-
PDF
- Split View
-
Views
-
Cite
Cite
Joel Guertin, Pavel Chrobak, Clémence Meunier, Cassandra M Thomson, Zaher Hanna, Paul Jolicoeur, HIV Nef disrupts Lck signaling by inducing aberrant phosphorylation of its substrates, ImmunoHorizons, Volume 9, Issue 6, June 2025, vlaf016, https://doi.org/10.1093/immhor/vlaf016
Close - Share Icon Share
Abstract
Human in vitro studies of HIV Nef on TcR proximal signaling have been controversial and have not provided an integrated picture of its impact. Tyrosine (Y) phosphorylation (pY) of Lck and its substrates (CD3ζ, Zap-70) was investigated in vivo, in Nef-expressing transgenic (Tg) thymocytes. In Tg cells, Lck was mis-localized and activated, but the pY-CD3ζ levels were unexpectedly lower, both constitutively and after anti-CD3ε Ab stimulation. Nef also favors the hyperphosphorylation of the Lck Y505 site and the accumulation of doubly phosphorylated (Y394, Y505) Lck. In contrast, after anti-CD3ε+anti-CD4 Ab stimulation, Nef decreased Lck activity and Lck was deprived of its pY partners. In Nef and LckY505F Tg thymocytes, Lck had similar activity but distinct LckY505 levels, Zap-70 pY phosphorylation, and Zap-70 activity, suggesting a different mode of Lck activation. Western blot analysis of Zap-70 with pY site-specific mAb showed modest enhanced levels of Zap-70pY292 and Zap-70pY493 (the latter required for its full activation) constitutively and after anti-CD3ε Ab stimulation, consistent with elevated Tg LATpY and suggesting a semiactive kinase. In fact, phenotypes of Nef Tg mice are very similar to those of mice harboring semiactive Zap-70 mutants. After anti-CD3ε+anti-CD4 stimulation, Tg Zap-70 activity and Zap-70pY493 levels were severely decreased, but Zap-70pY292 and Zap-70pY319 levels were barely affected, suggesting qualitative Lck defect. Rescue of Nef-mediated CD4+ T-cell loss with LckY505F in double (Nef × LckY505F) Tg mice correlated with greatly enhanced levels of Zap-70pY and Zap-70 activity. Thus, Nef impacts Lck in a unique way, triggering it to mis-phosphorylate its substrates.
Introduction
Single-positive (SP) CD4+ T cells are generated in the thymus through selection, lineage commitment, and differentiation of their precursors, the double-positive CD4+CD8+ thymocytes.1 This process is largely determined by T-cell antigen receptor (TcR) signaling that is initiated through interaction of CD4 or CD8 with MHC class II or class I, respectively. The cytoplasmic tail of CD4 and CD8 binds Lck, a member of the Src family of protein tyrosine (Y) kinases.2,3 Lck activity is largely determined by the phosphorylation of two Y residues, Y394 and Y505 (reviewed in4). Phosphorylation of Y394 favors, but phosphorylation of C-terminal Y505 negatively regulates, its activity.
Lck is a key player in TcR proximal signaling. Strong evidence indicates that it is activated by TcR engagement.5–7 Activated Lck initiates a cascade of tyrosine phosphorylation (pY) events leading to activation of several signaling pathways and the generation of an appropriate T-cell response. Briefly, activated Lck phosphorylates pY sites of its initial substrate, the cytoplasmic tail of the ITAMs of CD3 or ζ chains. Phosphorylated CD3ζ homodimers become a docking site for Zap-70, a cytoplasmic tyrosine kinase, which acquires a new conformation relieving its autoinhibition. Zap-70, recruited at the plasma membrane, is then phosphorylated by Lck at specific sites (Y292, Y315/318, Y493) leading to its full activation. Activation of Zap-70 leads to pY phosphorylation of one of its best characterized substrates, linker for activation of T cells (LAT). Subsequently, this pY phosphorylation cascade leads to the activation of downstream pathways, such as the MAPK pathway, cytokine production, and calcium mobilization.
HIV-1 infects human T-cell thymocytes8 and mature human CD4+ T cells,9 typically leading to their depletion. The consequences of HIV infection on TcR signaling were mainly studied in human T-cell lines or in circulating primary human CD4+ T cells in vitro. Among the HIV genes, nef has been the most investigated for its impact on TcR signalization, because of its profound effects reported early and its known interaction with members of the src-related kinases, with the highest affinity for Hck and Lyn.10 Nef was also found to bind to Lck,11–13 although not by all groups.14
Using distal readouts (NF-κB, NFAT, IL-2, CD69, actin remodeling) of TcR signaling in human cells, discordant results on the effects of Nef on this pathway were published (reviewed in 15,16), some groups finding activation17–23 and others reporting inhibition12,24–30 or no effect.31–33 Similarly, the impact of Nef expression on proximal TcR effectors, including Lck, led to discordant results (reviewed in 16), either activation23,34,35 or inhibition12,26–28,36–38 being reported or Nef effect depending on its intracellular localization39 or on the state of T-cell activation.17,18,21,40 Only a few groups studied pY phosphorylation of well-identified proximal TcR signal molecules in Nef-expressing human T cells. Nef was found to have either no effect on pLck,40 pLckY394, and pLckY505,22 or to favor accumulation of pLckY394,35 pZap-70Y319,23,35 and pTcRζY142,23 but decrease of pLck36 and Lck kinase activity.12 Many factors are likely to contribute to such discordant results in human T cells in vitro. The use of in vivo–infected human CD4+ T cells would bypass many of these problems, but we are unaware of such study.
However, it has been possible to investigate the impact of Nef on TcR signaling in an in vivo setting, using transgenic (Tg) animals expressing Nef in T cells, thus avoiding the use of transformed cells and in vitro manipulations for transducing nef. Nef-expressing peripheral CD4+ T cells of CD2/Nef41 and CD4C/Nef42,43 Tg mice or HIVΔGag/Pol Tg rats44 were reported to have a poor response to TcR engagement. Similarly, thymocytes of CD2/Nef Tg mice showed reduced proliferation after TcR stimulation.41,45 In contrast, thymocytes from CD3/Nef46 and CD4C/Nef42,47 Tg mice were hyperresponsive to TcR signals.
Our group used these latter CD4C/Nef Tg mice to study the involvement of Nef in the pathogenesis of AIDS in vivo. These Tg mice express Nef under the control of the human CD4 gene regulatory elements in cells that are targeted by HIV-1 in humans, ie immature and mature CD4+ T cells, macrophages, and dendritic cells.42 These mice develop a very severe AIDS-like disease that closely resembles human pediatric AIDS. In particular, they exhibit depletion of peripheral CD4+ T cells, associated with their activation and apoptosis and lower helper function.43,48,49 In addition, they show depletion of double positive (DP) CD4+CD8+ and single positive (SP) CD4+CD8- thymocytes by distinct mechanisms.47 Lck is activated in these Tg thymocytes both constitutively and after stimulation with anti-CD3ε mAbs.47 We could also document that LAT pY phosphorylation (a readout for Zap-70 activity) was enhanced following anti-CD3ε stimulation.42 Interestingly, CD4+ SP thymocyte depletion could be rescued by enhancing TcR affinity or preventing CD4 downregulation or by providing a constitutively activated LckY505F transgene.47 This latter result suggested that TcR signaling may be impaired in these Tg T cells, despite their elevated levels of activated Lck.
In the present work, we further analyzed the impact of Nef on the TcR signal pathway, in thymocytes of the CD4C/Nef Tg mice. Because limited information is available on Nef-mediated pY modifications of specific TcR proximal molecules in any of the human in vitro CD4+ T-cell models discussed above, we focused our study on pY phosphorylation of such specific TcR proximal effectors.
Materials and methods
Mice
The CD4C/Nef (previously designated as CD4C/HIVMutG or CD4C/HIVNef)42 and DLGF (line A16924)50 Tg mice have been described. The DLGF Tg mice express the constitutively active LckY505F mutant driven by the distal Lck promoter and were obtained from Dr. Jose Alberola Ila (Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma). Mice were bred on C3H (H-2k) background (Harlan Laboratories, Indianapolis, Indiana). Mice were kept in a specific pathogen-free animal facility. Animal studies followed guidelines set by the Canadian Council on Animal Care and were approved by the Clinical Research Institute of Montreal Animal Care Committee.
Abs and reagents
For flow cytometry, PE-, CyCh-, biotin-, allophycocyanin-, or FITC-conjugated Abs against mouse CD4, CD8, TCRβ, CD62, CD44, and human CD4, as well as isotypic control Abs rat IgG2a, rat IgG2b, Armenian hamster IgG1, streptavidin PE-, PE-Cy7-, or allophycocyanin-conjugated were purchased from Cedarlane Laboratories (Burlington, Ontario, Canada) or BD Biosciences (San Jose, California). Abs used for immunofluorescence (IF)/confocal microscopy were anti-Lck (3A5) (Santa Cruz Biotechnology, Santa Cruz, California), anti-Rab11A47 (Abcam, Cambridge, Massachusetts), anti-c-Cbl (7G10) (Upstate Biotechnology), and anti-HIV Gag p24 (384) (NIH AIDS Research and Reference Reagent Program). Purified (hybridoma) or biotinylated (BD Biosciences) anti-CD3ε (145-2C11), anti-CD4 biotin (GK1.5, BD Biosciences), and streptavidin (Zymed, San Francisco, California) were used for thymocyte stimulation. Abs used for immunoblotting were: anti-pY (4G10) (Upstate Biotechnology), anti-Cbl (clone 7G10) (Upstate Biotechnology), anti-Lck (3A5) mAb (Santa Cruz Biotechnology, Santa Cruz, California), anti-SrcpY416 (Cell Signaling Technology, Beverly, Massachusetts), anti-SrcpY505 (Cell Signaling Technology, Beverly, Massachusetts), anti-Zap-70 (clone 99F2) (Cell Signaling Technology, Beverly, Massachusetts), anti-CD3ζ (6B10.2) (Santa Cruz Biotechnology, Santa Cruz, California), anti-Zap-70pY292 (Invitrogen), anti-Zap-70pY319 (Cell Signaling Technology, Beverly, Massachusetts), anti-Zap-70pY493 (Cell Signaling Technology, Beverly, Massachusetts), anti-Nef clone NF2-B2 (NIH AIDS Research and Reference Reagent Program), anti-GAPDH (6C5) mAb (Abcam, Cambridge, Massachusetts), anti-actin (Sigma-Aldrich, St. Louis, Missouri), goat anti-mouse IRDye 800 (LI-COR, Lincoln, Nebraska), anti-mouse IgG (H+L) Alexa Fluor 680 and 555 (Cedarlane Laboratories) and IgG anti-rabbit (H+L) Alexa Fluor 680, 488, and 633. For Lck in vitro kinase assay (IVKA), anti-Lck (3A5) mAb and polyclonal anti-Lck serum were used for immunoprecipitation and Western blotting, respectively. PP2 inhibitor was from Calbiochem (San Diego, California). Cholera toxin (B subunit)–biotin was from Molecular Probes, 7-amino-actinomycin D (7-AAD) from eBioscience, and DAPI from Vector Laboratories, Inc. (Burlingame, California).
FACS analysis
Flow cytometry was performed as previously published.47 For intracellular Ag detection, a protocol from BD Biosciences was followed, using Cytofix/Cytoperm and Perm/Wash solutions (BD Biosciences). Acquisition was performed on a FACScan or FACSCalibur (BD Biosciences). Data were analyzed using the CellQuest Pro (BD Biosciences) software. Cell sorting was performed on a MoFlo cell sorter (DakoCytomation, Carpinteria, California).
Thymocyte stimulation in vitro
To assess tyrosine phosphorylation, thymocytes (50 × 106 cells/ml) were incubated in RPMI-1640 medium/5% fetal bovine serum with anti-CD3ε (145-2C11) biotin and/or anti-CD4 (GK1.5) biotin mAbs (both 10 µg/ml) on ice for 10 min. Subsequently, cross-linking was done at 37 °C for 5 min in the same medium containing prewarmed streptavidin (20 µg/ml) before cell lysis. Cell extracts were either directly analyzed by Western blot or immunoprecipitated with Ab. Immunoprecipitates were analyzed by Western blot.
Immunofluorescence and confocal microscopy
A technique similar to that previously published48 was used. Stimulated or unstimulated thymocytes (1 × 106) were washed 1× in PBS and fixed with 4% paraformaldehyde for 30 min at room temperature, recovered by centrifugation, washed 2× with PBS, resuspended in PBS 0.5% BSA and cytospotted onto glass slides by centrifugation. Cells were then permeabilized in PBS/0.1% Triton X-100 for 6 min, washed 2× with PBS and incubated in PBS 5% BSA for 1.5 h (for blocking) followed by incubation with primary Ab (diluted in PBS 2.5% BSA) for 1.5 h and washing 3× with PBS for 5 min. Fluorochrome-conjugated secondary Ab or cholera toxin (B subunit)–biotin (diluted in PBS 2.5% BSA) was added for 1 h before washing with PBS for 5 min and addition of DAPI and streptavidin Alexa Fluor 488 (for cholera toxin–treated slides). Immunofluorescence staining was analyzed by confocal microscopy using LSM 700, LSM 710 and Zen software.
Cell lysis and immunoprecipitation
Cells were lysed in a buffer containing 50 mM Tris-HCl pH 7.8, 150 mM NaCl, 1% Triton, 2 mM EDTA, 4 mM sodium orthovanadate (Na3VO4), 10 mM NaF, 1 mM PMSF and a protease inhibitor cocktail. Cell extracts were either directly loaded on SDS gels or subjected to immunoprecipitation with specific Ab for 2 h at 4 °C. Protein A-Sepharose (50 µl) was added to the protein/Ab mix (50 μl) and incubation continued for 30 min at 4 °C. Immunoprecipitates with Protein A-Sepharose were then collected by centrifugation (1,000 rpm), washed in lysis buffer, and loaded on SDS gels or washed for IVKA.
In vitro kinase assay
IVKA was carried out essentially as previously described.47 Briefly, the mix containing the target proteins, the Ab, and Protein A-Sepharose was first washed 1× in the “kinase extraction buffer” (KEB) (50 mM Tris-HCl pH 7.8, 150 mM NaCl, 0,5% Triton, 2 mM EDTA) at 4 °C and then 2X in the “kinase activation buffer” (KAB) (50 mM Tris-HCl pH 7.8, 100 mM NaCl, 0.5% Triton, 10 mM MgCl2) at 4 °C and collected by centrifugation. The pellet was resuspended in KAB containing [γ-32P]ATP and incubated for 6.5 min at room temperature. The reaction was stopped by transferring the tubes onto ice, and the pellet washed 1× in KEB before loading on SDS gels.
Western blot analysis
Immunoblot analysis was performed as reported previously.47 Protein extracts were loaded on 8% to 12% SDS gels. Separated proteins in gels were transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Massachusetts). Blocking was performed in 5% BSA at 4 °C. Incubation of membranes with primary Abs was done for 2 h at room temperature in TBS containing 0.1% Tween-20 and 2.5% BSA and was followed by incubation with secondary Abs coupled to Alexa Fluor 680 or 800. Signals were revealed using the Odyssey Infrared Imaging System (LI-COR, Lincoln, Nebraska).
Analysis of lipid rafts
Thymocytes (15 × 107) were washed in cold PBS and resuspended in 1 ml lysis buffer [50 mM HEPES, pH 7.6, 150 mM NaCl, 2 mM EDTA, 2 mM sodium orthovanadate (Na3VO4), 10 mM NaF, 1 mM PMSF, a cocktail of protease inhibitors, and 1% Brij 98] for 5 min at 37 °C, before being mixed with an equal volume of 80% sucrose in centrifuge tubes. Then, 2 ml 30% sucrose and 1 ml 5% sucrose were added on top for a total volume of 5 ml. Sucrose solutions were made with lysis buffer without detergent. Samples were centrifuged at 40,000 x g for 18 to 24 h in SW55Ti rotor at 4 °C; fractions (0.5 ml) collected, run on SDS gels; and GM1 ganglioside detected with cholera toxin (subunit B)–biotin/streptavidin.
Quantification
Protein bands were quantitated on the Odyssey LI-COR scanner using the Odyssey software and results normalized to control actin or GAPDH levels or to levels of total unphosphorylated specific protein (Lck, Zap-70) for analysis of pY proteins.
Statistical analysis
Statistical analysis was performed with the software GraphPad Prism using the paired 2-tailed Student’s t test. Results are expressed as mean ± standard deviation and represent at least 3 independent experiments. Significance differences are illustrated: *P < 0.05; **P < 0.01; ***P < 0.001.
Results
Nef interferes with proper Lck localization in CD4+ T cells of Nef Tg mice
Lck is bound to the CD4 cytoplasmic tail at the cell membrane and its activity is much influenced by its cellular localization,2 (reviewed in3). The downregulation of CD4 by Nef,51 including in Tg thymocytes,42 and the poor phosphorylation of Lck substrates in Tg thymocytes after anti-CD4 stimulation47 suggested that Lck may not be attached to the CD4 tail and that its localization may be altered by Nef. We investigated Lck localization in Tg CD4+ T cells, using IF confocal microscopy. In normal thymocytes, Lck was mainly detected at the plasma membrane, as GM-1, a membrane marker (Fig. 1A). In contrast, in Tg thymocytes, Lck tends to be less associated with GM1 and a large proportion of Lck is in the intracellular compartment (Fig. 1A) in ∼70% of the analyzed cells (Fig. 1B). Similar Lck intracellular accumulation, was also observed in Tg Nef-expressing peripheral CD4+ T cells (Fig. S1). These results confirm earlier reports on Nef-expressing Tg thymocytes45 or human T cells.22,27,38
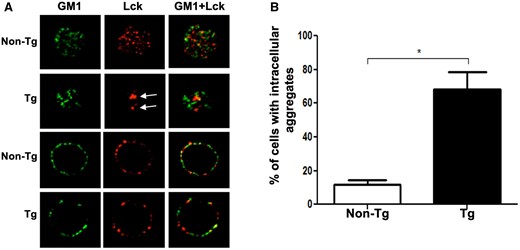
Nef interferes with proper Lck localization in thymocytes of Nef Tg mice. Immunofluorescence (IF)/confocal microscopy analysis of isolated thymocytes of Nef Tg and non-Tg mice. (A) Cells were labeled with anti-Lck (3A5) (red) Ab and the microdomain membrane marker, cholera toxin subunit B, GM1 (green). Images showed staining in electronic slices located near the pole (upper panels) and equator (lower panels) of the cell. Arrows show intracellular Lck accumulation. (B) Quantification of percentage of cells showing intracellular Lck accumulation in (A). At least 20 cells counted in each of 3 independent experiments. Statistical comparison was performed using the Student’s t test. *P <0.05.
Nef favors the hyperphosphorylation of the Lck pY505 site and the accumulation of doubly phosphorylated Lck
Lck is present in 4 different molecular forms in T cells: a primed inactive Lck dephosphorylated at Y394 and Y505, a closed-inactive form phosphorylated at Y505, but not at Y394, an active-open form phosphorylated at Y394 but dephosphorylated at Y505, and a di-phosphorylated active form (DPho-Lck [doubly phosphorylated Lck]) phosphorylated at both Y394 and Y505.52 We previously reported47 and confirm here that constitutive levels of Lck (Fig. 2A, time 0) and of total activated Lck (pLckY394) (Fig. 2B, time 0) were modestly enhanced (∼1.3- to 1.5-fold) in Tg thymocytes.
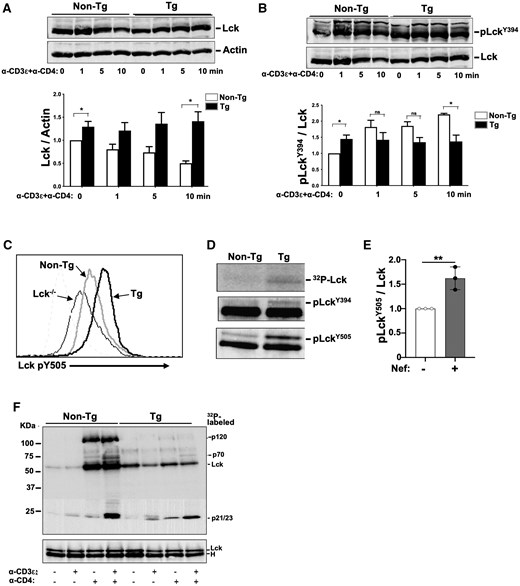
Higher Lck, pLckY394, and DPho-Lck constitutive levels, but impaired Lck degradation and activation in stimulated Nef Tg thymocytes. Isolated total thymocytes of Nef Tg and non-Tg mice were stimulated or not with anti-CD3ε or anti-CD4 or both anti-CD3ε+anti-CD4 Abs, for different times (0 to 10 min), as indicated, at 37 °C and analyzed. (A, B) Levels of total Lck proteins (A) or active phosphorylated Lck (pLckY394) (B) were determined respectively with anti-Lck (3A5) or anti-SrcpY416 (upper panels) Ab by Western blotting. Membranes were stripped and reacted with anti-actin (A) or anti-Lck (B) Ab (lower panels). For quantification (graphs), the intensities of Lck and pLckY394 were evaluated relative to those of actin or Lck, respectively, and compared to those in non-Tg mice (value = 1). Results from a single experiment are presented in panels A and B, and the same Western blot with anti-Lck Ab is shown in upper panel A and lower panel B, for clarity. Statistical comparisons were performed using the Student’s t test with data pooled from 3 independent experiments. *P < 0.05; ns, not significant. (C) Detection of LckpY505 levels. FACS analysis of unstimulated thymocytes from non-Tg (grey) and Nef Tg (black) mice. Cells were labeled with anti-CD4 and anti-CD8 Abs for extracellular cell surface staining and with phospho-specific anti-LckpY505 Ab for intracellular staining. Graph shows LckpY505-positive cells after gating on CD4+ T cells. Thymocytes from Lck-deficient non-Tg mice were used as negative controls. Note the enhanced levels of pY505 staining in Nef Tg thymocytes. Representative of 2 independent experiments. (D, E) Detection of DPho-Lck active form in Nef Tg and non-Tg thymocytes. Lck was first immunoprecipitated from unstimulated thymocyte extracts with anti-Lck, followed by a second immunoprecipitation with anti-pY505 Ab, and then analyzed in Western blot with anti-pY394 or anti-pY505 Ab and compared with 32P-Lck levels from IVKA from the same immunoprecipitates (D). For quantification (E), the intensity of the p-LckY505 band was evaluated relative to that of the total Lck band (ratio p-LckY505/Lck) and compared to that in non-Tg extract (value = 1). Data pooled from 3 independent experiments. Statistical comparison was performed using the Student’s t test, **P < 0.01. (F) Lck tyrosine kinase activity in unstimulated (–) and stimulated (+) (5 min) Tg and non-Tg thymocytes. Total protein extracts were reacted with anti-Lck (3A5) Ab and immunoprecipitates used for IVKA at room temperature. Proteins were run on SDS-PAGE and transferred to membrane for detection of 32P-labeled proteins (upper panel) and for Western blotting with rabbit polyclonal anti-Lck Ab (lower panel). Representative experiment out of 3 performed.
We next determined whether Nef favors the accumulation of specific Lck forms. FACS analysis with anti-pY505-specific Ab showed that pLckY505 was increased in Nef Tg relative to non-Tg thymocytes (Fig. 2C), a change also observed in CD4+ T cells of HIV Tg rats.44 Because DPho-Lck is also phosphorylated at Y505, its levels were measured. Under basal conditions, its levels were higher in Tg than in non-Tg thymocytes (Fig. 2D, E). Thus, Nef favors the hyperphosphorylation of the Lck Y505 site and a modest accumulation of DPho-Lck.
TcR-mediated Lck activation and degradation are impaired in Nef Tg thymocytes
We next studied the Lck fate after TcR stimulation. Upon stimulation with anti-CD3ε+anti-CD4 Abs, non-Tg Lck levels decreased by ∼50%, whereas the higher levels of Tg Lck remained unchanged or modestly increased (Fig. 2A), suggesting that Nef delays Lck degradation. Levels of pLckY394 increased in non-Tg thymocytes after stimulation, as expected, but remained unchanged in Tg cells (Fig. 2B). This suggests that Nef somehow prevents further Lck activation.
To directly assess the enzymatic activity of Lck, we used an IVKA, relying mainly on the autophosphorylation of the immunoprecipitated Lck as a readout, since Nef may prevent association of Lck with its partners (see below). We confirmed our previous findings47 that Lck activity was significantly increased in Tg relative to non-Tg unstimulated thymocytes (Fig. 2F, lanes 1 and 5). Similarly, anti-CD3ε Ab stimulation led to a small increase of Lck activity in Tg relative to non-Tg thymocytes, with the appearance of p23 band, likely CD3ζ (Fig. 2F). Anti-CD4 or anti-CD3ε+anti-CD4 Ab stimulation led to a large increase of Lck activity in non-Tg, but not in Tg thymocytes (Fig. 2F), likely resulting from Nef-mediated CD4 downregulation well documented on these Tg thymocytes.42 The extracts from non-Tg cells showed a major p56 Lck autophosphorylated band and 3 major substrates, p120, p70, and p21/p23 (Fig. 2F), likely representing c-Cbl, Zap-70, and CD3ζ, respectively, and known to bind to Lck.53–55 Interestingly, p120 and p70 were barely detectable in Tg cells, suggesting that Nef may prevent their phosphorylation by and/or binding to Lck (see below). These IVKA data are in concordance with the levels of pLckY394 described above (Fig. 2B), and with levels of other phosphorylated substrates detected by Western blotting (Fig. S2). The lower Lck activity observed in fully stimulated Nef Tg mouse cells has previously been observed by some investigators in HIV-infected or Nef-expressing human T cells in vitro.12,36,39
Nef enhances tyrosine phosphorylation of c-Cbl and alters its localization in Tg thymocytes
Our findings that Lck levels were higher and that its degradation was impaired in anti-CD3ε+anti-CD4 Ab-stimulated Tg thymocytes (Fig. 2A) led us to examine c-Cbl. Indeed, c-Cbl is the main negative regulator of Lck54 and its pY phosphorylation by Zap-70 is Lck dependent.56
We first measured the levels of c-Cbl by Western blotting with anti-Cbl Ab. Levels of c-Cbl were not significantly different in Nef Tg versus non-Tg thymocytes (Fig. 3A). We also studied the phosphorylation of c-Cbl by performing its immunoprecipitation followed by Western blotting with anti-pY Ab. This experiment revealed a 2-fold increase of constitutive c-CblpY in Tg compared to non-Tg thymocytes (Fig. 3B), confirming results in Jurkat cells.57 Upon stimulation by anti-CD3ε+anti-CD4 Ab, the levels of c-CblpY were significantly enhanced in Tg, but not as much as in non-Tg thymocytes (Fig. 3B), indicating that Tg cells remain responsive to anti-CD4 stimulation despite the downregulation of CD4 on their surface.42 This c-Cbl pY pattern is consistent with lower pLckY394 levels (Fig. 2B) and Lck activity (Fig. 2F) in doubly stimulated Tg cells, since low Lck levels have been reported to reduce c-Cbl pY phosphorylation.58 Thus, the sub-optimal levels of c-CblpY in stimulated Tg thymocytes may explain the delay of Lck degradation following stimulation (Fig. 2A).
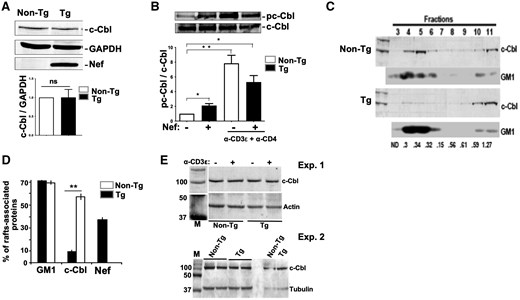
Alteration of c-Cbl phosphorylation and localization in Nef Tg thymocytes. (A) Levels of total c-Cbl and Nef proteins from Nef Tg and non-Tg thymocyte extracts were measured by Western blot with respectively anti-c-Cbl (7G10) or anti-Nef Ab. Protein loading was determined with anti-GAPDH Ab. For quantification (graph), the intensity of the c-Cbl band was evaluated relative to that of the GAPDH band (ratio c-Cbl/GAPDH) and compared to that in non-Tg extract (value = 1). Data pooled from 3 independent experiments. Statistical comparison was performed using the Student’s t test. ns, nonsignificant. (B) Total thymocytes of Nef Tg and non-Tg mice were stimulated or not with anti-CD3ε+anti-CD4 Abs for 5 min at 37 °C. Cell extracts were immunoprecipitated with anti-c-Cbl Ab (7G10), and phosphorylated c-Cbl (pc-Cbl) was measured by Western blot with anti-pY 4G10 Ab (upper panel). Protein loading was determined by evaluating the levels of precipitated c-Cbl with anti-c-Cbl Ab (lower panel). For quantification (graph), the intensity of pc-Cbl was evaluated relative to that of c-Cbl (ratio p-cCbl/c-Cbl) and compared to that in unstimulated non-Tg thymocytes (value = 1). Data pooled from 3 independent experiments. Statistical comparisons were performed using the Student’s t test. *P < 0.05; **P < 0.01. (C–E) Reduction of c-Cbl associated with lipid rafts in Nef Tg thymocytes. Plasma membranes from Tg and non-Tg thymocytes were prepared and fractionated by sucrose density gradient centrifugation, as described in Materials and methods. (C) Fractions were collected and either subjected to SDS-PAGE and Western blotting with anti-c-Cbl Ab or spotted onto nitrocellulose and probed for GM1. One experiment is shown. (D) Quantification of c-Cbl proteins (graph) in low-density gradient fractions 3 to 6, corresponding to lipid rafts, was determined relative to total proteins loaded onto the gradient. (E) Blotting of total thymocytes lysates with anti-c-Cbl (upper panels) and after stripping with anti-actin or anti-tubulin (lower panels) Abs. Note that equivalent levels of c-Cbl were found in Nef Tg (+) and non-Tg (–) thymocytes. Two independent experiments are shown. M, markers.
We next explored c-Cbl localization using a biochemical approach, assessing the presence of c-Cbl in GM1-positive lipid rafts. c-Cbl was dramatically decreased in lipid rafts of Tg relative to non-Tg thymocytes (Fig. 3C, D), although total c-Cbl levels were similar (Fig. 3A, E). Such mis-localization of c-Cbl is expected to affect its interaction with its partners and/or the ubiquitination of its substrates. In particular, the sustained high levels of Lck observed in Tg cells after anti-CD3ε+anti-CD4 Ab stimulation (Fig. 2A) may result from its poor proximity with c-Cbl, known to be responsible for its degradation.54,59
Nef enhances or decreases pY phosphorylation of several Lck substrates depending on the state of T-cell activation
In view of high constitutive Lck activity in Tg thymocytes, we investigated its pY substrates. We previously reported that a number of downstream TcR molecules (p37, p56, and p70) were constitutively hyperphosphorylated (as detected with anti-pY 4G10 Ab) in Tg relative to non-Tg thymocytes.47 We extended these findings here to p76, p95, and p120 proteins (Fig. S2). After anti-CD3ε, but not after anti-CD4, Ab stimulation, levels of all these phosphoproteins were also higher in Tg than in non-Tg cells (Fig. S2). However, after anti-CD3ε+anti-CD4 Ab stimulation, they all remained less phosphorylated in Tg than in non-Tg thymocytes (Fig. S2), consistent with previous results.47 Low response to anti-CD4 Ab stimulation likely reflects the downregulation of CD4 on Tg thymocytes.42 This pattern of phosphorylation correlates with that of the Lck activity described above (Fig. 2F), suggesting that Lck is mediating these pY phosphorylations directly or indirectly. Also consistent with this interpretation, PP2, a Src-kinase inhibitor, was found to reduce Lck autophosphorylation and the phosphorylation of most of its substrates, including Zap-70Y319, both in Tg and non-Tg thymocytes (Fig. S3), suggesting that TcR stimulation remains dependent on Lck in Tg T cells.
The effect of Nef on pY phosphorylation of CD3ζ appears to be different from that on other proximal Lck substrates
We next examined the phosphorylation of CD3ζ, one of the most proximal substrates of Lck,60 using Western blotting with anti-pY 4G10 Ab or immunoprecipitation of CD3ζ followed by immunoblotting with anti-pY 4G10 Ab. In absence of stimulation, CD3ζ p21 was surprisingly hypophosphorylated in Tg relative to non-Tg thymocytes (Fig. 4A, time 0). Upon stimulation with anti-CD3ε Ab, CD3ζ p21pY remained lower (Fig. 4A–C), whereas CD3ζ p23pY (Fig. 4A, B) was higher in Tg than in non-Tg cells. This latter CD3ε-mediated enhanced p23 phosphorylation could also be seen on IVKA of anti-Lck precipitates (Fig. 2F, lane 6). These quantitated data (Fig. 4D) confirm earlier results42,43 and suggest that the overactivated Lck in unstimulated and anti-CD3ε Ab-stimulated Tg cells is less efficient at phosphorylating its most proximal substrate (CD3ζ p21), but remains quite capable of overphosphorylating other substrates, such as p37 (LAT) (Figs. 4B, S2), p70 (Zap-70), p76, p95, p120 (c-Cbl)47 (Fig. S2), as well as c-Cbl (through Zap-70) (Fig. 3B) and Zap-70 (see below; Fig. 5C, D). This suggests a qualitative defect of Lck activity in the presence of Nef.
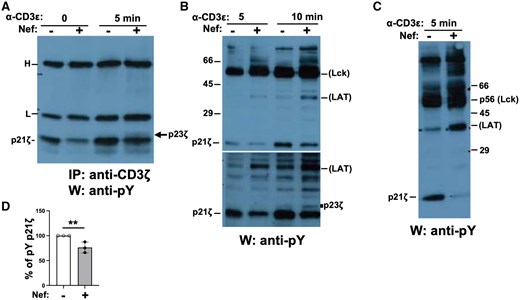
Decreased tyrosine phosphorylation of CD3ζ in Nef Tg thymocytes. Thymocytes of Nef Tg (+) and non-Tg (–) mice were stimulated or not with anti-CD3ε Ab. (A) Cell extracts were immunoprecipitated with anti-CD3ζ Ab, and phosphorylated CD3ζ detected in Western blot with anti-pY 4G10 Ab. H, L, heavy and light chains of immunoglobulin, respectively. (B, C) Total protein extracts of thymocytes were run on SDS-PAGE and transferred to membranes for Western blotting with anti-pY 4G10 Ab for detection of total phosphoproteins. Two independent experiments are shown. Note the increase of Tg p23 CD3ζ and p37 (likely LAT) and decrease of p21 CD3ζ phosphorylation after stimulation. This latter decrease was observed in 8 independent experiments. In (B), membrane was exposed for a short (upper panel) and longer (lower panel) time. (D) Quantification of phosphorylated (pY) p21 CD3ζ proteins (graph). Percentage of pY p21 CD3ζ levels in Tg cells relative to those in non-Tg cells (value = 100%) in 3 independent experiments. Student’s t test, **P < 0.01.
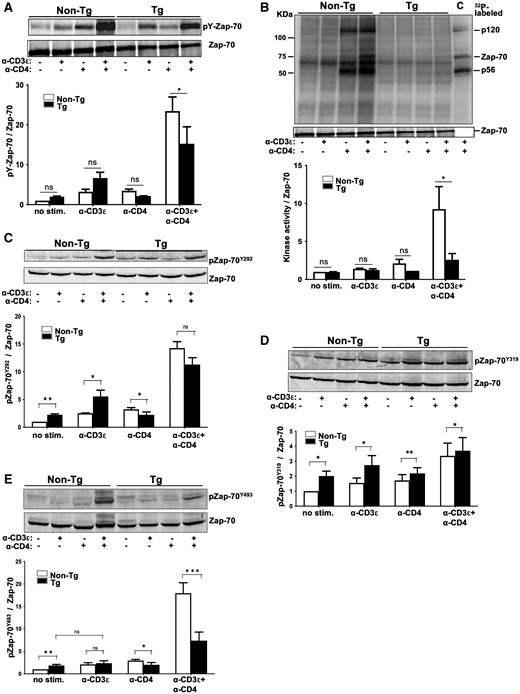
Altered tyrosine phosphorylation of Zap-70 in Nef Tg thymocytes. Total thymocytes of Nef Tg and non-Tg mice were subjected (+) or not (–) to stimulation with anti-CD3ε or anti-CD4 or both anti-CD3ε+anti-CD4 Abs for 5 min at 37 °C. (A) Cell extracts were immunoprecipitated with rabbit polyclonal anti-Zap-70 Ab and phosphorylated Zap-70 (pY-Zap-70) analyzed in Western blot with anti-pY 4G10 Ab (upper panel). For protein loading, membrane was stripped and reacted with anti-Zap-70 (99F2) Ab (lower panel). For quantification (graph), the intensities of pY-Zap-70 were evaluated relative to those of Zap-70 and compared to those in unstimulated non-Tg cells (value = 1). Data pooled from 3 independent experiments. Note that the pY-Zap-70 band shown (upper panel) is the same as the one shown in Fig. 6 and cropped from it. The Zap-70 band (lower panel) is also the same as the one shown in Fig. 6 and Fig. 5B, because the experiment was done on the same extracts. (B) Zap-70 kinase activity was measured, as described above in the legend of Fig. 2F, on rabbit polyclonal anti-Zap-70 Ab immunoprecipitates (same extracts as in Fig. 6), using an IVKA. 32P-labeled proteins, separated on SDS-PAGE, are shown in upper panel. Western blotting with anti-Zap-70 Ab (lower panel). Quantification (graph) was performed as described above in Fig. 5A. Data pooled from 3 independent experiments. C, control representing IVKA on anti-Lck immunoprecipitates of non-Tg thymocyte extracts. Note that the lower panel (Zap-70) is the same as the one in Fig. 5A and Fig. 6, because the experiment was done on the same extracts. (C–E) Zap-70 phosphorylation at Y292, Y319, and Y493 pY sites. Levels of phosphorylated pZap-70Y292 (C), pZap-70Y319 (D), and pZap-70Y493 (E) were determined with phospho-specific, site-specific anti-ZAP-70Y292, ZAP-70Y319, ZAP-70Y493 Abs, respectively (upper panels). Membranes were stripped and reacted with anti-Zap-70 (99F2) Ab (lower panels). Representative images from one out of three independent experiments. Quantification (graphs) was performed as described above in Fig. 5A, by pooling data from three independent experiments. Statistical comparisons were performed using the Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
Zap70 pY phosphorylation is altered in Nef Tg thymocytes
In normal cells, CD3ζpY binds Zap-70, which is then phosphorylated by Lck.60 The abnormal pY phosphorylation of CD3ζ in Nef Tg cells may impair its binding to ZAP-70 and the subsequent phosphorylation of Zap-70 by Lck. Thus, Zap-70 phosphorylation was investigated. The levels of total phosphorylated p70 (presumably Zap-70) (Fig. S2)47 or immunoprecipitated pY Zap-70 itself (Fig. 5A) were slightly higher in Tg than non-Tg unstimulated cells. Stimulation with anti-CD3ε or anti-CD4 Ab had little effect on pZap-70 levels (Fig. 5A). However, stimulation with anti-CD3ε+anti-CD4 Abs significantly enhanced pY Zap-70 levels in non-Tg cells, as expected, but intriguingly, also in Tg thymocytes, although at lower levels than in non-Tg cells (Fig. 5A). These enhanced Tg pY Zap-70 levels are in line with the enhanced p70 detected in doubly stimulated cells (Fig. S2), but contrast with the inability of such double stimulation to further enhance pLckY394 levels (Fig. 2B) and Lck activity (Fig. 2F) in Tg cells over what was observed with anti-CD3ε Ab stimulation alone. Thus, the anti-CD3ε+anti-CD4 Abs costimulation appears to especially favor phosphorylation of Zap-70, in absence of detectable enhanced Lck activity and despite downregulation of cell surface CD4 on Tg thymocytes.42
Because previous studies showed a correlation between Lck-dependent Zap-70 phosphorylation and its catalytic activity, following TcR/CD4 engagement,61 we determined whether pZap-70 levels reflect its kinase activity in Tg cells, by performing IVKA on anti-Zap-70 immunoprecipitates relying mainly on the autophosphorylation of the immunoprecipitated Zap-70 as a readout, since Nef seems to prevent association of Zap-70 with its partners (see below). In absence of stimulation or after anti-CD3ε or anti-CD4 Ab stimulation, Zap-70 activity was low and comparable in Tg and non-Tg thymocytes (Fig. 5B). Stimulation with anti-CD3ε+anti-CD4 Abs significantly enhanced Zap-70 activity (including its autophosphorylation) in non-Tg thymocytes, but only modestly so in Tg thymocytes (Fig. 5B), despite the quite high Tg pY Zap-70 levels after such stimulation (Fig. 5A). This apparent discordance (high pY Zap-70 levels but modest activity) indicates that its high pY content does not favor its activity and is unlikely to result from Zap-70 autophosphorylation and suggests that Zap-70 may not be phosphorylated properly by Lck in the presence of Nef.
Phosphorylation at some pY sites (Y292, Y315/Y319, Y493) are known to regulate Zap-70 catalytic activity.61,62 Phosphorylation at Y292 is involved in mitigating Zap-70 activity and specifically serves as recruitment for c-Cbl.63 Residue Y319, located in Zap-70 regulatory domain and binding Lck,64 becomes rapidly phosphorylated following TcR/CD4 engagement, an event relieving autoinhibition and promoting Zap-70 activity.65 Subsequently, Y493, located in the catalytic domain of Zap-70, is phosphorylated by Lck or transphosphorylated by Zap-70, an event which stabilizes Zap-70 in an open conformation favoring its full enzymatic activity.61,66,67 Using phospho-specific antibodies to each of these 3 pY sites, we next studied Zap-70 phosphorylation in thymocytes. Western blot analysis showed that levels of phosphorylated Zap-70 at all 3 sites were constitutively higher in Tg than in non-Tg thymocytes (Fig. 5C–E), consistent with higher activated Lck (Fig. 2B) and Lck activity (Fig. 2F), in unstimulated Tg cells. After anti-CD3ε Ab stimulation, higher levels of pY292 and pY319 pZAP-70 were measured in Tg than in non-Tg cells (Fig. 5C, D), as reported in Nef-expressing human Jurkat cells.68 However, pZAP-70Y493 in Tg cells remains intriguingly at similar levels as in non-Tg cells (Fig. 5E). After stimulation with anti-CD3ε+anti-CD4 Abs, levels of pZap-70Y493 were much lower in Tg than in non-Tg cells (Fig. 5E), as found with other Lck substrates, such as p70, p76, and p120, in these conditions (Fig. S2).47 Since the Zap-70 Y493 site can be phosphorylated by Lck and/or transphosphorylated by Zap-70,67,69 these low pZap-70Y493 levels are consistent with lower pLckY394 levels (Fig. 2B), Lck activity (Fig. 2F), and Zap-70 activity (Fig. 5B) in doubly stimulated Tg than in non-Tg cells. In contrast, upon the same anti-CD3ε+anti-CD4 Abs stimulation, the pY292 and pY319 sites were phosphorylated at nearly equal levels as in non-Tg cells (Fig. 5C, D). Thus, Nef appears to modify the phosphorylation of specific Zap-70 pY sites. This differential phosphorylation pattern of Zap-70 in the presence of Nef suggests a quantitative and qualitative impairment of Lck, the main enzyme responsible for Zap-70 pY phosphorylation.67 Such aberrant Zap-70 phosphorylation in doubly stimulated Tg cells, especially the much lower phosphorylation of Y493, may explain its sub-optimal activity (Fig. 5B), since phosphorylation of Zap-70 at Y493 is known to favor its activity.61,67 Also, the abnormal phosphorylation of Zap-70 may negatively affect its interaction with its partners.
Nef prevents Zap-70 from forming complexes with its partners
We next examined whether the altered Zap-70 phosphorylation would prevent its interaction with other phosphoproteins. The IVKA on anti-Zap-70 immunoprecipitates already suggested that p56 (likely Lck) and p120 were not bound to Zap-70 in Tg thymocytes as they are in non-Tg ones, after anti-CD4 or anti-CD3ε+anti-CD4 Abs stimulation (Fig. 5B). Also, only very small amounts of p70 (likely Zap-70) and p120 were found in the IVKA on anti-Lck precipitates of doubly stimulated Tg cells, relative to control non-Tg cells (Fig. 2F). To extend these results, we carried out Western blotting on Zap-70 immunoprecipitates from Tg and non-Tg thymocytes, using anti-pY (4G10) Ab to detect tyrosine phosphoproteins interacting with Zap-70. A number of easily detectable phosphoproteins (p120, p95, p76, and p56) were coprecipitated with Zap-70 and were best observed after stimulation with anti-CD3ε+anti-CD4 Abs in non-Tg samples, as expected, but were almost nondetectable in extracts from stimulated Tg cells (Fig. 6). The p120 band possibly represents c-Cbl, reported to associate with Zap-70.56 Likewise, p56 likely represents Lck, known to form a complex with pZap-70 in normal cells.53 In fact, p70 (likely Zap-70) was also detected in the IVKA anti-Lck precipitates of doubly stimulated normal thymocytes, but barely so in Tg ones (Fig. 2F).
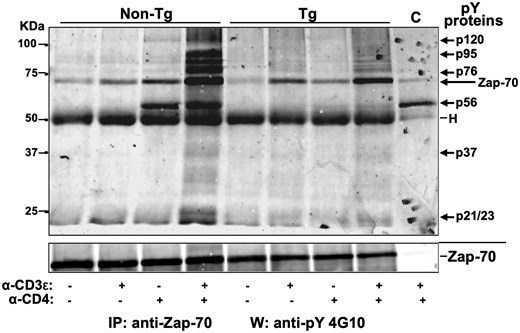
Nef prevents binding of tyrosine phosphorylated partners to Zap-70. Total thymocytes of Nef Tg and non-Tg mice were subjected (+) or not (–) to stimulation with anti-CD3ε or anti-CD4 or both anti-CD3ε+anti-CD4 Abs for 5 min at 37 °C. Cell extracts were immunoprecipitated with rabbit polyclonal anti-Zap-70 Ab (same extracts as in Figs. 5A and 5B) and the phosphorylated proteins co-immunoprecipitating with Zap-70 were analyzed in Western blot with anti-pY 4G10 Ab (upper panel). For protein loading, membrane was stripped and reacted with anti-Zap-70 (99F2) Ab (lower panel). Representative data for one out of three independent experiments are shown. C, control representing anti-Lck immunoprecipitates from non-Tg thymocytes reacted with anti-pY G410 Ab in Western blot. Note that the pY-Zap-70 band shown in upper panel is the same as the one shown in Fig. 5A after being cropped. The lower panel (Zap-70) is also the same as the one in Fig. 5A and Fig. 5B, because the experiment was done on the same extracts.
Thus, pY mis-phosphorylation of Zap-70 or of its partners may explain their failure to form complexes.
Despite similar constitutively elevated Lck kinase activity in Nef and in LckY505F Tg mice, phosphorylation of Zap-70 is higher in thymocytes of LckY505F Tg mice
Zap-70 is a major substrate of Lck.67,69 In view of the apparent pY mis-phosphorylation of Zap-70 (Fig. 5) and the enhanced levels of the DPho-Lck (Fig. 2D, E) in Nef Tg mice, we explored whether the higher Lck kinase activity in Nef Tg thymocytes was comparable to that of the classical active-open form of Lck. To do so, we directly compared pY phosphorylation of Lck and some of its substrates in Nef Tg and in mutant LckY505F (DLGF) Tg thymocytes. DLGF Tg thymocytes express low levels of Lck mutated and dephosphorylated at its Y505 site, thus representing an active-open form of Lck, phosphorylated at Y394 (LckY394). We previously studied these latter mice and showed that levels of Lck, activated LckY394, Lck kinase activity, and some pY phosphoproteins (p56, p80) were similarly elevated in LckY505F Tg and Nef Tg thymocytes relative to those in non-Tg mice.47 Here, we show that p-LckY505 levels were higher in LckY505F Tg than in Nef Tg thymocytes (Fig. 7A), but levels of DPho-Lck (Fig. 7B), of Lck aggregates (Fig. 7C), and of c-Cbl (Fig. 7D) were comparable in both Tg strains.
![Mutant LckY505F enhances LckY394 levels and decreases c-Cbl levels in Nef-expressing thymocytes. Thymocytes of single Nef, single LckY505F, and double (Nef × LckY505F) Tg and non-Tg mice were analyzed. (A) Detection of LckpY505 levels by FACS analysis. Unstimulated thymocytes from non-Tg (green) and Nef Tg (dark black), LckY505F Tg (red) and double (Nef × LckY505F) Tg (dotted line) mice were incubated with anti-CD4 and anti-CD8 Ab for extracellular cell surface labeling and with phospho-specific anti-pY505-Lck Ab for intracellular staining. Graph shows LckpY505-positive cells after gating on CD4+ T cells. Thymocytes from Lck-deficient (–/–) and normal non-Tg mice treated with H2O2 were used as negative and positive controls, respectively. Note the enhanced levels of LckpY505 staining in double Tg mice. (B) Detection of DPho-Lck active form. Lck was first immunoprecipitated from unstimulated thymocyte extracts with anti-Lck Ab, followed by a second immunoprecipitation with anti-LckpY505 Ab, and then analyzed in Western blot with anti-pSrcY416 or anti-LckpY505 Ab or subjected to an IVKA with 32P-γ ATP. Note that lanes 1 (non-Tg [–]) and 2 (Nef Tg [+]) are the same as those presented in Fig. 2D and are shown here for comparison. Representative data from one out of three independent experiments, with similar results, are shown. (C) Quantification of percentage of thymocytes showing intracellular Lck accumulation. Cells were labeled with anti-Lck Abs and assessed as described in legend to Fig. 1. (D) Levels of total c-Cbl and Nef proteins were determined by Western blotting with respectively anti-c-Cbl or anti-Nef Ab, and protein loading was determined with anti-GAPDH Ab. For quantification (graph), the intensities of c-Cbl were evaluated relative to those of GAPDH and compared to those in non-Tg mice (value = 1). Data pooled from 3 independent experiments. Note that the lanes 1 (non-Tg [–]) and 2 (Nef Tg [+]) are the same as those presented in Fig. 3A and are shown here for comparison. Statistical comparisons were performed using the Student’s t test. **P < 0.01; ns, not significant.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/immunohorizons/9/6/10.1093_immhor_vlaf016/2/m_vlaf016f7.jpeg?Expires=1750779928&Signature=l4AWe7CRk9AVKfNGX5M2F3bWh-QuPtyR8WmGgDhrWlKqUcSwFeOHdyfBkJRx1WvTQlvjUPXqgv1LRIIzgygxYYfwgJqmcVEvVNExe7k-E5APUbQYtScka8HJrRgn1ZGZze0kQd7eThPaOvW~8Hc-HDIe1tbOPyg8ZrsFFvYoHl-Op-x1sF9rIZWT4Jij~n4YGyqol-lOWHJcU3GkHbBNIYr9JDge~6Iw9MQKHlSTPvmh6w-~Ugi4nAfkeDpkGxGZ~PJAlMSRrQj-CENJ6VknWWFgt4W3ZWwc87SVEPC4Oq-ZtPmKyYF2z3JMB2mtIK8SNY72rMhTKaKoamaZ38jC1A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Mutant LckY505F enhances LckY394 levels and decreases c-Cbl levels in Nef-expressing thymocytes. Thymocytes of single Nef, single LckY505F, and double (Nef × LckY505F) Tg and non-Tg mice were analyzed. (A) Detection of LckpY505 levels by FACS analysis. Unstimulated thymocytes from non-Tg (green) and Nef Tg (dark black), LckY505F Tg (red) and double (Nef × LckY505F) Tg (dotted line) mice were incubated with anti-CD4 and anti-CD8 Ab for extracellular cell surface labeling and with phospho-specific anti-pY505-Lck Ab for intracellular staining. Graph shows LckpY505-positive cells after gating on CD4+ T cells. Thymocytes from Lck-deficient (–/–) and normal non-Tg mice treated with H2O2 were used as negative and positive controls, respectively. Note the enhanced levels of LckpY505 staining in double Tg mice. (B) Detection of DPho-Lck active form. Lck was first immunoprecipitated from unstimulated thymocyte extracts with anti-Lck Ab, followed by a second immunoprecipitation with anti-LckpY505 Ab, and then analyzed in Western blot with anti-pSrcY416 or anti-LckpY505 Ab or subjected to an IVKA with 32P-γ ATP. Note that lanes 1 (non-Tg [–]) and 2 (Nef Tg [+]) are the same as those presented in Fig. 2D and are shown here for comparison. Representative data from one out of three independent experiments, with similar results, are shown. (C) Quantification of percentage of thymocytes showing intracellular Lck accumulation. Cells were labeled with anti-Lck Abs and assessed as described in legend to Fig. 1. (D) Levels of total c-Cbl and Nef proteins were determined by Western blotting with respectively anti-c-Cbl or anti-Nef Ab, and protein loading was determined with anti-GAPDH Ab. For quantification (graph), the intensities of c-Cbl were evaluated relative to those of GAPDH and compared to those in non-Tg mice (value = 1). Data pooled from 3 independent experiments. Note that the lanes 1 (non-Tg [–]) and 2 (Nef Tg [+]) are the same as those presented in Fig. 3A and are shown here for comparison. Statistical comparisons were performed using the Student’s t test. **P < 0.01; ns, not significant.
We next studied Zap-70 phosphorylation in these Tg mice. For this analysis, we used anti-pY 4G10 Ab and site- and phospho-specific Abs, as described above (Fig. 5). Interestingly, the total pY phosphorylation of Zap-70 (Fig. 8A) and its phosphorylation at Y292 (Fig. 8B) and Y493 (Fig. 8C), but not at Y319 (Fig. 8D), were higher in LckY505F Tg than in Nef Tg thymocytes. Zap-70 kinase activity (measured by IVKA) was higher in thymocytes of LckY505F Tg than in Nef Tg thymocytes (Fig. 8E). Thus, with nearly identical levels of Lck activity in Nef and LckY505F Tg thymocytes, LckY505F shows nevertheless a higher capacity to phosphorylate Zap-70, in particular at pY493 whose phosphorylation is required for its full catalytic activity.61,66,67 These results suggest that p-LckY394, the major active-open mutated form present in LckY505F Tg mice, may not be the predominant active form in Nef Tg thymocytes. They also suggest that Dpho-Lck, which is elevated in Nef Tg mice (Fig. 2D, E), may be less efficient than LckY505F at phosphorylating Zap-70 properly, especially at the Y493 site, a result consistent with the low phosphorylation of Zap-70 Y493 detected in stimulated Nef Tg thymocytes relative to non-Tg ones (Fig. 5E).

Rescue of Nef-mediated major signaling thymic defects downstream of Lck by mutant LckY505F. Thymocytes of single Nef (+), single LckY505F (+), and double (Nef × LckY505F) Tg and non-Tg mice (–) were analyzed. (A) Cell extracts were immunoprecipitated with rabbit polyclonal anti-Zap-70 Ab and immunoprecipitates run on SDS-PAGE followed by Western blotting using phospho-specific anti-pY 4G10 (upper panel) or anti-Zap-70 (99F2) (lower panel) Ab. (B–D) Proteins from cell extracts were separated by SDS-PAGE and analyzed by Western blotting with phospho-specific, site-specific anti-pY292 (B), anti-pY493 (C), or anti-pY319 (D) Zap-70 Ab (upper panels). Protein loading was determined with anti-Zap-70 (99F2) Ab (lower panels). For quantification (graphs), the intensities of p-Zap-70 were evaluated relative to those of total Zap-70 and compared to those in non-Tg mice (value = 1). Data pooled from 3 independent experiments, except that shown in (A) performed twice. Statistical comparisons were performed using the Student’s t test. *P < 0.05; **P < 0.01; ***P < 0.001. (E) Zap-70 kinase activity. Total protein extracts were reacted with rabbit polyclonal anti-Zap-70 Ab, and immunoprecipitates were used for IVKA. Proteins were run on SDS-PAGE and transferred to membrane for detection of 32P-labeled proteins (upper panel) and for Western blotting with anti-Zap-70 (99F2) Ab (lower panel). Note that the anti-Zap-70 Ab lanes shown in lower panel are the same as those presented in Fig. 8A, because both experiments were carried out on the same extracts. Representative images of 2 experiments giving similar results.
Together, these results show that a failure of Lck to properly activate Zap-70 in the presence of Nef is a major signaling defect in Tg thymocytes.
Zap-70 phosphorylation defects in Nef Tg thymocytes can be rescued by expression of mutant LckY505F
We previously reported that the Nef-mediated impairment of CD4+ SP thymocyte maturation and depletion of peripheral CD4+ T cells could be reversed by LckY505F in double (Nef × LckY505F) Tg mice.47 The enhanced number of apoptotic (Fig. S4A, B) and activated effector/memory (CD62Llo CD44hi) (Fig. S4C) peripheral CD4+ T cells are also normalized in double Tg mice. Double Tg thymocytes harbor significantly higher levels of Lck, activated pLckY394, Lck activity, and pY phosphoproteins (p56, p80) than non-Tg or single Tg ones.47 We show here that levels of pLckY505 (Fig. 7A) and DPho-Lck (Fig. 7B) are also highly increased in double Tg thymocytes, DPho-Lck becoming a major form of Lck in these latter Tg cells.
Because c-Cbl is responsible for Lck degradation,54,59 we also explored its status in double Tg mice. Levels of c-Cbl were significantly decreased in thymocytes from double relative to single Tg mice (Fig. 7D). Thus, the enhanced Lck levels in double Tg thymocytes47 may result from decreased degradation caused by lower c-Cbl levels.
We next checked the impact of the very high Lck activity in double Tg thymocytes,47 on Zap-70 pY phosphorylation, using the same approach as described above (Fig. 5). In double (Nef × LckY505F) Tg thymocytes, levels of total Zap-70 pY phosphorylation (Fig. 8A), phosphorylation of the 3 Zap-70 sites (Y292, Y319, and Y493) (Fig. 8B–D), and Zap-70 kinase activity (Fig. 8E) were highly increased relative to those in single Nef or single LckY505F Tg or in non-Tg thymocytes.
Discussion
Nef promotes Lck mis-localization and accumulation of Dpho-Lck
We previously reported that levels of Lck, pLckY394, and Lck activity were constitutively increased in Nef Tg relative to non-Tg thymocytes.47 In the present work, we further studied Lck biology. Lck was found to be mis-localized in unstimulated Tg immature DP and mature CD4+ T cells, confirming earlier studies in human T cells22,27,28,38 and extending them to unmanipulated primary mouse thymocytes, a population not easy to study in humans. The Nef-mediated downregulation of CD4 in Tg CD4+ T cells42 most likely contributes to the intracellular redistribution of Lck, as Lck binds to CD4 cytoplasmic tail2 and may be outcompeted by Nef binding to CD4, as shown before.70,71 Mis-localization of Lck is likely to be important to promote its abnormal function. Indeed, CD4+ SP thymocyte maturation and peripheral CD4+ T-cell depletion induced by Nef were less severe in absence of CD4 downregulation, in Nef Tg mice expressing CD4 truncated of its intracellular domain (CD4tailless), as we previously reported.47 It is possible that Lck mis-localization also contributes to its activation, since the free Lck pool shows enhanced pY394 phosphorylation and kinase activity in some conditions.72
Another Lck abnormality associated with Nef expression was the enhanced phosphorylation of its Y505 site and the elevated levels of active DPho-Lck. Phosphorylation of LckY505 has long been thought to inhibit its activity.73 However, DPho-Lck is activated.52 Interestingly, the enhanced levels of DPho-Lck in Nef Tg thymocytes are reminiscent of the Nef-mediated activation of Hck, in absence of dephosphorylation of its pY501 site, and thus dually pY phosphorylated (at Y390 and Y501).74,75 Such Lck activation by Nef could not previously be observed in vitro,74 in Rat-2 fibroblasts14 or in Saccharomyces cerevisiae,76 suggesting that a T-cell milieu may be required for its occurence. Also, it may originate, by a positive feedback, from pY changes in Zap-70, in particular from the higher levels of pZap-70Y319 detected in these Tg cells (Fig. 5D). Indeed, pZap-70Y319 is a docking site for Lck64 and such association has been reported to promote Lck activation.67
High Lck activity in Nef-expressing thymocytes induces constitutive hyperphosphorylation of some, but not all, Lck substrates
As expected from the constitutive higher levels of activated Lck detected in Nef Tg thymocytes, our analysis revealed that known substrates of Lck showed higher pY phosphorylation in unstimulated Tg relative to non-Tg thymocytes. This was observed for Lck itself (autophosphorylation), for Zap-70 (at total pY and at its pY292, pY319, pY493 sites), for c-Cbl, and for other non-identified substrates (p36, p76, p95, and p120). Phosphorylations of Lck, ZAP-70, and c-Cbl (through Zap-7056) are known to be Lck-dependent in normal cells.6 This Lck dependency is also apparent in Tg cells, as PP2, an inhibitor of members of the Src family kinase and of Lck, abolished the Nef-induced hyperphosphorylation of most of them.
Interestingly however, an intriguing anomaly of the most proximal substrate of Lck, CD3ζ p21, was noted. Despite enhanced constitutive Lck activity, it was unexpectedly less phosphorylated in the presence of Nef, reminiscent of what was found in anti-CD3-stimulated Lck-depleted T cells.77 In these conditions, hypophosphorylated CD3ζ binds to novel effectors, such as Grb2/SOS, resulting in activation of Erk1/2.77 The high levels of activated pMAPK (Erk1/2) in Nef Tg thymocytes42 may be induced by a similar mechanism, although this remains to be established.
Lck activation by Nef appears to be different than its activation by dephosphorylation of its Y505 site
We reported previously that constitutive levels of activated Lck were comparable (as judged by pLckY394 levels and IVKA Lck activity) in Nef Tg and mutant LckY505F Tg thymocytes.47 Interestingly, comparison of Lck substrates showed higher levels of Zap-70pY, Zap-70pY292, Zap-70pY493 and of Zap-70 activity in mutant LckY505F Tg than in Nef Tg thymocytes (Fig. 8), suggesting that the Nef-mediated activation of Lck is distinct from its classical activation by Y505F mutation. It also suggests that Nef-induced Lck dysfunction cannot be attributed only to its high kinase activity similar in both Tg strains, but may reflect qualitative differences at phosphorylating specific pY sites (see below).
Unmasking additional Nef-mediated Lck abnormalities after stimulation of TcR/CD4 receptors
Upon α-CD3ε Ab stimulation, we previously reported that levels of a number of tyrosine phosphoproteins, including p70 (possibly Zap-70), p37,47 and LAT,42 were increased in Tg thymocytes. We confirmed and extended these findings here. However, upon α-CD3ε + α-CD4 Ab stimulation, all these substrates remain much less phosphorylated in Tg than in non-Tg cells. Also, after α-CD3ε or α-CD3ε + α-CD4 Ab stimulation, phosphorylation of Zap-70Y319 was higher in Nef Tg than in non-Tg thymocytes, but Zap-70Y493 was not, and showed a sub-optimal phosphorylation (Fig. 5), strongly suggesting that Lck exhibits a selective defect at phosphorylating Zap-70Y493 adequately in the presence of Nef. Consistent with our findings, pLckY394 and pZap-70Y319 were among the most enhanced following HIV infection of human cells in vitro, an effect mediated by Nef.35 Also, Nef was found to favor accumulation of pZap-70Y319 with endosomal Lck23 and to enhance pZap-70Y292 and pZap-70Y318 levels in the presence of Hck.68 The sub-optimal levels of Tg Zap-70 pY493 phosphorylation likely explains why ZAP-70 kinase activity is blunted in Tg thymocytes, since phosphorylation of Y493 is required for its full kinase activity61,66,67 (reviewed in4).
Interestingly, despite the documented downregulation of cell surface CD4 on Nef Tg thymocytes,42 stimulation with α-CD3ε + α-CD4 Abs unmasked additional Lck defects in Tg cells. First, some phosphorylated binding partners of Lck present in anti-Lck immunoprecipitates of stimulated non-Tg thymocytes (specially p70 [likely Zap-70] and p120 [likely c-Cbl]) were almost totally absent in Tg ones (Fig. 2F). Consistent with this latter result, p56 (likely Lck) was absent in anti-Zap-70 immunoprecipitates of Tg thymocytes, but present, as expected,53 in non-Tg ones (Fig. 6). Despite being stripped of its phosphorylated partners, Lck maintains its capacity to phosphorylate some of its substrates (Zap-70Y319 [Fig. 5D], p37, p95 [Fig. S2]). Second, Lck degradation is much delayed in doubly stimulated Tg thymocytes (Fig. 2A), likely reflecting lower p-Cbl levels, and inhibition of c-Cbl activity by Nef.78 Third, pLckY394, Lck kinase activity, p76, and p95 are much higher in doubly stimulated than unstimulated non-Tg cells (Fig. 2, S2), but not in Tg cells. Yet and paradoxically, some of the known Lck substrates (pZAP-70 [Fig. 5A], p21/23 [Fig. 2F], p37, p70, and p120 [Fig. S2]) are overphosphorylated in doubly stimulated, relative to unstimulated, Tg cells, despite comparable Lck activity. This indicates that Tg thymocytes remain responsive to anti-CD4 stimulation, despite CD4 downregulation. This latter Lck response may reflect the action of DPho-Lck which is overrepresented in Nef-expressing cells. Alternatively, TcR/CD4 engagement may unmask novel pY sites in these substrates in the presence of Nef and allow them to be hyperphosphorylated by the already high levels of activated Lck in Tg cells. However, despite Zap-70 overphosphorylation, Zap-70 kinase activity remained comparable in α-CD3ε + α-CD4-stimulated and unstimulated Tg thymocytes, consistent with the poor phosphorylation of Y493 in these conditions.
Together, these observations highlight the presence of a very selective qualitative defect of Lck in Nef Tg T cells, leading to mis-phosphorylation of its substrates, in particular of Zap-70.
Semiactive Zap-70 in Nef Tg mice and its possible involvement in the development of many immune phenotypes
Thus Zap-70, being a critical downstream effector of Lck, was expected to be activated in Tg cells. We were unable to detect an enhanced Zap-70 kinase activity relative to control by IVKA in unstimulated or anti-CD3ε-stimulated Tg thymocytes, possibly because of a too modest activation and the poor sensitivity of this method. However, phosphorylation of Zap-70Y493 (Fig. 5E), thought to be essential for its activation61,66,67 and that of its substrate c-Cbl56 (Fig. 3B), was elevated in unstimulated Tg thymocytes. Similarly, following anti-CD3ε stimulation, pY phosphorylation of LAT (a substrate of Zap-70 kinase activity), detected here as p37 (Fig. 4B, 4C, S2B) or previously with anti-LAT Ab42 was higher in Tg than non-Tg thymocytes. Together, these results suggest a modest activation of Zap-70 kinase activity in Tg cells.
This semiactive Zap-70 in Nef Tg CD4+ T cells is likely to be involved in the development of the phenotypes of these mice. Indeed, C57BL/6 mice harboring the Zap-70W131A or Zap-70W163C mutant allele, with a semiactive kinase, show increased constitutive TcR signaling,79,80 as Nef Tg mice42,47 (Figs. 2, S2) and develop many T- and B-cell phenotypes79,81 (reviewed in82) very similar to those reported in Nef Tg mice, namely decreased number of total and SP CD4+ T thymocytes and peripheral CD4+ T cells,42,47 cell-autonomous decreased of SP thymic and peripheral CD4+ T cells,47 increased of activated effector/memory43,83 (Fig. S4) and of apoptotic49,83 (Fig. S4) CD4+ T cells, decreased response of peripheral CD4+ T cells to Ag stimulation,43,48 rescue of SP CD4+ thymocytes by Bcl-2,49 enhanced Foxp3+ T reg cells,84 hypergammaglobulinemia, and autoantibodies.48 In addition, Zap-70W163C81 and Nef Tg mice share other phenotypes: tissue deposition of immunoglobulins,85,86 increase of serum IgM, of anti-DNA Ab, of PNA-binding spleen cells, of spleen B cells,48 and of IL-17-producing cells.87 The remarkable similarity of so many phenotypes suggests a common pathway, ie the presence of a semiactive Zap-70 in Nef Tg mice.
Moreover, Nef-mediated changes in proximal TcR effectors, including those of Zap-70, are expected to impact more distal effectors of TcR signaling. In line with this, we previously reported the activation of CD69,43,83,88 of MAPK,42 and of PAK2,89 the increased expression of FasL,49 and the decreased cell proliferation42 in Nef Tg thymocytes. These effectors are known to be distal to TcR activation.22,33,90–92
It is worth noting that the numerous and severe T-cell phenotypes observed in these Nef Tg mice seem to be induced by relatively modest increase of pY phosphorylation and/or activity of Lck and Zap-70. However, pY changes on the effectors studied are also qualitative, suggesting that such qualitatively-modified effectors may affect T-cell functions without necessarily having to be expressed at high levels. Consistent with our results, heterozygous Lck+/- mutant mice (2-fold decrease of endogenous Lck) show a consistent CD4 downregulation.93 And Tg mice expressing mutant LckY505F at the same or lower levels than endogenous levels exhibit a very profound loss of CD3+ thymocytes.94
Rescue of Nef-induced Lck defects by LckY505F, an active-open form of Lck
Because Lck showed defects in Nef Tg mice, we previously attempted to correct these defects by breeding Nef Tg with LckY505F (DLGF) Tg mice. Depletion of mature CD4+ T cells was almost completely rescued in double Tg mice, and their thymocytes showed much higher levels of Lck, LckY394, Lck activity than single Nef or single LckY505F Tg mice, possibly related to their TcRhigh phenotype.47 We show here that the high percentage of effector/memory CD62LlowCD44hi peripheral CD4+ T cells are normalized in double Tg mice. We also present evidence that constitutive levels of DPho-Lck are much increased and those of c-Cbl are largely decreased in double relative to single Nef or single LckY505F Tg mice. Such low levels of c-Cbl may explain the very high levels of active Lck,47 including DPho-Lck (Fig. 7B) in double Tg thymocytes, consistent with the accumulation of Lck observed in c-Cbl-deficient thymocytes.54,95 Other phenotypes of double (Nef × LckY505F) Tg mice (enhanced CD4+ T-cell selection and increased number of peripheral CD4+ T cells47) are also similar to those found in c-Cbl-deficient mice.96
Study of Zap-70 in this context was rewarding. In double Tg mice, levels of the 3 pY ZAP-70 sites studied and ZAP-70 activity are much higher than in single Nef and single LckY505F Tg mice. Sub-optimal ZAP-70 kinase activity being one of the major proximal defects documented in Nef Tg CD4+ T cells, it is tempting to suggest that the rescue of ZAP-70 activity, through higher Lck activity, may be largely responsible for the rescue of mature CD4+ T-cell loss in these double Tg mice. Activation of ZAP-70 has indeed been shown to be required for differentiation of DP into SP thymocytes.97,98 Moreover, as reported previously,47 loss of DP thymocytes was not rescued in double (Nef × LckY505F) Tg mice, despite high ZAP-70 activity, a finding consistent with maintenance of DP thymocytes in absence of Zap-7097 and with the involvement of the thymic stroma in their depletion in Nef Tg mice.47 Thus, Zap-70 is an interesting candidate, but the contribution of other effector(s) downstream of mutated LckY505F in the rescue of Tg phenotypes remains to be elucidated.
This study of double (Nef × LckY505F) Tg mice strongly suggests that LckY505F and Nef (or the activated Lck it mediates) cooperate. The molecular mechanism of this cooperation is unclear. The activated mutant Tg LckY505F may transphosphorylate the Y394 site of both endogenous Lck inactive forms, namely primed Lck (unphosphorylated at both Y394 and Y505) and closed Lck (unphosphorylated at Y394 but phosphorylated at Y505), thus converting them into active LckY394 and DPho-Lck, respectively. This scenario would explain the higher levels of DPho-Lck in thymocytes of these double Tg mice and is compatible with the ability of Lck to autophosphorylate its Y394 site in trans.99
Conclusion
Analysis of Lck and of several Lck-dependent tyrosine phosphorylation events in Nef Tg thymocytes revealed that Lck is constitutively activated, but paradoxically remains poorly activated in stimulated cells. Importantly, some of its phosphorylation activity are somehow perverted and redirected at abnormal sites (mis-phosphorylation). This leads to selective defects, both at the molecular level (hypophosphorylated CD3ζ p21, sub-optimal ZAP-70 kinase activity) and at the cellular level (block of SP CD4+ T-cell maturation).47 In CD4+ SP thymocytes, this cascade is very likely initiated by the mis-localization of Lck, partly the consequence of the Nef-mediated downregulation of CD4. The capacity of Lck to aberrantly phosphorylate some of its substrates in Tg thymocytes may be related to the higher levels of a unique form of Lck (DPho-Lck) detected. It probably also reflects its lower turnover. Indeed, mislocalization of c-Cbl, involved in its normal degradation, likely favors the Lck accumulation observed in these Tg cells. The decreased phosphorylation by Lck of p21 CD3ζ, one of its most proximal substrates, is likely of major importance, because pCD3ζ is thought to serve as a platform for the recruitment of Zap-70 and its subsequent phosphorylation and activation by Lck. We show here that Zap-70 activation is sub-optimal in Nef-expressing Tg thymocytes. However, Zap-70 activation is well restored in double (Nef × LckY505F) Tg thymocytes in which SP CD4+ T-cell maturation is also rescued, strongly suggesting that higher ZAP-70 activity is contributing to maturation of SP CD4+ T cells in these double Tg mice. Thus, Nef appears to critically affect Zap-70 functions, likely as a consequence of its effects on Lck.
Although our results are similar to some findings in Nef-expressing human T cells, as noted above, full parallels remain difficult to establish because studies in human T cells on the effects of Nef on pY phosphorylation of Lck and its substrates have been contradictory/controversial (see Introduction). These discordant results are likely to be related to the different T cells used in each laboratory. These are derived from tumors or cultured from different patients with distinct stimuli for various times and subjected to different nef transducing protocols. Our work highlights the importance to work with a single source of Nef-expressing primary T cells that do not require gene transduction. It also emphasizes the critical role of stimulation (with anti-CD3ε, anti-CD4, or anti-CD3ε+anti-CD4 mAbs) of such primary T cells on effector responses. In view of the high conservation of proximal TcR signaling, including of Lck and many of its effectors, in mouse and human,100 Nef would be expected to have very similar effects in primary human CD4+ T cells as those described here.
Acknowledgments
We thank Dominic Filion (Microscopy and Imaging Core), Eric Massicotte, Julie Lord, Martine Dupuis (Flow Cytometry Core) for assistance in microscopy/image analysis and cell sorting/flow cytometry, respectively. We are grateful to Drs. Dominique Davidson and André Veillette for reagents (rabbit polyclonal Abs). We thank Ginette Massé, Benoît Laganière, and Nathalie Bouchard for excellent technical assistance. We also thank Stéphanie Lemay, Isabelle Corbin, Raphael Moreault-Truchon, and Jean-Rene Sylvestre for taking care of mice.
Author contributions
Joel Guertin (Conceptualization [Equal], Data curation [Lead], Formal analysis [Lead], Investigation [Lead], Methodology [Lead], Validation [Lead], Writing—original draft [Lead]), Pavel Chrobak (Investigation [Supporting], Methodology [Equal], Validation [Supporting], Writing—original draft [Supporting]), Clémence Meunier (Data curation [Supporting], Formal analysis [Supporting], Investigation [Supporting], Methodology [Supporting], Validation [Supporting], Visualization [Supporting]), Cassandra M. Thomson (Data curation [Supporting], Formal analysis [Supporting], Investigation [Supporting], Validation [Supporting]), Zaher Hanna (Conceptualization [Equal], Data curation [Supporting], Formal analysis [Supporting], Supervision [Equal], Writing—original draft [Equal]), and Paul Jolicoeur (Conceptualization [Lead], Funding acquisition [Lead], Project administration [Equal], Supervision [Supporting], Writing—review & editing [Lead])
Supplementary material
Supplementary material is available at ImmunoHorizons online.
Funding
This work was supported by grants from the Canadian Institute of Health Research to P.J. (MT-10313) and Z.H. (191117).
Conflicts of interest
The authors have declared that no conflict of interest exists.
Data availability
The relevant data are available in the article and its supplementary materials. Additional information and requests for reagents used in this study are also available from the corresponding author.



