-
PDF
- Split View
-
Views
-
Cite
Cite
Jesper Bogefors, Anne Månsson Kvarnhammar, Camilla Rydberg Millrud, Susanna Kumlien Georén, Lars Olaf Cardell, LEAP-2, LL-37 and RNase7 in tonsillar tissue: downregulated expression in seasonal allergic rhinitis, Pathogens and Disease, Volume 72, Issue 1, October 2014, Pages 55–60, https://doi.org/10.1111/2049-632X.12183
Close - Share Icon Share
Abstract
In the upper airway, the production of antimicrobial peptides (AMPs) protects against bacteria, viruses and fungi. Previous investigations have revealed downregulated expression of AMPs in different manifestations of allergic disease. In this study, we examined the expression of LL-37, Ribonuclease7 (RNase7) and Liver expressed antimicrobial peptide 2 (LEAP-2) in tonsillar tissue and studied a possible relation to seasonal allergic rhinitis (SAR). Tonsils, obtained from patients with SAR and nonallergic controls, were examined for the occurrence of LL-37, RNase7 and LEAP-2 with real-time RT-PCR and immunohistochemistry. Tonsillar mononuclear cells were cultured in presence or absence of LEAP-2 or LL-37 and analyzed for cytokine levels using ELISA. mRNA and protein for LL-37, RNase 7 and LEAP-2 were found in all tonsils. Immunohistochemistry revealed prominent staining for LL-37 and RNase7 in the tonsillar epithelium, whereas a moderate staining was seen with LEAP-2. Real-time RT-PCR showed a downregulation of RNase7 and LEAP-2 in the allergic as compared to the nonallergic group. Mononuclear cells cultured in presence of LEAP-2 or LL-37 demonstrated reduced levels of IL-10. The present study demonstrates the presence and function of LEAP-2, LL-37 and RNase7 in tonsils. Moreover, a downregulation of LEAP-2 and RNase7 is seen in SAR patients, indicating that allergic individuals may be more susceptible to respiratory tract infections due to an impaired antimicrobial defense.

Allergic individuals may be more susceptible to respiratory tract infections due to downregulation of antimicrobial peptides.
Introduction
In the upper airway epithelium, a barrier against invading microbes is provided through tight junctions and mucociliary activity. The production of antimicrobial peptides (AMPs) further increases the protection against bacteria, viruses and fungi. These short cationic peptides not only directly kill pathogens, but also induce the production of cytokines (Boniotto et al., 2006). Previous investigations of a group of AMPs, the human beta defensins (HBDs) have been performed in the upper respiratory tract. In these studies, a link between allergic status and expression of HBDs was revealed (Bogefors et al., 2013). Similar connections were observed in other AMPs, such as S100A7 (also known as psoriasin; Bryborn et al., 2008). The palatine tonsils are optimally located for immunological detection of airborne antigens. They have a reticulated epithelium acting as a barrier, but also as a site of interaction between pathogens and the innate and adaptive immune system (Karchev, 1988). Based on previous findings of HBDs in the upper airway in relation to inflammatory disease, we were interested in further examination of other AMPs.
LL 37 is an antibacterial peptide cleaved from the human cathelicidin hCAP 18, an 18 kDa propeptide packaged in secondary granules of neutrophils and proteolytically cleaved to generate the mature AMP (Dixon et al., 2012). It has broad antimicrobial activity toward both Gram negative and Gram positive bacteria and shows synergistic antibacterial effects with the defensins. LL 37 also acts as a chemotactic agent for neutrophils and monocytes, and might be involved in healing processes, especially cutaneous wound angiogenesis and cell growth (Sorensen et al., 2001; Koczulla et al., 2003). The LL 37 protein is expressed by a wide array of immune cells, and tissues that are constantly exposed to microbes, including the skin, mouth, airways, and intestines (Bals et al., 1998; Frohm Nilsson et al., 1999). In the oral cavity, it seems to have a strong activity against S. mutans, the major etiological agent of dental caries and against S. aureus, which often colonizes the nasopharynx (Leszczynska et al., 2013). LL 37 is expressed in the palatine tonsils, and one study reported that recurrent throat infections upregulated the levels of LL 37 (Song et al., 2006). In contrast, another study found reduced levels in recurrent acute tonsillitis (Ball et al., 2007).
Ribonuclease7 (RNase7) is classified as a member of the RNase A family. Although first recognized for its ribonucleolytic activity, this small (14.5 kDa), positively charged protein is able to permeate the bacterial membrane and kills Gram positive and Gram negative bacteria as well as yeast but has no antiviral activity. The bactericidal mechanism of RNase7 is not dependent on its RNase enzymatic activity but on direct membrane disruptive action (Torrent et al., 2010). It is extensively studied in the skin and has a strong antimicrobial activity against S. aureus. Low concentrations of RNase7 seem to be associated with higher susceptibility to skin infections (Wiesner & Vilcinskas, 2010; Zanger et al., 2009). Not much is known about RNase7 in the upper airway. However, one study found its presence in nasal secretions and biopsies (Laudien et al., 2011).
Another interesting AMP that was recently discovered is the Liver expressed antimicrobial peptide 2 (LEAP 2). First described in 2003, LEAP 2 is a cysteine rich, 40 residue cationic AMP (Krause et al., 2003). The biological role of LEAP 2 is unclear. However, LEAP 2 exhibited in vitro antimicrobial activities against several Gram positive bacteria (Hocquellet et al., 2010). In dry eye conditions, conjunctival and corneal impression cytology reveals elevated levels of LEAP 2 (Mohammed et al., 2011). Protein and gene expression of LEAP 2 are previously described in tonsillar tissue (Ball et al., 2007).
The purpose of the present study was to characterize the expression of LL 37, RNase7, and LEAP 2 in tonsils from healthy subjects and patients with seasonal allergic rhinitis (SAR) and to investigate the role of these AMPs in tonsillar mononuclear cells.
Materials and methods
Subjects and tissue collection
Forty pairs of tonsils from non smoking patients were collected from individuals between 2 and 44 years of age. Immediately after removal, samples for microbial examination were taken from the surface and the core of the tonsils. All tonsils included had a negative culture test (except from the normal oral flora). Blood Phadiatop testing was obtained from all participants. If positive, it was followed by specific RAST for pollen (birch, timothy, and artemisia). Patients included in the allergic group (n = 19; eight females, 11 males with a mean age of 19; range 4–40) were all classified as class 3 or higher on the RAST scale and had a history of allergic rhinoconjunctivitis. Patients included in the control group (n = 21; 16 females, five males with a mean age of 20; range 2–44) had a negative phadiatop test and no symptoms of allergy. Directly after surgery, a piece of tonsillar tissue (2–4 mm) was placed in RNAlater (Qiagen, Hilden, Germany) for 24 h and then kept at −80 °C until use. Another piece was fixed in a 4% solution of formaldehyde in 0.1% phosphate buffer (pH 7.0), embedded in paraffin, then cut in 3 μm sections, mounted on glass slides and stored at −80 °C until use. The tonsillectomy was performed as a consequence of idiopathic tonsillar hypertrophy, and the indications were the same for both allergic and non allergic individuals. All tonsils were obtained outside pollen season. None of the subjects displayed any signs of acute infection at the time of surgery or received antibiotic treatment for at least 1 month prior to surgery. Apart from the tonsillar symptoms, all subjects were healthy and did not receive any medications. Four additional tonsils were obtained for in vitro experiments. These tonsils were not characterized according to infectious or allergic status. The study was approved by the local Ethics Committee, and an informed consent was obtained from all participants.
RNA extraction and real time RT PCR
RNA was extracted from homogenized tonsils using the RNeasy Mini Kit (Qiagen). The quality and quantity of the RNA was assessed by spectrophotometry based on the A260/A280 ratio (between 1.8 and 2.0 in all preparations). Reverse transcription of total RNA into cDNA was carried out using Omniscript™ reverse transcriptase kit (Qiagen) with oligo(dT)16 (DNA Technology A/S, Aarhus, Denmark) in a Mastercycler personal PCR machine (Eppendorf AG, Hamburg, Germany) in a final volume of 20 μL at 37 °C for 1 h.
Intron spanning oligonucleotide primers for detection of LL 37, RNase7, LEAP 2, and β actin were designed to generate a PCR product between 100 and 150 bp using Prime Express® 2.0 software (Applied Biosystems, Foster City, CA) and synthesized by DNA Technology A/S (Aarhus, Denmark). The following primers were used for LL 37, RNase7 and LEAP 2 and β actin:
RNase7 fwd:GGA GTC ACA GCA CGA AGA CCA AGC GCA AAG
RNase7 rev:CAT GGC TGA GTT GCA TGC TTG A
LEAP 2 fwd:CCT CAG GCC TAT TGG AGC C
LEAP 2 rev:CTG TCC TTT CTT TCC CTG GTA TGT AC
LL 37 fwd:GTC CTC GGA TGC TAA CCT CTA CC
LL 37 rev:GCA CAC TGT CTC CTT CAC TGT GA
For comparisons of LL 37, RNase7, and LEAP 2 levels in tonsils from allergic and control subjects, real time PCRs were performed on a Stratagene Mx3000P (Agilent Technologies, Santa Clara, CA) using the Stratagene Brilliant SYBR® Green QPCR Mastermix in a final volume of 20 μL. The thermal cycler was set to perform 95 °C for 15 min, followed by 46 cycles of 94 °C for 30 s and 55 °C for 60 s (initially 65 °C, followed by a 2 °C decrease for the six first cycles). Melting curve analysis was performed to ensure specificity of the amplified PCR products. The mRNA expression was assessed using the comparative cycle threshold (Ct) method where the relative amounts of mRNA for LL 37, RNase7, and LEAP 2 were determined by subtracting the Ct value for these genes with the Ct value for β actin (ΔCt). The amount of mRNA is expressed as  × 105.
× 105.
Immunohistochemistry
The morphological localization of LL 37, RNase7, and LEAP 2 proteins in tonsils was assessed using immunohistochemistry. Immunostaining was performed according to the Envision+ System horseradish peroxidise (HRP) kit (Dako, Copenhagen, Denmark). Briefly, the sections were incubated overnight in 4 °C with a rabbit antihuman pAb to LL 37 (Abcam, Cambridge, UK), a rabbit antihuman pAb to RNase7 (Abcam) and a rabbit antihuman pAb to LEAP 2 (Abcam). The antibodies were diluted: LL 37 1:400, RNase7 1:200, and LEAP 2 1:20 in antibody diluent from Dako. Thereafter, the sections were incubated with HRP labeled goat antirabbit or goat antimouse polymer for 30 min, followed by 3, 3 diaminobenzidine substrate chromogen for 5 min. Finally, the slides were mounted in Faramount aqueous mounting medium (Dako). As negative controls, N series universal negative control reagents against mouse and rabbit (both from Dako) or antibody diluent were utilized. Tris buffered saline (TBS; pH 7.6) supplemented with 0.05% Tween 20 was used for all washing steps.
In vitro culture of tonsillar mononuclear cells
Fresh tonsils were minced in complete RPMI 1640 medium supplemented with 0.3 g L−1 L glutamine (Gibco, Paisly, UK), 10% FBS (Gibco), 100 U mL−1 penicillin/100 μg mL−1 streptomycin, and 50 μg mL−1 gentamicin (Gibco, Grand Island, NY). The cells were cultured at 37 °C in a humidified 5% CO2 air atmosphere (4 × 106 cells mL−1) in 500 μL medium in 24 well plates in the absence or presence of LEAP 2 (USCN Life Science, Wuhan, China) or LL 37 (Hycult Biotech, Uden, The Netherlands). After 72 and 96 h in culture, cell free supernatants were analyzed for levels of IL 10 by ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). Data are expressed as mean ± SEM or % reduction compared with control. Statistical differences were analyzed using Mann–Whitney t test or one way Repeated Measures anova. n equals the number of subjects and a P value ≤ 0.05 was considered statistically significant.
Results
The expression of LL 37, RNase7, and LEAP 2 was investigated by real time RT PCR. mRNA for all AMPs were found in all tonsils. RNase7 and LEAP 2 were downregulated in the allergic group (P = 0.0030 and P = 0.0062, respectively), whereas no difference was seen with LL 37 (Fig. 1).
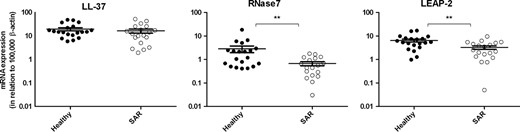
Expression of LL 37, RNase 7 and LEAP 2 in tonsils. Levels of LL 37, RNase7, and LEAP 2 in tonsils from control subjects (n = 21) and patients with seasonal allergic rhinitis (SAR; n = 19) was determined by real time RT PCR. Data are presented in relation to β actin as  × 105 and depicted as mean ± SEM. **P < 0.01.
× 105 and depicted as mean ± SEM. **P < 0.01.
To confirm and complement the mRNA data, immunohistochemical staining was performed to demonstrate the morphological localization of the AMPs. An intense immunostaining of LL 37 and RNase7 was observed in the surface epithelium, whereas a moderate staining was seen for LEAP 2 (Fig. 2). When the primary specific antibodies were omitted, a complete loss of staining was seen.
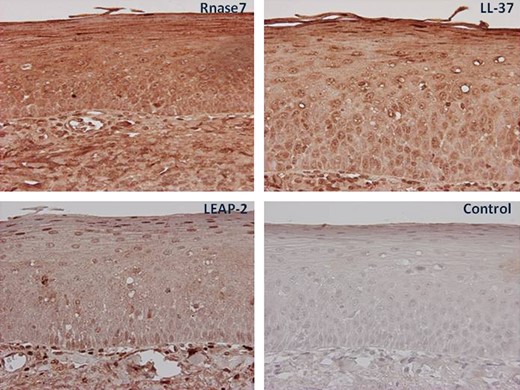
Immunohistochemical staining of the tonsils. Tonsillar tissue slides obtained from non allergic individuals and patients with allergic rhinitis were incubated with antibodies against RNase7, LL 37, and LEAP 2 and visualized by 3,3 diaminobenzidine (brown). All slides were counterstained with hematoxylin (blue). In control slides, N series universal negative control reagent was used. Magnification 40–100×.
Next, we wanted to investigate the role of these AMPs on immune cells from tonsils. For this purpose, tonsillar mononuclear cells were isolated and cultured for 72 and 96 h in presence of LEAP 2 or LL 37. However, despite great efforts, we were not able to get RNase7 synthesized. The cell free supernatants were analyzed for levels of IL 10 by ELISA. A clear dose dependent reduction in IL 10 levels was seen with both AMPs after 72 (Fig. 3) as well as after 96 h (data not shown).

Tonsillar mononuclear cells were cultured (4 × 106 cells mL−1) in 500 μL medium in 24 well plates in the absence or presence of LEAP 2 or LL 37. After 72 h in culture, cell free supernatants were analyzed for levels of IL 10 by ELISA. Data are presented as % reduction compared with control. **, P < 0.01; ***, P < 0.001 (one way Repeated Measures anova).
Discussion
In this study, we continue to explore the expression and function of AMPs in the upper respiratory tract. No examinations of LL 37, RNase7, and LEAP 2 have previously been performed in relation to allergic conditions in the upper airway. mRNA and protein expression of LL 37, RNase7, and LEAP 2 were found in tonsils and a marked downregulation of RNase7 and LEAP 2 was observed in allergic individuals. Tonsillar mononuclear cells cultured in presence of LEAP 2 or LL 37 demonstrated reduced levels of IL 10 as compared to controls.
The presence of LL 37, RNase7, and LEAP 2 was expected in tonsils as we recently found the expression of HBDs in tonsillar tissue (Bogefors et al., 2012). In accordance with our study, the expression of LEAP 2 and LL 37 has previously been observed in tonsils (Bell et al., 2012; Bals et al., 1998). Not much is known about RNase7 in the upper airway, but one report found presence of RNase7 in nasal secretions and biopsies (Laudien et al., 2011). As the tonsils are key players for microbial recognition and trigger the immune system in the upper airway, we have given these tissues special attention.
Patients with allergic rhinitis were found to have a low tonsillar expression of RNase7 and LEAP 2. In accordance, studies in patients with allergic rhinitis, asthma, and atopic dermatitis have further reported downregulated levels of AMPs, as for instance LL 37 and HBDs, in these patients. This has been proposed to be due to the dominant T helper (Th2) cytokine milieu present in patients with allergies (Wollenberg et al., 2011; Ong et al., 2002; Howell et al., 2006; Bogefors et al., 2012). In the present study, the tonsils were obtained from patients with allergic rhinitis outside pollen season. High levels of allergic mediators as for instance Th2 cytokines have also been found in patients with allergic rhinitis outside pollen season. This could indicate that the low expression of RNase7 and LEAP 2 in tonsils from patients with allergic rhinitis found in this study is in fact a result of an elevated level of Th2 cytokines. Hence, the Th2 cytokine milieu may be detrimental for the antimicrobial defense provided by the epithelial cells.
In contrast to RNase7 and LEAP 2, no differences were found in the levels of LL 37 between the allergic patients and the healthy individuals. The expression and role of LL 37 in allergies are contradictory. LL 37 have been shown to be decreased in sputum of asthmatic patients and atopic dermatitis, whereas other reports have demonstrated that LL 37 have a pro asthmatic role and to be elevated in eosinophils from asthmatic patients (Ong et al., 2002; Xiao et al., 2005; Sun et al., 2013). The differences in results might be due to the different cellular composition and inflammatory states investigated in these various studies. Thus, the different AMPs might be controlled and affected differently.
Furthermore, a synergistic or additive antibacterial effect of HBDs and LL 37 against S. aureus has been observed in the skin (Chen et al., 2005). AMPs contribute to host defense by disrupting the cytoplasmic membrane of microorganisms. However, recent studies have shown that AMPs have multiple roles in host defense. AMPs have been attributed immunomodulatory roles with both pro and anti inflammatory functions. HBDs and cathelicidins are for instance known to attract neutrophils, monocytes, and T cells (Chaly et al., 2000; Biragyn et al., 2001). AMPs have also been demonstrated to modulate the inflammatory responses through regulation of cytokine production. One study reported that defensins from neutrophils increase the levels of the pro inflammatory factors IL 1 and TNFα, and suppress the levels of IL 10 at the site of microbial infection (Chaly et al., 2000). This is in analogy with our findings, that LL 37 and LEAP 2 downregulated the release of IL 10 in mononuclear cells. It is known that patients with allergies display a decreased level of IL 10 in comparison with healthy individuals (Genc et al., 2012; Baumann et al., 2013). Therefore, it seems that the AMPs do not to have any impact on the level of IL 10 in patients with allergic rhinitis as the level of AMPs was low among these patients. Nonetheless, these observations support the idea that AMPs provide signals to bridge innate and adaptive immunity.
We have previously reported reduced levels of HBDs in tonsils from patients with allergic rhinitis. In the same study, we found a diminished secretion of HBDs by epithelial cells after stimulation with IL 4, IL 5, and histamine (Bogefors et al., 2012). Further, in nasal lavage fluids obtained from patients before and after allergen specific immunotherapy (ASIT), an increase in HBD release was seen after completion of treatment (Bogefors et al., 2012). This suggests that the absence of the allergic Th2 phenotype induced by ASIT leads to restored levels of AMPs in these patients. Moreover, reduced levels of S100A7 have been seen in both tonsils and nasal lavage fluid from SAR patients (Bryborn et al., 2005, 2008). Thus, several studies point toward a tendency of suppressed AMPs in the presence of allergic inflammation. In the present study, the marked downregulation of RNase7 and LEAP 2 found in allergic individuals further strengthens this theory. These findings indicate that allergic individuals may be more susceptible to respiratory tract infections due to an impaired antimicrobial defense.
Acknowledgements
We express our gratitude to Ingegerd Larsson for helpful assistance during the study. We also acknowledge the support from the Swedish Medical Research Council, Karolinska Institute, and the Karolinska University Hospital. This study was supported by grants from the Swedish Medical Research Council and the Swedish Heart–Lung foundation.
References
This paper describes a changed innate immunity profile in allergic rhinitis patients that can predispose to reduced infection resistance.
Editor: Willem van Eden



