-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Lucia Taylor, Guillermo M. Ruíz-Palacios, María Rocío Reyes-Montes, Gabriela Rodríguez-Arellanes, Laura E. Carreto-Binaghi, Esperanza Duarte-Escalante, Aurora Hernández-Ramírez, Armando Pérez, Roberto O. Suárez-Alvarez, Yuri A. Roldán-Aragón, Rafael Romero-Martínez, Jorge H. Sahaza-Cardona, José Sifuentes-Osornio, Luis E. Soto-Ramírez, Gabriela R. Peña-Sandoval, Identification of the infectious source of an unusual outbreak of histoplasmosis, in a hotel in Acapulco, state of Guerrero, Mexico, FEMS Immunology & Medical Microbiology, Volume 45, Issue 3, September 2005, Pages 435–441, https://doi.org/10.1016/j.femsim.2005.05.017
Close - Share Icon Share
Abstract
Three isolates of Histoplasma capsulatum were identified from mice lung, liver, and spleen inoculated with soil samples of the X hotel's ornamental potted plants that had been fertilized with organic material known as compost. The presence of H. capsulatum in the original compost was detected using the dot-enzyme-linked immunosorbent assay. Nested-PCR, using a specific protein Hcp100 coding gene sequence, confirmed the fungal identification associated with an unusual histoplasmosis outbreak in Acapulco. Although, diversity between the H. capsulatum isolate from the hotel and some clinical isolates from Guerrero (positive controls) was observed using random amplification of polymorphic DNA based-PCR, sequence analyses of H-anti and ole fragment genes revealed a high homology (92–99%) between them.
1 Introduction
In its natural environment, the dimorphic fungus Histoplasma capsulatum var. capsulatum (Darling, 1906) grows favorably in bat and/or bird guano producing a multicellular filamentous form, characteristic of its mycelial-phase, which presents macro- and microconidia. Microconidia together with small hyphal fragments are the main fungal structures that infect susceptible hosts through their inhalation in contaminated places, starting the process of histoplasmosis infection [1].
In Mexico, this disease represents an occupational and environmental health problem, and people in the countryside are particularly affected, especially miners, peasants, and guano collectors, due to their work in given enclosed spaces where guano accumulates [2–6]. Histoplasmosis has been registered in every state of the country, and when an outbreak suddenly appears, the lethality percentage gets higher. However, the presence of fungus propagules in urban areas may be also important to explain clinical cases that do not refer any exposure to high risk infection sites [2].
In 2001, the Department of Health of the USA notified the Centers for Disease Control and Prevention (CDC) of an outbreak of histoplasmosis affecting American tourists in Acapulco. Morgan et al. [7] reported a cohort study of this outbreak of acute pulmonary histoplasmosis among American travelers, mostly college students, who were vacationing during their spring break in Acapulco, Mexico, from March to May 2001. Frequent use of the hotel's stairwells, where construction was ongoing, was associated with increased risk of illness. However, the source of infection of the outbreak was not accurately confirmed.
The present work reports the identification of the source of infection in an outbreak that occurred in September 2001, in a hotel in Acapulco, Guerrero (GR), Mexico, which most likely was the same source of infection of the outbreaks that occurred in March and May in the same hotel in 2001.
2 Materials and methods
2.1 Samples
Nineteen environmental samples from different places of the X hotel in Acapulco, GR, and three compost (organic fertilizer) samples from a commercial plant nursery, located near the Acapulco airport, which supplied compost material to the hotel during 2001, were collected (Table 1) in October. Each sample (1 g) was prepared in 10 ml phosphate buffered saline (PBS), pH 7.2, supplemented with 50 µg ml−1 streptomycin and 100 IU ml−1 penicillin (Lakeside, Mexico City, MX), and allowed to precipitate. Finally, 0.5 ml of each suspension was intraperitoneally inoculated into inbred male BALB/c mice (4-weeks old), using five mice per sample.
| Number | Type | Place of collection | Dot-ELISA |
| S-158 | Guano (marine birds) | Rock in the sea | Negative |
| S-159 | Soil (planter) | Restaurant A (1st planter) | Positive |
| S-160 | Soil (planter) | Restaurant A (4th planter) | Positive |
| S-161 | Soil (planter) | Restaurant A (6th planter) | Positive |
| S-162 | Soil (planter) | Restaurant A (exterior planter) | Positive |
| S-163 | Soil (planter) | Auditorium (ornamental potted plants) | Positive |
| S-164 | Ventilator dust | Restaurant A (5th ventilator) | Negative |
| S-165 | Soil (planter) | Restaurant A (1st planter) | Negative |
| S-166 | Leaves (ornamental plants) | Restaurant A (1st planter) | Negative |
| S-167 | Leaves (ornamental plants) | Restaurant A (2nd planter) | Negative |
| S-168 | Leaves (ornamental plants) | Restaurant A (3rd planter) | Negative |
| S-169 | Dust (air conditioner) | Restaurant B | Negative |
| S-170 | Guano (pigeon) | Swimming pool (planter) | Negative |
| S-171 | Mold (wall) | Right lateral hotel wall | Negative |
| S-172 | Leaves (ornamental plants) | Swimming pool (planter) | Negative |
| S-175 | Sand | Restaurant B (path) | Negative |
| S-176 | Bat guano (scarce) | Restaurant B (ceiling) | Negative |
| S-177 | Bat guano (scarce) | Elevator shaft | Negative |
| S-178 | Dust (air conditioner) | Restaurant B | Negative |
| S-180 | Compost (in the shade) | Plant nursery | Positive |
| S-181 | Compost (in the sun) | Plant nursery | Negative |
| S-182 | Compost/sand (mixture) | Plant nursery | Positive |
| Number | Type | Place of collection | Dot-ELISA |
| S-158 | Guano (marine birds) | Rock in the sea | Negative |
| S-159 | Soil (planter) | Restaurant A (1st planter) | Positive |
| S-160 | Soil (planter) | Restaurant A (4th planter) | Positive |
| S-161 | Soil (planter) | Restaurant A (6th planter) | Positive |
| S-162 | Soil (planter) | Restaurant A (exterior planter) | Positive |
| S-163 | Soil (planter) | Auditorium (ornamental potted plants) | Positive |
| S-164 | Ventilator dust | Restaurant A (5th ventilator) | Negative |
| S-165 | Soil (planter) | Restaurant A (1st planter) | Negative |
| S-166 | Leaves (ornamental plants) | Restaurant A (1st planter) | Negative |
| S-167 | Leaves (ornamental plants) | Restaurant A (2nd planter) | Negative |
| S-168 | Leaves (ornamental plants) | Restaurant A (3rd planter) | Negative |
| S-169 | Dust (air conditioner) | Restaurant B | Negative |
| S-170 | Guano (pigeon) | Swimming pool (planter) | Negative |
| S-171 | Mold (wall) | Right lateral hotel wall | Negative |
| S-172 | Leaves (ornamental plants) | Swimming pool (planter) | Negative |
| S-175 | Sand | Restaurant B (path) | Negative |
| S-176 | Bat guano (scarce) | Restaurant B (ceiling) | Negative |
| S-177 | Bat guano (scarce) | Elevator shaft | Negative |
| S-178 | Dust (air conditioner) | Restaurant B | Negative |
| S-180 | Compost (in the shade) | Plant nursery | Positive |
| S-181 | Compost (in the sun) | Plant nursery | Negative |
| S-182 | Compost/sand (mixture) | Plant nursery | Positive |
| Number | Type | Place of collection | Dot-ELISA |
| S-158 | Guano (marine birds) | Rock in the sea | Negative |
| S-159 | Soil (planter) | Restaurant A (1st planter) | Positive |
| S-160 | Soil (planter) | Restaurant A (4th planter) | Positive |
| S-161 | Soil (planter) | Restaurant A (6th planter) | Positive |
| S-162 | Soil (planter) | Restaurant A (exterior planter) | Positive |
| S-163 | Soil (planter) | Auditorium (ornamental potted plants) | Positive |
| S-164 | Ventilator dust | Restaurant A (5th ventilator) | Negative |
| S-165 | Soil (planter) | Restaurant A (1st planter) | Negative |
| S-166 | Leaves (ornamental plants) | Restaurant A (1st planter) | Negative |
| S-167 | Leaves (ornamental plants) | Restaurant A (2nd planter) | Negative |
| S-168 | Leaves (ornamental plants) | Restaurant A (3rd planter) | Negative |
| S-169 | Dust (air conditioner) | Restaurant B | Negative |
| S-170 | Guano (pigeon) | Swimming pool (planter) | Negative |
| S-171 | Mold (wall) | Right lateral hotel wall | Negative |
| S-172 | Leaves (ornamental plants) | Swimming pool (planter) | Negative |
| S-175 | Sand | Restaurant B (path) | Negative |
| S-176 | Bat guano (scarce) | Restaurant B (ceiling) | Negative |
| S-177 | Bat guano (scarce) | Elevator shaft | Negative |
| S-178 | Dust (air conditioner) | Restaurant B | Negative |
| S-180 | Compost (in the shade) | Plant nursery | Positive |
| S-181 | Compost (in the sun) | Plant nursery | Negative |
| S-182 | Compost/sand (mixture) | Plant nursery | Positive |
| Number | Type | Place of collection | Dot-ELISA |
| S-158 | Guano (marine birds) | Rock in the sea | Negative |
| S-159 | Soil (planter) | Restaurant A (1st planter) | Positive |
| S-160 | Soil (planter) | Restaurant A (4th planter) | Positive |
| S-161 | Soil (planter) | Restaurant A (6th planter) | Positive |
| S-162 | Soil (planter) | Restaurant A (exterior planter) | Positive |
| S-163 | Soil (planter) | Auditorium (ornamental potted plants) | Positive |
| S-164 | Ventilator dust | Restaurant A (5th ventilator) | Negative |
| S-165 | Soil (planter) | Restaurant A (1st planter) | Negative |
| S-166 | Leaves (ornamental plants) | Restaurant A (1st planter) | Negative |
| S-167 | Leaves (ornamental plants) | Restaurant A (2nd planter) | Negative |
| S-168 | Leaves (ornamental plants) | Restaurant A (3rd planter) | Negative |
| S-169 | Dust (air conditioner) | Restaurant B | Negative |
| S-170 | Guano (pigeon) | Swimming pool (planter) | Negative |
| S-171 | Mold (wall) | Right lateral hotel wall | Negative |
| S-172 | Leaves (ornamental plants) | Swimming pool (planter) | Negative |
| S-175 | Sand | Restaurant B (path) | Negative |
| S-176 | Bat guano (scarce) | Restaurant B (ceiling) | Negative |
| S-177 | Bat guano (scarce) | Elevator shaft | Negative |
| S-178 | Dust (air conditioner) | Restaurant B | Negative |
| S-180 | Compost (in the shade) | Plant nursery | Positive |
| S-181 | Compost (in the sun) | Plant nursery | Negative |
| S-182 | Compost/sand (mixture) | Plant nursery | Positive |
2.2 Bio-collector mice and mice processing
Sixty BALB/c mice were used as targets of infection and distributed, in special boxes, in 12 different sites of the hotel to expose them to natural infection overnight. One box of these different sites was randomly chosen as control (see Table 2).
Serum samples were obtained on days 10 and 17 after exposing mice to natural infection.
Restaurant B (central table) was chosen as a control because it had very few reports of infected people, when a cohort study was made by Mexican Epidemiologists (unpublished data).
Serum samples were obtained on days 10 and 17 after exposing mice to natural infection.
Restaurant B (central table) was chosen as a control because it had very few reports of infected people, when a cohort study was made by Mexican Epidemiologists (unpublished data).
All mice were kept under observation to find histoplasmosis signs, and retro-orbital bleeding was performed on days 10 and 17 to separate sera to detected anti-H. capsulatum antibodies using dot-enzyme-linked immunosorbent assay (dot-ELISA), as described by Papas et al. [8]; after the last bleeding, mice were killed for fungal isolation and histopathology assessment.
2.3 Fungal isolation and Histoplasma capsulatum identification
The guts, lungs, livers, and spleens were processed for fungal isolation, as previously described by Taylor et al. [9]. Briefly, homogenates of each organ sample were obtained under sterile conditions, and mixed in 5 ml PBS, pH 7.2, supplemented with antibiotics. Tissue homogenates were centrifuged at 300g for 15 min, and 0.1 ml supernatant of each homogenate sample was inoculated in Petri-plates with Mycobiotic agar and Brain Heart Infusion (BHI) agar (Bioxón, Mexico City, MX) supplemented with 0.05% cycloheximide and 0.005% chloramphenicol. Plates were incubated at 28 °C and checked daily for fungal growth during 2 to 3 weeks; suspected colonies of H. capsulatum were cultured after serial dilutions to separate and isolate each one.
Once the macro and microscopic morphology of H. capsulatum had been observed, the mycelia-to-yeast conversion was processed, at 37 °C, in synthetic-liquid medium [10] and in BHI broth (Bioxón) supplemented with 0.1%l-cysteine and 1% glucose. Finally, the exoantigen test of Kaufman and Standard [11] was performed by the double immunodiffusion method [12] to confirm antigenic identity of the fungus. A positive rabbit hyperimmune serum and a standardized reference exoantigen were used.
2.4 Histopathology
The organs from each inoculated mice were fixed with Zamboni and De Martino solution [13] during 24 h at least, and then processed to be paraffin embedded. Tissue sections of 6 µm were stained for fungi by means of the Grocott method.
2.5 Dot-enzyme-linked immunosorbent assay
A deproteinized polysaccharide-protein complex of Histoplasma (DPPC-Histo), as described elsewhere [14], was used as antigen for dot-ELISA. The purified DPPC-Histo (50 ng 20 µl−1 of protein) was applied in separate wells to a 0.45 µm nitrocellulose membrane narrow strip (BioTrace, GelmanSciences, Inc. MI, USA) using a 96-well microfiltration Bio-Dot apparatus (Bio-Rad, Richmond, CA, USA). DPPC-Histo binding to the membrane was favored by vacuum filtration, during 15 min. The membrane was blocked with PBS, pH 7.2, plus 3% bovine serum albumin (PBS-A) for 1 h, to prevent non-specific binding. The membrane was washed with 0.05% Tween 20-PBS, and 1:50 or 1:100 PBS-A-diluted, of each mouse serum sample, was applied to different membrane narrow strips. Membranes were incubated 20 h at 4 °C. After membrane washing, 1:500 dilution of a biotinylated anti-mouse IgG antibody produced in goat (Sigma Chemical Co., St. Louis, MO, USA) was added and incubation was allowed during 1 h. After washing membranes again, they were incubated for another hour with streptavidin-peroxidase conjugate. Membranes were again washed and immersed in a fresh mixture of 25 mg of 3′,3′-diaminobenzidine-4HCl (Sigma) in 50 ml of 0.1 M Tris–HCl buffer, pH 7.5, plus 50 µl of 30% H2O2. After enzyme-substrate reaction, membranes were washed and dried. Positive enzymatic reactions were visible as brown spots. Sera from infected and non-infected mice were processed as positive and negative controls. Criteria to define positive reaction in sera from tested mice were standardized taking into account that three out of five mice revealed brown spots using the dot-ELISA.
2.6 DNA sampling and nested-PCR assay
The H. capsulatum isolate recovered from the X hotel, where several histoplasmosis outbreaks were detected, was characterized by nested-PCR, random amplification of polymorphic DNA based-PCR (RAPD-PCR), and sequencing assays. DNA samples were extracted from one H. capsulatum isolate (EH-554B) recovered from an infected mouse inoculated with contaminated planter soil collected in the hotel and from six H. capsulatum clinical isolates from different geographic origins in America (EH-46, EH-316, and EH-557 from GR-Mexico; 01558 from Argentina; WCh from Colombia; and H.1.02.W from Guatemala) used as positive controls, as well as from Sporothrix schenckii (DNA negative control of non-related strain). Isolates were taken from the Fungal Immunology Laboratory Culture Collection of the Department of Microbiology and Parasitology, School of Medicine-UNAM. Procedures for isolation of whole-cell DNA were performed as described elsewhere by Reyes-Montes et al. [15]. Each purified DNA was centrifuged at 15 000g for 5 min, and the pellet was washed with 70% ethanol, dried and resuspended in 100 µl of water. The DNA was quantified fluorometrically and checked against standard concentrations using agarose gel electrophoresis. Finally, it was frozen at −20 °C until required.
Nested-PCR, with minor modifications, was performed as described by Bialek et al. [16]. DNA samples, from the EH-554B isolate and from six positive H. capsulatum controls, as well as from one clinical isolate of sporotrichosis (negative control), were processed. Two sets of primers were used, corresponding to gene encoding for a 100-kDa protein (Hcp100) unique to H. capsulatum[17]. The outer primer set Hc I (5′-GCGTTCCGAGCCTTCCACCTCAAC-3′) and Hc II (5′-ATGTCCCATCGGGCGCCGTGTAGT-3′) delimit a 391 nucleotides sequence of the gene. The inner primers Hc III (5′-GAGATCTAGTCGCGGCCAGGTTCA-3′) and Hc IV (5′-AGGAGAGAACTGTATCGGTGGCTTG-3′) delimit a specific 210-nucleotides sequence, the primers were supplied by Operon Technologies Inc. (Alameda, CA, USA). DNA amplification was performed on a Perkin–Elmer Cetus DNA thermal cycler (Emeryville, CA, USA) and the first PCR was performed in a 50 µl reaction mixture containing 200 µM of dNTPs (Applied Biosystems Inc., Foster City, CA, USA), 1 mM MgCl2, 100 pmol of each outer primer, 1.5 U Taq DNA polymerase (Applied Biosystems), and 10 ng DNA. Cycling conditions were as follow: one cycle at 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 50 °C for 30 s and 72 °C for 1 min, one cycle at 72 °C for 5 min. For the second (nested) PCR, the mixture was 50 µM dNTPs, 0.25 mM MgCl2, 50 pmol of each primer, 1 U Taq DNA polymerase, and 1 µl of the first reaction product. Cycling conditions were as follows: one cycle at 94 °C for 5 min; 30 cycles at 94 °C for 30 s, 65 °C for 30 s, 72 °C for 1 min, and one cycle at 72 °C for 5 min.
Amplification products were electrophoresed through 1.5% agarose in Tris-borate-EDTA 0.5× buffer. Electrophoresis was conducted at 90 V for 60 min. The 123 bp DNA Ladder (Gibco Laboratories, Grand Island, NY, USA) was used as molecular marker. The bands were visualized with a UV transilluminator after ethidium bromide (0.5 µg ml−1) staining. They were captured with a documentation system (GeneCam; Syngene, Cambridge, MA, USA) and printed with a thermal printer (Sony 650, Tokyo Japan).
2.7 Two-primer RAPD-PCR assay
This assay was performed as described by Hu et al. [18] using only H. capsulatum DNA samples in a 20 µl reaction, using 10 ng H. capsulatum DNA, 2.5 mM MgCl2, 200 µM of each dNTPs (Applied Biosystems), 15 pmol of each primer 1281 (5′-AACGCGCAAC-3′) and 1283 (5′-GCGATCCCCA-3′) supplied by Operon Technologies, and 1 U Taq DNA polymerase (Applied Biosystems). PCR amplification was performed in a thermal cycler, programmed as follows: one cycle of 7 min at 94 °C followed by 45 cycles of 1 min at 92 °C; 1 min at 35 °C; and 1 min at 72 °C. A final cycle of 5 min at 72 °C ensured extension of all amplified products, and samples were maintained at 4 °C. The RAPD-PCR patterns were analyzed through digital images of ethidium bromide-stained agarose gels (1.5%) captured with a documentation system (GeneCam).
2.8 DNA sequencing and data analysis
DNA fragments of two H. capsulatum nuclear genes were compared between the EH-554B H. capsulatum isolate from the X hotel and all clinical isolates from GR (EH-46, EH-316, and EH-557) and were PCR-amplified as described by Kasuga et al. [19]. Selected primers (Operon Technologies) were: For H-anti gene (H antigen precursor), H-anti3 (5′-CGCAGTCACCTCCATACTATC-3′) and H-anti4 (5′-GCGCCGACATTAACCC-3′); for ole gene (delta-9 fatty acid desaturase), ole3 (5′-TTTAAACGAAGCCCCCACGG-3′) and ole4 (5′-CACCACCTCCAACAGCAGCA-3′). The PCR products were sent to the Institute of Cellular Physiology, UNAM-Mexico, for sequencing in an ABI-automated DNA sequencer (Applied Biosystems). Sequences were generated for a single strand. They were edited, aligned, and compared by means of the BLAST program, version 2.0 [20] (National Center for Biotechnology Information-NCBI- Databases).
3 Results and discussion
3.1 Histoplasma capsulatum identification
Three isolates were obtained from lung, liver, and spleen of a mouse inoculated with the S-162 sample from the exterior planter of restaurant A (Table 1). Isolates developed brownish colonies with slow and limited growth. Microscopic morphology revealed the presence of thin hyphae, microconidia and the typical H. capsulatum tuberculate macroconidia (Fig. 1A–C); all isolates converted to yeast-phase in 1–3 weeks, at 37 °C, in synthetic-liquid medium or in supplemented BHI broth. Finally, exoantigen production confirmed the identification of H. capsulatum isolates by the presence of specific H and M precipitation lines, when exoantigens were reacted with a rabbit hyperimmune serum in the double immunodiffusion test. A register number was assigned to each isolate, EH-554P, EH-554H, and EH-554B, respectively, in order to incorporate them to the Histoplasma capsulatum Culture Collection of Fungal Immunology Laboratory, School of Medicine, UNAM.
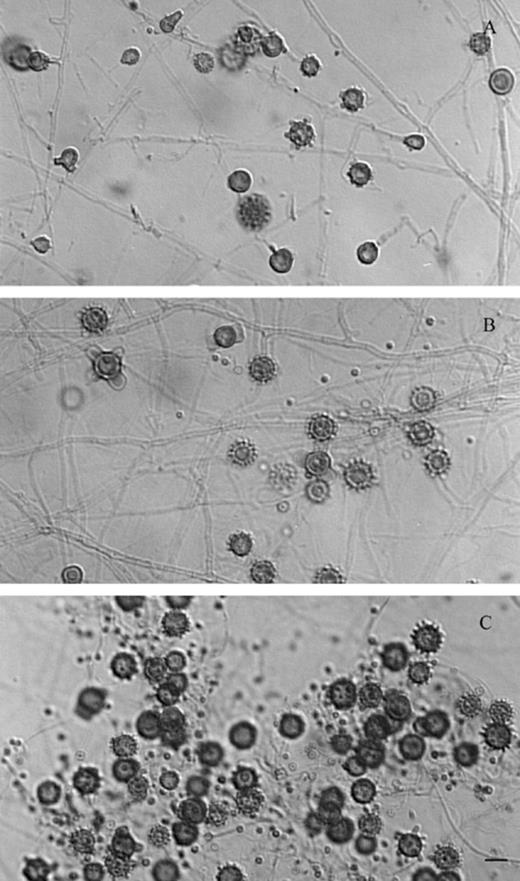
Microscopic morphology of H. capsulatum isolated from a BALB/c mouse inoculated with a sample from a planter (S-162) of restaurant A. (A) EH-554P from lung; (B) EH-554H from liver; (C) EH-554B from spleen. Bar = 10 µm.
3.2 Histopathology
Yeast cells were found in different organs of the mice inoculated with the S-160 sample (Table 1). Intracellular yeasts compatible with H. capsulatum were observed inside Kupffer's cells (Fig. 2A and B), as well as in bronchiolar epithelia (Fig. 2C and D). Inflammatory responses associated to granulomas were mainly observed in the liver (data not shown).
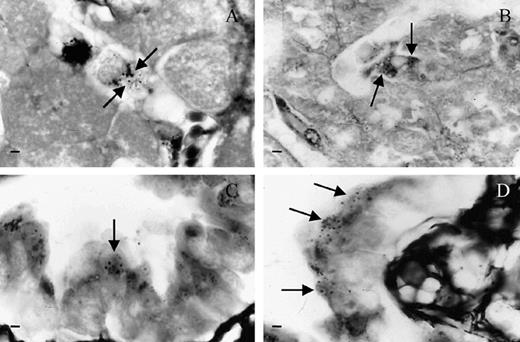
Histopathological findings of mice inoculated with the S-160 sample. (A, B) Yeast-cells within Kupffer's cells; (C, D) Yeast-cells within epithelial cells from the bronchiole. Arrows indicate intracellular yeasts stained using the Grocott method. Bar = 10 µm.
3.3 Dot-ELISA
The presence of anti-H. capsulatum antibodies in sera of mice inoculated with different samples collected in the X hotel (Table 1), as well as in sera of bio-collector mice placed at strategic points that could be associated with the source of infection (Table 2), were monitored using the dot-ELISA. Results suggest that dot-ELISA was a useful tool to detect infectious sources in the hotel planters and that the probable origin of infection was associated with the compost, from a plant nursery, which was prepared to fertilize the hotel planters (Table 1), and explain the fungal isolation from organic material contained in the S-162 planter sample (Fig. 1A–C). Results of sera from bio-collector mice also support the identification of the source of infection in planters with soil and compost materials (Table 2). In addition, it is important to emphasize that the analyzed compost samples (S-180 to S-182) were taken from the plant nursery that supplied the hotel during 2001.
3.4 Nested-PCR
The products revealed by the nested-PCR assay, targeting the Hcp100 protein gene, showed that the H. capsulatum isolate from the hotel planters shares the same bands (0.391 and 0.210 Kb in the first and nested amplifications, respectively) with six clinical isolates from different geographic origins (Fig. 3A and B). DNA of S. schenckii, used as negative control, did not amplify. This type of PCR has been reported as sensitive and highly specific for H. capsulatum. Although it is more frequently used in samples where fungi are scarcely found, its employ in Histoplasma DNA from cultures was useful to confirm the molecular identification of the H. capsulatum isolate sampled from the hotel planters.
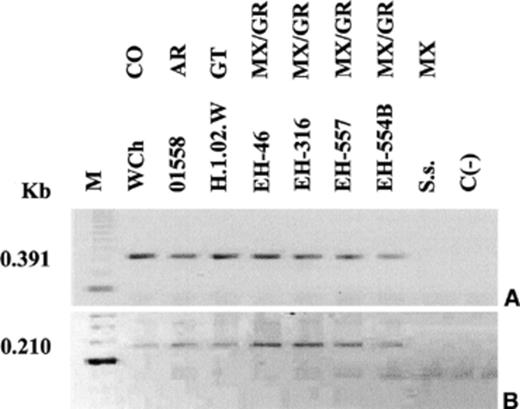
Identification of H. capsulatum by nested-PCR of fungal DNA samples. The assay was performed with two sets of fungal-specific primers of the 100-kDa protein gene of H. capsulatum (see details under Section 2). PCR products were analyzed by electrophoresis through 1.5% agarose gels containing ethidium bromide. First (A) and nested (B) PCR reactions. M — molecular weight marker was estimated from 123 bp DNA Ladder; WCh, 01558, H.1.02.W, EH-46, EH-316, and EH-557 –H. capsulatum clinical isolates from different geographic origins of the American Continent; EH-554B –H. capsulatum isolate from the X hotel; Ss –S. schenckii (DNA negative control); C (−) Negative control of the system. Abbreviations: CO — Colombia; AR — Argentina; GT; Guatemala; MX/GR — Mexico/Guerrero.
3.5 RAPD-PCR and DNA sequences of two protein-coding genes
Fig. 4 shows distinct RAPD banding patterns among the studied H. capsulatum isolates. The EH-554B isolate that was recovered from a mouse inoculated with soil sample from a planter of the X hotel differed undoubtedly from the other RAPD patterns associated with isolates from the GR clinical cases, and also differed from the DNA banding patterns of clinical isolates from CO, AR, and GT.
Sequence comparison among H. capsulatum isolates from GR was done with H-anti and ole genes, selected due to their best homology with the H. capsulatum isolates from our laboratory. The DNA of each H. capsulatum isolate was amplified and sequenced. High similarity for the two genes tested, ranging from 92% to 99%, was found among the compared isolates and few insertions and/or deletions (gaps) were observed, revealing no critical genome changes in the partial sequences of the two genes studied (Table 3).
| Isolates | H-anti | ole | ||
| Identities (%) | Gaps (%) | Identities (%) | Gaps (%) | |
| EH-554B and EH-46 | 92 | 2 | 96 | 2 |
| EH-554B and EH-316 | 94 | 2 | 98 | 0 |
| EH-554B and EH-557 | 95 | 2 | 96 | 1 |
| EH-46 and EH-316 | 96 | 0 | 98 | 0 |
| EH-46 and EH-557 | 96 | 0 | 97 | (−) |
| EH-316 and EH-557 | 99 | 0 | 98 | 0 |
| Isolates | H-anti | ole | ||
| Identities (%) | Gaps (%) | Identities (%) | Gaps (%) | |
| EH-554B and EH-46 | 92 | 2 | 96 | 2 |
| EH-554B and EH-316 | 94 | 2 | 98 | 0 |
| EH-554B and EH-557 | 95 | 2 | 96 | 1 |
| EH-46 and EH-316 | 96 | 0 | 98 | 0 |
| EH-46 and EH-557 | 96 | 0 | 97 | (−) |
| EH-316 and EH-557 | 99 | 0 | 98 | 0 |
The PCR products were sequenced taking into account the conditions described under Section 2. (−) Negative.
| Isolates | H-anti | ole | ||
| Identities (%) | Gaps (%) | Identities (%) | Gaps (%) | |
| EH-554B and EH-46 | 92 | 2 | 96 | 2 |
| EH-554B and EH-316 | 94 | 2 | 98 | 0 |
| EH-554B and EH-557 | 95 | 2 | 96 | 1 |
| EH-46 and EH-316 | 96 | 0 | 98 | 0 |
| EH-46 and EH-557 | 96 | 0 | 97 | (−) |
| EH-316 and EH-557 | 99 | 0 | 98 | 0 |
| Isolates | H-anti | ole | ||
| Identities (%) | Gaps (%) | Identities (%) | Gaps (%) | |
| EH-554B and EH-46 | 92 | 2 | 96 | 2 |
| EH-554B and EH-316 | 94 | 2 | 98 | 0 |
| EH-554B and EH-557 | 95 | 2 | 96 | 1 |
| EH-46 and EH-316 | 96 | 0 | 98 | 0 |
| EH-46 and EH-557 | 96 | 0 | 97 | (−) |
| EH-316 and EH-557 | 99 | 0 | 98 | 0 |
The PCR products were sequenced taking into account the conditions described under Section 2. (−) Negative.
The RAPD-PCR and DNA sequence analyses of two nuclear gene loci distinguished between the H. capsulatum isolate from the hotel planters and the clinical strains from other geographic origins, including clinical isolates recovered from three patients from Acapulco, GR. This finding suggests that there could be more than one molecular pattern of H. capsulatum in GR.
References




