-
PDF
- Split View
-
Views
-
Cite
Cite
Qilin Yu, Xiaohui Ding, Bing Zhang, Ning Xu, Chang Jia, Jiwei Mao, Biao Zhang, Laijun Xing, Mingchun Li, Inhibitory effect of verapamil on Candida albicans hyphal development, adhesion and gastrointestinal colonization, FEMS Yeast Research, Volume 14, Issue 4, June 2014, Pages 633–641, https://doi.org/10.1111/1567-1364.12150
Close - Share Icon Share
Abstract
Candida albicans morphogenesis and gastrointestinal colonization are closely associated with the pathogenicity of this pathogen. This study investigated the in vitro and in vivo effect of verapamil, a calcium channel blocker, on these processes. Exposure to ≥ 10 μg mL−1 verapamil led to a significant decrease of C. albicans hyphal cells. The ability to adhere to a polystyrene surface and buccal epithelial cells was inhibited by exposure to ≥ 20 μg mL−1 verapamil. Detection of the Hwp1–green fluorescent protein fusion protein showed that verapamil inhibited expression and transport of Hwp1, indicating its activity against both the regulation network of morphogenesis-associated proteins and the secretory pathway in C. albicans. Moreover, treatment with verapamil at 10 mg (kg day)−1 led to a remarkable decrease in gastrointestinal-colonizing fungal cells. This study revealed the inhibitory effect of verapamil on C. albicans hyphal development, adhesion and gastrointestinal colonization, which is relevant to decreased expression and abnormal transport of the proteins required for morphogenesis. Therefore, verapamil may be taken into account when choosing an antifungal therapy against C. albicans colonization and infection.

Verapamil inhibits Candida albicans morphogenesis, adhesion and gastrointestinal colonization, and influences both expression and transport of morphogenesis-related proteins.
Introduction
Candida albicans is one of the most prevalent fungal pathogens. It can cause not only superficial mucosal candidiasis, but also life-threatening systemic infections in immunocompromised patients (Klepser, 2006; Odds et al., 2007). In particular, C. albicans colonization is frequently a risk in intensive care unit (ICU) patients, and infections caused by this pathogen in this population are of growing interest (Charles et al., 2005). An important feature of this pathogen is its ability to switch between different morphological forms, including yeast, pseudohyphae and true hyphae (Sudbery, 2011). Abundant evidence has shown that the ability to make morphogenetic switches is involved in adhesion and invasion of host tissues, and is a crucial virulence attribute of this pathogen (Gow et al., 2011). Besides being an opportunistic pathogen, C. albicans is commonly found in the gastrointestinal tract (Odds, 1987), which is thought to be a frequent source of invasive candidiasis (Cole et al., 1996; Blijlevens et al., 2002). To achieve efficient gastrointestinal colonization, this fungus possesses complicated adaptation capacities to sense host niches, adhere to the host mucosal surface and successfully colonize this unique environment. All of these capacities are also related to morphogenetic processes (White et al., 2007). Therefore, strategies that inhibit morphogenesis of this fungus may also have an impact on relevant physiological processes, such as adhesion, colonization and invasion of host tissues, and may be used for antifungal drugs against its infections.
Candida albicans morphogenetic processes depend on its mechanisms of sensing and responding to environmental cues by sophisticated signal transduction pathways leading to expression of morphogenesis-related genes, and functions of the products encoded by these genes (Gow et al., 2011; Sudbery, 2011). Among these functional factors, Hwp1, a well-known morphopathogenic determinant, plays an important role in morphogenesis, including hyphal development, biofilm formation, adhesion and mating (Daniels et al., 2003; Nobile et al., 2006). During these processes, Hwp1 is highly expressed, linked to the glycosylphosphatidylinositol anchor in the endoplasmic reticulum (ER) and transported to the cell surface by the secretory pathway (Chaffin, 2008; Plaine et al., 2008; Gaillardin et al., 2008). Hence, deficiency in Hwp1 expression or transport will influence the morphogenetic ability of this fungus.
In C. albicans, calcium signalling functions not only in stress response, but also in morphogenesis (Sanglard et al., 2003; Bates et al., 2005; Wang et al., 2011), which is dependent on the calcium homeostasis system (Karababa et al., 2006). Our previous studies also revealed that several members of the calcium homeostasis system, such as the high-affinity calcium influx system (HACS, Cch1–Mid1 complex) and Spf1, play an important role in morphogenesis (Yu et al.,2012a , b). These findings indicated that agents interrupting the calcium homeostasis system have potential inhibitory effects on C. albicans morphogenesis.
Verapamil, a typical calcium channel blocker of the phenylalkylamine class, is widely used in the treatment of angina pectoris and hypertension (Strigun et al., 2011). This drug, as an L-type voltage-gated calcium channel (VGCC) blocker, was shown to partially inhibit the function of the HACS, resulting in decreased calcium influx under normal growth conditions in Saccharomyces cerevisiae (Teng et al., 2008). A recent study further revealed that it also inhibits C. albicans calcium uptake when the cells are confronted with alkaline stimulus, indicating its activity against calcium homeostasis in fungal cells (our unpublished data). Most importantly, this drug has in vitro activity against C. albicans biofilm formation and maintenance (Yu et al., 2013). Because of the relationship among biofilm formation, hyphal development, adhesion and colonization, we evaluated the effect of verapamil on these processes, and investigated whether this effect is associated with abnormal expression and transport of Hwp1, the factor related to morphogenesis.
Materials and methods
Strains
Strains used in this study are listed in Table 1. For construction of strain NKF152 for indicating expression of the morphogenesis-associated gene HWP1 and Hwp1 localization, a GF-URA3-FP cassette was amplified from plasmid pMG2082 (Gerami-Nejad et al., 2009) with the following primers: 5′-AGGAAGCTCTTATTCAAAAGAGATCTTATGATTACTATCAAGAACCATGTGATGATTACCCACAACAATCTAAAGGTGAAGAATTATTC-3′, 5′-TGTAGAAATAGGAGCGACACTTGAGTAATTGGCAGATGGTTGCATGAGTGGAACTGATTCTAATGTAGTTTTGTACAATTCATCCATAC-3′. This cassette was then transformed into strain DAY1 (Wilson et al., 1999), to give strain NKF151 in which the incomplete copy of GFP flanking the URA3 marker was inserted at the HWP1 locus. Cells of strain NKF151 were cultured on SC medium plates (0.67% yeast nitrogen base, 0.2% amino acid mixture, 2% glucose, 2% agar) containing 0.1% 5-fluoroorotic acid, generating strain NKF152. In this strain, the URA3 sequence was deleted, and a functional GFP gene was consequently in-frame with the HWP1 gene (Gerami-Nejad et al., 2009). In addition, expression of the fusion gene HWP1–GFP is controlled by the HWP1 promoter.
| Strain | Genotype | Source |
| SC5314 | Wild-type | American type culture collection |
| DAY1 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Dana Davis (Wilson et al., 1999) |
| NKF151 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GF-URA3-FP | This study |
| NKF152 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GFP | This study |
| Strain | Genotype | Source |
| SC5314 | Wild-type | American type culture collection |
| DAY1 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Dana Davis (Wilson et al., 1999) |
| NKF151 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GF-URA3-FP | This study |
| NKF152 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GFP | This study |
| Strain | Genotype | Source |
| SC5314 | Wild-type | American type culture collection |
| DAY1 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Dana Davis (Wilson et al., 1999) |
| NKF151 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GF-URA3-FP | This study |
| NKF152 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GFP | This study |
| Strain | Genotype | Source |
| SC5314 | Wild-type | American type culture collection |
| DAY1 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG | Dana Davis (Wilson et al., 1999) |
| NKF151 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GF-URA3-FP | This study |
| NKF152 | ura3Δ::λimm434/ura3Δ::λimm434 his1::hisG/his1::hisG arg4::hisG/arg4::hisG HWP1-GFP | This study |
Hyphal induction
Candida albicans hyphae were induced in liquid RPMI-1640 medium. Overnight cultured cells of strain SC5314 or NKF152 were harvested, washed with phosphate-buffered saline (PBS), and diluted with RPMI-1640 medium to an optical absorbance at 600 nm (A600 nm) of 0.1. Verapamil was then added at 0, 10, 20, 40, 80, 160, 320 or 640 μg mL−1. The cell suspensions were cultured at 37 ℃ with shaking for the indicated times. Cells were centrifuged, fixed with 4% formaldehyde and observed with a light microscope (Olympus). Both hyphal cells and total cells were counted, and the percentage of hyphal cells was calculated as the number of hyphal cells divided by the number of total cells. At least 10 microscopic fields were detected.
Adhesion to polystyrene
Overnight cultured cells were diluted with RPMI-1640 medium as described above, and the suspension was added into 24-well polystyrene microplates. Verapamil was supplemented into the wells as in hyphal induction. The plates were incubated at 37 ℃ for 4 h, washed with PBS buffer, and stained using 0.1% crystal violet for 5 min. The wells were then washed again with PBS buffer, and 500 μL of 10% acetic acid was added to extract the dye. The optical absorbance at 595 nm (A595 nm) of the extract was determined using a spectrophotometer (Bio-Rad). The percentage of adhering cells were calculated as the A595 nm of each group divided by the A595 nm of the control group × 100%. The cells adhering to the well surface were also examined by light microscopy.
Adhesion to buccal epithelial cells (BECs)
Adhesion to BECs was assessed according to Bates et al. (2005). BECs were collected from healthy individuals, washed with sterilized physiological saline and suspended in the same solution at 2.5 × 105 mL−1. Cells of C. albicans strain SC5314 or NKF152 were cultured overnight in modified NGY medium (0.1% peptone, 0.1% yeast extract, 0.4% glucose), harvested, washed with saline and suspended in the same solution at 2.5 × 106 yeast cells mL−1. Then, 100 μL of BECs and 100 μL of yeast cells were mixed. Verapamil was added to the mixtures as described for hyphal induction. The mixture was incubated with shaking at 30 ℃ for 1 h, washed with saline, fixed with 4% formaldehyde and observed. The percentage of fungus-adhered BECs was calculated as the number of fungus-adhered BECs divided by the number of total BECs × 100%.
Quantification of green fluorescent protein (GFP) fluorescence
Fluorescence intensities of the fusion protein Hwp1–GFP expressed in strain NKF152 were detected to assess expression levels of HWP1. Hyphae of NKF152 were induced for the indicated time, collected, washed with PBS buffer and fixed with 4% formaldehyde. The fluorescence intensities (excitation wave 488 nm, emission wave 520 nm) of the hyphal suspension were determined using a fluorescent microplate reader (PerkinElmer).
Fluorescence microscopy
NKF152 hyphal cells in RPMI-1640 or yeast cells adhering to BECs were fixed. The localization of Hwp1–GFP was observed by fluorescence microscopy (Olympus) with the GFP filter set.
Measurement of gastrointestinal colonization
Gastrointestinal colonization of strain SC4314 was assessed by a modified method of White et al. (2007). Candida albicans cells were cultured overnight in liquid YPD medium, washed with sterilized saline, and adjusted with the same solution to 2.5 × 108 cells mL−1. Forty female Institute of Cancer Research mice (18–20 g) were equally divided into four groups, two antibiotic-free groups and two antibiotic-treated groups. In the antibiotic-free groups, the mice were inoculated with 200 μL of yeast cells by oral gavage, and then treated without verapamil (the antibiotic-free control group) or with verapamil [10 mg (kg day)−1, the verapamil group] in their drinking water throughout the experiment. In the antibiotic-treated groups, the mice were treated with streptomycin (2 mg mL−1), tetracycline (1 mg mL−1) and gentamycin (0.1 mg mL−1) in their drinking water throughout the experiment beginning 3 days prior to yeast inoculation. After being inoculated with yeast cells, the mice were treated without verapamil (the antibiotic-treated control group) or with verapamil as the antibiotic-free groups. To monitor gastrointestinal colonization, fresh fecal pellets (produced within 5 min prior to collection) were collected on the 7th and 14th days, and C. albicans concentrations in the pellets were measured by plating pellet homogenates on YPD SAK plates (1% yeast extract, 2% peptone, 2% glucose, 2% agar, 100 μg mL−1 streptomycin, 100 μg mL−1 ampicillin and 50 μg mL−1 kanamycin). The animal experiments were approved by the Institutional Animal Care and Use Committee of Nankai University.
Statistic analysis
Each experiment was performed with five replicates for each treatment. The effect of verapamil on hyphal development, adhesion and colonization was compared with the control (no verapamil treatment), and the significance of the difference (P < 0.05) was assessed using Student's t-test using spss v19.0 software (IBM).
Results
Verapamil inhibits C. albicans hyphal development
Our previous study revealed that disruption of the genes encoding calcium channels resulted in abnormal hyphal formation of C. albicans. As verapamil is a potential calcium channel blocker, we evaluated the inhibitory effect of this drug on hyphal development. When induced for 2 h, 95% of cells formed true hyphae in liquid drug-free RPMI-1640 medium. In contrast, only 70% of cells produced hyphae by exposure to 10 μg mL−1 verapamil (Fig. 1). Increasing the concentration of verapamil resulted in drastic reduction of hyphal cells, and the percentage of hyphal cells deceased to < 15% by exposure to 320 μg mL−1. Similarly, when induced for prolonged periods, treatment of verapamil with the tested concentrations led to a significant decrease of hypahl cells as compared with the control. Notably, the proportion of hyphal cells decreased from 60% to only 20% by exposure to 10 μg mL−1 verapamil for 6 h (Fig. 1). Therefore, verapamil showed a remarkable inhibitory effect on C. albicans hyphal development.
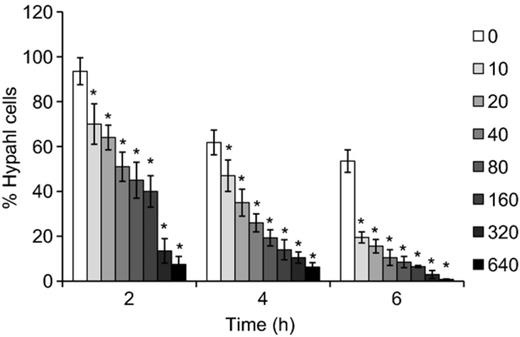
Verapamil inhibits Candida albicans hyphal development. Candida albicans cells were incubated in RPMI-1640 medium containing different concentrations of verapamil (μg mL−1). Both the total cells and the hyphal cells were counted for the indicated times. *Significant difference between the control group (0) and the verapamil-treated groups (P< 0.05).
Verapamil inhibits C. albicans adherence
Another factor that allows C. albicans to invade host tissues is its ability to adhere to host cells. Here we further assess the effect of verapamil on C. albicans adherence to polystyrene and BECs. When incubated on the surface of polystyrene microplates, 20 μg mL−1 or higher concentrations of verapamil resulted in a significant decrease of cell attachment. A strong negative correlation was seen between verapamil levels and the biomass of adhering cells. The proportion of adherent cells reduced from 65% to 10% as the concentrations of verapamil increased from 20 to 640 μg mL−1 (Fig. 2a). Exposure of verapamil also led to a significant decrease of C. albicans attachment to BECs when the drug concentration reached 20 μg mL−1. Interestingly, the proportion of adhering BECs remained c. 30% as the verapamil concentrations increased from 80 to 640 μg mL−1 (Fig. 2b). Moreover, while most of the control cells adhered to the well surface by hyphae, abundant adherent fungal cells exposed to verapamil displayed yeast-like morphology, indicating that this drug also inhibits hyphal development during adhesion. Thus, verapamil also inhibits C. albicans adherence to both polystyrene and host epithelial cells.
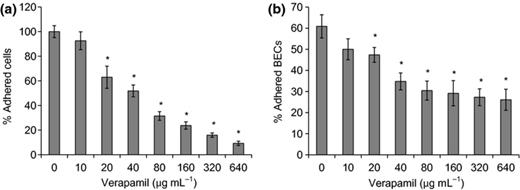
Verapamil inhibits adherence of Candida albicans to polystyrene surface (a) and BECs (b). (a) Candida albicans cells were suspended in RPMI-1640 medium with different levels of verapamil, and added to polystyrene microplates. The biomass of C. albicans adhering to the polystyrene surface was quantified by crystal violet staining. The percentage of adherent cells were calculated as the A595 nm of each group divided by the A595 nm of the control group (0) × 100%. (b) BECs and C. albicans were coincubated for 1 h, and fungus-adhered and total BECs were counted. The percentage of fungus-adhered BECs was calculated as the number of fungus-adhered BECs divided by the number of total BECs × 100%. *Significant difference between the control group (0) and the verapamil-treated groups (P< 0.05).
Verapamil inhibits expression of HWP1 associated with hyphal development
Studies have suggested that the adhesin HWP1 is closely associated with hyphal development and adhesion in C. albicans, and is upregulated during these processes. Therefore, we tested if the inhibitory effects of verapamil on morphogenesis are relevant to its abnormal expression. We tagged Hwp1 with GFP, and evaluated its expression levels by GFP fluorescence assays. Cells treated with ≥ 20 μg mL−1 verapamil had significantly lower fluorescence density than the control within the tested time. Notably, GFP fluorescence decreased by 50% when the concentration of verapamil increased to 160 μg mL−1 (Fig. 3). This result demonstrated that verapamil indeed inhibits the expression of HWP1 under hyphal induction, which may contribute to its inhibitory effect on morphogenesis.

Verapamil inhibits expression of HWP1. Strain NKF152 was incubated in RPMI-1640 medium containing different concentrations of verapamil (μg mL−1), and GFP fluorescence density of the culture was determined for the indicated times. *Significant difference between the control group (0) and the verapamil-treated groups (P< 0.05).
Verapamil inhibits Hwp1 transport
Hwp1, as a hyphal cell-wall protein, has to be transported to the cell surface through the secretory pathway for its functions during cell-surface adhesion. Using the Hwp1–GFP system, we further investigated whether verapamil also affected the transport of Hwp1 during morphogenesis. As expected, the control cells formed regular hyphae after 2 h of hyphal induction, and Hwp1–GFP was localized not only in the cytoplasm, but also on the cell surface and the hyphal tip (Fig. 4a, its localization on the cell surface and the hyphal tip is indicated by white arrows). This observation implied normal synthesis and cytoplasm-to-surface transport of Hwp1–GFP in the control cells. In contrast, few hyphal cells were observed when exposed to 80 μg mL−1 verapamil, and the fusion protein of the treated cells was localized predominately in the cytoplasm rather than the cell surface, suggesting that transport of the synthesized protein from the cytoplasm to the cell surface was inhibited by verapamil.

Verapamil inhibits the transport of Hwp1 to the cell surface and the hyphal tip during hyphal induction (a) and adhering to BECs (b). (a) Candida albicans was cultured in RPMI-1640 medium containing 80 μg mL−1 verapamil or without verapamil for 2 h, and the cells were observed. Arrows indicate surface and tip localization of Hwp1–GFP. (b) BECs and the fungus were coincubated as described in Fig. 2, and the cells were observed. Scale bar = 10 μm.
We obtained similar results when using the BEC model to evaluate Hwp1 localization. Most of the control C. albicans cells adhered to BECs, with regular surface localization of Hwp1–GFP in abundant fungal cells, although a few cells showed both a cytoplasm and surface localization of this protein. However, the cells treated with verapamil at the same concentration displayed only cytoplasm localization of the fusion protein, and decreased adhering ability to BECs (Fig. 4b). These observations suggested that verapamil also has an impact on Hwp1 transport from the cytoplasm to the cell surface, which may be also linked to its effect on morphogenesis.
Verapamil inhibits C. albicans gastrointestinal colonization
We further investigated the in vivo effect of verapamil on C. albicans morphogenesis using two gastrointestinal colonization models. In the antibiotic-free model, CFU of the verapamil group [by exposure to 10 mg (kg day)−1 verapamil] from the faecal pellets decreased c. twofold on the 7th day post-inoculation, and decreased more than 15-fold on the 14th day compared with the control group (Fig. 5a). Similarly, in the antibiotic-treated model, a significant decrease of CFU in the verapamil group was observed on the tested days (Fig. 5b). Thus, verapamil displayed an inhibitory effect on C. albicans gastrointestinal colonization, which may be associated with its effect on hyphal development and adhesion of this pathogen.

Verapamil inhibits gastrointestinal colonization of Candida albicans Mice with no antibiotic treatment (a) or with antibiotic treatment (b) were gavaged with the fungus, and then treated with 10 mg (kg day)−1 verapamil or without verapamil in drinking water. CFU of faecal pellets were determined on the YPD + SAK plates for the indicated times. *Significant difference between the control group and the verapamil-treated groups (P< 0.05).
Discussion
The cytoplasmic membrane HACS, composed of Cch1 and Mid1, was shown to play a role in morphogenesis (Brand & Gow, 2009). The system mediates calcium influx and leads to the elevation of tip Ca2+ in the hyphal apex that is required for determination of tropism (Brand et al., 2007). It also functions in hyphal maintenance rather than hyphal formation, which is associated with the transcription regulation of morphogenesis-associated genes (Yu et al., 2012b). In this study, we demonstrate that verapamil, an efficient blocker of the HACS, has an inhibitory effect on hyphal development, further confirming the role of calcium channels in C. albicans morphogenesis. Therefore, calcium channels may be a potential target against morphogenesis of C. albicans.
Given the importance of Hwp1 in C. albicans morphogenesis, we investigated the effect of verapamil on expression and transport of this protein. On the one hand, GFP fluorescence detection showed that treatment with verapamil caused a significant decrease of HWP1 expression, indicating the inhibitory activity of this drug against expression of morphogenesis-associated genes. This observation is consistent with our previous findings that deletion of genes encoding calcium channels led to decreased expression of these genes (Yu et al., 2012b). On the other hand, verapamil also has a remarkable impact on Hwp1 transport towards to the cell surface and the hyphal tip. As a typical glycosylphosphatidylinositol-anchored protein, Hwp1 is synthesized and modified with the glycosylphosphatidylinositol anchor in the ER lumen, and then follows the secretory pathway to the cell surface (Richard & Plaine, 2007). In particular, during polarized hyphal growth, this protein, together with other components required for hyphal extension, were packaged in secretory vesicles and transported along the actin cables towards the hyphal tip (Sudbery, 2011). In many fungi (including C. albicans), a cytoplasmic Ca2+ gradient, which is determined by extracellular calcium influx or intracellular calcium release from calcium stores, exists along the hyphae with a high concentration at the hyphal tip during hyphal extension, and the absence of this gradient leads to a defect in polarized growth (Silverman-Gavrila & Lew, 2002, 2003; Brand et al., 2007). We propose that the effect of verapamil on Hwp1 transport is attributed to its inhibitory ability against calcium channels, resulting in a lack of the calcium gradient and consequent defect in Hwp1 transport. Hence, both decreased expression of morphogenesis-associated genes and abnormal transport of their products were supposed to be involved in the inhibitory activity of verapamil against morphogenesis.
Due to the significant effect of verapamil on C. albicans morphogenesis, it is not difficult to understand the observation that this drug causes a decreased ability of gastrointestinal colonization. Several factors, including hyphal development, adhesion and survival in environmental stress such as host immune attack, are related to efficient gastrointestinal colonization of this pathogen (Rosenbach et al., 2010). To successfully survive under stress conditions, C. albicans evolves a dedicated signalling transduction network to sense and respond to these stresses, and the cell calcium survival (CCS) pathway is a matter of great concern in this network (LaFayette et al., 2010). In the CCS pathway, the HACS is a crucial component, mediating cell responds to abundant stress factors, such as antifungal drugs, oxidative agents and high pH (Sanglard et al., 2003; Wang et al., 2011). Therefore, verapamil was proposed to reduce gastrointestinal colonization by inhibiting both morphogenesis, adhesion and stress response. In addition, the observation that verapamil inhibits C. albicans gastrointestinal colonization indicates a possible role of Hwp1 in this process. Although Hwp1 has been demonstrated to be important for morphogenesis and adhesion, its role in gastrointestinal colonization remains to be investigated. Herein, verapamil-caused deficiency of Hwp1 expression and cytoplasm-to-surface transport may contribute to the decreased ability of gastrointestinal colonization. This suggested a possible link between Hwp1 functions and gastrointestinal colonization.
Increasing evidence supports the view that Candida colonization, especially its gastrointestinal colonization, is a major risk factor for invasive fungal infection in critically ill patients (Charles et al., 2005; Agvald-Ohman et al., 2008; Caggiano et al., 2011). Moreover, its colonization favours the development of pathogenic bacteria in host niches, such as Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli, promoting bacterial infections (Ricard & Roux, 2012). Therefore, inhibition of its colonization will be an efficient strategy to protect individuals from invasive candidiasis and Candida-related bacterial infections. In this study, verapamil showed strong activity against C. albicans gastrointestinal colonization, indicating that it may be used as a drug to prevent these infections in patients, especially those infected by HIV, admitted to the ICU and suffering from severe neutropenia or cancer.
In conclusion, this study has revealed the inhibitory effect of verapamil on C. albicans hyphal development, adhesion and gastrointestinal colonization, and this effect is relevant to decreased expression and abnormal transport of the proteins required for morphogenesis. Further investigation into the mechanisms by which calcium channel blockers inhibit C. albicans morphogenesis and the synergy between this drug and other antifungal agents is likely to have an impact on the development of novel and effective therapies against C. albicans infections.
Acknowledgements
We thank Prof. Dana Davis and Judith Berman (University of Minnesota, Minneapolis, MN) for generously providing C. albicans strain DAY1 and the plasmid pMG2082, respectively. We are grateful to Prof. Jiatong Chen for fluorescence microscopy, and to Zonglin Liu for microplate reading. This work was supported by the National Natural Science Foundation of China (81171541, 31070126) and Natural Science Foundation of Tianjin (13JCYBJC20700). The authors have declared no conflict of interest.
References
Author notes
Editor: Richard Calderone



