-
PDF
- Split View
-
Views
-
Cite
Cite
Stefano Giulieri, Katia Jaton, Alain Cometta, Laurence T. Trellu, Gilbert Greub, Development of a duplex real time PCR for the detection of Rickettsia spp. and typhus group rickettsia in clinical samples, FEMS Immunology & Medical Microbiology, Volume 64, Issue 1, February 2012, Pages 92–97, https://doi.org/10.1111/j.1574-695X.2011.00910.x
Close - Share Icon Share
Abstract
Molecular diagnosis using real-time polymerase chain reaction (PCR) may allow earlier diagnosis of rickettsiosis. We developed a duplex real-time PCR that amplifies (1) DNA of any rickettsial species and (2) DNA of both typhus group rickettsia, that is, Rickettsia prowazekii and Rickettsia typhi. Primers and probes were selected to amplify a segment of the 16S rRNA gene of Rickettsia spp. for the pan-rickettsial PCR and the citrate synthase gene (gltA) for the typhus group rickettsia PCR. Analytical sensitivity was 10 copies of control plasmid DNA per reaction. No cross-amplification was observed when testing human DNA and 22 pathogens or skin commensals. Real-time PCR was applied to 16 clinical samples. Rickettsial DNA was detected in the skin biopsies of three patients. In one patient with severe murine typhus, the typhus group PCR was positive in a skin biopsy from a petechial lesion and seroconversion was later documented. The two other patients with negative typhus group PCR suffered from Mediterranean and African spotted fever, respectively; in both cases, skin biopsy was performed on the eschar. Our duplex real-time PCR showed a good analytical sensitivity and specificity, allowing early diagnosis of rickettsiosis among three patients, and recognition of typhus in one of them.
Introduction
Rickettsial diseases are worldwide emerging arthropod-borne zoonoses that are caused by small obligate intracellular gram-negative rods. They are traditionally divided into the spotted fever group, the typhus group, and the scrub typhus group (Parola et al., 2005).
Microbiological diagnosis of rickettsiosis is usually established by serology, as isolation in cell culture or animals is difficult and dangerous for the laboratory personnel, and immunohistochemistry is not widely available. However, as IgM increase takes 15–26 days, serological diagnosis is usually retrospective, thus limiting the clinical impact of diagnosis (Fournier et al., 2002). Moreover species identification is limited by cross-reactions.
Molecular diagnosis using polymerase chain reaction (PCR) allows earlier diagnosis of rickettsiosis and species identification. Thus, several PCR assays targeting various rickettsial genes have been developed to accelerate the diagnosis of rickettsiosis. While some targeted several species (Leitner et al., 2002; Fournier & Raoult, 2004), other were designed to detect only a single rickettsial species (Choi et al., 2005; Karpathy et al., 2009). As several rickettsiae can be responsible of the same clinical syndrome, a broader spectrum is warranted. In addition, the biodiversity of rickettsial species is likely underestimated and some yet unknown species might also be pathogenic (Parola et al., 2005). Moreover, the recognition of typhus group rickettsiosis is clinically and epidemiologically relevant, as these infections may be associated with a worse prognosis than spotted fevers (Dumler et al., 1991; Bechah et al., 2008). We therefore developed a duplex real-time PCR that amplifies DNA of any rickettsial species and both typhus group rickettsia, that is, Rickettsia prowazekii and Rickettsia typhi.
Materials and methods
Development of the real-time PCR
Primers and probes were designed using Primer3 software (Rozen & Skaletsky, 2000) starting from alignments of the 16S rRNA gene and of the citrate synthase (gltA) genes obtained for the different rickettsial species available in the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/).
For the Rickettsia spp. PCR, a forward primer Rsp-F1 (5′-CGCAACCCTCATTCTTATTTGC-3′), a second forward primer Rsp-F2 (5′-CGCAACCCTTATTCTTATTTGC-3′), a reverse primer Rsp-R (5′-CCTCTGTAAACACCATTGTAGCA-3′), and a MGB probe (minor-groove binder) labeled with 5′FAM (6-carboxyfluorescein) Rsp-Probe (5′-FAM-TAAGAAAACTGCCGGTGATAAGCCGGAG-BHQ-3′) were designed to amplify a 149-bp fragment of the 16S rRNA gene of all Rickettsia spp. This fragment was chosen because several rickettsial species including Rickettsia conorii, Rickettsia africae, Rickettsia rickettsii, Rickettsia slovaca, and Rickettsia akari show an identical sequence in the selected 16S rRNA gene fragment (Fournier et al., 2003). As typhus group rickettsia differs from other rickettsial species at position 1097, the second forward primer was added.
For the typhus group rickettsia PCR, the citrate synthase gene gltA was targeted, as it is less conserved among rickettsial species. To amplify R. prowazekii DNA, the forward primer Rtp-F (5′-TTCGGATTGCTGGCTCATCA-3′) and the reverse primer Rtp-R (5′-AAATGGATCATTCTTATCTTTAGCTTTAGC-3′) were designed. To amplify R. typhi DNA, the primers Rtt-F (5′-TACGAATTGCTGGCTCATCA-3′) and Rtt-R (5′-AAATGGATCATTTTTGTCTTTAGCTTTAGC-3′) were added. Only one MGB probe was designed labeled with 5′TET (tetrachlorofluorescein phosphoramidite), Rt-Probe (5′-TET — ATCCTTTTGCATGTATTAGCACTGGTATTGCATCA–BHQ-3′), to detect both species. All primers and probes were prepared by Eurogentec (Belgium).
PCR amplification and products detection were performed with ABI Prism 7900 Sequence Detection system (Applied Biosystems, Rotkreuz, Switzerland) during 45 cycles. The reactions were performed with 0.2 µM of each primer, 0.1 µM of probe and 10 µL 2× TaqMan universal master Mix (Applied Biosystem) and 5 µL DNA sample (final volume 20 µL). Cycling conditions were 2 min at 50 °C, 10 min at 95 °C, followed by 45 cycles during 15 s at 95 °C and 1 min at 60 °C.
To obtain positive controls for both PCR and to allow quantification, four plasmids were constructed using rickettsial DNA from three species: DNA from a skin biopsy positive for R. conorii ssp. israelensis (Boillat et al., 2008) and R. prowazekii DNA and R. typhi DNA extracted from two strains grown in cell culture (kindly provided by Prof. Didier Raoult, Université de la Méditerranée, Marseille, France). Thus, for each PCR, we obtained two positive control plasmids: (1) R. conorii and R. typhi for the pan-rickettsial real-time PCR; (2) R. typhi and R. prowazekii controls for the typhus group real-time PCR. The genomic DNA was amplified using the polymerase AmpliTaq Gold (Applied Biosystems, Zug, Switzerland). PCR products were cloned using the TOPO TA Cloning® kit (Invitrogen, Basel, Switzerland). After isolation of plasmidic DNA using the QIAprep Spin Miniprep Kit (Qiagen, Kombrechtikon, Switzerland), quantification was performed on a Nanodrop ND-1000 (Witech, Littau, Switzerland).
Analytical sensitivity, reproducibility, and specificity
To assess analytical sensitivity of both real-time PCR, 10-fold dilutions of the four positive control plasmids were tested in five independent runs and in five replicates. Intra- and inter-run reproducibility was assessed by comparing mean threshold cycle (Ct) and standard error of the mean of replicates obtained in the five runs. Analytical specificity of the pan-rickettsial PCR was tested using DNA extracted from 22 pathogens and skin commensals, including bacteria, fungi, and virus (Table 1). In addition, the broad range of the pan-rickettsial PCR was investigated using the following rickettsial DNA (grown in cell culture and provided by Prof. Didier Raoult, Université de la Méditerranée, Marseille, France): R. africae, R. conorii, R. felis, R. rickettsii, R. slovaca, R. prowazekii and R. typhi.
List of DNA of strains tested to investigate the specificity of the rickettsial PCR
| Species | Source/strain |
| Aspergillus fumigatus | Clinical specimen |
| Aspergillus terreus | Clinical specimen |
| Candida albicans | ATCC 90028 |
| Candida glabrata | Clinical specimen |
| Chlamydia pneumoniae | Clinical specimen |
| Chlamydia trachomatis | Clinical specimen |
| Corynebacterium pyogenes | Clinical specimen |
| Escherichia coli | ATCC 25922 |
| Herpes simplex virus 1 | Clinical specimen |
| Herpes simplex virus 2 | Clinical specimen |
| Kingella kingae | Clinical specimen |
| Lactobacillus spp. | Clinical specimen |
| Mycoplasma pneumoniae | Clinical specimen |
| Neisseria lactamica | Clinical specimen |
| Neisseria subflava | Clinical specimen |
| Neisseria weaveri | Clinical specimen |
| Pseudomonas aeruginosa | ATCC 27853 |
| Staphylococcus aureus | ATCC 43300 |
| Staphylococcus epidermidis | Clinical specimen |
| Streptococcus mitis | Clinical specimen |
| Streptococcus pyogenes | Clinical specimen |
| Varicella zoster virus | Clinical specimen |
| Human DNA | Human Genomic DNA (Roche Diagnostics, Basel, Switzerland) |
| Species | Source/strain |
| Aspergillus fumigatus | Clinical specimen |
| Aspergillus terreus | Clinical specimen |
| Candida albicans | ATCC 90028 |
| Candida glabrata | Clinical specimen |
| Chlamydia pneumoniae | Clinical specimen |
| Chlamydia trachomatis | Clinical specimen |
| Corynebacterium pyogenes | Clinical specimen |
| Escherichia coli | ATCC 25922 |
| Herpes simplex virus 1 | Clinical specimen |
| Herpes simplex virus 2 | Clinical specimen |
| Kingella kingae | Clinical specimen |
| Lactobacillus spp. | Clinical specimen |
| Mycoplasma pneumoniae | Clinical specimen |
| Neisseria lactamica | Clinical specimen |
| Neisseria subflava | Clinical specimen |
| Neisseria weaveri | Clinical specimen |
| Pseudomonas aeruginosa | ATCC 27853 |
| Staphylococcus aureus | ATCC 43300 |
| Staphylococcus epidermidis | Clinical specimen |
| Streptococcus mitis | Clinical specimen |
| Streptococcus pyogenes | Clinical specimen |
| Varicella zoster virus | Clinical specimen |
| Human DNA | Human Genomic DNA (Roche Diagnostics, Basel, Switzerland) |
List of DNA of strains tested to investigate the specificity of the rickettsial PCR
| Species | Source/strain |
| Aspergillus fumigatus | Clinical specimen |
| Aspergillus terreus | Clinical specimen |
| Candida albicans | ATCC 90028 |
| Candida glabrata | Clinical specimen |
| Chlamydia pneumoniae | Clinical specimen |
| Chlamydia trachomatis | Clinical specimen |
| Corynebacterium pyogenes | Clinical specimen |
| Escherichia coli | ATCC 25922 |
| Herpes simplex virus 1 | Clinical specimen |
| Herpes simplex virus 2 | Clinical specimen |
| Kingella kingae | Clinical specimen |
| Lactobacillus spp. | Clinical specimen |
| Mycoplasma pneumoniae | Clinical specimen |
| Neisseria lactamica | Clinical specimen |
| Neisseria subflava | Clinical specimen |
| Neisseria weaveri | Clinical specimen |
| Pseudomonas aeruginosa | ATCC 27853 |
| Staphylococcus aureus | ATCC 43300 |
| Staphylococcus epidermidis | Clinical specimen |
| Streptococcus mitis | Clinical specimen |
| Streptococcus pyogenes | Clinical specimen |
| Varicella zoster virus | Clinical specimen |
| Human DNA | Human Genomic DNA (Roche Diagnostics, Basel, Switzerland) |
| Species | Source/strain |
| Aspergillus fumigatus | Clinical specimen |
| Aspergillus terreus | Clinical specimen |
| Candida albicans | ATCC 90028 |
| Candida glabrata | Clinical specimen |
| Chlamydia pneumoniae | Clinical specimen |
| Chlamydia trachomatis | Clinical specimen |
| Corynebacterium pyogenes | Clinical specimen |
| Escherichia coli | ATCC 25922 |
| Herpes simplex virus 1 | Clinical specimen |
| Herpes simplex virus 2 | Clinical specimen |
| Kingella kingae | Clinical specimen |
| Lactobacillus spp. | Clinical specimen |
| Mycoplasma pneumoniae | Clinical specimen |
| Neisseria lactamica | Clinical specimen |
| Neisseria subflava | Clinical specimen |
| Neisseria weaveri | Clinical specimen |
| Pseudomonas aeruginosa | ATCC 27853 |
| Staphylococcus aureus | ATCC 43300 |
| Staphylococcus epidermidis | Clinical specimen |
| Streptococcus mitis | Clinical specimen |
| Streptococcus pyogenes | Clinical specimen |
| Varicella zoster virus | Clinical specimen |
| Human DNA | Human Genomic DNA (Roche Diagnostics, Basel, Switzerland) |
Conversely, the specificity of the typhus group PCR was confirmed using the DNA from the spotted-group rickettsiae.
Clinical samples
The new rickettsia duplex real-time PCR, that is, the pan-rickettsial PCR and the typhus group rickettsia PCR, was applied to various samples taken from patients with clinical suspicion of rickettsiosis. With one exception, tissue samples were not fixed. DNA was extracted from clinical samples using MagNA Pure LC automated system (Roche) with the MagNA Pure LC DNA isolation kit I (Roche). DNA was extracted from 200 µL of sample and eluted in a final volume of 100 µL.
Results
Analytical sensitivity, reproducibility, and specificity
Sensitivity and reproducibility of the pan-rickettsial real-time PCR are shown in Fig. 1. Fourteen of 25 replicates (56%) were positive with a R. conorii plasmid positive control concentration of one copy per reaction, and all replicates were positive at a concentration of 10 copies. Similar results were obtained with the R. typhi positive control (data not shown). Intra- and inter-run reproducibility was high for both R. conorii and R. typhi positive controls (Figs 1b and 2b). The average difference between tenfold dilutions was 3.24 and 3.17 cycles when testing the R. conorii and R. typhi plasmid, respectively. No cross-amplification was observed with the different microorganisms tested (Table 1). Moreover, with this PCR, we obtained an excellent positive amplification with all the different rickettsial species investigated: R. africae, R. conorii, R. felis, R. rickettsii, R. slovaca, R. prowazekii, and R. typhi (with Ct values ranging from 18.8 to 24.8). Sensitivity of typhus group PCR was 100% at a positive control concentration of 10 copies per reaction for both plasmids (Fig. 2a). Specificity was high, as there was no amplification when testing DNA of R. africae, R. conorii, R. felis, R. rickettsii, and R. slovaca.

Sensitivity and reproducibility of the pan-rickettsial real-time PCR (Rickettsia conorii positive control plasmid). (a) Analytical sensitivity. (b) Inter-run and intra-run reproducibility assessed using 101–104 positive control plasmid copies per reaction (copies per 5 µL) in five independent runs. Error bars represent the standard error of the mean of replicates.
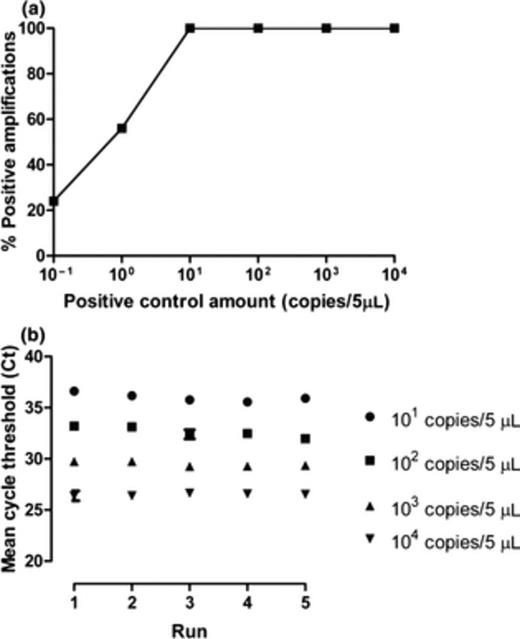
Sensitivity and reproducibility of the typhus group rickettsia real-time PCR (Rickettsia typhi positive control plasmid). (a) Analytical sensitivity. (b) Inter-run and intra-run reproducibility assessed using 101–104 positive control plasmid copies per 5 µL in five independent runs. Error bars represent the standard error of the mean of replicates.
Clinical application
The duplex real-time PCR was applied to 16 specimens taken from 13 patients: eight skin biopsies, four EDTA blood samples, three cerebrospinal fluids (CSF), and one pericardial fluid. Rickettsial DNA was detected in three samples from three different patients, whose clinical characteristics are summarized in Table 2. In one patient, typhus group rickettsia real-time PCR was also positive. This 43-year-old man presented with fever, petechiae, pulmonary infiltrates, acute renal failure, encephalopathy, and hyperbilirubinemia after a stay in Tunisia, where he was in contact with animals in his family farm. At admission, both leptospirosis and rickettsiosis were suspected and he was promptly treated with ceftriaxone and doxycycline. Our duplex real-time PCR performed on a biopsy of a petechial cutaneous lesion (taken 2 days after treatment start) allowed diagnosis of typhus. By contrast, EDTA blood tested negative for rickettsial DNA. During the course of illness, renal-replacement therapy was necessary and he developed heart failure with an ejection fraction of 25%. After 14 days of treatment, he recovered without sequelae. Seroconversion against Rickettsia spp. was documented 17 and 37 days after the onset of symptoms for IgM (titer 1/1024) and IgG (titer 1/128), respectively, but because of cross-reaction, identification at species level was not possible. A presumptive diagnosis of murine typhus was considered based on the clinical presentation, the zoonotic exposure, and the PCR results.
Clinical characteristics and results of laboratory investigations of the three patients with positive PCR
| Patient no. | Age | Sex | Clinical presentation | Laboratory findings | Complications | Travel history | Sample | Rickettsia spp. PCR, DNA copies mL−1 (mean Ct value) | Typhus group PCR, DNA copies mL−1 (mean Ct value) | Serology | Final diagnosis |
| 1 | 35 | F | Fever Headache Rash Eschar | Leucopenia Thrombocytopenia ELT | None | Italy | Skin biopsy | 7886 (31.4) | Negative | NA | MSF |
| 2 | 41 | F | Fever Headache Eschar | NA | None | South Africa | Skin biopsy | 791 (35.9) | Negative | Negative | ATBF |
| 3 | 43 | M | Fever Headache Myalgia Dysphagia Cough Rash | Thrombocytopenia Elevated creatinine ELT Hyperbilirubinemia | Acute renal failure Encephalopathy Myocarditis | Tunisia | Skin biopsy | 137 (39.5) | 171 (40.1) | Seroconversion after 17 days | Murine typhus |
| Patient no. | Age | Sex | Clinical presentation | Laboratory findings | Complications | Travel history | Sample | Rickettsia spp. PCR, DNA copies mL−1 (mean Ct value) | Typhus group PCR, DNA copies mL−1 (mean Ct value) | Serology | Final diagnosis |
| 1 | 35 | F | Fever Headache Rash Eschar | Leucopenia Thrombocytopenia ELT | None | Italy | Skin biopsy | 7886 (31.4) | Negative | NA | MSF |
| 2 | 41 | F | Fever Headache Eschar | NA | None | South Africa | Skin biopsy | 791 (35.9) | Negative | Negative | ATBF |
| 3 | 43 | M | Fever Headache Myalgia Dysphagia Cough Rash | Thrombocytopenia Elevated creatinine ELT Hyperbilirubinemia | Acute renal failure Encephalopathy Myocarditis | Tunisia | Skin biopsy | 137 (39.5) | 171 (40.1) | Seroconversion after 17 days | Murine typhus |
ELT, elevated liver enzymes; NA, not available; MSF, Mediterranean spotted fever; ATBF, African tick bite fever.
Clinical characteristics and results of laboratory investigations of the three patients with positive PCR
| Patient no. | Age | Sex | Clinical presentation | Laboratory findings | Complications | Travel history | Sample | Rickettsia spp. PCR, DNA copies mL−1 (mean Ct value) | Typhus group PCR, DNA copies mL−1 (mean Ct value) | Serology | Final diagnosis |
| 1 | 35 | F | Fever Headache Rash Eschar | Leucopenia Thrombocytopenia ELT | None | Italy | Skin biopsy | 7886 (31.4) | Negative | NA | MSF |
| 2 | 41 | F | Fever Headache Eschar | NA | None | South Africa | Skin biopsy | 791 (35.9) | Negative | Negative | ATBF |
| 3 | 43 | M | Fever Headache Myalgia Dysphagia Cough Rash | Thrombocytopenia Elevated creatinine ELT Hyperbilirubinemia | Acute renal failure Encephalopathy Myocarditis | Tunisia | Skin biopsy | 137 (39.5) | 171 (40.1) | Seroconversion after 17 days | Murine typhus |
| Patient no. | Age | Sex | Clinical presentation | Laboratory findings | Complications | Travel history | Sample | Rickettsia spp. PCR, DNA copies mL−1 (mean Ct value) | Typhus group PCR, DNA copies mL−1 (mean Ct value) | Serology | Final diagnosis |
| 1 | 35 | F | Fever Headache Rash Eschar | Leucopenia Thrombocytopenia ELT | None | Italy | Skin biopsy | 7886 (31.4) | Negative | NA | MSF |
| 2 | 41 | F | Fever Headache Eschar | NA | None | South Africa | Skin biopsy | 791 (35.9) | Negative | Negative | ATBF |
| 3 | 43 | M | Fever Headache Myalgia Dysphagia Cough Rash | Thrombocytopenia Elevated creatinine ELT Hyperbilirubinemia | Acute renal failure Encephalopathy Myocarditis | Tunisia | Skin biopsy | 137 (39.5) | 171 (40.1) | Seroconversion after 17 days | Murine typhus |
ELT, elevated liver enzymes; NA, not available; MSF, Mediterranean spotted fever; ATBF, African tick bite fever.
The two other patients with a positive pan-rickettsial PCR and negative typhus group PCR were clinically and epidemiologically diagnosed with Mediterranean spotted fever and African tick bite fever, after a travel to Sardinia (Southern Italy) and South Africa, respectively. Both presented with fever, headache, and an inoculation eschar, while only the patient with Mediterranean spotted fever exhibited a rash and a severe disease requiring hospitalization. Diagnosis was established in both patients by a PCR performed on the biopsy of the inoculation eschar.
Ten patients with negative duplex real-time PCR results presented with various clinical syndromes: encephalitis (2), fever and rash (1), fever in a returning traveler (1), fever of unknown origin in a HIV-positive patient (1), hemophagocytosis syndrome (1), acute liver failure and pericardial tamponade (1), skin nodules (1), and bilateral lung infiltrates (1). In one case, no clinical information was available. Five patients had a history of travel to an endemic region. Serology for Rickettsia spp. was performed in six cases and was negative or showed a pattern of past infection.
Discussion
A duplex real-time PCR targeting all rickettsial species and the typhus group rickettsiae was developed to detect rickettsial DNA in clinical samples and to identify agents of typhus. The test was sensitive for at least 10 DNA copies per reaction and exhibited a good reproducibility. Its application to clinical samples (skin biopsies) from patients with clinical suspicion of rickettsiosis allowed diagnosis of spotted fever in two cases and recognition of murine typhus in another case.
Timely diagnosis of rickettsiosis can be challenging, as seroconversion occurs usually in the convalescent phase (Brouqui et al., 2004). For example, using indirect fluorescent antibody assay, diagnostic titers of R. typhi antibodies are found 15 days after the onset of symptoms (Dumler et al., 1991). For R. conori and R. africae, median time to IgM seroconversion is even longer (16 and 25 days, respectively) (Fournier et al., 2002). Therefore, PCR represents an interesting alternative diagnostic approach. Since the first report of use of molecular methods for the detection of rickettsiosis (Tzianabos et al., 1989), several assays have been developed. Primers have usually targeted outer membrane protein genes ompA (Fournier & Raoult, 2004) and ompB (Paris et al., 2008), the citrate synthase gene gltA (Roux et al., 1997), and the 17-kD protein gene (Leitner et al., 2002). In this work, we selected the 16S rRNA gene for the Rickettsia spp. PCR and were able to target a gene region that was conserved among all rickettsial species. The gltA gene was chosen for the typhus group rickettsia, because of its higher variability.
Because of its broad spectrum and the low discriminative power of the 16S rRNA gene, our pan-rickettsial PCR is not able to precisely identify the rickettsia at species level. Species identification is warranted, as several species can be responsible of the same clinical picture. Practically, we recommend DNA amplification and sequencing of gltA and ompA genes (Fournier & Raoult, 2004), which allow identification at species and subspecies level (Boillat et al., 2008).
Despite the limited number of samples tested and their heterogeneity, the clinical experience with the new duplex real-time PCR is encouraging. Since its development in 2007, we could confirm the clinical suspicion of rickettsial infection in three cases. Moreover, the test allowed rapid identification of typhus in a patient with a severe febrile illness after a stay in Tunisia. Clinical presentation was nonspecific, and in particular, leptospirosis was suspected initially because of the triad of rash, hyperbilirubinemia, and acute renal failure. This case illustrates that clinical recognition of rickettsiosis may be difficult and that empirical treatment with doxycycline is indicated in case of severe illnesses in returning travelers.
It should be noted that only skin biopsies were positive in this series. PCR has been successfully applied to blood and serum samples (Leitner et al., 2002; Choi et al., 2005), arthropod vectors (Karpathy et al., 2009), but most studies have used skin biopsies (Fournier & Raoult, 2004). Biopsies of the inoculation eschar, when present, have the best diagnostic efficiency (Fournier & Raoult, 2004). In case of rickettsioses that are not associated with an eschar (e.g. murine typhus), skin biopsy should be performed on skin lesions (maculopapular or petechial lesions), as endothelial cells are the site of multiplication of rickettsiae (Walker et al., 2003) and as these skin lesions generally result from local rickettsial multiplication. As highlighted in this case, PCR of a skin lesion may remain positive even after a few days of doxycycline treatment.
In conclusion, we have developed a duplex real-time PCR for the direct detection of rickettsial DNA and for the identification of typhus group rickettsia. Optimal use of the assay includes its application to skin biopsy of patients presenting a clinical picture and epidemiological features compatible with a rickettsial infection. Furthermore, the broad-range format of the pan-rickettsial PCR may allow the identification of new rickettsial species.
References
Author notes
Editor: Achilles Gikas



