T emerged in the patient at failure, combined with pre-existing NS5B polymorphisms at T179A, I293M, M434L and H479P, which were previously reported to be associated with GT2a resistance to sofusbuvir.33 These polymorphisms represent the consensus substitutions for GT2b. A hybrid GT1b (Con1) replicon harbouring a patient-derived GT2b NS5B sequence34 with these substitutions exhibited a 3-fold increase in susceptibility for sofusbuvir compared with a GT2a replicon (N. Zhou, unpublished data). The unfit signature sofusbuvir-resistant variant NS5B-S282T was not detected by population-based sequencing (≥20% sequencing cut-off) or by next-generation sequencing (≥1% sequencing cut-off using the Illumina platform; F. McPhee, unpublished data). The patient also had decompensated liver cirrhosis (Child–Pugh class C) that has been reported as a factor for failure of anti-HCV treatment.21,35
Daclatasvir activity against NS5A variants observed in patients with HCV GT2 failing daclatasvir-based regimens
| Patient . | GT . | Virologic failure . | Visit . | Variants . | EC90 (nM) in reference sequencea . | EC90 (nM) in patient sequence . |
|---|---|---|---|---|---|---|
| DCV 60 mg + SOF + RBV (12 week) | ||||||
| F1 | 2b | relapse | BL | L31M | 99 | ND |
| FUWK12 | L31M | 99 | ND | |||
| DCV 60 mg + pegIFNλ + RBV (12 week) | ||||||
| F2 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK1 | F28C,L31M | 392 | ND | |||
| F3 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK4 | T24S,F28G | >5000 | ND | |||
| F4 | 2a | relapse | BL | F28C,L31M | 392 | ND |
| FUWK4 | F28C,L31M | 392 | ND | |||
| F5 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F6 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F7 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M,C92S | 1377 | ND | |||
| F8 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M | 187 | ND | |||
| F9 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK48 | L31M | 25 | 71 | |||
| F10 | 2b | relapse | BL | F28L,L31M,C92S | 206 | ND |
| FUWK4 | F28L,L31M,C92S | 206 | ND | |||
| F11 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F12 | 2b | relapse | BL | F28L,L31M | 20 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F13 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | F28L,L31M | 20 | 93 | |||
| F14 | 2i | relapse | BL | T24S,L31M | 187 | ND |
| FUWK12 | T24S,L31M | 187b | 177 | |||
| Patient . | GT . | Virologic failure . | Visit . | Variants . | EC90 (nM) in reference sequencea . | EC90 (nM) in patient sequence . |
|---|---|---|---|---|---|---|
| DCV 60 mg + SOF + RBV (12 week) | ||||||
| F1 | 2b | relapse | BL | L31M | 99 | ND |
| FUWK12 | L31M | 99 | ND | |||
| DCV 60 mg + pegIFNλ + RBV (12 week) | ||||||
| F2 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK1 | F28C,L31M | 392 | ND | |||
| F3 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK4 | T24S,F28G | >5000 | ND | |||
| F4 | 2a | relapse | BL | F28C,L31M | 392 | ND |
| FUWK4 | F28C,L31M | 392 | ND | |||
| F5 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F6 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F7 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M,C92S | 1377 | ND | |||
| F8 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M | 187 | ND | |||
| F9 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK48 | L31M | 25 | 71 | |||
| F10 | 2b | relapse | BL | F28L,L31M,C92S | 206 | ND |
| FUWK4 | F28L,L31M,C92S | 206 | ND | |||
| F11 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F12 | 2b | relapse | BL | F28L,L31M | 20 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F13 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | F28L,L31M | 20 | 93 | |||
| F14 | 2i | relapse | BL | T24S,L31M | 187 | ND |
| FUWK12 | T24S,L31M | 187b | 177 | |||
DCV, daclatasvir; FUWK, follow-up week; pegIFN, peginterferon; RBV, ribavirin; SOF, sofosbuvir.
aDCV susceptibility of GT2a variants was determined using the JFH-1 replicon and GT2b variants using the JFH-2b NS5A hybrid replicon. EC90 values are the average of two independent experiments.
bEC90 value derived from JFH-1 replicon harbouring the substitutions.
Daclatasvir activity against NS5A variants observed in patients with HCV GT2 failing daclatasvir-based regimens
| Patient . | GT . | Virologic failure . | Visit . | Variants . | EC90 (nM) in reference sequencea . | EC90 (nM) in patient sequence . |
|---|---|---|---|---|---|---|
| DCV 60 mg + SOF + RBV (12 week) | ||||||
| F1 | 2b | relapse | BL | L31M | 99 | ND |
| FUWK12 | L31M | 99 | ND | |||
| DCV 60 mg + pegIFNλ + RBV (12 week) | ||||||
| F2 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK1 | F28C,L31M | 392 | ND | |||
| F3 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK4 | T24S,F28G | >5000 | ND | |||
| F4 | 2a | relapse | BL | F28C,L31M | 392 | ND |
| FUWK4 | F28C,L31M | 392 | ND | |||
| F5 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F6 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F7 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M,C92S | 1377 | ND | |||
| F8 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M | 187 | ND | |||
| F9 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK48 | L31M | 25 | 71 | |||
| F10 | 2b | relapse | BL | F28L,L31M,C92S | 206 | ND |
| FUWK4 | F28L,L31M,C92S | 206 | ND | |||
| F11 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F12 | 2b | relapse | BL | F28L,L31M | 20 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F13 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | F28L,L31M | 20 | 93 | |||
| F14 | 2i | relapse | BL | T24S,L31M | 187 | ND |
| FUWK12 | T24S,L31M | 187b | 177 | |||
| Patient . | GT . | Virologic failure . | Visit . | Variants . | EC90 (nM) in reference sequencea . | EC90 (nM) in patient sequence . |
|---|---|---|---|---|---|---|
| DCV 60 mg + SOF + RBV (12 week) | ||||||
| F1 | 2b | relapse | BL | L31M | 99 | ND |
| FUWK12 | L31M | 99 | ND | |||
| DCV 60 mg + pegIFNλ + RBV (12 week) | ||||||
| F2 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK1 | F28C,L31M | 392 | ND | |||
| F3 | 2a | breakthrough | BL | L31M | 25 | ND |
| WK4 | T24S,F28G | >5000 | ND | |||
| F4 | 2a | relapse | BL | F28C,L31M | 392 | ND |
| FUWK4 | F28C,L31M | 392 | ND | |||
| F5 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F6 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | F28S,L31M | 4513 | ND | |||
| F7 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M,C92S | 1377 | ND | |||
| F8 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK4 | T24S,L31M | 187 | ND | |||
| F9 | 2a | relapse | BL | L31M | 25 | ND |
| FUWK48 | L31M | 25 | 71 | |||
| F10 | 2b | relapse | BL | F28L,L31M,C92S | 206 | ND |
| FUWK4 | F28L,L31M,C92S | 206 | ND | |||
| F11 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F12 | 2b | relapse | BL | F28L,L31M | 20 | ND |
| FUWK4 | L31M | 99 | ND | |||
| F13 | 2b | relapse | BL | F28L | 0.03 | ND |
| FUWK4 | F28L,L31M | 20 | 93 | |||
| F14 | 2i | relapse | BL | T24S,L31M | 187 | ND |
| FUWK12 | T24S,L31M | 187b | 177 | |||
DCV, daclatasvir; FUWK, follow-up week; pegIFN, peginterferon; RBV, ribavirin; SOF, sofosbuvir.
aDCV susceptibility of GT2a variants was determined using the JFH-1 replicon and GT2b variants using the JFH-2b NS5A hybrid replicon. EC90 values are the average of two independent experiments.
bEC90 value derived from JFH-1 replicon harbouring the substitutions.
In 40 patients infected with GT2 (9 GT2a, 23 GT2b, 4 GT2c, 1 GT2k and 3 unassigned GT2 subtype) and treated with DCV/pegIFNα/RBV for 12 or 16 weeks (Table S1), 95% (38/40) harboured NS5A polymorphisms at daclatasvir-resistant positions including F28C (n = 3), F28L (n = 14), L31M (n = 12) and F28L-L31M (n = 9). None of these GT2 patients failed treatment (Table 3).
In 170 patients infected with GT2 (89 GT2a, 56 GT2b, 23 GT2c, 1 GT2i and 1 unassigned GT2 subtype) and treated with DCV/pegIFN-lambda-1a (pegIFNλ)/RBV for 12 weeks (Table S1), 90% (153/170) harboured polymorphisms at daclatasvir-resistant positions, including F28C (n = 3), F28L (n = 32), K30R (n = 3), L31M (n = 86), F28C-L31M (n = 3), F28L-L31M (n = 21), F28L-L31I/M (n = 1), K30R-L31M (n = 2), F28L-K30R-L31M/V (n = 1), and F28L-L31M-C92S (n = 1). The majority of patients (92%, 157/170) achieved SVR including 91% (110/121) of patients with noted NS5A polymorphisms (except F28L) at baseline (Table 3). There was no significant difference in SVR rates between patients infected with GT2a (91% [81/89]) or GT2b (93% [52/56]). Thirteen patients with GT2 failed DCV/pegIFNλ/RBV treatment; this included 9% (8/89) with GT2a, 7% (4/56) with GT2b and 100% (1/1) with GT2i. Ten of the 13 failures (patients F2, F3, F5–F9, and F11–F13) had daclatasvir EC90 values ranging from 0.03 to 25 nM (Table 4) or ≥9-fold below the mean trough daclatasvir exposures in HCV-infected patients.32 Three of the 13 patients (patients F4, F10 and F14) had baseline NS5A RAVs (F28C-L31M, F28L-L31M-C92S or T24S-L31M) with daclatasvir EC90 values ranging from 187 to 392 nM (Table 4), values that were similar to or higher than the mean trough daclatasvir exposures in HCV-infected patients.32 The T24S substitution was observed in a patient with GT2i; this substitution, when combined with L31M, conferred 25-fold daclatasvir resistance when tested in the GT2a (JFH-1) replicon although no resistance was observed in a GT2b NS5A-T24S hybrid replicon (data not shown). NS5A-T24S was not detected in any of the baseline or database GT2a (0/137) NS5A sequences, although it was observed in all GT2b (203/203) and GT2c (42/42) patient-derived baseline and database NS5A sequences.
Of the 13 DCV/pegIFNλ/RBV treatment failures, 8 (patients F2, F3, F5–8, F11 and F13) had emergent NS5A RAVs at failure: T24S, F28C/G/S, L31M or C92S (Table 4). One patient with GT2b (F12) had F28L-L31M at baseline and only L31M at failure. Four patients (patients F4, F9, F10 and F14) had the same RAVs detected at baseline and failure. Analysis of daclatasvir activity against NS5A RAVs detected at failure indicated that 11 of 13 patients (patients F2–F8, F10–F12 and F14) had RAVs conferring daclatasvir EC90 values similar to (<2-fold) or greater than the mean trough daclatasvir exposures in HCV-infected patients.32 NS5A RAVs observed in two patients (patients F9 and F13) at relapse did not explain virologic failure. The daclatasvir EC90 values for patient-derived NS5A population sequences from F9 and F13 were 2.8- and 4.7-fold less potent, respectively, compared with the EC90 values for reference replicons harbouring the respective NS5A RAVs: GT2a L31M and GT2b F28L-L31M (Table 4). Clonal analysis of the NS5A population sequence from F13 identified additional NS5A RAVs: F28L-L31M-C92S (1/8 clones; EC90 = 206 nM) and F28L-L31M-Y93N (2/8 clones; EC90 = 450 nM) while no additional RAVs were identified from F9.
Comparison of NS5A inhibitor activity against HCV GT2 NS5A substitutions and NS5A patient-derived sequences
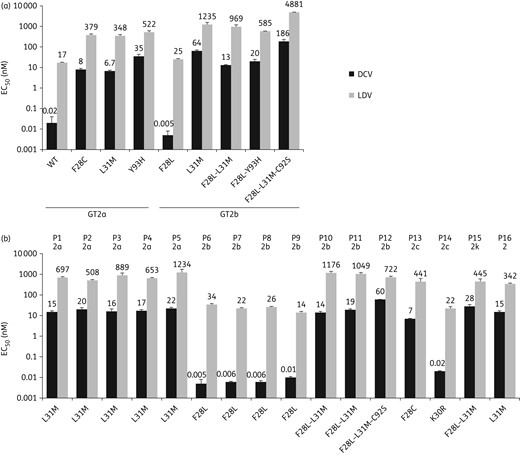
Effect of HCV GT2 NS5A resistance-associated variants on NS5A inhibitor activity. Bars indicate the 50% effective concentration (EC50) ± SD for daclatasvir (DCV) and ledipasvir (LDV), respectively, against HCV GT2 replicons harbouring the indicated NS5A substitutions or patient-derived NS5A sequences. EC50 values represent the average of three independent experiments. (a) Substitutions in GT2a and GT2b NS5A replicon reference sequences (see Methods section). (b) Patient-derived NS5A sequences with observed substitutions at positions of interest; patient numbers (P1–P16) above the bars reflect NS5A sequence clusters identified in the phylogenetic analysis (Figure 1) and GT2 subtypes are also shown.
Daclatasvir EC50 values ranged from 0.005 to 60 nM against 16 GT2 patient-derived baseline NS5A sequences representing different phylogenetic clusters (Figures 1 and 3b), and no association between daclatasvir potency and specific phylogenetic clusters was observed. All 16 patient-derived baseline GT2 NS5A sequences had ≥1 polymorphism at amino acid positions 28, 30, 31 or 92, and 10 of these NS5A sequences harboured L31M. Daclatasvir EC50 values for the patient-derived NS5A sequences were comparable (within 3-fold) to those obtained against respective substitutions in reference GT2 sequences. Daclatasvir was ≥1100-fold more potent than ledipasvir against five patient-derived NS5A sequences and 12- to 84-fold more potent for the remaining 11 patient-derived NS5A sequences.
Discussion
HCV GT2 NS5A sequencing data were obtained from five daclatasvir-based clinical studies and the usHCVdb. Comparison of HCV genotyping results obtained from the LiPA analysis of clinical samples and NS5A sequencing alignment analyses indicate that the LiPA assay is generally less specific and sometimes unreliable for GT2 subtype determination. A recent report highlighted the unreliability of LiPA in the identification of patients infected with multiple inter-genotypic HCV GT2 recombinants with other genotypes; an example being patients infected with HCV sequences that represented GT2k and GT1b.36,37 If the GT2 subtype is required to determine treatment options, sequencing of drug-targeted genes may be more appropriate.
Predominance of GT2 subtype depended on geography. In the Americas and Asia, GT2b was predominant (59% and 51%, respectively); however, GT2a was also common (23% and 47%, respectively). The most prevalent subtype in Europe was GT2c (48%), whereas GT2a and GT2b were not common (≤8%). In agreement with a previous report from northern Italy,38 GT2c predominated (100% [18/18]) in Italian patients included in our study.
Subtype- and region-specific distributions of NS5A polymorphisms at some daclatasvir resistance-associated positions were observed. In GT2a and GT2b, L31M was predominant (88% and 59%, respectively), while it was uncommon in GT2c NS5A sequences (10%). NS5A-F28L was only predominant in GT2b and was not observed in GT2a. Thus, NS5A polymorphisms at 28 and 31 may be among the GT2 subtype determinants. Interestingly, the prevalence of L31M in GT2a and GT2b NS5A sequences from Asia was higher than in other geographical regions.
Additionally, baseline NS5A polymorphisms conferring high-level daclatasvir resistance were uncommon: 96% of GT2 NS5A sequences had polymorphisms with EC90 values ranging from 0.03 to 27 nM in vitro. Plasma trough concentrations at the recommended daclatasvir dose of 60 mg (221 nM)32 would therefore be expected to suppress viruses harbouring these NS5A polymorphisms. Regarding the 4% of NS5A sequences harbouring polymorphisms that conferred high EC90 values (≥99 nM), the majority were usHCVdb NS5A sequences with unknown patient treatment history.
HCV GT2 has been reported to have a low daclatasvir resistance barrier in vitro, especially when L31M was present in NS5A.39 However, the meaning of this finding clinically was not assessed. In our study, baseline NS5A polymorphisms did not appear to impact SVR rates in patients with HCV GT2 who were treated with daclatasvir-based regimens. Although pre-existence of polymorphisms conferring low-level daclatasvir resistance can potentially lower the barrier to subsequent acquisition of high-level resistance causing virologic failure, there has been no evidence of this in patients infected with HCV GT2 treated with daclatasvir-based regimens. This illustrates the importance of combination regimens.
In contrast to other HCV genotypes, such as GT1b and GT3 where NS5A polymorphisms at Y93 have been reported to pre-exist at 11% and 8%, respectively,40,41 NS5A-Y93 polymorphisms were not observed in the GT2 sequences assessed in this study. The replication ability (fitness) of a GT2a (JFH-1) replicon harbouring the NS5A-Y93H substitution was only slightly reduced (81% replication level) when compared with a wild-type GT2a (JFH-1) replicon, while a dramatic reduction (4% replication level) was observed for GT2a (JFH-1) virus harbouring NS5A-Y93H.31 Thus, HCV GT2 virus harbouring Y93H may also be too unfit in an infected patient to emerge as the predominant viral population.
For patients treated with DCV/pegIFNλ/RBV for 12 weeks,25 an association between the level of daclatasvir resistance of baseline NS5A polymorphisms and an impact on response rates was not apparent as evidenced by two patients with F28C-L31M at baseline achieving SVR; these substitutions conferred high-level daclatasvir resistance. In contrast, ten patients with NS5A polymorphisms conferring low resistance to daclatasvir (EC90 values ≥9-fold below mean trough daclatasvir exposures) failed treatment. The majority (62%) of DCV/pegIFNλ/RBV treatment failures had previously documented emergent daclatasvir-resistant variants.19,30,42 NS5A-T24S emerged in three patients with GT2a and pre-existed in one GT2i patient, and was shown to confer resistance to daclatasvir. Substitutions at NS5A position 24 have been associated with NS5A inhibitor resistance in patients with GT1 and GT4;42–45 however, substitutions at this position have conferred no or minimal resistance to daclatasvir (data not shown). The susceptibility of NS5A-T24S to daclatasvir in GT2 was subtype-dependent as T24S was present as the consensus NS5A sequence in GT2b and conferred no resistance to daclatasvir, although it exhibited 25-fold resistance in GT2a. Similarly, the presence of NS5A-F28L reduced resistance to daclatasvir when combined with L31M in GT2b (5-fold), while daclatasvir resistance was slightly increased when it was combined with L31M in GT2a (2-fold; data not shown). It should be noted that F28L-L31M was not observed in GT2a sequences examined in this study.
Treatment failure with DCV/pegIFNλ/RBV could be explained by the presence of variants highly resistant to daclatasvir in the majority (85%) of patients with GT2 at failure. For two patients where this was not the case, minor variants conferring high-level daclatasvir resistance were detected in one (F13) while the reason for failure in the other patient (F9) was unclear, since other factors such as IL28B genotype and compliance were favourable.
Previously, we had reported on potency comparisons between daclatasvir and ledipasvir against GT3 and GT4 NS5A polymorphisms and patient-derived NS5A sequences.41,46 As observed against GT3 and GT4 NS5A sequences, daclatasvir was more potent than ledipasvir against GT2a (850-fold) and GT2b (5000-fold) NS5A reference sequences in vitro. In addition, most of the frequently observed NS5A polymorphisms, whether assessed using reference or patient-derived NS5A sequences, showed that ledipasvir was less potent than daclatasvir. The potential relevance of these potency differences between daclatasvir and ledipasvir, as well as the impact of GT2 subtype polymorphisms on SVR rates, would need to be assessed in the context of sofusbuvir-based regimens containing these agents. The GT2 subtype polymorphism effects on the in vitro potency of other approved NS5A inhibitors (ombitasvir, elbasvir)47–49 have not been assessed in this study.
Sofusbuvir/ribavirin for ≥12 weeks is approved for the treatment of HCV GT2 infection.16 Real-world SVR rates have been lower (79%) than reported in controlled clinical trials (95%).50 This discrepancy was partly due to early discontinuations and to patients with prior interferon-based treatment experience or more advanced liver disease being less likely to achieve SVR. For these patients, addition of a DAA with potency against GT2 would be expected to enhance SVR rates. The efficacy of DCV/SOF ± RBV has been examined in difficult-to-treat patient populations, including patients with cirrhosis and/or coinfection with/without prior treatment experience.21,22 Although patient sample size has been limited, response rates have been promising irrespective of the presence of baseline NS5A polymorphisms.
It should be noted that only 13% (33/246) of patients treated with daclatasvir-based regimens in this report were infected with HCV GT2 subtypes other than GT2a or GT2b, and the majority (82%; 27/33) of those with GT2 minor subtypes were infected with HCV GT2c. The prevalence of GT2 minor subtypes observed in the NS5A sequence analysis was 20% (Table 1). Despite the small number of patients infected with GT2 minor subtypes, the majority (97%; 32/33) achieved SVR except for one patient infected with HCV GT2i who also had baseline NS5A-L31M. This patient was treated with DCV/pegIFNλ/RBV. None of the patients treated with DCV/SOF ± RBV were infected with GT2 minor subtypes, therefore the response rates in this group would require further exploration.
In summary, the substantial diversity of circulating GT2 subtypes was confirmed in this analysis. Geographical differences in the distribution of GT2 subtypes and NS5A polymorphisms were observed. The most prevalent daclatasvir-resistant NS5A polymorphism was L31M, while RAVs at NS5A positions 28, 30 and 92 were of low prevalence, and polymorphisms at Y93 were not observed. Overall response rates in these patients infected with GT2 treated with different daclatasvir-based regimens were not notably impacted by GT2 subtype or baseline NS5A RAVs, with up to 100% achieving SVR.
Funding
This analysis was funded by Bristol-Myers Squibb.
Transparency declarations
This study was designed, executed and analysed by Bristol-Myers Squibb.
N. Z., D. H. and F. M. are employees of Bristol-Myers Squibb and obtain stock as partial compensation. Z. H., S. H.-N., B. D., J. U and V. V. are employees of Aerotek Recruiting Agency and contracted to work with Bristol-Myers Squibb.
Author contributions
N. Z. and F. M. designed the experiments and wrote the manuscript. N. Z., Z. H., S. H.-N., B. D., J. U., V. V. and D. H. performed the research. N. Z., D. H. and F. M. analysed the data. F. M. provided critical review and revision for important intellectual content. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, were fully responsible for all content and editorial decisions, and were involved at all stages of manuscript development.
Acknowledgements
The authors would like to thank Xiaoyan Yang for assistance with the phenotyping assays.
References



