-
PDF
- Split View
-
Views
-
Cite
Cite
Hongyu Qiu, Zack Li, Rhonda KuoLee, Greg Harris, Xiaoling Gao, Hongbin Yan, H. Howard Xu, Wangxue Chen, Host resistance to intranasal Acinetobacter baumannii reinfection in mice, Pathogens and Disease, Volume 74, Issue 5, July 2016, ftw048, https://doi.org/10.1093/femspd/ftw048
Close - Share Icon Share
Acinetobacter baumannii is a major causative agent of healthcare-associated infection and develops multidrug resistance rapidly. However, little is known in the host defense mechanisms against this infection. In this study, we examined if mice recovered from a previous intranasal A. baumannii infection (recovered mice) are fully protected against a subsequent reinfection. We found that, despite the presence of specific serum IgG and mucosal IgA responses prior to the reinfection, the recovered mice were only marginally better protected against intranasal challenge with low doses of homologous or heterologous A. baumannii strains than the naïve mice. Post-challenge immune and inflammatory (cells and cytokines) responses were generally comparable between recovered and naïve mice although the recovered mice produced significantly higher amounts of IFN-ó and IL-17 and had higher percentages and numbers of resident lung CD44hiCD62LâÂÂCD4+ and CD19+ B lymphocytes. Taken together, our results suggest that mice recovered from a previous A. baumannii infection remain susceptible to reinfection, indicating the complexity of immune protection mechanism for this Gram-negative, multidrug-resistant emerging pathogen.
Acinetobacter baumannii infection has emerged as an important cause of both nosocomial and community-associated infections worldwide (Gaynes and Edwards 2005; Fournier and Richet 2006; Peleg, Seifert and Paterson 2008). Nosocomial A. baumannii infection mainly affects ICU patients, and manifested as pneumonia and bacteremia with mortality as high as 50% (Dijkshoorn, Nemec and Seifert 2007; Kuo etÃÂ al.2007; Peleg, Seifert and Paterson 2008). Moreover, A. baumannii infections have become increasingly difficult to treat because of its rapid development of resistance to almost all classes of current clinically prescribed antibiotics (Fournier and Richet 2006; Dijkshoorn, Nemec and Seifert 2007; Gootz and Marra 2008; Peleg, Seifert and Paterson 2008). Recurrence (relapse and reinfection) of Acinetobacter infections is a recognized clinical problem and can occur in >5% A. baumannii-infected patients in certain clinical settings (Lai etÃÂ al.2012). In addition, A. baumannii isolates recovered from the recurrence patients were generally more antibiotic resistant (Lai etÃÂ al.2012). However, there are no studies on the relative resistance of the host to reinfection with A. baumannii. Such information would be important for clinical management of patients with A. baumannii recurrence, and will also provide insight for the rational design and development of novel vaccine and immunotherapeutics. In this study, we examined if mice that had recovered from a previous A. baumannii infection (recovered mice) are fully protected against a subsequent intranasal (i.n.) challenge with homologous or heterologous strains of A. baumannii.
Six- to ten-week old, specific-pathogen-free female C57BL/6 (B6) and BALB/c mice were purchased from Charles Rivers Laboratories (St. Constant, Que.). The animals were used in accordance with the recommendations of the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals. All animal-use protocols were approved by the Institute for Biological Sciences (National Research Council Canada) Animal Care Committee. The general experimental design for the reinfection model is illustrated in Fig. S1 (Supporting Information). Mice were i.n. inoculated with the designated doses of freshly grown A. baumannii ATCC17961 or LAC-4 (a clinical strain showing hypervirulence in mice) (Harris etàal.2013) in 50 ül saline under isoflurane inhalation anesthesia. The clinical signs were monitored daily and scored as previously described (Harris etàal.2013). At predetermined time points, groups of three to five mice were sacrificed for blood, bronchoalveolar lavage (BAL) fluid and tissue (lungs and spleen) collection. For quantitative bacteriology, aliquots (100 ül) of 10-fold serial dilutions of tissue homogenates and heparinized blood were cultured on brainâÂÂheart infusion agar plates supplemented with 50 ügàmlâÂÂ1 streptomycin (van Faassen etàal.2007). Serum and BAL levels of A. baumannii-specific immunoglobulin G1 (IgG1), IgG2a and IgA were measured by ELISA (KuoLee etàal.2015). Cytokine and chemokine levels were determined using the mouse panel of Fluorokine MAP Multiplex Kits (R & D Systems, Inc. Minneapolis, MN) on a Luminexî 100IS system (Luminex, Austin, TX) (van Faassen etàal.2007). The phenotypes of the lung-resident lymphocytes were determined by the FACS analysis (Yan etàal.2009). To assess in vitro cytokine and chemokine responses to A. baumannii stimulation, tracheobronchial lymph node (TBLN) cells collected from naïve and infected mice 6 weeks after the initial infection were stimulated with formalin-fixed A. baumannii cells (ffAb) or medium alone. Culture supernatants were collected 72 h later for cytokine assays (KuoLee etàal.2007).
As reported previously (Joly-Guillou etàal.1997; Branger etàal.2004; Renckens etàal.2006; van Faassen etàal.2007), sublethal i.n. inoculation with A. baumannii ATCC17961 (2.9 à107 CFU) results in acute but self-limiting infection in mice. Log10 5.88 ñ 0.20 and 2.86 ñ 0.66 CFU of A. baumannii were cultured from the lung and spleen, respectively, at 24 h post-inoculation (hpi), but no bacteria were recovered at either tissue at day 4 (â¤1.3 logs) or 7 (â¤1.0 logs) (Fig. S2, Supporting Information). The numbers of total and differential BAL cells 6 weeks after the infection were very comparable between the recovered and naïve mice of both BALB/c and B6 background with the majority (>95%) of them being alveolar macrophages. In addition, both strains of mice produced large amounts of serum A. baumannii-specific antibodies (IgG1 and IgG2a) as well as IgA in the serum and BAL fluid (Fig. S3, Supporting Information).
To assess if recovered mice are more resistance to the reinfection than the age-matched naïve mice, the recovered BALB/c and B6 mice were challenged 6 weeks after the primary infection by i.n. with either 108 or 109 CFU of a homologous A. baumannii strain (ATCC17961). There were no significant differences in the clinical scores, body weight or survival rates between recovered and naive mice in that all mice succumbed to 109 CFU challenge by dpi 3 whereas the majority of mice survived 108 CFU challenge (Fig. 1A), although significantly lesser numbers of bacteria were cultured from the BAL (4, 24 and 72 h) and lungs (24 and 72 h) of both strains of recovered mice (Fig. 1B). Similarly, the recovered mice showed significantly lesser body weight loss and lower tissue (the lung and spleen) bacterial burdens than the naïve mice 24 h after i.n. challenge with 8.5 à107 CFU of a heterologous A. baumannii strain (LAC-4) (Fig. S4, Supporting Information).
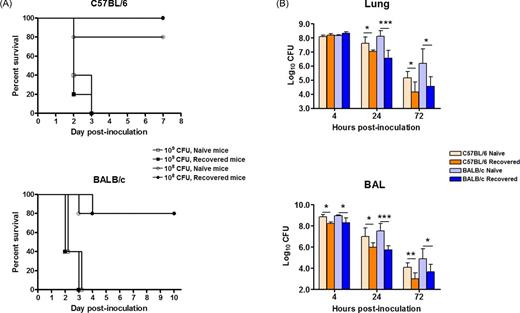
Survival rate (A) and bacterial burdens in the lung and BAL fluid (B) of C57Bl/6 (B6) and BALB/c mice after i.n. reinfection with A. baumannii. Groups of 5 B6 or BALB/c mice were i.n infected with 2 à107 CFU A. baumannii. Six weeks later, the completely recovered mice and naïve control mice were i.n. challenged with 107, 108 or 109 CFU A. baumannii ATCC17961. The survival rates of the high (109) and medium (108) dose-infected mice were monitored daily for 7âÂÂ10 days (A). The bacterial burdens in the lung and BAL fluid of the medium dose (108) infected mice were determined by quantitative bacteriology at the indicated hours after inoculation (B). The data are presented as mean ñ SD (n = 5) and represent one of at least two experiments with similar results. The detection limit for bacterial burdens was 1.3 log10 CFU/organ. *P<0.05, **P<0.01, or ***P<0.001 naïve vs recovered mice.
Comparative analysis of the local inflammatory and immunological responses showed that the kinetics and numbers of neutrophils and other inflammatory cells in the BAL fluid were similar between reinfected and primarily infected mice (data not shown). There was no significant difference in the BAL levels of a panel of 11 proinflammatory cytokines and chemokines that have previously been implicated in the host defense against A. baumannii infection (Knapp etàal.2006; Renckens etàal.2006; van Faassen etàal.2007; Harris etàal.2013), apart from that IL-17 (4, 24 and 72 hpi), and IFN-ó (24 hpi) levels were significantly higher in recovered than naïve mice (Fig. S5, Supporting Information). Although recovered BALB/c and B6 mice were completely free of A. baumannii 5 weeks after the infection, the numbers of resident pulmonary T (both CD4+ and CD8ñ+ T cells) and B lymphocytes (CD19+ cells) were significantly higher in recovered mice than in naïve mice (Fig. S6A, Supporting Information). There were also significantly more B cells and CD4+ T lymphocytes in the lungs of recovered BALB/c mice than recovered B6 mice (Fig. S6A, Supporting Information). Moreover, the numbers of effector memory CD4+ and CD8ñ+ T cells (CD44hiCD62LâÂÂ) were significantly increased in the lungs of recovered BALB/c mice (Fig. S6B, P<0.001), but only slightly increased in the recovered B6 mice as compared to naïve BALB/c and B6 mice, respectively. Sublethal A. baumannii infection also primed the mononuclear cells from TBLNs, the major draining lymph nodes of the lower respiratory tract in mice, of recovered mice to produce more antigen-specific IFN-ó, IL-17 and IL-10 than those of naïve mice (Fig. S7, Supporting Information). In contrast, the level of IL-12p40, a cytokine promoting the production of IFN-ó by T lymphocytes and NK cells (Chan etàal.1991; Trinchieri 1995; Matikainen etàal.2001; Kusaba etàal.2005) did not change at all (Fig. S7, Supporting Information), suggesting that the highly elevated IFN-ó production may come from the stimulation of some other cytokines such as IL-18 (Micallef etàal.1996; Fantuzzi etàal.1998; Ngoumou etàal.2004). However, despite the presence of cell-mediated immunity, the recovered mice remain largely susceptible to A. baumannii reinfection (Fig. 1), suggesting that T cells may not be activated and migrated to tissues quickly enough to recruit neutrophils in such an acute infection and re-emphasis of the importance of the host innate immunity, with the help of antigen-specific antibody from prior exposure, in the clearance of the bacterium and survival from infection.
In conclusion, we found that mice recovered from a previous i.n. A. baumannii infection were not well protected against a moderate dose of reinfection with homologous or heterologous A. baumannii strains than naïve mice, despite the presence of specific serum IgG and mucosal IgA responses in recovered mice prior to the reinfection. These results suggest the complexity of immune protection for this Gram-negative multidrug-resistant bacterium and could have important implication in the rational design and development of novel prophylactic and therapeutic (immunotherapy) vaccines for A. baumannii infection.
SUPPLEMENTARY DATA
FUNDING
This work was partially supported by the National Research Council (NRC) Canada (A-base), the NRC Vaccine Program, a joint research collaboration project between NRC and Taiwan Ministry of Science and Technology, and a grant fromArmy Research Office of U.S. Department of Defense (W911NF-12-1-0059).
Conflict of interest. None declared.
REFERENCES



