-
PDF
- Split View
-
Views
-
Cite
Cite
Zuzana Sekeyova, Geetha Subramanian, Oleg Mediannikov, Marco Quevedo Diaz, Alexander Nyitray, Hana Blaskovicova, Didier Raoult, Evaluation of clinical specimens for Rickettsia, Bartonella, Borrelia, Coxiella, Anaplasma, Franciscella and Diplorickettsia positivity using serological and molecular biology methods, FEMS Immunology & Medical Microbiology, Volume 64, Issue 1, February 2012, Pages 82–91, https://doi.org/10.1111/j.1574-695X.2011.00907.x
Close - Share Icon Share
Abstract
We monitored clinical samples from patients of different age groups from selected regions in Slovakia. Overall seroprevalence evaluated by immunofluorescence (IFA) using nine Bartonella, two Borrelia, six rickettsial (spotted fever and typhus group), two Coxiella, and one human granulocytic ehrlichiosis Anaplasma, Franciscella tularensis and Diplorickettsia massiliensis antigens, in rural and city populations of Slovak Republic, was found to be 32% positive for spotted fever group rickettsiae. Only five (10%) of the rickettsia-positive cases evaluated by IFA were confirmed by polymerase chain reaction. Rickettsia helvetica, Rickettsia slovaca, and Rickettsia raoultii infection appear to be prevalent in Slovakia. Furthermore, Coxiella burnetii, Borrelia and, for the first time, Bartonella elisabethae were confirmed in Slovakia.
Introduction
The manifestation of clinical symptoms after a tick or insect bite, for example high fever, vomiting, diarrhea and headache, can probably be considered partly specific for hourly studied diseases. Nevertheless, similar or the same symptoms manifest in several other diseases, including colds or flu, and thus can easily imitate the origin of the disease.
Immunofluorescent antibody assay (IFA) using acute phase sera is generally regarded as the most convenient and sensitive serological procedure to identify bacteria (Philip et al., 1978; Kovacova et al., 1994; McGill et al., 2001; Houhamdi & Raoult, 2005). The method can detect immunoglobulin G (IgG) and IgM antibodies with a sensitivity rate of 84–100% (Beati et al., 1992; Teysseire & Raoult, 1992). However, even this technique can be limited by possible cross-reactions, as nonspecific lipopolysaccharide reactions have been found to involve immunoglobulin M (IgM) antibodies. A possibility of reduced species specificity can be circumvented by using a multiple-antigen IFA (Jensenius et al., 2004), and precision can be increased by the application of molecular genetic methods.
We have used IFA to evaluate clinical specimens for Rickettsia, Bartonella, Borrelia, Coxiella, Anaplasma, Franciscella and Diplorickettsia. All serum samples included in this study were obtained from hospitalized patients with ‘a disease of unknown etiology’ which had tested negative for viral infections. We have meticulously chosen the list of bacteria to test.
Rickettsia are common tick parasites causing severe human diseases (Sekeyova et al., 1998; Kovacova et al., 2006; Santibanez et al., 2006; Sreter-Lancz et al., 2006; Spitalska et al., 2008; Chmielewski et al., 2009; Dobler & Wolfel, 2009), and Bartonella, which has been recovered from the blood of humans, is quite common in Europe (Vinson & Fuller, 1961; Chomel et al., 1997; Piemont & Heller, 1998, 1999; La et al., 2002). We have included also a ‘Pandora's Box’ — expected pathogens in Ixodes ricinus ticks in Central Europe that have a high infectivity in the human population, for example Borrelia (Bhide et al., 2005; Derdakova & Lencakova, 2005), Anaplasma (Stanek, 2009) and Franciscella (Gurycova et al., 2001, 2010). Coxiella is one of the bacteria that may trigger severe epidemics in Europe (Serbezov et al., 1999; Kovacova & Kazar, 2002; Delsing & Kullberg, 2008). Franciscella tularensis, known to be present in Czechoslovakia at least since 1967 (Lukas, 1967), was isolated for the first time in 1996 (Gurycova, 1998). No data are available about Diplorickettsia massiliensis in relation to humans (Mediannikov et al., 2010).
In this study we screened serum samples with IFA, polymerase chain reaction (PCR) and sequencing, to identify precisely human infections of bacterial origin that are circulating in Slovakia.
Materials and methods
Antigens used in IFA
A complete inventory of antigens applied in the IFA together with the origin of the strains and isolates are listed in Table 1. They were prepared as described previously (Teysseire & Raoult, 1992; Cardenosa et al., 2003; Rolain et al., 2003).
| No. of patient | Name of the antigen | Collection No. or source |
| 1 | Bartonella henselae (Houston-1T) | ATCC 49882 |
| 2 | Bartonella henselae (Marseille) | RCR (Drancourt et al., 1996) |
| 3 | Bartonella quintana (OklahomaT) | CDC |
| 4 | Bartonella alsatica (IBS382T) | CIP 105477 |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | ATCC 51672 |
| 6 | Bartonella ‘weissi’ (FC7049UT) | Bermond et al. (2002) |
| 7 | Bartonella grahamii (V2T) | NCTC 12860 |
| 8 | Bartonella elizabethae (F9251T) | ATCC 49927 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | ATCC 700727 |
| 10 | Rickettsia slovaca, strain B | Kindly provided by Brezina, Bratislava, VU SAS |
| 11 | Rickettesia conorii, strain Malish | ATCC VR-613T |
| 12 | Rickettsia raoultii, strain Khabarovsk | VR-1596 (T) RCR |
| 13 | Rickettsia mongolotimonae, strain HA-91 | RCR (Fournier et al., 2005) |
| 14 | Rickettsia helvetica, strain C3 | Kindly provided by RML |
| 15 | Rickettsia felis, strain URRWXCal2 | ATTC VR-1525 |
| 16 | Borrelia burgdorferi | ATTC 35210 |
| 17 | Borrelia recurrentis | ATTC700241 |
| 18 | Coxiella burnetii (phase I) Nine Mile | RSA 493 |
| 19 | Coxiella burnetii (phase II) Nine Mile | ATCC VR 615 |
| 20 | Diplorickettsia massiliensis | RCR |
| 21 | HGE Anaplasma, strain Webster | Kindly provided by J. S. Dumler, Baltimore |
| 22 | Francisella tularensis | URFT1 (Fournier et al., 1998a) |
| No. of patient | Name of the antigen | Collection No. or source |
| 1 | Bartonella henselae (Houston-1T) | ATCC 49882 |
| 2 | Bartonella henselae (Marseille) | RCR (Drancourt et al., 1996) |
| 3 | Bartonella quintana (OklahomaT) | CDC |
| 4 | Bartonella alsatica (IBS382T) | CIP 105477 |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | ATCC 51672 |
| 6 | Bartonella ‘weissi’ (FC7049UT) | Bermond et al. (2002) |
| 7 | Bartonella grahamii (V2T) | NCTC 12860 |
| 8 | Bartonella elizabethae (F9251T) | ATCC 49927 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | ATCC 700727 |
| 10 | Rickettsia slovaca, strain B | Kindly provided by Brezina, Bratislava, VU SAS |
| 11 | Rickettesia conorii, strain Malish | ATCC VR-613T |
| 12 | Rickettsia raoultii, strain Khabarovsk | VR-1596 (T) RCR |
| 13 | Rickettsia mongolotimonae, strain HA-91 | RCR (Fournier et al., 2005) |
| 14 | Rickettsia helvetica, strain C3 | Kindly provided by RML |
| 15 | Rickettsia felis, strain URRWXCal2 | ATTC VR-1525 |
| 16 | Borrelia burgdorferi | ATTC 35210 |
| 17 | Borrelia recurrentis | ATTC700241 |
| 18 | Coxiella burnetii (phase I) Nine Mile | RSA 493 |
| 19 | Coxiella burnetii (phase II) Nine Mile | ATCC VR 615 |
| 20 | Diplorickettsia massiliensis | RCR |
| 21 | HGE Anaplasma, strain Webster | Kindly provided by J. S. Dumler, Baltimore |
| 22 | Francisella tularensis | URFT1 (Fournier et al., 1998a) |
CIP, Collection de l'Institut Pasteur; Paris, France; ATCC, American Type Culture Collection, Manassas, VA; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom; CDC, Centers for Disease Control and Prevention, Atlanta, Ga; IV SAS, Virological Institute Slovak Academy of Sciences, Bratioslava Slovakia; RCR, Reference Center for Rickettsioses, Faculty of Medicine, Marseille, France; RML, Rocky Mountain Laboratories, Hamilton, MT.
| No. of patient | Name of the antigen | Collection No. or source |
| 1 | Bartonella henselae (Houston-1T) | ATCC 49882 |
| 2 | Bartonella henselae (Marseille) | RCR (Drancourt et al., 1996) |
| 3 | Bartonella quintana (OklahomaT) | CDC |
| 4 | Bartonella alsatica (IBS382T) | CIP 105477 |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | ATCC 51672 |
| 6 | Bartonella ‘weissi’ (FC7049UT) | Bermond et al. (2002) |
| 7 | Bartonella grahamii (V2T) | NCTC 12860 |
| 8 | Bartonella elizabethae (F9251T) | ATCC 49927 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | ATCC 700727 |
| 10 | Rickettsia slovaca, strain B | Kindly provided by Brezina, Bratislava, VU SAS |
| 11 | Rickettesia conorii, strain Malish | ATCC VR-613T |
| 12 | Rickettsia raoultii, strain Khabarovsk | VR-1596 (T) RCR |
| 13 | Rickettsia mongolotimonae, strain HA-91 | RCR (Fournier et al., 2005) |
| 14 | Rickettsia helvetica, strain C3 | Kindly provided by RML |
| 15 | Rickettsia felis, strain URRWXCal2 | ATTC VR-1525 |
| 16 | Borrelia burgdorferi | ATTC 35210 |
| 17 | Borrelia recurrentis | ATTC700241 |
| 18 | Coxiella burnetii (phase I) Nine Mile | RSA 493 |
| 19 | Coxiella burnetii (phase II) Nine Mile | ATCC VR 615 |
| 20 | Diplorickettsia massiliensis | RCR |
| 21 | HGE Anaplasma, strain Webster | Kindly provided by J. S. Dumler, Baltimore |
| 22 | Francisella tularensis | URFT1 (Fournier et al., 1998a) |
| No. of patient | Name of the antigen | Collection No. or source |
| 1 | Bartonella henselae (Houston-1T) | ATCC 49882 |
| 2 | Bartonella henselae (Marseille) | RCR (Drancourt et al., 1996) |
| 3 | Bartonella quintana (OklahomaT) | CDC |
| 4 | Bartonella alsatica (IBS382T) | CIP 105477 |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | ATCC 51672 |
| 6 | Bartonella ‘weissi’ (FC7049UT) | Bermond et al. (2002) |
| 7 | Bartonella grahamii (V2T) | NCTC 12860 |
| 8 | Bartonella elizabethae (F9251T) | ATCC 49927 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | ATCC 700727 |
| 10 | Rickettsia slovaca, strain B | Kindly provided by Brezina, Bratislava, VU SAS |
| 11 | Rickettesia conorii, strain Malish | ATCC VR-613T |
| 12 | Rickettsia raoultii, strain Khabarovsk | VR-1596 (T) RCR |
| 13 | Rickettsia mongolotimonae, strain HA-91 | RCR (Fournier et al., 2005) |
| 14 | Rickettsia helvetica, strain C3 | Kindly provided by RML |
| 15 | Rickettsia felis, strain URRWXCal2 | ATTC VR-1525 |
| 16 | Borrelia burgdorferi | ATTC 35210 |
| 17 | Borrelia recurrentis | ATTC700241 |
| 18 | Coxiella burnetii (phase I) Nine Mile | RSA 493 |
| 19 | Coxiella burnetii (phase II) Nine Mile | ATCC VR 615 |
| 20 | Diplorickettsia massiliensis | RCR |
| 21 | HGE Anaplasma, strain Webster | Kindly provided by J. S. Dumler, Baltimore |
| 22 | Francisella tularensis | URFT1 (Fournier et al., 1998a) |
CIP, Collection de l'Institut Pasteur; Paris, France; ATCC, American Type Culture Collection, Manassas, VA; NCTC, National Collection of Type Cultures, Central Public Health Laboratory, London, United Kingdom; CDC, Centers for Disease Control and Prevention, Atlanta, Ga; IV SAS, Virological Institute Slovak Academy of Sciences, Bratioslava Slovakia; RCR, Reference Center for Rickettsioses, Faculty of Medicine, Marseille, France; RML, Rocky Mountain Laboratories, Hamilton, MT.
Origin of patient sera
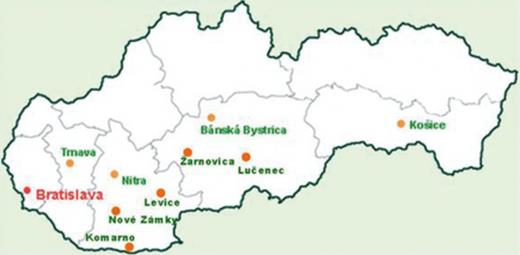
We tested 50 serum samples from patients with suspected tick-borne diseases received in Department of Rickettsiology (Bratislava, Slovakia) in the year 2009. Sera were obtained from hospitalized patients in southeastern regions of Slovakia (Table 3).
The sera included into this study were selected and obtained from the ‘bank of sera’ from patients that were sent to the Public Health Authority, Center of Infectology, based on the diagnoses provided by local doctors (hospitalized following tick or insect bite), and originated from localities that were monitored because several cases of ‘undetermined’ zoonoses had occurred.
Immunofluorescence
Serum specimens were tested with IFA using a large panel of antigens: D. massiliensis, Coxiella burnetii, Rickettsia spp., Bartonella sp., Borrelia sp., Anaplasma phagocytophillum and F. tularensis.
In total, 50 serum samples were screened by IFA in three dilutions (1/25, 1/50 and 1/100) for the presence of total IG, IgG and IgM against the listed bacteria. IgG titers of ≥ 1 : 50 were considered ‘suspicious’, and IgG of ≥ 1 : 100 and IgM titers of ≥ 1 : 50 were considered positive. The studies were approved by the local ethical committee. An unrelated bacterium was used as negative control, for example members of the unrelated families Anaplasmataceae, Bartonellaceae and Coxiellaceae, non-rickettsial agents, served as negative controls for rickettsiae.
IFA samples of ≥ 1 : 50 were tested further by PCR using bacteria-specific primers.
Isolation of DNA
Genomic DNA was extracted using Qiagen columns (QIAamp tissue kit; Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Polymerase chain reaction
To perform the PCR amplifications, we chose a universal 16S DNA gene (Roux & Raoult, 1995a). PCRs were carried out in a Peltier Thermal Cycler PTC-200 (MJ Research, Inc., Watertown, MA). The individual primer sets were as follows: (GCT TAA CAC ATG CAA G) and (CCA TTG TAG CAC GCG T).
Rickettsia-specific PCR amplifications were performed with primers CS-877 (GGG GGC CTG CTC ACG GCG G) and CS-1273 (CAT AAC CAG TGT AAA GCT G) obtained from the gene coding for Rickettsia citrate synthase (gltA) (Regnery et al., 1991; Roux et al., 1997).
To amplify a 70-bp fragment targeting C. burnetii insertion element IS1111 (Denison et al., 2007), we applied a forward primer AAA ACG GAT AAA AAG AGT CTG TGG TT and a reverse primer CCA CAC AAG CGC GAT TCA T.
The primers QHVE1 (TTC AGA TGA TGA TCC CAA) and QHVE3 (GAT ATA TTC AGA CAT GTT), which amplified a fragment of variable size of the 16S–23S rRNA intergenic spacer (ITS) region, were used for confirmation of Bartonella (Roux & Raoult, 1995b).
Borrelia was specified with 16S rRNA-encoding gene (Raoult et al., 1998). Primers Bf1 (GCT GGC AGT GCG TCT TAA GC) and Br1 (GCT TCG GGT ATC CTC AAC TC) were functional testing samples.
The positivity of the amplification was confirmed by electrophoresis in a 1% agarose gel. The sizes of the PCR amplification products were determined by comparison with the molecular weight standard marker VI (Boehringer).
If the amplification was positive, the PCR products were purified with Qiagen columns (QIAquick Spin PCR purification kit; Qiagen) and subsequently sequenced.
Results
Fifty serum samples were collected between days 1 and 45 after the onset of symptoms, selected from a prospective cohort study of severe affection after a tick or insect bite from 150 consecutive patients assigned with ‘unknown etiology’, obtained from various rural localities in the southeastern part of Slovakia (results shown in Table 2, Fig. 3). After excluding viral infection (tick-borne encephalitis, haemorrhagic fever), we tested them to examine the possibility of a bacterial origin of the disease. The selection for bacterial infections was done according to disease symptoms, epidemiological and clinical criteria, including myalgia and fever commencing no later than 10 days after a bite. Twenty-seven (54%) female patients and 23 (46%) males of different age groups (from a 3-year-old child to an adult of 79 years) were included in the study. Forty-five patients were treated with antibiotics (tetracycline or doxycycline), one (no. 37) had a complicated course of illness (sarcoid myocarditis), and all of patients were hospitalized.
Immunofluorescence
All 50 serum samples were examined with the 22-antigen IFA (Tables 2 and 3). A multiple-antigen IFA was performed as previously reported (Fournier et al., 1998b), using three IgG and/or IgM titers of ≥ 1 : 25, ≥ 1: 50, ≥ 1 : 100 against any of the tested species. We detected 16 (32%) rickettsia-positive cases. IgG titers ≥ 1 : 100 in two cases were considered serological evidence of rickettsial infection, which was triggered by Rickettsia helvetica (no. 25, village Horča), and Rickettsia raoultii (no. 46, county of Lučenec). We identified sera from eight patients with a titer of ≥ 1 : 50 against R. helvetica [from the city of Levice (Nos 3, 5, 13), the villages of Kukučínov (no. 23) and Ondrejovce (no. 24) from the county of Levice, the villages of Mankovce (no. 31) and Svodín (Nos 32, 33) from the county of Zlaté Moravce], and sera from two patients against Rickettsia slovaca (Nos 3 and 11) and one against Rickettsia conorii (no. 11), both from Levice (Table 1). A certain cross-reactivity with other rickettsia-tested bacteria was detected, for example samples Nos 3, 5, 23, and 32, which also reacted with Bartonella and Borrelia antigens.
| No. of patient | Organism | Total IG No. of positive/titer/sample | IgM No. of positive/titer/sample | IgG No. of positive/titer/sample |
| 1 | Bartonella henselae (Houston-1T) | 1/1 : 50/2 | 1/1 : 50/2 | – |
| 2 | Bartonella henselae (Marseille) | – | – | – |
| 3 | Bartonella quintana (OklahomaT) | 2/1 : 50/3, 18 | 2/1 : 50/3, 18 | – |
| 4 | Bartonella alsatica (IBS382T) | – | – | – |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | – | – | – |
| 6 | Bartonella ‘weissi’ (FC7049UT) | – | – | – |
| 7 | Bartonella grahamii (V2T) | 2/1 : 50/3, 23 | 3/1 : 50/34, 3, 23 | 3/1 : 50/34, 3, 23 |
| 8 | Bartonella elizabethae (F9251T) | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | – | – | – |
| 10 | Rickettsia slovaca, strain B | 1/1 : 50/11 | 2/1 : 50/3, 11 | 2/1 : 50/3, 11 |
| 11 | Rickettsia conorii, strain Malish | 1/1 : 50/11 | 1/1 : 50/11 | 1/1 : 50/11 |
| 12 | Rickettsia raoultii, strain Khabarovsk | 3/1 : 50/11, 25, 46 | 1/1 : 50/11 | 2/1 : 100/25, 46 |
| 13 | Rickettsia mongolotimonae, strain HA-91 | – | – | – |
| 14 | Rickettsia helvetica, strain C3 | 10/1 : 50 | 8/1 : 50/3, 5, 13, 23, 24, 31, 32, 33 | 2/1 : 100/25, 46 |
| 15 | Rickettsia felis, strain URRWXCal2 | – | – | – |
| 16 | Borrelia burgdorferi | 1/1 : 50/464 | – | – |
| 17 | Borrelia recurentis | 2/1 : 50/5, 18 | 1/1 : 50/5 | 1/1 : 50/5, 18 |
| 18 | Coxiella burnetii (phase I) Nine Mile | 1/1 : 50/37 | 1/1 : 50/37 | – |
| 19 | Coxiella burnetii (phase II) Nine Mile | 2/1 : 50/37, 47 | 2/1 : 50/37, 47 | 1/1 : 50/37 |
| 20 | Diplorickettsia massiliensis | – | – | – |
| 21 | HGE Anaplasma | – | – | – |
| 22 | Francisella tularensis | – | 1/1 : 50/2 | – |
| No. of patient | Organism | Total IG No. of positive/titer/sample | IgM No. of positive/titer/sample | IgG No. of positive/titer/sample |
| 1 | Bartonella henselae (Houston-1T) | 1/1 : 50/2 | 1/1 : 50/2 | – |
| 2 | Bartonella henselae (Marseille) | – | – | – |
| 3 | Bartonella quintana (OklahomaT) | 2/1 : 50/3, 18 | 2/1 : 50/3, 18 | – |
| 4 | Bartonella alsatica (IBS382T) | – | – | – |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | – | – | – |
| 6 | Bartonella ‘weissi’ (FC7049UT) | – | – | – |
| 7 | Bartonella grahamii (V2T) | 2/1 : 50/3, 23 | 3/1 : 50/34, 3, 23 | 3/1 : 50/34, 3, 23 |
| 8 | Bartonella elizabethae (F9251T) | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | – | – | – |
| 10 | Rickettsia slovaca, strain B | 1/1 : 50/11 | 2/1 : 50/3, 11 | 2/1 : 50/3, 11 |
| 11 | Rickettsia conorii, strain Malish | 1/1 : 50/11 | 1/1 : 50/11 | 1/1 : 50/11 |
| 12 | Rickettsia raoultii, strain Khabarovsk | 3/1 : 50/11, 25, 46 | 1/1 : 50/11 | 2/1 : 100/25, 46 |
| 13 | Rickettsia mongolotimonae, strain HA-91 | – | – | – |
| 14 | Rickettsia helvetica, strain C3 | 10/1 : 50 | 8/1 : 50/3, 5, 13, 23, 24, 31, 32, 33 | 2/1 : 100/25, 46 |
| 15 | Rickettsia felis, strain URRWXCal2 | – | – | – |
| 16 | Borrelia burgdorferi | 1/1 : 50/464 | – | – |
| 17 | Borrelia recurentis | 2/1 : 50/5, 18 | 1/1 : 50/5 | 1/1 : 50/5, 18 |
| 18 | Coxiella burnetii (phase I) Nine Mile | 1/1 : 50/37 | 1/1 : 50/37 | – |
| 19 | Coxiella burnetii (phase II) Nine Mile | 2/1 : 50/37, 47 | 2/1 : 50/37, 47 | 1/1 : 50/37 |
| 20 | Diplorickettsia massiliensis | – | – | – |
| 21 | HGE Anaplasma | – | – | – |
| 22 | Francisella tularensis | – | 1/1 : 50/2 | – |
| No. of patient | Organism | Total IG No. of positive/titer/sample | IgM No. of positive/titer/sample | IgG No. of positive/titer/sample |
| 1 | Bartonella henselae (Houston-1T) | 1/1 : 50/2 | 1/1 : 50/2 | – |
| 2 | Bartonella henselae (Marseille) | – | – | – |
| 3 | Bartonella quintana (OklahomaT) | 2/1 : 50/3, 18 | 2/1 : 50/3, 18 | – |
| 4 | Bartonella alsatica (IBS382T) | – | – | – |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | – | – | – |
| 6 | Bartonella ‘weissi’ (FC7049UT) | – | – | – |
| 7 | Bartonella grahamii (V2T) | 2/1 : 50/3, 23 | 3/1 : 50/34, 3, 23 | 3/1 : 50/34, 3, 23 |
| 8 | Bartonella elizabethae (F9251T) | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | – | – | – |
| 10 | Rickettsia slovaca, strain B | 1/1 : 50/11 | 2/1 : 50/3, 11 | 2/1 : 50/3, 11 |
| 11 | Rickettsia conorii, strain Malish | 1/1 : 50/11 | 1/1 : 50/11 | 1/1 : 50/11 |
| 12 | Rickettsia raoultii, strain Khabarovsk | 3/1 : 50/11, 25, 46 | 1/1 : 50/11 | 2/1 : 100/25, 46 |
| 13 | Rickettsia mongolotimonae, strain HA-91 | – | – | – |
| 14 | Rickettsia helvetica, strain C3 | 10/1 : 50 | 8/1 : 50/3, 5, 13, 23, 24, 31, 32, 33 | 2/1 : 100/25, 46 |
| 15 | Rickettsia felis, strain URRWXCal2 | – | – | – |
| 16 | Borrelia burgdorferi | 1/1 : 50/464 | – | – |
| 17 | Borrelia recurentis | 2/1 : 50/5, 18 | 1/1 : 50/5 | 1/1 : 50/5, 18 |
| 18 | Coxiella burnetii (phase I) Nine Mile | 1/1 : 50/37 | 1/1 : 50/37 | – |
| 19 | Coxiella burnetii (phase II) Nine Mile | 2/1 : 50/37, 47 | 2/1 : 50/37, 47 | 1/1 : 50/37 |
| 20 | Diplorickettsia massiliensis | – | – | – |
| 21 | HGE Anaplasma | – | – | – |
| 22 | Francisella tularensis | – | 1/1 : 50/2 | – |
| No. of patient | Organism | Total IG No. of positive/titer/sample | IgM No. of positive/titer/sample | IgG No. of positive/titer/sample |
| 1 | Bartonella henselae (Houston-1T) | 1/1 : 50/2 | 1/1 : 50/2 | – |
| 2 | Bartonella henselae (Marseille) | – | – | – |
| 3 | Bartonella quintana (OklahomaT) | 2/1 : 50/3, 18 | 2/1 : 50/3, 18 | – |
| 4 | Bartonella alsatica (IBS382T) | – | – | – |
| 5 | Bartonella vinsonii ssp. berkhoffii (93-CO1T) | – | – | – |
| 6 | Bartonella ‘weissi’ (FC7049UT) | – | – | – |
| 7 | Bartonella grahamii (V2T) | 2/1 : 50/3, 23 | 3/1 : 50/34, 3, 23 | 3/1 : 50/34, 3, 23 |
| 8 | Bartonella elizabethae (F9251T) | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 | 4/1 : 50/3, 23, 32, 34 |
| 9 | Bartonella vinsonii ssp. arupensis (OK 94-513T) | – | – | – |
| 10 | Rickettsia slovaca, strain B | 1/1 : 50/11 | 2/1 : 50/3, 11 | 2/1 : 50/3, 11 |
| 11 | Rickettsia conorii, strain Malish | 1/1 : 50/11 | 1/1 : 50/11 | 1/1 : 50/11 |
| 12 | Rickettsia raoultii, strain Khabarovsk | 3/1 : 50/11, 25, 46 | 1/1 : 50/11 | 2/1 : 100/25, 46 |
| 13 | Rickettsia mongolotimonae, strain HA-91 | – | – | – |
| 14 | Rickettsia helvetica, strain C3 | 10/1 : 50 | 8/1 : 50/3, 5, 13, 23, 24, 31, 32, 33 | 2/1 : 100/25, 46 |
| 15 | Rickettsia felis, strain URRWXCal2 | – | – | – |
| 16 | Borrelia burgdorferi | 1/1 : 50/464 | – | – |
| 17 | Borrelia recurentis | 2/1 : 50/5, 18 | 1/1 : 50/5 | 1/1 : 50/5, 18 |
| 18 | Coxiella burnetii (phase I) Nine Mile | 1/1 : 50/37 | 1/1 : 50/37 | – |
| 19 | Coxiella burnetii (phase II) Nine Mile | 2/1 : 50/37, 47 | 2/1 : 50/37, 47 | 1/1 : 50/37 |
| 20 | Diplorickettsia massiliensis | – | – | – |
| 21 | HGE Anaplasma | – | – | – |
| 22 | Francisella tularensis | – | 1/1 : 50/2 | – |
| Origin of patient | Sex | ||||||||
| No. of serum | City | County (district) | Village | Sample name | Date of serum | ♀ | ♂ | IFA organism detected | PCR bacteria detected/GenBank accession number |
| 1 | Nitra | Vráble | 509 Bra V | 2008 | 1 | n | n | ||
| 2 | Levice | 451 Ben S | 1985 | 1 | Francisella tularensis | ||||
| 3 | 452 Pri M | 1990 | 1 | Rickettsia helvetica, Rickettsia slovaca, Rickettsia quintana, Bartonella elizabethae, Bartonella grahamii | Rickettsia helvetica/U59 723.1 | ||||
| 4 | 453 Has J | 1950 | 1 | n | |||||
| 5 | 464 Fer I | 1981 | 1 | Borrelia recurentis, Rickettsia helvetica, Borrelia burgdorferi | Bartonella burgdorferi? | ||||
| 6 | 465 Med P | 1977 | 1 | n | n | ||||
| 7 | 466 Pag V | 1954 | 1 | n | n | ||||
| 8 | 580 Tur J | 1972 | 1 | n | n | ||||
| 9 | 599 Kla L | 1970 | 1 | n | n | ||||
| 10 | 431 Stu I | 1971 | 1 | n | n | ||||
| 11 | 690 Pas E | 1960 | 1 | R. slovaca, R. helvetica, R. raoultii, R. conorii | Rickettsia slovaca/U59 725.1 | ||||
| 12 | 503 Med M | 1981 | 1 | n | n | ||||
| 13 | 715 Tak M | 1977 | 1 | R. helvetica | n | ||||
| 14 | 728 Dek J | 1944 | 1 | n | n | ||||
| 15 | 760 Cso V | 1954 | 1 | n | n | ||||
| 16 | 776 Sip I | 1994 | 1 | n | n | ||||
| 17 | Levice | Farná | 350 Ban R | 1997 | 1 | n | n | ||
| 18 | Plášt'ovce | 351 Kor A | 1933 | 1 | Borrelia recurentis, Bartonella quintana, Bartonella henselae | ||||
| 19 | Žemberovce | 582 Sik O | 1945 | 1 | n | n | |||
| 20 | Žemberovce | 605 Ruf V | 1953 | 1 | n | n | |||
| 21 | Slatina | 604 Pat M | 1961 | 1 | n | n | |||
| 22 | Bory | 630 Lip P | 1993 | 1 | n | n | |||
| 23 | Kukučínov | 700 Srn M | 1993 | 1 | Rickettsia helvetica, Bartonella elizabethae, Bartonella grahamii | n | |||
| 24 | Ondrejovce | 702 Uhn A | 1947 | 1 | Rickettsia helvetica | n | |||
| 25 | Horča | 711 Mra R | 1973 | 1 | Rickettsia helvetica, Rickettsia raoultii | Rickettsia helvetica/U59 723.1 | |||
| 26 | Hronské Kl'ačany | 714 Kra A | 1956 | 1 | n | n | |||
| 27 | Kural'any | 729 Bie M | 1945 | 1 | n | n | |||
| 28 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 29 | Santovka | 786 Dek M | 1952 | 1 | n | n | |||
| 30 | Čaka | 793 Bie Z | 1965 | 1 | n | n | |||
| 31 | Zlaté Moravce | Mankovce | 719 Pau M | 1989 | 1 | Rickettsia helvetica | Rickettsia helvetica/U59723.1 | ||
| 32 | Svodín | 568 Mer M | 2001 | 1 | Rickettsia helvetica, Bartonella elizabethae | n | |||
| 33 | Svodín | 477 Jon D | 1990 | 1 | Rickettsia helvetica | n | |||
| 34 | Nové Zámky | 364 Var M | 1931 | 1 | Bartonella elizabethae, Bartonella grahamii | Bartonella elizabethae/L35103.1 | |||
| 35 | 731 Sche I | 1980 | 1 | n | n | ||||
| 36 | Nové Zámky | Nové Zámky | Palárikovo | 750 Fil T | 1990 | 1 | n | n | |
| 37 | Zemné | 595 Nag H | 1951 | 1 | Coxiella burnetii Ph I, Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |||
| 38 | Komárno | 396 Jan A | 1963 | 1 | n | n | |||
| 39 | 564 Oll V | 1958 | 1 | n | n | ||||
| 40 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 41 | Vráble | 509 Bra V | 2008 | 1 | n | n | |||
| 42 | Banská Bystrica | Žarnovica | Nová Baňa | 709 Bra J | 1957 | 1 | n | n | |
| 43 | Ostrý Grúň | 583 Vid S | 1947 | 1 | n | n | |||
| 44 | Banská Bystrica | Lučenec | 262 Jel G | 1983 | 1 | n | n | ||
| 45 | 264 Neu M | 1942 | 1 | n | n | ||||
| 46 | 747 Rad J | 1956 | 1 | Rickettsia raoultii, Rickettsia helvetica | Rickettsia raoultii/EU036985 | ||||
| 47 | Banská Bystrica | Vel'ký Krtíš | Vinica | 782 Tre A | 1957 | 1 | Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |
| 48 | Trnava | Galanta | 758 Kor D | 2003 | 1 | n | n | ||
| 49 | Košice | 337 Gaj S | 1936 | 1 | n | n | |||
| 50 | 338 Sal M | 1947 | 1 | n | n | ||||
| Origin of patient | Sex | ||||||||
| No. of serum | City | County (district) | Village | Sample name | Date of serum | ♀ | ♂ | IFA organism detected | PCR bacteria detected/GenBank accession number |
| 1 | Nitra | Vráble | 509 Bra V | 2008 | 1 | n | n | ||
| 2 | Levice | 451 Ben S | 1985 | 1 | Francisella tularensis | ||||
| 3 | 452 Pri M | 1990 | 1 | Rickettsia helvetica, Rickettsia slovaca, Rickettsia quintana, Bartonella elizabethae, Bartonella grahamii | Rickettsia helvetica/U59 723.1 | ||||
| 4 | 453 Has J | 1950 | 1 | n | |||||
| 5 | 464 Fer I | 1981 | 1 | Borrelia recurentis, Rickettsia helvetica, Borrelia burgdorferi | Bartonella burgdorferi? | ||||
| 6 | 465 Med P | 1977 | 1 | n | n | ||||
| 7 | 466 Pag V | 1954 | 1 | n | n | ||||
| 8 | 580 Tur J | 1972 | 1 | n | n | ||||
| 9 | 599 Kla L | 1970 | 1 | n | n | ||||
| 10 | 431 Stu I | 1971 | 1 | n | n | ||||
| 11 | 690 Pas E | 1960 | 1 | R. slovaca, R. helvetica, R. raoultii, R. conorii | Rickettsia slovaca/U59 725.1 | ||||
| 12 | 503 Med M | 1981 | 1 | n | n | ||||
| 13 | 715 Tak M | 1977 | 1 | R. helvetica | n | ||||
| 14 | 728 Dek J | 1944 | 1 | n | n | ||||
| 15 | 760 Cso V | 1954 | 1 | n | n | ||||
| 16 | 776 Sip I | 1994 | 1 | n | n | ||||
| 17 | Levice | Farná | 350 Ban R | 1997 | 1 | n | n | ||
| 18 | Plášt'ovce | 351 Kor A | 1933 | 1 | Borrelia recurentis, Bartonella quintana, Bartonella henselae | ||||
| 19 | Žemberovce | 582 Sik O | 1945 | 1 | n | n | |||
| 20 | Žemberovce | 605 Ruf V | 1953 | 1 | n | n | |||
| 21 | Slatina | 604 Pat M | 1961 | 1 | n | n | |||
| 22 | Bory | 630 Lip P | 1993 | 1 | n | n | |||
| 23 | Kukučínov | 700 Srn M | 1993 | 1 | Rickettsia helvetica, Bartonella elizabethae, Bartonella grahamii | n | |||
| 24 | Ondrejovce | 702 Uhn A | 1947 | 1 | Rickettsia helvetica | n | |||
| 25 | Horča | 711 Mra R | 1973 | 1 | Rickettsia helvetica, Rickettsia raoultii | Rickettsia helvetica/U59 723.1 | |||
| 26 | Hronské Kl'ačany | 714 Kra A | 1956 | 1 | n | n | |||
| 27 | Kural'any | 729 Bie M | 1945 | 1 | n | n | |||
| 28 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 29 | Santovka | 786 Dek M | 1952 | 1 | n | n | |||
| 30 | Čaka | 793 Bie Z | 1965 | 1 | n | n | |||
| 31 | Zlaté Moravce | Mankovce | 719 Pau M | 1989 | 1 | Rickettsia helvetica | Rickettsia helvetica/U59723.1 | ||
| 32 | Svodín | 568 Mer M | 2001 | 1 | Rickettsia helvetica, Bartonella elizabethae | n | |||
| 33 | Svodín | 477 Jon D | 1990 | 1 | Rickettsia helvetica | n | |||
| 34 | Nové Zámky | 364 Var M | 1931 | 1 | Bartonella elizabethae, Bartonella grahamii | Bartonella elizabethae/L35103.1 | |||
| 35 | 731 Sche I | 1980 | 1 | n | n | ||||
| 36 | Nové Zámky | Nové Zámky | Palárikovo | 750 Fil T | 1990 | 1 | n | n | |
| 37 | Zemné | 595 Nag H | 1951 | 1 | Coxiella burnetii Ph I, Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |||
| 38 | Komárno | 396 Jan A | 1963 | 1 | n | n | |||
| 39 | 564 Oll V | 1958 | 1 | n | n | ||||
| 40 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 41 | Vráble | 509 Bra V | 2008 | 1 | n | n | |||
| 42 | Banská Bystrica | Žarnovica | Nová Baňa | 709 Bra J | 1957 | 1 | n | n | |
| 43 | Ostrý Grúň | 583 Vid S | 1947 | 1 | n | n | |||
| 44 | Banská Bystrica | Lučenec | 262 Jel G | 1983 | 1 | n | n | ||
| 45 | 264 Neu M | 1942 | 1 | n | n | ||||
| 46 | 747 Rad J | 1956 | 1 | Rickettsia raoultii, Rickettsia helvetica | Rickettsia raoultii/EU036985 | ||||
| 47 | Banská Bystrica | Vel'ký Krtíš | Vinica | 782 Tre A | 1957 | 1 | Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |
| 48 | Trnava | Galanta | 758 Kor D | 2003 | 1 | n | n | ||
| 49 | Košice | 337 Gaj S | 1936 | 1 | n | n | |||
| 50 | 338 Sal M | 1947 | 1 | n | n | ||||
n, negative serum sample; ♀, female, ♂, male.
Geographical origin for the disease corresponding to the bacterial disease agent are highlighted in bold.
| Origin of patient | Sex | ||||||||
| No. of serum | City | County (district) | Village | Sample name | Date of serum | ♀ | ♂ | IFA organism detected | PCR bacteria detected/GenBank accession number |
| 1 | Nitra | Vráble | 509 Bra V | 2008 | 1 | n | n | ||
| 2 | Levice | 451 Ben S | 1985 | 1 | Francisella tularensis | ||||
| 3 | 452 Pri M | 1990 | 1 | Rickettsia helvetica, Rickettsia slovaca, Rickettsia quintana, Bartonella elizabethae, Bartonella grahamii | Rickettsia helvetica/U59 723.1 | ||||
| 4 | 453 Has J | 1950 | 1 | n | |||||
| 5 | 464 Fer I | 1981 | 1 | Borrelia recurentis, Rickettsia helvetica, Borrelia burgdorferi | Bartonella burgdorferi? | ||||
| 6 | 465 Med P | 1977 | 1 | n | n | ||||
| 7 | 466 Pag V | 1954 | 1 | n | n | ||||
| 8 | 580 Tur J | 1972 | 1 | n | n | ||||
| 9 | 599 Kla L | 1970 | 1 | n | n | ||||
| 10 | 431 Stu I | 1971 | 1 | n | n | ||||
| 11 | 690 Pas E | 1960 | 1 | R. slovaca, R. helvetica, R. raoultii, R. conorii | Rickettsia slovaca/U59 725.1 | ||||
| 12 | 503 Med M | 1981 | 1 | n | n | ||||
| 13 | 715 Tak M | 1977 | 1 | R. helvetica | n | ||||
| 14 | 728 Dek J | 1944 | 1 | n | n | ||||
| 15 | 760 Cso V | 1954 | 1 | n | n | ||||
| 16 | 776 Sip I | 1994 | 1 | n | n | ||||
| 17 | Levice | Farná | 350 Ban R | 1997 | 1 | n | n | ||
| 18 | Plášt'ovce | 351 Kor A | 1933 | 1 | Borrelia recurentis, Bartonella quintana, Bartonella henselae | ||||
| 19 | Žemberovce | 582 Sik O | 1945 | 1 | n | n | |||
| 20 | Žemberovce | 605 Ruf V | 1953 | 1 | n | n | |||
| 21 | Slatina | 604 Pat M | 1961 | 1 | n | n | |||
| 22 | Bory | 630 Lip P | 1993 | 1 | n | n | |||
| 23 | Kukučínov | 700 Srn M | 1993 | 1 | Rickettsia helvetica, Bartonella elizabethae, Bartonella grahamii | n | |||
| 24 | Ondrejovce | 702 Uhn A | 1947 | 1 | Rickettsia helvetica | n | |||
| 25 | Horča | 711 Mra R | 1973 | 1 | Rickettsia helvetica, Rickettsia raoultii | Rickettsia helvetica/U59 723.1 | |||
| 26 | Hronské Kl'ačany | 714 Kra A | 1956 | 1 | n | n | |||
| 27 | Kural'any | 729 Bie M | 1945 | 1 | n | n | |||
| 28 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 29 | Santovka | 786 Dek M | 1952 | 1 | n | n | |||
| 30 | Čaka | 793 Bie Z | 1965 | 1 | n | n | |||
| 31 | Zlaté Moravce | Mankovce | 719 Pau M | 1989 | 1 | Rickettsia helvetica | Rickettsia helvetica/U59723.1 | ||
| 32 | Svodín | 568 Mer M | 2001 | 1 | Rickettsia helvetica, Bartonella elizabethae | n | |||
| 33 | Svodín | 477 Jon D | 1990 | 1 | Rickettsia helvetica | n | |||
| 34 | Nové Zámky | 364 Var M | 1931 | 1 | Bartonella elizabethae, Bartonella grahamii | Bartonella elizabethae/L35103.1 | |||
| 35 | 731 Sche I | 1980 | 1 | n | n | ||||
| 36 | Nové Zámky | Nové Zámky | Palárikovo | 750 Fil T | 1990 | 1 | n | n | |
| 37 | Zemné | 595 Nag H | 1951 | 1 | Coxiella burnetii Ph I, Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |||
| 38 | Komárno | 396 Jan A | 1963 | 1 | n | n | |||
| 39 | 564 Oll V | 1958 | 1 | n | n | ||||
| 40 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 41 | Vráble | 509 Bra V | 2008 | 1 | n | n | |||
| 42 | Banská Bystrica | Žarnovica | Nová Baňa | 709 Bra J | 1957 | 1 | n | n | |
| 43 | Ostrý Grúň | 583 Vid S | 1947 | 1 | n | n | |||
| 44 | Banská Bystrica | Lučenec | 262 Jel G | 1983 | 1 | n | n | ||
| 45 | 264 Neu M | 1942 | 1 | n | n | ||||
| 46 | 747 Rad J | 1956 | 1 | Rickettsia raoultii, Rickettsia helvetica | Rickettsia raoultii/EU036985 | ||||
| 47 | Banská Bystrica | Vel'ký Krtíš | Vinica | 782 Tre A | 1957 | 1 | Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |
| 48 | Trnava | Galanta | 758 Kor D | 2003 | 1 | n | n | ||
| 49 | Košice | 337 Gaj S | 1936 | 1 | n | n | |||
| 50 | 338 Sal M | 1947 | 1 | n | n | ||||
| Origin of patient | Sex | ||||||||
| No. of serum | City | County (district) | Village | Sample name | Date of serum | ♀ | ♂ | IFA organism detected | PCR bacteria detected/GenBank accession number |
| 1 | Nitra | Vráble | 509 Bra V | 2008 | 1 | n | n | ||
| 2 | Levice | 451 Ben S | 1985 | 1 | Francisella tularensis | ||||
| 3 | 452 Pri M | 1990 | 1 | Rickettsia helvetica, Rickettsia slovaca, Rickettsia quintana, Bartonella elizabethae, Bartonella grahamii | Rickettsia helvetica/U59 723.1 | ||||
| 4 | 453 Has J | 1950 | 1 | n | |||||
| 5 | 464 Fer I | 1981 | 1 | Borrelia recurentis, Rickettsia helvetica, Borrelia burgdorferi | Bartonella burgdorferi? | ||||
| 6 | 465 Med P | 1977 | 1 | n | n | ||||
| 7 | 466 Pag V | 1954 | 1 | n | n | ||||
| 8 | 580 Tur J | 1972 | 1 | n | n | ||||
| 9 | 599 Kla L | 1970 | 1 | n | n | ||||
| 10 | 431 Stu I | 1971 | 1 | n | n | ||||
| 11 | 690 Pas E | 1960 | 1 | R. slovaca, R. helvetica, R. raoultii, R. conorii | Rickettsia slovaca/U59 725.1 | ||||
| 12 | 503 Med M | 1981 | 1 | n | n | ||||
| 13 | 715 Tak M | 1977 | 1 | R. helvetica | n | ||||
| 14 | 728 Dek J | 1944 | 1 | n | n | ||||
| 15 | 760 Cso V | 1954 | 1 | n | n | ||||
| 16 | 776 Sip I | 1994 | 1 | n | n | ||||
| 17 | Levice | Farná | 350 Ban R | 1997 | 1 | n | n | ||
| 18 | Plášt'ovce | 351 Kor A | 1933 | 1 | Borrelia recurentis, Bartonella quintana, Bartonella henselae | ||||
| 19 | Žemberovce | 582 Sik O | 1945 | 1 | n | n | |||
| 20 | Žemberovce | 605 Ruf V | 1953 | 1 | n | n | |||
| 21 | Slatina | 604 Pat M | 1961 | 1 | n | n | |||
| 22 | Bory | 630 Lip P | 1993 | 1 | n | n | |||
| 23 | Kukučínov | 700 Srn M | 1993 | 1 | Rickettsia helvetica, Bartonella elizabethae, Bartonella grahamii | n | |||
| 24 | Ondrejovce | 702 Uhn A | 1947 | 1 | Rickettsia helvetica | n | |||
| 25 | Horča | 711 Mra R | 1973 | 1 | Rickettsia helvetica, Rickettsia raoultii | Rickettsia helvetica/U59 723.1 | |||
| 26 | Hronské Kl'ačany | 714 Kra A | 1956 | 1 | n | n | |||
| 27 | Kural'any | 729 Bie M | 1945 | 1 | n | n | |||
| 28 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 29 | Santovka | 786 Dek M | 1952 | 1 | n | n | |||
| 30 | Čaka | 793 Bie Z | 1965 | 1 | n | n | |||
| 31 | Zlaté Moravce | Mankovce | 719 Pau M | 1989 | 1 | Rickettsia helvetica | Rickettsia helvetica/U59723.1 | ||
| 32 | Svodín | 568 Mer M | 2001 | 1 | Rickettsia helvetica, Bartonella elizabethae | n | |||
| 33 | Svodín | 477 Jon D | 1990 | 1 | Rickettsia helvetica | n | |||
| 34 | Nové Zámky | 364 Var M | 1931 | 1 | Bartonella elizabethae, Bartonella grahamii | Bartonella elizabethae/L35103.1 | |||
| 35 | 731 Sche I | 1980 | 1 | n | n | ||||
| 36 | Nové Zámky | Nové Zámky | Palárikovo | 750 Fil T | 1990 | 1 | n | n | |
| 37 | Zemné | 595 Nag H | 1951 | 1 | Coxiella burnetii Ph I, Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |||
| 38 | Komárno | 396 Jan A | 1963 | 1 | n | n | |||
| 39 | 564 Oll V | 1958 | 1 | n | n | ||||
| 40 | Tlmače | 757 Sve M | 1963 | 1 | n | n | |||
| 41 | Vráble | 509 Bra V | 2008 | 1 | n | n | |||
| 42 | Banská Bystrica | Žarnovica | Nová Baňa | 709 Bra J | 1957 | 1 | n | n | |
| 43 | Ostrý Grúň | 583 Vid S | 1947 | 1 | n | n | |||
| 44 | Banská Bystrica | Lučenec | 262 Jel G | 1983 | 1 | n | n | ||
| 45 | 264 Neu M | 1942 | 1 | n | n | ||||
| 46 | 747 Rad J | 1956 | 1 | Rickettsia raoultii, Rickettsia helvetica | Rickettsia raoultii/EU036985 | ||||
| 47 | Banská Bystrica | Vel'ký Krtíš | Vinica | 782 Tre A | 1957 | 1 | Coxiella burnetii PhII | Coxiella burnetii/AE016828 | |
| 48 | Trnava | Galanta | 758 Kor D | 2003 | 1 | n | n | ||
| 49 | Košice | 337 Gaj S | 1936 | 1 | n | n | |||
| 50 | 338 Sal M | 1947 | 1 | n | n | ||||
n, negative serum sample; ♀, female, ♂, male.
Geographical origin for the disease corresponding to the bacterial disease agent are highlighted in bold.
However, the spectrum of detected bacteria was larger: one Bartonella henselae (no. 2, from the village of Plášt'ovce), two Bartonella quintana (no. 3 from the city of Levice and no. 2 from Plášt'ovce), three Bartonella grahamii (no. 2 from Levice, no. 23 from Kukučínov, and no. 34 from Nové Zámky,) and four Bartonella elisabethae (no. 3 from Levice, no. 23 from Kukučínov, no. 32 from Svodín, and no. 34 from Nové Zámky) cases supposedly had positive IFA titers (≥ 1 : 50) (Fig. 1). In one serum of a patient from the city of Levice (no. 5, Fig. 2) both Borrelia burgdorferi and Borrelia recurrentis antigens were recognized. Cross-reaction with Borrelia and Bartonella was seen in case no. 18 from Plášt'ovce.
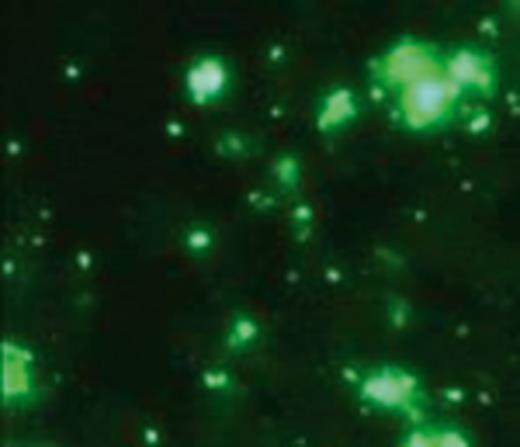
Bartonella elizabethae visualized in IFA assay. Magnification ×100.

The same titer range as above was used to detect two C. burnetii-specific cases identified with phase I and phase II antigens (no. 37 from the village of Zemné, county of Nové Zámky, and no. 47 from the village of Vinice, county of Vel'ký Krtíš). The only Franciscella-positive serum sample originated from the city of Levice (no. 2).
Polymerase chain reaction
The problems of interpreting conventional diagnostic serology results highlight the need for diagnostics with genetic and/or antigenic targets. PCR amplification of blood samples has the advantage of being able to detect infection if a seroconversion has occurred, and is especially important in endemic areas where high levels of background antibodies pose a challenge for serology. The rationale for selecting the IFA-positive samples for the PCR analysis included the presence of IgM antibodies with titers around 1 : 50 against any of the tested spotted fever group rickettsial antigens in the samples. Bacteria-specific PCR was used as a verification tool after IFA to diagnose the illness, although conflicting sensitivities were expected (Fournier & Raoult, 2003). Indeed, the results obtained by IFA were only partly confirmed by PCR, which confirmed five of 16 in IFA-positive rickettsial cases. Use of 16S rRNA genes and rickettsia-specific gltA genes enabled us to identify three R. helvetica-positive patient sera (no. 3 from Levice, no. 25 from Horča and no. 31 from Mankovce), one R. slovaca (no. 11 from the city of Levice), and one R. raoultii case (no. 46, from the county of Lučenec).
Amplification of the fragment of the 16S–23S rRNA gene ITS region verified Ba. elisabethae in the serum of the patient no. 34 from Nové Zámky.
Borrelia identified in serum by IFA (no. 5) was confirmed in PCR with primers Bf1 and Br1. However, species specificity (Bo. recurrentis ssp. A1, or Bo. burgdorferi) could not be satisfactorily distinguished. The single F. tularensis ssp. tularensis sample (no. 2), also obtained from the city of Levice, was detected by IFA only.
The presence of the insertion element IS1111 of the C. burnetii genome in multiple copies has ensured detection of the bacteria. Designed PCR provided evidence of two C. burnetii-positive serum samples, no. 37 from Zemné and no. 47 from Vinice.
Although in the course of testing we ended up with several unreadable results, all positive samples were reliably and repeatedly detected, and underwent PCR detection in duplicate. Non-bacteria-positive, for example non-rickettsial, non-template controls, gave negative results in all runs performed. Furthermore, the clinical picture of the patients (A. Nyitray, unpublished data) endorses our results.
Regardless of the applied method, we did not detect any case of Rickettsia mongolotimonae infection, which is known to cause lymphangitis-associated rickettsiosis (Fournier et al., 2005), nor did we find Rickettsia felis. However, reports of human infection with R. felis are rare (Renvoise et al., 2009). Similarly, some of Bartonella species used in this study remain undetected, for example Ba. henselae (Marseille), Bartonella alsatica, Bartonella vinsonii ssp. berkhoffii, Bartonella ‘weissi’, and Ba. vinsonii ssp. Arupensis. No infection with human granulocytic ehrlichiosis (HGE) Anaplasma, or D. massiliensis was confirmed either.
Discussion
The use of two complementary methods, IFA and PCR, allowed us to show Rickettsia, Borrelia, Bartonella, Coxiella and Franciscella as possible sources of human infections in Slovakia. Not all serologically detected cases could be confirmed with PCR (Table 3). We are aware of certain limits of the PCR with a single template assay, as the number of organisms found in the blood can be quite low. Detection limits for amplification of 47-kDa gltA and ompB gene targets of certain rickettsial strains are known be 2, 1 and 1 µL−1 in single template format, respectively (Paris et al., 2008). As few as seven copies of the 16S rRNA gene of R. helvetica could be detected in 200 µL of serum sample in another study (Choi et al., 2005). However, the use of two complementary tests, IFA and PCR, enabled the bacteria to be verified. Five of 16 rickettsial cases detected by IFA were confirmed by PCR. Rickettsiae have been detected in Slovakia previously (Rehacek et al., 1975; Kovacova et al., 2006), and R. slovaca (Sekeyova et al., 1998), R. helvetica (Spitalska et al., 2008) and R. raoultii (Boldis et al., 2008) are ‘domestic’ and are frequently neglected by the local medical community. On the other hand, R. conorii serum reactivity in IFA (not confirmed with PCR) is questionable. This agent has never before been identified in Slovakia due to a missing corresponding tick vector (Rhipicephalus sanguineus). Rickettsiae need specific invertebrates as vectors or hosts (ticks, lice and fleas). Thus, together with other detected bacterial agents (Subramanian et al., 2011) they are probably one of the most important causes of systemic febrile illness in Europe (Parola & Raoult, 2001; Chmielewski et al., 2009; Silaghi et al., 2011). Our results on the distribution of pathogenic rickettsiae in patients showed that the rural population is at risk for tick-borne rickettsioses.
Using IFA, we identified F. tularensis ssp. tularensis (biogroup palearctica) as a possible origin of the disease of a man (no. 2) from the city of Levice. He was clinically diagnosed as suffering from rickettsiosis, which gave certain evidence of disease symptom similarities to disease caused by these two representatives. A comparable case was described in France (Fournier et al., 1998a).
We also detected serum reactive to Bo. burgdorferi and Bo. recurrentis using IFA (Nos 5 and 18). Borrelia burgdorferi antibodies are commonly found in a defined group of patients depending on the circulation in individual regions in Slovakia (Trnovcova et al., 2007). Conversely, Bo. recurrentis is endemic in Ethiopia and Sudan. It is the agent that can cause a louse-borne relapsing fever in humans (Burgess, 1995), a rapidly progressive and severe septic disease (Raoult & Roux, 1999; Roux & Raoult, 1999). Transmission to humans occurs via infected lice (Buxton, 1940), a parasite that is frequently found in certain populations with poor sanitary conditions. Minor differences among Borrelia species based on rrs gene sequences limit the value of the discrimination of species for genotypic purposes. Nevertheless, we consider that Bo. burgdorferi is a possible source of infection in middle Europe.
In this study we provide the first evidence of Ba. elisabethae disease (no. 32 in Zlaté Moravce and no. 34 in Nové Zámky) in humans in Slovakia. Bartonella spp. have already been described in rodents and mice (Spitalska et al., 2008; Karbowiak et al., 2010); however, there are few studies of Ba. elisabethae in humans. This agent was isolated for the first time in Massachusetts (Daly et al., 1993) and was serologically detected in Maryland (Comer et al., 1996) and confirmed in Stockholm (Ehrenborg et al., 2008) and Spain (deSousa et al., 2006).
Another bacterial agent identified in this study, which infects a whole range of reservoirs and hosts (mammals, birds and arthropods), is C. burnetii, a Gram-negative gamma bacteria responsible for Q fever in humans (Seshadri et al., 2003). We confirmed two C. burnetii cases (Nos 37 and 47). One of them was a severe case with sarcoid myocarditis. Coxiella has been studied and detected in Slovakia for a long time (Brezina & Taborska, 1956, 1957; Kovacova et al., 1998; Vadovic et al., 2005; Toman et al., 2009; Skultety et al., 2011).
We are aware of certain discrepancies between IFA and PCR results. These may due to sensitivity linked to time of collection of serum samples. We are also conscious of certain cross-reactions of human sera in IFA which have been described previously. Nevertheless, we have verified that essentially Rickettsia, but also Franciscella, Borrelia and Coxiella, are domestic in Slovakia and, to our knowledge, we provide the first evidence of a human case of Ba. elisabethae. In the future, we would like to proceed with screening of a larger cohort of sera from incriminated regions to prove the possible incidence or persistence of the identified bacteria.
Acknowledgements
This work was partly supported by grant VEGA no. 2/0031/11, 2/0156/11, and 2/0065/09 from the Slovak Academy of Sciences, Bratislava, Slovakia, as well as bilateral Slovak (SAS) — French (CNRS) Research and Developmental Cooperation no. SK-FR-0007-11.
References
Author notes
Editor: Gilbert Greub



