-
PDF
- Split View
-
Views
-
Cite
Cite
Clara Ballesté-Delpierre, Anna Fàbrega, Mario Ferrer-Navarro, Ramkumar Mathur, Sankar Ghosh, Jordi Vila, Attenuation of in vitro host–pathogen interactions in quinolone-resistant Salmonella Typhi mutants, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 1, January 2016, Pages 111–122, https://doi.org/10.1093/jac/dkv299
Close - Share Icon Share
Abstract
The relationship between quinolone resistance acquisition and invasion impairment has been studied in some Salmonella enterica serovars. However, little information has been reported regarding the invasive human-restricted pathogen Salmonella Typhi. The aim of this study was to investigate the molecular mechanisms of quinolone resistance acquisition and its impact on virulence in this serovar.
Two antibiotic-resistant mutants (Ty_c1 and Ty_c2) were generated from a Salmonella Typhi clinical isolate (Ty_wt). The three strains were compared in terms of antimicrobial susceptibility, molecular mechanisms of resistance, gene expression of virulence-related factors, ability to invade eukaryotic cells (human epithelial cells and macrophages) and cytokine production.
Multidrug resistance in Ty_c2 was attributed to AcrAB/TolC overproduction, decreased OmpF (both mediated by the mar regulon) and decreased OmpC. The two mutants showed a gradually reduced expression of virulence-related genes (invA, hilA, hilD, fliC and fimA), correlating with decreased motility, reduced infection of HeLa cells and impaired uptake by and intracellular survival in human macrophages. Moreover, Ty_c2 also showed reduced tviA expression. Additionally, we revealed a significant reduction in TNF-α and IL-1β production and decreased NF-κB activation.
In this study, we provide an in-depth characterization of the molecular mechanisms of antibiotic resistance in the Salmonella Typhi serovar and evidence that acquisition of antimicrobial resistance is concomitantly detected with a loss of virulence (epithelial cell invasion, macrophage phagocytosis and cytokine production). We suggest that the low prevalence of clinical isolates of Salmonella Typhi highly resistant to ciprofloxacin is due to poor immunogenicity and impaired dissemination ability of these isolates.
Introduction
Typhoid fever is a human-restricted systemic infection caused by Salmonella enterica serovar Typhi. This pathogen causes ∼21 million infections causing typhoid fever each year, leading to ∼200 000 deaths.1 The highest incidence rates are seen in Asia, where this pathogen remains a major public health problem. The transmission route is through exposure to food and water contaminated with human faeces. Although it belongs to the same species as S. enterica serovar Typhimurium, their pathogenesis substantially differs. While salmonellosis caused by Salmonella Typhimurium infection is usually self-limiting, typhoid fever is a systemic infectious disease with much more severe consequences. Once bacteria reach the small intestine, they adhere to the mucosa and mostly invade M cells, which mediate internalization of the pathogen and allow transportation to Peyer's patches. This process is followed by phagocytosis by dendritic cells and macrophages, thus favouring dissemination through the mesenteric lymph nodes and eventual spread to the liver, spleen and bone marrow where Salmonella Typhi is able to survive and replicate.2–4
The treatment of typhoid fever has been changing and adapting together with the spread of drug-resistant strains. Chloramphenicol, trimethoprim/sulfamethoxazole and ampicillin were initially used until the emergence and spread of plasmid-mediated resistance to these antimicrobial agents. As a consequence, these drugs were replaced by ceftriaxone and ciprofloxacin as well as other fluoroquinolones such as ofloxacin.5 However, dissemination in the past decades, mostly in Asia, of strains showing both resistance to nalidixic acid (MIC >256 mg/L) and decreased ciprofloxacin susceptibility (MIC range, 0.125–1 mg/L) has occurred.5–7 In addition, some reports have already shown the emergence of highly fluoroquinolone-resistant isolates.8–10 This scenario is forcing the introduction of new-generation fluoroquinolones (e.g. gatifloxacin) and alternative drugs such as azithromycin.5,11
Quinolones act through inhibition of DNA gyrase and topoisomerase IV, hampering DNA replication and transcription as well as interfering with cell division. In S. enterica, resistance to quinolones is mainly due to point mutations in the quinolone resistance-determining regions (QRDRs) of the target genes, with amino acid substitutions at positions 83 and 87 of GyrA being the most frequently reported.4,12,13 Mutations affecting the internal accumulation of the drug, by decreasing the expression of porins (e.g. OmpF and OmpC) and/or increasing the expression of efflux pumps such as AcrAB/TolC,13–15 are also of great concern. Expression of the efflux pump AcrAB is controlled by its local repressor AcrR and by three homologous transcriptional activators, MarA, SoxS and RamA. The contribution of these regulators to the MDR phenotype has been reported in laboratory mutants and clinical isolates of S. enterica.16–20 There is only a single exception, since results concerning the role of MarA have been demonstrated in Escherichia coli,21–23 but not yet in clinical isolates of Salmonella. Similarly, activation of the transcription of micF, an antisense RNA that inhibits synthesis of the outer membrane porin OmpF,24 has yet to be supported with clinical data for Salmonella. In addition, plasmid-encoded genes [e.g. aac(6′)-Ib-cr and the qnr genes] are also responsible for quinolone resistance in Salmonella spp.13
Pathogenicity is primarily mediated by a number of virulence factors, the genes of which are organized within particular regions of the genome, named Salmonella pathogenicity islands (SPIs). To date, 15 SPIs have been identified in Salmonella Typhi, among which SPI-1, homologous to that described in Salmonella Typhimurium, contains genes encoding the type III secretion system-1 (T3SS-1) and secreted effector proteins needed for the invasion process.25–27 Furthermore, Salmonella Typhi-specific SPI-7 contains genes involved in the biosynthesis, regulation and export of the Vi capsular antigen. This virulence factor plays a key role in the attempt of Salmonella Typhi to avoid host defences and is therefore important in enhancing infectivity and virulence.28 Moreover, innate immune responses such as inflammation are also activated through the interaction of pathogen-associated molecular patterns, e.g. LPS and flagellin, with the Toll-like receptors (TLRs) TLR-4 and TLR-5, respectively.4,27,29 Stimulation of TLRs induces activation of the transcriptional regulator NF-κB, thus triggering the production of proinflammatory cytokines such as TNF-α, IL-6 and IL-1β.30
Previous reports conducted in non-Typhi S. enterica have shown that acquisition of quinolone resistance is related to decreased expression of virulence factors and impaired adherence to and invasion into the host cell.31,32 Even though there is an epidemiological relatedness between Typhi and non-Typhi serovars, many differences have been reported in terms of virulence and colonization behaviour, suggesting that adequate experiments should be conducted to address this relationship in Salmonella Typhi. Thus, the main objective of this study was to investigate if this loss of virulence, in terms of invasion and induction of host immune responses, also occurred in Salmonella Typhi upon acquisition of quinolone resistance. Moreover, despite the above-mentioned mechanisms of quinolone resistance being well studied in non-typhoidal Salmonella, an in-depth characterization of the molecular mechanisms involved in antibiotic resistance of two Salmonella Typhi mutants was also carried out.
Materials and methods
Strains
A clinical isolate of Salmonella Typhi was recovered from a patient diagnosed with spondylodiscitis at the Hospital Clínic of Barcelona (Spain). This isolate (Ty_wt) was used to generate in vitro antibiotic-resistant mutants in a multistep selection process, as previously described,31 by exposure of the bacteria to doubling concentrations of ciprofloxacin, starting at 0.25 mg/L. The mutants studied in this work were selected at 1 and 2 mg/L ciprofloxacin, named Ty_c1 and Ty_c2, respectively. When overnight cultures were required, strains were grown for 16–18 h.
Antimicrobial susceptibility
Antimicrobial susceptibility to nalidixic acid, ciprofloxacin, norfloxacin, ampicillin, ceftriaxone, cefoxitin, erythromycin, chloramphenicol, tetracycline, trimethoprim and kanamycin was assessed using Etests (bioMérieux) on Mueller–Hinton II plates (Becton Dickinson) following the manufacturer's recommendations. At least three replicates of each susceptibility test were performed.
Sequencing of quinolone resistance genes
DNA amplification of the target genes gyrA, gyrB, parC and parE as well as the global regulators acrR, marRAB, soxRS and ramR was performed using the primers listed in Table S1 (available as Supplementary data at JAC Online). Sequencing was performed by Beckman Coulter Genomics (Essex, UK) and the sequences analysed by alignment with the template sequence of Salmonella Typhi (RefSeq NC 016832.1).
Bacterial growth
Fresh cultures were grown at 37°C with shaking, as previously described,31 and OD readings at 600 nm (OD600) were done every 15 min for 24 h by means of an iEMS Multiskan Reader MF (Thermo Fisher Scientific). Each plate included four replicates of each sample and the assay was repeated three times.
Motility
Bacterial cultures were grown overnight in LB medium at 37°C with shaking and 10 μL was ‘stab inoculated’ into soft agar plates containing 0.5% agar. Plates were incubated for 7 days at 37°C in a humid chamber. Motility was assessed by measuring and comparing the growth diameter of the three tested strains.
Relative expression of resistance and virulence genes
RNA extraction from exponential cultures of Ty_wt, Ty_c1 and Ty_c2 was performed as reported by Fàbrega et al.31 A two-step real-time PCR was performed as previously described by our group33 following the 2−ΔΔCT method. Briefly, the obtained RNA was reverse transcribed to cDNA using the PrimeScript RT Reagent Kit (Takara) followed by PCR (SYBR® Premix Ex Taq Tli RNase H Plus Kit, Takara) under universal thermal cycling conditions. Expression of the efflux-related genes acrB, tolC, emrB and acrF, the marA regulator, the ompF porin and the virulence-related genes hilA, hilD, invA, fliC, fimA and tviA was studied. The primers used (Supplementary Data) were designed using Primer Express® software (Applied Biosystems). The 2−ΔΔCT method was applied to measure the gene expression, defined as relative quantification (RQ) of the target genes in the two mutant strains. The 16S rRNA was used as the reference gene for normalization and Ty_wt was the calibrator strain. Five independent extractions were performed and differences of >2-fold were considered relevant. Standard deviation was calculated and only reported when this value was >0.1.
Epithelial cell invasion assay
The invasion assay was performed according to Fàbrega et al.31 with some modifications. Briefly, HeLa cells (ECACC 84211901) were seeded into 6-well tissue culture-treated plates (Corning) in order to obtain a monolayer corresponding to ∼5 × 105 cells/well at 37°C in a 5% CO2 atmosphere. Infection was then performed with bacteria grown overnight at 37°C without shaking at an moi of 100. Plates were incubated at 37°C/5% CO2 for 2 h followed by an additional 2 h of incubation in the presence of 100 mg/L gentamicin (Life Technologies) to kill extracellular bacteria. Wells were then washed and 1 mL of chilled sterile water was added and kept at 4°C for 30 min to lyse the cells. This volume was recovered for intracellular bacterial counting by plating several dilutions in LB agar. At least three independent experiments were performed with intraexperiment duplicates. The invasion ability of each strain was determined by calculating the ratio between the number of intracellular bacteria and the inoculum.
Human macrophage infection assay
Human-derived monocytes (U-937 cell line) were used upon differentiation into adherent macrophages with 50 μg/L phorbol 12-myristate 13-acetate. A total of 5 × 105 cells/well were seeded into 24-well plates (Becton Dickinson) and incubated at 37°C/5% CO2 with RPMI-1640 (Life Technologies) supplemented with 10% FBS (Life Technologies). After 24 h, wells were washed three times with PBS (Life Technologies) and new medium was added in order to eliminate the non-adhered cells. Infection was carried out with Salmonella Typhi strains grown overnight at 37°C without shaking at an moi of 50. Plates were then centrifuged for 5 min at 2500 g at room temperature and incubated for 20 min at 37°C/5% CO2. Afterwards, cells were washed again and new medium containing 100 mg/L gentamicin was added before incubating for 2 h under the same conditions as described above. After this time, bacterial entry into host cells was determined by eliminating the supernatant, incubating the cells for 15 min at 4°C with 1 mL of chilled sterile water and then plating the appropriate dilutions on LB agar plates in order to allow bacterial counting. Alternatively, to determine the uptake rate, after 2 h of incubation with 100 mg/L gentamicin, the medium was replaced with new RPMI containing a lower dose of gentamicin (12 mg/L) and maintained for 24 h. Then, wells were washed and treated with 1 mL of chilled sterile water for cell lysis and bacterial counting. The intracellular survival ability was determined by calculating the ratio between the number of bacteria recovered at 24 and 2 h post-infection.
Cytokine assay
Production levels of TNF-α and IL-1β were measured in the supernatants during the human macrophage infection assay. After the two incubation periods (2 and 24 h), supernatants were collected and levels of TNF-α (from the 2 h post-infection plates) and IL-1β (from the 24 h post-infection plates) were measured by ELISA according to the manufacturer's recommendations (BD OptEIA).
NF-κB luciferase assay
HEK293 cells stably transfected with an NF-κB luciferase reporter construct, previously obtained by Koblansky et al.,34 were plated onto 48-well plates. After 24 h of incubation at 37°C/5% CO2, cells were stimulated with heat-killed Salmonella Typhi as well as with 0.2 μg/L TNF-α as a positive control for 6 h. Luciferase activity was measured with the Luciferase Reporter Assay System (Promega). Heat-killed bacteria were prepared from an overnight culture adjusted to OD600 = 0.7. An 800 μL aliquot of the bacterial culture was centrifuged at 13 500 g for 10 min, washed twice with PBS and resuspended in 1 mL of the same solution. Then, following 1 h of incubation at 65°C, the bacterial solution was vortexed for 3 min and centrifuged for 5 min at 2500 g to discard protein debris. For stimulation, 25 μL of the supernatant was added to each well. Each sample was tested in triplicate and three independent assays were performed.
Outer membrane protein analysis
Extraction of outer membrane proteins was performed with N-lauroyl sarcosinate. Briefly, bacteria were harvested from 200 mL of exponential culture (OD600 = 0.6) by centrifugation at 4°C and 3500 g. Cells were washed twice in PBS in order to remove any residual medium. Then, cells were resuspended in 6 mL of 10 mM Tris, pH 8.0/1% NaCl. At this point, cells were sonicated for 15 min (cycles of 59 s ‘on’ and 59 s ‘off’). After cell disruption, samples were centrifuged at 4°C and 3500 g in order to remove any cell debris. Supernatants were collected and transferred into ultracentrifugation tubes and samples were centrifuged at 100 000 g for 1 h at 4°C in a Sorvall MS-150 microultracentrifuge (Thermo Scientific) using an S50-ST rotor. After this centrifugation step, the supernatant was discarded and the pellet resuspended in 1% sarcosyl solution and incubated for 60 min at room temperature with gentle agitation. After incubation, samples were again centrifuged at 100 000 g for 1 h at 4°C and the resulting pellet was cleaned twice with 10 mM Tris, pH 8.0/1% NaCl. Finally, the pellet was carefully resuspended in 500 mL of milliQ water and the protein concentration estimated with the 2D-Quant Kit (GE Healthcare). Protein extracts were separated by SDS–PAGE on 12.5% isocratic Laemmli gels using a mid-size gel casting system (Hoefer SE600 Chroma). Gels were run at 25 mA following the manufacturer's recommendations. Bands showing differential expression between strains were recovered and further identified through MALDI-TOF MS performed using an Ultraflex instrument (Bruker Daltonics).
Statistics
Data were analysed using IBM SPSS Statistics 20 software. As data were normally distributed, multiple comparisons were performed using the one-way ANOVA test. P values <0.05 were considered to be significant.
Results
Selection of the strains
A Salmonella Typhi clinical isolate (Ty_wt) was selected to generate mutants able to grow at inhibitory concentrations of ciprofloxacin. First, Ty_wt was plated onto MacConkey agar plates containing 0.25 mg/L ciprofloxacin, corresponding to 0.5× its MIC (0.5 mg/L). From this point onwards, the concentration of ciprofloxacin was doubled at each step, in which a single colony was selected and plated again in the following selection stage, reaching a ciprofloxacin concentration of 16 mg/L. The mutants selected for the study were Ty_c1 and Ty_c2, which corresponded to colonies grown on plates containing 1 and 2 mg/L ciprofloxacin with MICs of ciprofloxacin of 1 and 8 mg/L, respectively. The mutant recovered at 4 mg/L ciprofloxacin, with an MIC of 8 mg/L, was not considered for the study as it showed the same antimicrobial susceptibility profile as Ty_c2, suggesting that no additional resistance mechanisms were selected. Mutants able to grow at greater concentrations (8 and 16 mg/L) were also recovered (MICs 12–24 mg/L), although they could not be further studied as they showed important growth impairment. Resistant colonies appeared at frequencies of 2 × 10−6 and 7 × 10−6 mutants per cfu for Ty_c1 and Ty_c2, respectively. When bacteria were exposed to a concentration of 8 mg/L ciprofloxacin, the frequency of selection decreased to 5.5 × 10−7 and in the presence of 16 mg/L it was further reduced to 6 × 10−8 mutants per cfu.
To assess reversion of the resistance phenotype, the selected resistant mutants Ty_c1 and Ty_c2 were plated onto MacConkey agar plates without antibiotic. However, no revertant colonies were obtained after 35 consecutive passages.
Bacterial growth
A link between fluoroquinolone resistance and fitness has been stated previously. Several studies carried out in Salmonella Typhimurium and S. enterica serovar Enteritidis have shown that acquisition of high levels of fluoroquinolone resistance have a fitness cost.31,35,36 In order to evaluate this feature in our strains, bacterial growth was examined. No differences between the three strains were observed, indicating that acquisition of antimicrobial resistance in these mutants (ciprofloxacin MICs of 1 and 8 mg/L) did not impose an energy cost on bacterial growth. However, the mutants with ciprofloxacin MICs of 12 and 32 mg/L (named Ty_c8 and Ty_c32) showed a much longer lag phase than the previous strains and Ty_c32 did not reach the same OD in the stationary phase as the previous mutants (Figure 1). Moreover, these mutants reached stationary phase (OD600 = 0.6) after 8 h of incubation at 37°C with shaking, compared with 3 h for Ty_wt, Ty_c1 and Ty_c2. In addition to the altered bacterial growth, the colonies were phenotypically different from the others: they were much smaller and did not grow well on MacConkey agar plates. Taking into account all these observations, Ty_c8 and Ty_c32 were excluded from the present study.
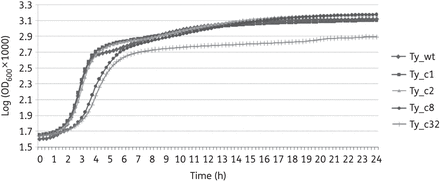
Mutants Ty_c8 and Ty_c32 show impaired growth. Bacterial growth of all the mutants obtained was assessed. The most resistant mutants (Ty_c8 and Ty_c32) were rejected as they presented growth deficiencies. Results correspond to the mean of three independent experiments.
Characterization of the antimicrobial resistance mechanisms
Quinolone resistance profile
The MICs of nalidixic acid and the fluoroquinolones ciprofloxacin and norfloxacin were determined (Table 1). As expected, the MICs of the quinolones tested sequentially increased for the two mutants, except for the MIC of ciprofloxacin for which a slight difference was recorded between Ty_wt and Ty_c1, whereas the MIC for Ty_c2 increased 8-fold. In contrast, a 4- and 8-fold increase was seen for Ty_c1 in the MICs of nalidixic acid (MIC 1000 mg/L) and norfloxacin (MIC 8 mg/L), respectively, and a further 2- and 3-fold increase for Ty_c2.
| Strain . | MIC (mg/L) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAL . | CIP . | NOR . | ERY . | CHL . | TET . | TMP . | AMP . | FOX . | CRO . | KAN . | |
| Ty_wt | 250 | 0.5 | 1 | 96 | 4 | 0.5 | 0.094 | 0.094 | 1 | 0.064 | 1 |
| Ty_c1 | 1000 | 1 | 8 | 128 | 4 | 1.5 | 0.25 | 0.75 | 4 | 0.125 | 1 |
| Ty_c2 | 2000 | 8 | 24 | >256 | 128 | 4 | 0.75 | 4 | 128 | 0.5 | 1 |
| Strain . | MIC (mg/L) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAL . | CIP . | NOR . | ERY . | CHL . | TET . | TMP . | AMP . | FOX . | CRO . | KAN . | |
| Ty_wt | 250 | 0.5 | 1 | 96 | 4 | 0.5 | 0.094 | 0.094 | 1 | 0.064 | 1 |
| Ty_c1 | 1000 | 1 | 8 | 128 | 4 | 1.5 | 0.25 | 0.75 | 4 | 0.125 | 1 |
| Ty_c2 | 2000 | 8 | 24 | >256 | 128 | 4 | 0.75 | 4 | 128 | 0.5 | 1 |
NAL, nalidixic acid; CIP, ciprofloxacin; NOR, norfloxacin; ERY, erythromycin; CHL, chloramphenicol; TET, tetracycline; TMP, trimethoprim; AMP, ampicillin; FOX, cefoxitin; CRO, ceftriaxone; KAN, kanamycin.
| Strain . | MIC (mg/L) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAL . | CIP . | NOR . | ERY . | CHL . | TET . | TMP . | AMP . | FOX . | CRO . | KAN . | |
| Ty_wt | 250 | 0.5 | 1 | 96 | 4 | 0.5 | 0.094 | 0.094 | 1 | 0.064 | 1 |
| Ty_c1 | 1000 | 1 | 8 | 128 | 4 | 1.5 | 0.25 | 0.75 | 4 | 0.125 | 1 |
| Ty_c2 | 2000 | 8 | 24 | >256 | 128 | 4 | 0.75 | 4 | 128 | 0.5 | 1 |
| Strain . | MIC (mg/L) . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NAL . | CIP . | NOR . | ERY . | CHL . | TET . | TMP . | AMP . | FOX . | CRO . | KAN . | |
| Ty_wt | 250 | 0.5 | 1 | 96 | 4 | 0.5 | 0.094 | 0.094 | 1 | 0.064 | 1 |
| Ty_c1 | 1000 | 1 | 8 | 128 | 4 | 1.5 | 0.25 | 0.75 | 4 | 0.125 | 1 |
| Ty_c2 | 2000 | 8 | 24 | >256 | 128 | 4 | 0.75 | 4 | 128 | 0.5 | 1 |
NAL, nalidixic acid; CIP, ciprofloxacin; NOR, norfloxacin; ERY, erythromycin; CHL, chloramphenicol; TET, tetracycline; TMP, trimethoprim; AMP, ampicillin; FOX, cefoxitin; CRO, ceftriaxone; KAN, kanamycin.
In order to study the molecular mechanisms of quinolone resistance, sequencing of the QRDRs of the target genes gyrA, gyrB, parC and parE was carried out. The results revealed a single amino acid substitution in GyrA (S83F) in all three strains and no additional mutations were found in the QRDRs of the mutants. As mutations outside the QRDR region of gyrA were found in a previous study and were suggested to contribute to the quinolone resistance phenotype,37 sequencing of the entire target genes (gyrA, gyrB, parC and parE) was also performed, but no additional change was found.
Multidrug resistance profile
The absence of new mutations acquired in the target genes of the two mutants suggested the acquisition of broad-spectrum resistance mechanisms such as increased efflux. In this context, the antimicrobial susceptibility profile of Ty_wt, Ty_c1 and Ty_c2 was assessed against a broader collection of antibiotics including erythromycin, ampicillin, ceftriaxone, cefoxitin, chloramphenicol, tetracycline, trimethoprim and kanamycin. The results obtained, reported in Table 1, showed a progressive, but different, increase in the MIC of most of the antibiotics tested. In comparison with Ty_wt, Ty_c1 showed the highest increase in the MIC of ampicillin (8-fold), modest increments (2- to 4-fold) in the MICs of tetracycline, trimethoprim, cefoxitin and ceftriaxone, whereas no major change (<1.5-fold) was seen for chloramphenicol and erythromycin. Contrarily, a different response was observed for strain Ty_c2. This mutant could be classified as MDR according to the ECDC.38 It acquired resistance to cefoxitin and chloramphenicol with a 32-fold increase in both MICs compared with Ty_c1. Moreover, and even though the MICs of the remaining drugs tested did not reach the resistance breakpoints, increases of 3- to 5-fold were recorded in all cases. The only antibiotic tested for which no changes in susceptibility were seen in any of the mutants was kanamycin.
Efflux and permeability are altered in Ty_c2
In Salmonella Typhimurium, the predominant transport system involved in multidrug resistance is AcrAB/TolC.15 Other less frequently detected drug transporters such as AcrEF, highly homologous to AcrAB, and EmrAB have also been reported to extrude quinolones13,39 and have been shown to be overexpressed in quinolone-resistant Salmonella Typhimurium mutants.40 Expression of the acrB, tolC, acrF, emrB and ompF genes was tested in the three strains by real-time PCR. Results revealed overexpression of acrB and tolC only in Ty_c2 (RQ = 3.91 for acrB and RQ = 3.42 for tolC). Surprisingly, the other efflux-related genes, acrF and emrB, were repressed in this strain compared with Ty_wt (3.06- and 3.96-fold, respectively). Additionally, the porin ompF was >6-fold repressed in Ty_c2. In the case of Ty_c1, despite the fact that statistically significant differences were seen for acrF, emrB and ompF, no relevant results were detected for any of these genes since all expression values were <2-fold. Thus, these results suggest the involvement of AcrAB/TolC and OmpF in the multidrug resistance profile of Ty_c2 (Table 2).
Transcriptional levels of genes involved in antimicrobial resistance and virulence
| Strain . | Efflux-related genes . | Regulatory genes . | Virulence genes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acrB . | tolC . | acrF . | emrB . | ompF . | marA . | soxS . | ramA . | acrR . | hilD . | hilA . | invA . | tviA . | fimA . | fliC . | |
| Ty_wt | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ty_c1 | −1.08 | −1.64 | −1.60* | −1.90** | −1.20* | −1.28 | 1.25 | −1.32* | −1.89** | −2.50** | −2.59** | −3.75** | −1.26* | −3.22** | −9.89** |
| Ty_c2 | 3.91** | 3.42** | −3.06* | −3.96** | −6.37** | 108.19** | −2.38** | −2.70** | −5.88** | −9.19** | −5.04** | −8.55** | −27.85** | −6.09** | −121.09** |
| Strain . | Efflux-related genes . | Regulatory genes . | Virulence genes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acrB . | tolC . | acrF . | emrB . | ompF . | marA . | soxS . | ramA . | acrR . | hilD . | hilA . | invA . | tviA . | fimA . | fliC . | |
| Ty_wt | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ty_c1 | −1.08 | −1.64 | −1.60* | −1.90** | −1.20* | −1.28 | 1.25 | −1.32* | −1.89** | −2.50** | −2.59** | −3.75** | −1.26* | −3.22** | −9.89** |
| Ty_c2 | 3.91** | 3.42** | −3.06* | −3.96** | −6.37** | 108.19** | −2.38** | −2.70** | −5.88** | −9.19** | −5.04** | −8.55** | −27.85** | −6.09** | −121.09** |
*P < 0.5 and **P < 0.01.
Transcriptional levels of genes involved in antimicrobial resistance and virulence
| Strain . | Efflux-related genes . | Regulatory genes . | Virulence genes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acrB . | tolC . | acrF . | emrB . | ompF . | marA . | soxS . | ramA . | acrR . | hilD . | hilA . | invA . | tviA . | fimA . | fliC . | |
| Ty_wt | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ty_c1 | −1.08 | −1.64 | −1.60* | −1.90** | −1.20* | −1.28 | 1.25 | −1.32* | −1.89** | −2.50** | −2.59** | −3.75** | −1.26* | −3.22** | −9.89** |
| Ty_c2 | 3.91** | 3.42** | −3.06* | −3.96** | −6.37** | 108.19** | −2.38** | −2.70** | −5.88** | −9.19** | −5.04** | −8.55** | −27.85** | −6.09** | −121.09** |
| Strain . | Efflux-related genes . | Regulatory genes . | Virulence genes . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| acrB . | tolC . | acrF . | emrB . | ompF . | marA . | soxS . | ramA . | acrR . | hilD . | hilA . | invA . | tviA . | fimA . | fliC . | |
| Ty_wt | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ty_c1 | −1.08 | −1.64 | −1.60* | −1.90** | −1.20* | −1.28 | 1.25 | −1.32* | −1.89** | −2.50** | −2.59** | −3.75** | −1.26* | −3.22** | −9.89** |
| Ty_c2 | 3.91** | 3.42** | −3.06* | −3.96** | −6.37** | 108.19** | −2.38** | −2.70** | −5.88** | −9.19** | −5.04** | −8.55** | −27.85** | −6.09** | −121.09** |
*P < 0.5 and **P < 0.01.
Analysis of the bacterial outer membrane proteins further confirmed part of these changes in gene expression: two bands identified as AcrA and TolC were overproduced only in Ty_c2, together with a decreased production of the outer membrane protein OmpC (Figure 2).
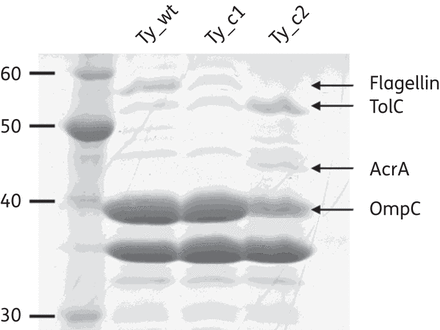
Ty_wt, Ty_c1 and Ty_c2 have different outer membrane protein profiles. Proteins showing differential expression are indicated by arrows.
Role of MarA in the antimicrobial resistance profile of Ty_c2
Mutations in AcrR have been shown to impair its repressive effect, hence leading to derepression of the acrAB genes.16–18 The repressors MarR and RamR and the activator SoxR are regulators of transcriptional factors MarA, RamA and SoxS, respectively. Mutations in RamR and SoxR have been reported in different Salmonella serovars to increase expression of the homologue activators and, therefore, increase protein levels of AcrAB and down-regulate OmpF.16–20 However, studies describing mutations in MarR in strains selected in the clinical setting have been reported for E. coli, but not for Salmonella spp.21,22
In this study, sequencing of the known regulatory regions of AcrAB expression (acrR, marRAB, soxRS and ramR) was carried out. Indeed, an important alteration in the marR locus was detected in the Ty_c2 mutant corresponding to a deletion of 445 nucleotides. The fragment deleted included the first 411 nucleotides of the marR gene and 34 nucleotides upstream of the gene (Figure 3). In E. coli, the existence of two binding sites (Site I and Site II) for MarR in the marO region has been reported.41 In Ty_c2, the entire Site II was deleted although Site I was completely preserved as well as the −35 and −10 elements of the promoter. Interpretation of this finding suggests that MarA expression is not only possible since the promoter is still intact, but also overexpression is likely to be detected due to a lack of the MarR protein. Consistently, real-time PCR transcription analysis of marA revealed high overexpression (RQ 108.19 ± 0.16) in the MDR strain Ty_c2 compared with Ty_wt. Nonetheless, despite no further changes being seen in the sequences of the other regulators, repression of acrR, soxS and ramA ranging from 2- to almost 6-fold was observed in Ty_c2 (Table 2). The results obtained in this section suggest that overexpression of MarA is responsible, at least in part, for the resistance phenotype observed in Ty_c2.
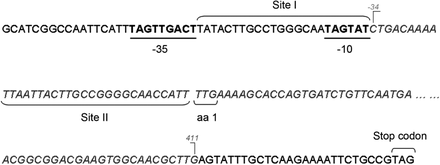
Schematic representation of the deleted region located in the marRAB regulatory region of Ty_c2. The predicted −35 and −10 promoter regions are shown in underlined bold letters. The two MarR-binding sites (Site I and Site II) are also indicated and extrapolated from E. coli.41 Letters in italics correspond to the deletion detected and numbers in grey indicate the first and last position of the deleted nucleotides.
Evaluation of virulence in the quinolone-resistant Salmonella Typhi mutants
Gradual reduction of motility is seen in Ty_c1 and Ty_c2
Intact motility of Salmonella Typhi has been determined to participate in the invasion process and is therefore related to the virulence potential of this pathogen.42,43 For this reason, the motility of the three strains was measured by inoculation into soft agar plates. Although the clinical strain (Ty_wt) was already poorly motile, with a colony diameter of 13 mm, a decrease in motility was seen for the mutants, showing diameters of 9 mm for Ty_c1 and 7 mm for Ty_c2 (data not shown).
As flagellin is the major component of the bacterial flagellar filament, the expression profile of the flagellin-encoding gene fliC was analysed by real-time PCR and revealed statistically significant differences between the parental strain and the mutants (Table 2). Ty_c1 showed a 10-fold reduction in fliC expression levels in comparison with Ty_wt and an additional >10-fold decreased expression was seen for Ty_c2 (RQ = 0.01). These results are in accordance with the phenotype observed in the soft agar assay.
In order to determine whether the differences in fliC expression could be attributed to the acquisition of mutations within the gene, fliC as well as the regulators fliA and flhDC, involved in flagella biosynthesis,35,44 were sequenced. Amino acid substitutions at particular positions of the conserved site of flagellin have been reported to be essential for protofilament assembly, bacterial motility and TLR-5 recognition.45 A single mutation in fliC was found in both Ty_c1 and Ty_c2 leading to the amino acid substitution T187I, whereas no mutations in any of the regulators were detected. This change detected in the mutants is located outside of the conserved region, likely suggesting it to be trivial for the function and structure of flagellin.45
Salmonella Typhi mutants are less able to invade epithelial cells
As described previously in several serovars of S. enterica,31,32,46 acquisition of quinolone resistance has been related to decreased invasion ability in an in vitro eukaryotic cell model. For this reason, in vitro invasion of HeLa cells was examined with the three studied strains (Ty_wt, Ty_c1 and Ty_c2) and revealed a gradual reduction in the invasion ability of the mutants compared with the parental isolate. The invasion rate of Ty_wt was 7.31%, that of Ty_c1 was 1.69% (∼4 times lower) and the lowest rate of invasion (0.27%) was reported for Ty_c2, which was 6 times lower than the Ty_c1 mutant and 27 times lower than Ty_wt (Figure 4a).
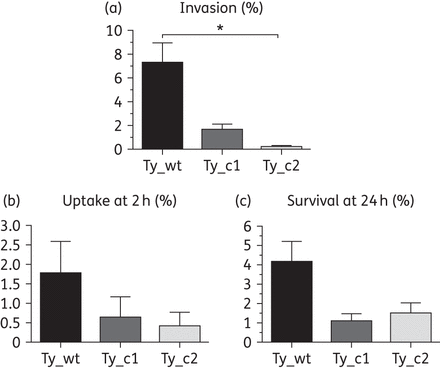
Ability to invade HeLa cells and uptake by and survival in macrophages is compromised upon infection with Ty_c1 and Ty_c2. Invasion was performed by infecting HeLa cells with the different bacteria (a). Uptake at 2 h (b) and survival at 24 h (c) was assessed by infecting human differentiated macrophages with the three strains studied. Results correspond to the mean of three independent experiments ± standard error. Differences between groups were assessed using the one-way ANOVA test. *P < 0.05.
Salmonella Typhi mutants show impaired internalization and survival in macrophages
Internalization of Salmonella Typhi by phagocytes once Salmonella Typhi has reached the submucosa as well as survival of the pathogen inside these host cells are crucial processes for systemic infection.2 Thus, differentiated U-937 human macrophages were infected with the three strains. In order to study their ability to be phagocytosed by macrophages, the number of intracellular bacteria was determined at 2 h post-infection. Results were expressed as percentage values of recovered bacteria with respect to the total number of infecting bacteria. A reduction of 2.75-fold in Ty_c1 compared with Ty_wt and an additional 1.6-fold for Ty_c2, which corresponded to 4.37 times less than Ty_wt, was seen (Figure 4b). Moreover, the intracellular survival rate was also determined and expressed as the percentage of bacterial count at 2 h versus bacterial load at 24 h post-infection. Similar results were observed for the two mutants and corresponded to a 4-fold reduction compared with the parental strain (Figure 4c). These results showed a gradual inability of the mutants to be recognized by macrophages and therefore be internalized. In addition, the internalized bacteria were less able to survive inside macrophages, showing no significant differences between the two mutants.
Transcription levels of virulence factors are low in Salmonella Typhi mutants
Among the virulence factors reported to play a key role in Salmonella Typhi–host interactions, several loci have been described. The invasion process of Salmonella is mainly driven by virulence determinants contained in SPI-1,25,26,36 such as the effector invA and the key regulators hilD and hilA.47 Type 1 fimbriae are important for adhesion to eukaryotic cells and are encoded by fim genes, with fimA being responsible for the production of the major fimbrial subunit.36 The Vi capsular antigen, a Salmonella Typhi-specific determinant encoded by the tviA gene located in SPI-7, is involved in systemic dissemination.25 In this study, the gene expression profiles of invA, hilD, hilA, fimA and tviA were evaluated through real-time PCR analysis (Table 2). The results reflect sequential gene repression in the two mutants compared with the original strain for almost all the genes tested. In Ty_c1, invA, hilD, hilA and fimA were 2.5- to 3.75-fold less expressed than in Ty_wt. On comparing Ty_c2 versus Ty_c1, expression of these genes was reduced by 1.89- to 3.67-fold. Contrary to the results observed for these genes, tviA expression in Ty_c1 was comparable to that seen for the parental strain (RQ = 0.79). However, almost 30-fold less expression was observed in Ty_c2 (Table 2). These results suggest involvement of invA, hilD, hilA and fimA in the progressive loss of virulence in the two mutants; however, as tviA only showed a decreased expression in Ty_c2, this gene would only be related to the phenotype of this latter mutant.
Induction of the immune response is compromised in Salmonella Typhi mutants
The levels of the proinflammatory cytokines TNF-α and IL-1β produced by infected U-937 macrophages were measured. Levels of TNF-α were checked in the supernatants collected after 2 h of infection and revealed a gradual and significant decrease in the two mutants compared with Ty_wt. The levels of this cytokine consisted of 5181 μg/mL in Ty_wt, almost 4000 μg/mL in Ty_c1 and 3256 μg/mL in Ty_c2 (Figure 5a). A greater reduction was seen for IL-1β, checked in the supernatants collected at 24 h post-infection: Ty_wt reached 3082 pg/mL, whereas the levels in Ty_c1 and Ty_c2 were reduced by >50%, showing values of 1167 and 1067 pg/mL, respectively (Figure 5b).
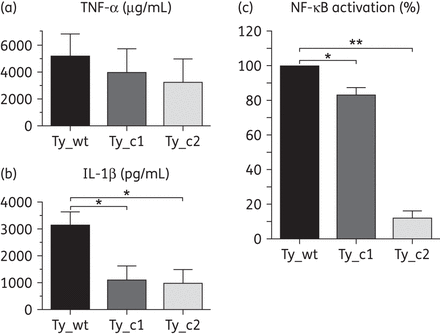
Salmonella Typhi mutants are less immunogenic than Ty_wt. Levels of TNF-α (a) and IL-1β (b) were measured in infected macrophages and activation of NF-κB (c) was assessed in stimulated HEK293 cells. Results correspond to the mean of three independent experiments ± standard error. Differences between groups were assessed using the one-way ANOVA test. *P < 0.05 and **P < 0.01.
Since transcription of these two cytokines is controlled by NF-κB,2,27 activation of this nuclear transcription factor was checked upon stimulation of HEK293 cells with heat-killed bacteria by means of a luciferase reporter assay. Activation of NF-κB significantly diminished in Ty_c1 compared with the parental strain and corresponded to a decrease of 16.67%. This ratio was much more significant for Ty_c2, with a 71.72% reduction of the NF-κB activity in comparison with Ty_c1 (Figure 5c). The results obtained in this section indicate that Salmonella Typhi mutants elicit a more discrete immunogenic reaction in vitro than the original clinical isolate, with Ty_c2 being significantly less reactive.
Discussion
In this study, we evaluated the process of quinolone resistance acquisition and the impact on virulence properties in the pathogen Salmonella Typhi. In the first part of the study, we characterized the molecular mechanisms of resistance since they have not been extensively studied in this Salmonella serovar. We obtained two mutants, Ty_c1 and Ty_c2, with increased MICs of ciprofloxacin (1 and 8 mg/L, respectively) selected from a clinical isolate (ciprofloxacin MIC of 0.5 mg/L). All studies reporting resistance or decreased susceptibility to ciprofloxacin (MIC >0.25 mg/L) in Salmonella Typhi associate this phenotype with mutations in the QRDRs of the target genes, particularly in gyrA.37,48 In this study, we found that Ty_wt already harboured a GyrA substitution (S83F), but no additional change was detected in the two mutants on sequencing the full locus of each quinolone target gene. The profile of increased MICs was different for the two mutants. A moderate increase in MICs of several unrelated compounds was seen in Ty_c1, whereas more important increases were reported for Ty_c2 affecting almost all compounds tested and leading to an MDR phenotype.
According to the results obtained from gene expression and protein analyses, we found that alteration of membrane permeability was seen in Ty_c2 due to a reduction in the production of OmpF and OmpC. Although this alteration in porin expression has been previously shown in E. coli and non-Typhi Salmonella,31,49,50 it has not been reported in Salmonella Typhi. More importantly, this MDR phenotype was primarily associated with hyperproduction of the major efflux pump AcrAB/TolC. Only a few studies, such as that of Chiou et al.,10 have previously reported increased efflux (based on the use of an efflux pump inhibitor) among Salmonella Typhi clinical isolates with resistance or decreased susceptibility to ciprofloxacin (MICs of 0.12–16 mg/L). Nonetheless, this is the first time that the efflux pump AcrAB/TolC has been characterized in this serovar. Our findings together with the data of Chiou et al.10 contrast with the results of Baucheron et al.,51 in which no involvement of efflux mechanisms was seen in a collection of Salmonella Typhi clinical isolates. It is worth mentioning that the majority of these latter strains showed MICs of ciprofloxacin ranging from 0.125 to 1 mg/L, although a single strain had a higher MIC of 8 mg/L, attributed to the acquisition of two further target gene mutations. In the study by Chiou et al.,10 clinical isolates for which increased efflux activity was reported had ciprofloxacin MICs of >4 mg/L. In our study, Ty_c2 had a ciprofloxacin MIC of 8 mg/L. Therefore, we suggest that selection of increased efflux is acquired at ciprofloxacin MICs of 4–8 mg/L as a general trend. To better confirm this hypothesis, characterization of larger collections of ciprofloxacin-resistant clinical isolates is needed to accurately evaluate the relevance and prevalence of this mechanism. In an attempt to understand the increased levels of AcrAB/TolC detected in Ty_c2, we found that this was due to alterations in the marRAB regulatory region. A deletion of almost the entire marR gene detected in this mutant likely resulted in the absence of transcription of the repressor, hence leading to hyperproduction of MarA. This phenotype, in addition to causing increased amounts of AcrAB/TolC, would also explain the down-regulation of the ompF gene detected in Ty_c2. This is the first demonstration in Salmonella Typhi of the molecular regulation of this efflux system, as it has been previously described only in non-typhoidal Salmonella.13,23,52 In addition, and to the best of our knowledge, it is also the first description of a marR mutation reported in S. enterica leading to increased AcrAB/TolC, since most of the reported mutations are located in ramR17,53 and, less frequently, in acrR54 and soxRS.19
Surprisingly, the transcription levels of the regulators acrR, soxS and ramA were repressed in Ty_c2. Additionally, the alternative efflux pump candidates emrB and acrF, reported to be overexpressed in quinolone-resistant Salmonella Typhimurium isolates,40 were also studied and revealed down-regulation only in this mutant. A similar observation has also been reported by Kang and Woo,55 where expression levels of efflux pump-related genes were tested in ciprofloxacin-resistant S. enterica serovar Istanbul mutants. In all of the mutants, not only soxS, but also acrF, were down-regulated compared with the parental strain, whereas acrB, ramA and/or marA were overexpressed. Despite another study conducted in E. coli56 reporting an increase in the expression of soxS in first-step mutants selected with tetracycline, a decrease in the expression levels of this gene was observed in the most resistant strain. Therefore, the authors suggested that the SoxS response could be activated at the early stages of antibiotic resistance adaptation. Considering this proposal, the decreased expression levels of soxS observed in Ty_c2 could be partly explained by this phenomenon. According to all these results, including the findings reported in the present study, it seems that overexpression of AcrAB may lead to reduced expression of some of their regulators and/or alternative transport systems to avoid redundancy in efflux transportation, resulting in a reduction of the energy cost of the bacteria.
Contrary to the scenario reported for Ty_c2, the increased MICs detected for the intermediate resistant mutant Ty_c1 could not be attributed to the enhanced expression of AcrAB/TolC. Moreover, despite statistically significant results being detected for acrF, emrB and ompF, these results were considered irrelevant as repression was <2-fold. These results suggest that unknown TolC-independent mechanisms of resistance with reduced impact are likely to be involved in antibiotic resistance.
In the second part of this work, the association between virulence and quinolone resistance was investigated. This relation has been studied based on data reporting a rise in the number of Salmonella Typhi clinical isolates with decreased ciprofloxacin susceptibility, considered endemic in countries such as India,57 but a lower incidence of strains showing high levels of ciprofloxacin resistance. A study conducted in the UK from 2001 to 2004 reported low incidence (1%–2%) of ciprofloxacin-resistant Salmonella Typhi isolates (MIC ≥1 mg/L) in contrast to an increase in the number of strains with decreased ciprofloxacin susceptibility (from 35% in 2001 to 47% in 2004).58 Nonetheless, some cases reporting high levels of ciprofloxacin resistance in Salmonella Typhi have already emerged.9,59
Moreover, previous studies conducted in different serovars of S. enterica have already associated quinolone resistance with a decreased invasion profile. Wang et al.32 reported a reduction in the expression of SPI-1 genes, decreased ability to invade epithelial cells and low replication inside macrophages for Salmonella Typhimurium and Salmonella Choleraesuis resistant mutants. Similarly, studies conducted with Salmonella Typhimurium and Salmonella Enteritidis also showed an association between resistance, reduced virulence and impaired expression of SPI-1 genes in quinolone-resistant mutants.16,31 Consistent with these findings, our results showed that upon acquisition of resistance to quinolones, the two mutants showed a progressively reduced ability to invade epithelial cells and macrophages. Moreover, the survival rate inside macrophages was also diminished. Regulation of these virulence determinants has been well established in Salmonella Typhimurium36 and demonstrated to be mostly homologous in Salmonella Typhi by Faucher et al.26 They identified that SPI-1 genes were involved in the invasion process when infecting macrophages. Moreover, Bishop et al.60 showed that Salmonella Typhi strains lacking invA were 1000-fold less able to invade epithelial cells. Thus, the results of our gene expression analysis agreed with the phenotype observed, since several SPI-1-related genes (invA, hilA and hilD) showed a gradual reduction in their expression levels.
In an attempt to find an explanation for this effect on virulence, several hypotheses should be considered. First, DNA supercoiling has been proposed to influence the transcription process.61 Thus, acquisition of mutations in DNA gyrase has been considered to impair regular supercoiling activity and hence modify gene expression patterns.32 Nonetheless, our results do not support this as our parental strain already had a mutation in GyrA, but no other QRDR mutation was acquired in the mutants. Second, impaired bacterial growth has been described for quinolone-resistant mutants of Salmonella Typhimurium and Salmonella Enteritidis.31,46,62 This reduced ability to grow may shut off dispensable bacterial functions such as virulence properties. Nonetheless, the three strains reported in this study did not show any difference in terms of bacterial growth. On the contrary, a recent study performed in Salmonella Typhi associated mutations in the QRDRs of the target genes with fitness benefits, with the S83F mutation in GyrA, which leads to decreased ciprofloxacin susceptibility, being the most advantageous.63 The presence of this mutation in our strains may explain the unchanged growth observed. Third, high production levels of AcrAB/TolC have been suggested to be the reason for impaired virulence.46 As quorum-sensing signal molecules have been shown to be extruded by efflux pumps64 and such molecules can activate virulence genes,65 an impaired quorum-sensing homeostasis triggered by increased efflux activity may lead to impaired gene transcription. To strengthen this hypothesis, a study conducted by Bailey et al.66 showed that high overexpression of ramA was accompanied by overexpression of efflux pumps as well as a decrease in the expression of virulence genes and impaired host–pathogen interactions. Taking into account these findings, we suggest that a similar situation could be happening in the present work triggered by the high overexpression of marA (>100-fold) in the Ty_c2 mutant.
Additional virulence genes were also studied in the present work. On the one hand, Salmonella Typhimurium and Salmonella Typhi have been demonstrated to share a number of pathogenesis determinants, such as the bacterial flagellum. Mutations in the flagellar regulatory genes flhDC and fliA have been associated with severely decreased entry into eukaryotic cells and reduced cytotoxicity in macrophages.67 Moreover, uptake and survival defects in macrophages have also been seen in fliC and flhCD mutants as reported by Sabbagh et al.68 A correlation between reduced motility, diminished fliC levels and impaired invasion of epithelial cells was seen for our mutants. On the other hand, Salmonella Typhi-specific virulence genes have also been identified. Among these factors, the most relevant are the genes located in the Salmonella Typhi-specific pathogenicity island SPI-7.25,69 The Vi capsular polysaccharide is encoded by the viaB locus, from SPI-7, and has been reported to be necessary for Salmonella Typhi survival in macrophages, serum resistance and systemic dissemination.25,70 An important repression of the tviA gene was reported in the MDR mutant Ty_c2. Thus, it seems reasonable that repression of these virulence factors (FliC and TviA) has also contributed to the impaired virulence phenotype observed in the two mutants, concerning invasion of epithelial cells as well as uptake by and survival inside human macrophages.
It has been previously reported that TviA represses important virulence factors including genes encoding flagella and T3SS-1 through the flhDC–fliZ–hilD–hilA axis.60,71 In our study, however, repressed transcription of tviA was only considered in Ty_c2, as values seen in Ty_c1 were <1.3, whereas the expression levels of fliC and the SPI-1 encoded genes, were already decreased in the Ty_c1 mutant. Thus, these results support the existence of a tviA-independent regulation of flagellin in Ty_c1, in accordance with the different regulators governing flagella expression reported in other Salmonella serovars. However, in Ty_c2, TviA certainly seems to play a role in fliC regulation.
In the last part of this work, we evaluated the ability of the mutants to trigger an immunogenic response when infecting human macrophages. The flagellin protein FliC has been reported to activate TLR-5 inside macrophages, thus decreasing the signalling pathway of NF-κB, which elicits cytokine production (TNF-α and IL-1β). Our results showed that Ty_c1 and Ty_c2 triggered a poor innate immune response as reflected by a decrease in the production of these two proinflammatory cytokines as well as in the nuclear transcriptional regulator NF-κB. This attenuated immunogenic profile is most likely linked to reduced levels of FliC, as described previously.72,73 Moreover, we attribute the more noticeable reduction observed for IL-1β (>2-fold), compared with TNF-α, to the additive effects derived from fliC repression, mediated by both the TLR-dependent pathway directly stimulated by fliC and the alternative TLR-independent secretion pathway through caspase-1 activation, which is also stimulated by flagellin.30
Conclusions
The results obtained in the present study provide evidence, for the first time in Salmonella Typhi, of a link between the MDR phenotype and increased levels of AcrAB/TolC caused by the hyperproduction of its regulator MarA. Moreover, we determined that when Salmonella Typhi acquires resistance to quinolones and other unrelated antibiotics it becomes less virulent. Concerning the hypothesis to explain this impaired virulence and host–pathogen interactions, our results rule out previously reported explanations such as acquisition of QRDR mutations or reduced growth. Our findings, particularly those obtained from Ty_c2, reinforce the idea that high efflux pump activity may affect quorum-sensing homeostasis, eventually leading to changes in virulence gene expression. Although more research is needed to clarify this phenomenon and consider also the situation reported for Ty_c1, our results contribute to the complex understanding of quinolone resistance and virulence. On the other hand, with the aim to explain the lower prevalence of ciprofloxacin-resistant Salmonella Typhi causing illness in the clinical setting, we hypothesize that, in comparison with susceptible bacteria, when quinolone-resistant Salmonella Typhi reaches the gut only a small proportion of the bacterial load is able to cross the mucosa and survive inside the host due to: (i) poor invasion of enterocytes due to a mild activation of the invasion machinery; (ii) reduced phagocytosis by dendritic cells and macrophages; and (iii) reduced survival rate inside macrophages. This situation, together with decreased activation of the innate immune response, may result in impaired dissemination of the resistant pathogen. However, the emergence of isolates able to overcome antibiotic pressure needs to be taken into account and efforts should be made to fight against them.
Funding
This study was supported by grant 2014SGR0653 from the Departament de Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya, by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, co-financed by European Regional Development Fund (ERDF) ‘A Way to Achieve Europe,’ the Spanish Network for Research in Infectious Diseases (REIPI RD12/0015), EUROSALUD (EUS2008-03616) and FIS 11/02024. We also thank the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica for the travel grant awarded to C. B.-D. that allowed her to perform the work at Columbia University of the City of New York. A. F. is sponsored by the Barcelona Institute for Global Health (ISGlobal).
Transparency declarations
None to declare.
Acknowledgements
We acknowledge Thomas Most for his advice on the macrophage infection procedure.
References



