-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer P. Wang, Tomoko Hayashi, Sandip K. Datta, Richard S. Kornbluth, Eyal Raz, Donald G. Guiney, CpG oligonucleotides partially inhibit growth of Mycobacterium tuberculosis, but not Salmonella or Listeria, in human monocyte-derived macrophages, FEMS Immunology & Medical Microbiology, Volume 45, Issue 2, August 2005, Pages 303–310, https://doi.org/10.1016/j.femsim.2005.05.007
Close - Share Icon Share
Abstract
Immunostimulatory DNA sequences and their synthetic oligonucleotide analogs (CpG-ODN) activate innate immunity and can stimulate antibacterial effects against numerous intracellular pathogens. While it has been shown previously that CpG-ODN inhibit growth of Mycobacterium avium in murine and human macrophages, we now report that Mycobacterium tuberculosis growth can be inhibited by CpG-ODN treatment of human monocyte-derived macrophages (hMDM). This inhibitory effect was reversed by IFN-γ, which has been shown repeatedly to enhance the growth of virulent M. tuberculosis in cultured hMDM. The antibacterial effect of CpG-ODN in human macrophages was specific for M. tuberculosis when compared to other intracellular pathogens including Listeria monocytogenes and Salmonella enterica serovar Dublin. These data indicate that CpG-ODN can improve the ability of hMDM to contain growth of virulent M. tuberculosis.
1 Introduction
Mycobacterium tuberculosis remains one of the most successful pathogens of humankind. Following initial infection, an effective immune response is able only to control bacterial growth and cannot eliminate the organism (for a comprehensive review of the immunology of M. tuberculosis infection, see [1]). The macrophage is a critical cell in the pathogenesis of M. tuberculosis and development of host resistance, for it represents the principal target cell for M. tuberculosis and the central effector cell of both the innate and acquired immune responses. A central paradigm of M. tuberculosis pathogenesis is that resting, differentiated macrophages have little ability to control bacterial growth. However, stimulation of macrophages with various effector molecules can lead to substantial increases in antimycobacterial activity. Treatment of infected human macrophages with Vitamin D3 has been demonstrated to decrease M. tuberculosis growth by a mechanism dependent on phosphatidylinositol 3-kinase and NADPH oxidase [2]. Activation of human monocytes with the 19-kDa lipoprotein of M. tuberculosis has been shown to reduce the growth of intracellular M. tuberculosis in a manner dependent on Toll-like receptor 2 (TLR2) activation [3]. However, the in vivo antimycobacterial effects of Vitamin D3 and TLR2 ligands have not been determined.
Recent attention has been placed on the role of immunostimulatory DNA sequences to activate the innate immune response to display antibacterial activity (for review, see [4]). The first evidence for immunostimulatory activity of bacterial DNA came from studies by Tokunaga et al. [5] who investigated DNA from Mycobacterium bovis Bacillus Calmette-Guerin (BCG). Krieg [6] later demonstrated that unmethylated CpG dinucleotides within a particular sequence context are responsible for the immunostimulatory properties of bacterial DNA. The effects of bacterial DNA can be mimicked through synthetic oligodeoxynucleotides (ODN) containing CpG motifs. CpG-ODN have been shown to facilitate the control of a variety of intracellular pathogens, including Listeria monocytogenes (L. monocytogenes), Leishmania major, and Francisella tularensis in murine models of infection [7–11]. Hayashi et al. [12] reported that Mycobacterium avium (M. avium) growth is significantly inhibited by CpG-ODN treatment of human and murine macrophages, and M. avium growth is inhibited in mice treated with CpG-ODN. In addition, recent studies have shown that CpG-ODN have the ability to enhance antimycobacterial activity in murine models of M. tuberculosis infection. Friedag et al. [13] reported that mice vaccinated with BCG plus CpG-ODN have a greater reduction in bacterial loads after infection with M. tuberculosis strain Erdman than those vaccinated with BCG alone. More recently, Juffermans et al. [14] found that mice infected with virulent M. tuberculosis are protected by CpG-ODN and that this effect is abrogated in IFN-γ gene-deficient mice. Previous results had indicated that IFN-γ knock-out mice were more susceptible to infections with M. tuberculosis.
In contrast to murine studies, IFN-γ treatment of human macrophages in vitro consistently produces a paradoxical stimulation in the intracellular growth of virulent M. tuberculosis strains [15,16]. This effect is surprising since IFN-γ has been found to directly activate human macrophage antimicrobial activity against a wide variety of pathogens in cell culture. However, human macrophages co-cultured with autologous lymphocytes, primed with IFN-γ plus M. tuberculosis antigen, and treated with IFN-γ are mycobactericidal [17]. These results suggest that IFN-γ may function as a co-stimulatory factor in the human immune response to M. tuberculosis, rather than as the primary activator of macrophage antimycobacterial activity. Auricchio et al. [18] observed that CpG-ODN treatment of human macrophages induced antimycobacterial activity in a manner dependent on phospholipase D. We report that CpG-ODN treatment of human monocyte-derived macrophages (hMDM) can partially inhibit growth of virulent M. tuberculosis in vitro and that the inhibitory effect of CpG-ODN on growth of M. tuberculosis is reversed in the presence of exogenous IFN-γ. Inhibition resulting from CpG-ODN treatment appears to be specific for M. tuberculosis when compared with other intracellular pathogens, including L. monocytogenes and Salmonella enterica serovar Dublin.
2 Materials and methods
2.1 Reagents and cytokines
Endotoxin-free, phosphorothioate, single-stranded oligodeoxynucleotides were obtained from Trilink Biotechnologies, San Diego, California. The sequence of the immunostimulatory CpG-ODN was 5′-TGACTGTGAACGTTCGAGATGA-3′. The sequence of the mutated ODN (M-ODN) was 5′-TGACTGTGAAGGTTAGAGATGA-3′ [underlining indicates immunostimulatory sequences]. We used 10 µg of CpG-ODN or M-ODN per ml unless otherwise noted. Polyinosine-polycytidylic acid [poly (I:C)] was purchased from Amersham (Piscataway, NJ) and used at 50 µg ml−1. Human recombinant IFN-γ was purchased from Calbiochem (La Jolla, CA) and used at a concentration of 50 ng ml−1. To make repurified LPS, LPS from Escherichia coli serotype O11:B4 (Sigma, St. Louis, MO) was phenol re-extracted to remove contaminating lipopeptides [19].
2.2 Preparation of human monocyte-derived macrophages
Human monocytes were isolated from the blood of healthy, tuberculin skin test-negative donors by the method of Freundlich and Audalovic [20]. Briefly, the mononuclear cell fraction was collected from IsoPrep (Robbins Scientific, Sunnyvale, CA) gradients, and monocytes were purified by differential adherence to fibronectin. Approximately 3 ×107 monocytes were isolated from 180 ml of peripheral blood. Monocytes were seeded into 24 well plates at a density of 5 ×105 cells per well, cultured in RPMI 1640 supplemented with 18% autologous serum, and allowed to differentiate for 4 days. To study the protective effect of CpG-ODN treatment before infection, hMDMs were then treated with CpG-ODN for 72 h prior to infection. Macrophages treated with M-ODN or with medium alone served as controls. In some experiments, macrophages were pretreated with human recombinant IFN-γ, LPS, or poly (I:C) for 72 h.
2.3 M. tuberculosis infection of macrophages in vitro
All manipulations with M. tuberculosis were performed under BL-3 laboratory conditions. M. tuberculosis Erdman was grown in Middlebrook 7H9 supplemented with ADC and 0.05% Tween 80 in a 5% CO2 incubator [21]. When the M. tuberculosis culture reached mid-log growth (OD580 of 0.5), a single-cell bacterial suspension was prepared by vortexing the bacteria with 2 mm glass beads, opsonizing in 50% autologous serum for 20 min, and then passing the suspension through a 5 µm filter. The concentration of bacteria was adjusted accordingly after counting bacterial particles using a Petroff–Hauser chamber and a darkfield microscope. The number of viable bacteria was subsequently confirmed by counting colony forming units (CFU). Macrophages were infected for 2 h with M. tuberculosis at a bacterium to macrophage ratio of 1:1, then were washed and subsequently cultured in fresh RPMI 1640 and 18% autologous serum without CpG-ODN. Intracellular growth of M. tuberculosis was determined on days 1, 4, and 7 after infection by lysing macrophages in 0.05% SDS and plating the lysate onto 7H10 agar (37 °C, 2 weeks).
2.4 Protein quantification
Total protein quantification was performed on lysed macrophages using the method of Bradford (BioRad, Hercules, CA) per manufacturer's protocol.
2.5 Listeria monocytogenes infection of macrophages in vitro
J. Fierer (UCSD) provided the pathogenic L. monocytogenes strain 10403S. Macrophages were pretreated with CpG-ODN for 72 h, then infected with L. monocytogenes at a bacterium to macrophage ratio of 1:1. After 15 min of uptake, cells were incubated for 45 min in medium containing 5 µg ml−1 gentamicin (GibcoBRL) to kill extracellular bacteria. This time point was considered 0 h. Infected cells were then incubated at 37 °C in medium containing gentamicin for 6 and 24 h, washed, lysed with 10% saponin in H2O, and serial dilutions were plated on Luria–Bertani agar (room temperature, 24 h). The number of live bacteria was estimated by counting CFU.
2.6 Salmonella enterica infection of macrophages in vitro
Macrophages were pretreated with CpG-ODN for 72 h, then infected with Salmonella enterica serovar Dublin Lane strain at a bacterium to macrophage ratio of 4:1. After 30 min of uptake, cells were incubated for 30 min in medium containing 20 µg ml−1 gentamicin (GibcoBRL) to kill extracellular bacteria. This time point was considered 0 h. Cultures were harvested at 0, 6, and 24 h after infection. Infected cells were lysed with 0.5% deoxycholate in phosphate-buffered saline and serial dilutions of this lysate were plated on Luria–Bertani agar for counting CFU.
2.7 Detection of TNF-α secretion by ELISA
Human macrophages were incubated with CpG-ODN, poly (I:C), or LPS for 72 h. The supernatant was measured for TNF-α secretion using a Quantikine human TNF-α colorimetric sandwich ELISA from R&D Systems (Minneapolis, MN). Results from a representative experiment are presented as means ± standard deviation (SD) of triplicate samples.
2.8 Statistical analysis
Results are expressed as means ± standard errors of the mean (SEM) unless otherwise noted, and statistical differences were determined with the non-paired Student's t test (two-tailed distribution).
3 Results
3.1 CpG-ODN pretreatment of human monocyte-derived macrophages causes a significant reduction in M. tuberculosis growth
To examine whether treatment with CpG-ODN stimulates human macrophages to inhibit the growth of M. tuberculosis, hMDM were treated with CpG-ODN or mutated-ODN (M-ODN) control at 10 µg ml−1 for 72 h and then infected with M. tuberculosis Erdman. We selected the particular CpG-ODN sequence and the 72 h duration of exposure based on prior successes in inhibiting M. avium growth in human macrophages [12]. The numbers of CFU were counted on days 1, 4, and 7 post-infection (Fig. 1(a)). By day 7, treatment with CpG-ODN inhibited intracellular growth of M. tuberculosis compared with no treatment or treatment with M-ODN. The inhibitory effects of CpG-ODN were reproducible in three individual donors in six independent experiments with an overall inhibition of growth of 50% at day 7 (Fig. 1(b)). No increase in inhibition was achieved using a higher concentration (50 µg ml−1) of CpG-ODN (data not shown). CpG-ODN did not appear to influence uptake of mycobacteria in that no statistical differences were seen in the number of CFU recovered from cells treated with CpG-ODN compared to those treated with M-ODN or medium alone at 24 h post-infection.

CpG-ODN pretreatment of hMDM can partially inhibit growth of M. tuberculosis. (a) Growth of M. tuberculosis is significantly diminished by day 7 in CpG-ODN (10 µg ml−1) pretreated cells in comparison to M-ODN (10 µg ml−1) pretreated cells and medium control. Error bars represent the standard error of the mean. *P < 0.01 when compared to medium control. (b) The average reduction of growth on day 7 post-infection was 50% in CpG-ODN-treated macrophages compared with untreated macrophages (P < 0.01). Normalized means were calculated from six independent experiments using macrophages from three human donors and are shown with the standard error of the mean. (c) Total protein from cell lysates was quantified to assess for preferential detachment of macrophages. No differences in total protein levels were observed in monolayers over the time course of infection and between the treatment groups (CpG-ODN) and medium control. Quantity of protein is reported as the measured optical density at 595 λ (BioRad Assay).
3.2 Assessment of macrophage detachment
Some CpG-ODN sequences have been reported to influence the adhesion of macrophages derived from human blood monocytes in vitro [22]. In order to confirm that differences in mycobacteria colony counts over time were not due to preferential detachment of cells in particular experimental groups, we used three methods for assessment. First, the number of macrophages in each monolayer was followed by counting every other day. Second, to confirm that preferential detachment of MDM was not occurring in infected cells, total protein in CpG-ODN-pretreated and in untreated macrophages was quantified on days 1, 4, and 7 after infection. No significant differences were observed between pretreated infected cells and untreated infected cells over the time course of infection (Fig. 1(c)). Finally, the number of CFUs in detached cells was determined by centrifuging cell supernatants, lysing pellets, and plating on 7H10 agar. No differences were seen in mycobacterial growth from supernatants of the treatment groups versus control groups. Total CFUs in the supernatants accounted for less than 10% of the total mycobacteria per well.
3.3 CpG effects on M. tuberculosis growth are reversed with IFN-γ
IFN-γ is known to be essential in providing host resistance to M. tuberculosis infection in mice [16], although the mechanism for the IFN-γ effect is not known. To further investigate the mechanism of the antimycobacterial effects of CpG-ODN, hMDM were pretreated with both IFN-γ and CpG-ODN. IFN-γ can activate macrophages to kill other intracellular pathogens, but cannot activate human macrophages to kill M. tuberculosis[23]. Instead, early intracellular growth of M. tuberculosis in hMDM is enhanced by IFN-γ[24]. We found that pretreatment of hMDM with 50 ng ml−1 of IFN-γ for 72 h prior to infection did not inhibit growth of M. tuberculosis. When hMDM were treated with both CpG-ODN and IFN-γ, the inhibition of M. tuberculosis growth observed with CpG-ODN treatment was abolished (Fig. 2). Similar results were obtained when a second human donor was used in a separate, otherwise identical experiment.
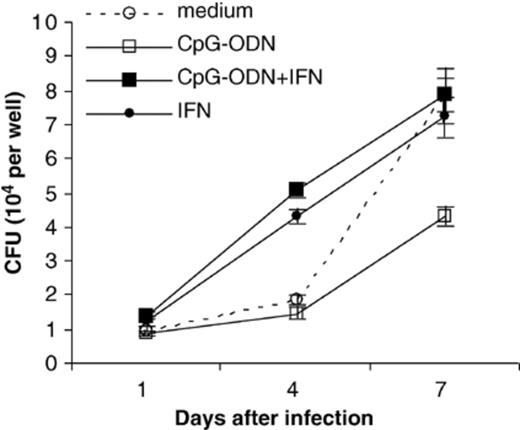
The CpG-ODN antimycobacterial effect is reversed in the presence of human recombinant IFN-γ. Results are shown for one experiment using hMDM pretreated with CpG-ODN (10 µg ml−1) and/or IFN-γ (50 ng ml−1). Similar results were obtained using a second human donor in an independent experiment. Error bars represent the standard error of the mean.
3.4 TNF-α is not induced by CpG-ODN in human monocyte-derived macrophages
Toll-like receptor 9 (TLR9) is reportedly involved in the recognition of CpG motifs in human cells [25,26]. Activation of TLRs on macrophages triggers the release of pro-inflammatory cytokines such as TNF-α. We were interested in finding out whether CpG-ODN treatment can induce the secretion of TNF-α in hMDM. We found that treatment of hMDM with 10 µg ml−1 of CpG-ODN did not induce the secretion of TNF-α above levels of unstimulated macrophages at 8, 24, or 72 h post-treatment (Fig. 3). Increasing the CpG-ODN concentration to 100 µg ml−1 did not affect this result. As a control, we treated human macrophages with repurified LPS, a TLR4 agonist. Treatment with 10 ng ml−1 of repurified LPS induced TNF-α at all three time points.
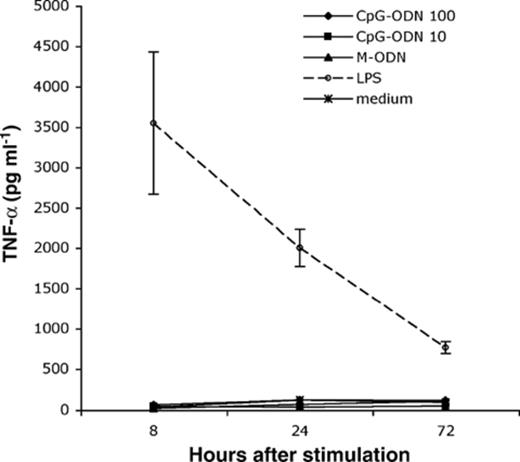
TNF-α is not induced after treating hMDM with CpG-ODN. Macrophages were stimulated with either CpG-ODN (10 or 100 µg ml−1), M-ODN (10 µg ml−1), or repurified LPS (10 ng ml−1). TNF-α was measured in supernatants collect at 8, 24, and 72 h after stimulation. Only repurified LPS stimulated TNF-α in hMDM. Error bars represent the standard deviation from three independent wells.
3.5 Pretreatment with poly (I:C) does not inhibit growth of M. tuberculosis
We were interested to know if TLR agonists other than the TLR2 ligand, 19-kDa lipoprotein [3], or the TLR9 ligand, CpG-ODN, could inhibit the growth of virulent M. tuberculosis in human macrophages. We pretreated hMDM with poly (I:C), a double-stranded RNA that acts as a ligand of TLR3 [27]. No toxicity was observed by counting adherent macrophages after poly (I:C) treatment. We stimulated hMDM with 50 µg ml−1 of poly (I:C) for 72 h prior to infecting with M. tuberculosis. By day 7 post-infection, M. tuberculosis growth was significantly higher in cells pretreated with poly (I:C) compared with medium control (P < 0.01) (Fig. 4). Similar results were obtained from an experiment using a second human donor. No differences were observed in uptake of mycobacteria for each treatment group based on CFU counts on day 1 post-infection.
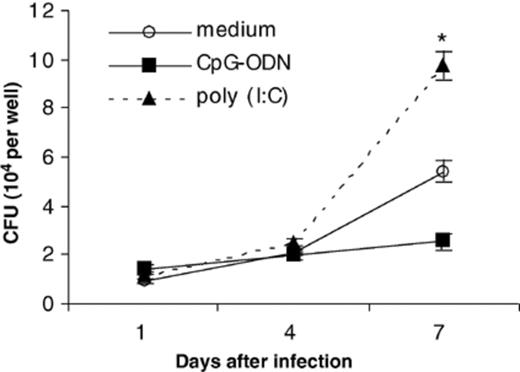
Pretreatment of hMDM with 50 µg ml−1 of poly (I:C), a ligand for TLR3, enhances the growth of M. tuberculosis. Results shown are representative of one experiment. Similar results were obtained using a second human donor in an independent experiment. Error bars represent the standard error of the mean. *P < 0.01 when compared to medium control.
3.6 The CpG-ODN effect in human macrophages is unique against mycobacteria compared to other intracellular pathogens
Because CpG-ODN were shown to inhibit growth of L. monocytogenes in mice in vivo, we examined if CpG-ODN could inhibit growth of L. monocytogenes in hMDM [7–9]. We pretreated macrophages with CpG-ODN for 72 h and subsequently infected with Listeria. As shown in Fig. 5 (a), bacteria grew exponentially during the 24 h following infection. In cells pretreated with IFN-γ for 72 h, growth of Listeria was significantly reduced at 24 h post-infection (P < 0.01). This inhibition of growth was consistent with prior literature reports [28,29]. However, CpG-ODN pretreatment of macrophages did not inhibit growth of L. monocytogenes over this same time course. Experiments were performed independently three times using three different donors. Of note, the same human blood donors were used for Listeria experiments as for M. tuberculosis experiments. Therefore, differences in growth between Listeria and mycobacteria in CpG-treated cells could not be attributed to genetic differences between human donors.
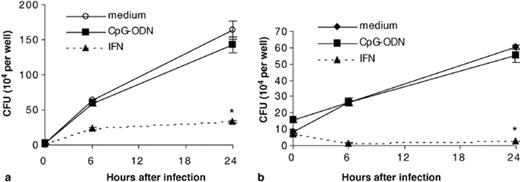
Intracellular growth of (a) Listeria monocytogenes and (b) Salmonella enterica serovar Dublin are not influenced by CpG-ODN (10 µg ml−1) pretreatment of hMDM over a 24 h time course. Cells pretreated with human recombinant IFN-γ at 50 ng ml−1 were able to significantly inhibit growth of bacteria. Similar data were obtained in three independent experiments using different human donors. Error bars represent the standard error of the mean. *P < 0.01 when compared to medium control.
We also obtained data to demonstrate that CpG-ODN pretreatment of macrophages does not inhibit growth of the intracellular pathogen Salmonella enterica serovar Dublin. IFN-γ pretreatment was found to significantly inhibit growth of Salmonella 6 and 24 h after infection (Fig. 5(b)). All experiments were performed under CpG-ODN pretreatment conditions identical to those used in M. tuberculosis studies and hMDM were obtained from the same donors as above.
4 Discussion
CpG-ODN have been shown to increase the resistance of mice to a variety of bacterial agents, including Listeria, Francisella, Mycobacterium, and agents of polymicrobial sepsis [7–14,30]. Recently, evidence was reported for a direct effect of CpG-ODN on the ability of murine and human macrophages to inhibit the growth of M. avium in cell culture. We have now found that the induction of antimycobacterial activity by CpG-ODN in human macrophages extends to virulent M. tuberculosis. These findings suggest that CpG-ODN induces a general antimycobacterial response in macrophages that is not specific for the host species and cannot be overcome by even the most pathogenic organisms, increasing the possibility that CpG-ODN could have therapeutic significance in tuberculosis.
Only a small number of agents have been shown to enhance the resistance of human macrophages to M. tuberculosis infection in cell culture, most notably Vitamin D3 and TLR2 ligands. The mechanism of the Vitamin D3 effect was shown to involve signal transduction through activation of PI-3 kinase and enhancement of the NADPH oxidase-dependent antimicrobial system [2]. In murine cells, TLR2 ligands increased nitric oxide-dependent antimycobacterial activity, but this system was shown not to be involved in the human macrophage system, and the mechanism of M. tuberculosis inhibition remains unknown [3]. Activation of phospholipase D has been demonstrated to participate in CpG-induced M. tuberculosis growth inhibition [16]. In the present study, we found that the mechanism of the CpG-induced inhibition of M. tuberculosis growth in human macrophages was not due to non-specific effects on cell viability or on phagocytosis. The CpG-ODN effect requires the CpG sequence motif, indicating a specific interaction with the macrophage. We considered the possibility that interleukin-12 (IL-12) induction might account for mycobacterial growth inhibition. CpG-ODN can induce mRNA expression of IL-12 in murine macrophage and human myeloma cell lines [25]. In Listeria experiments, mice injected with CpG-ODN respond by producing IL-12 [7]. With regard to M. tuberculosis infection, IL-12 has been shown to be important for resistance. IL-12 p40-gene deficient mice have increased susceptibility to mycobacterial infection and have decreased survival compared to control mice [31]. However, when we used ELISA to measure IL-12 protein levels in supernatants from human macrophages treated with CpG-ODN for 72 h, we were unable to detect its presence, which argues against direct influence of IL-12 on M. tuberculosis growth in this system (data not shown).
We found that the ability of CpG-ODN to inhibit M. tuberculosis growth in human macrophages was abolished by treatment with IFN-γ. We were able to confirm previous reports that IFN-γ alone has a paradoxical effect on human macrophages to increase the early intracellular growth rate of virulent M. tuberculosis strains [21,22]. Co-treatment with IFN-γ and CpG-ODN did not decrease this enhanced growth rate, suggesting that the IFN-γ effect dominates in this system. Control experiments showed that IFN-γ induced significant antibacterial activity against both Listeria and Salmonella in this same human macrophage system, indicating that stimulation of bacterial growth by IFN-γ is specific for M. tuberculosis infected cells. In fact, IFN-γ treated human macrophages are able to effectively kill virulent Salmonella. We postulate that the resistance of M. tuberculosis to IFN-induced antibacterial activity in human macrophages may represent a specific virulence mechanism for this host-adapted pathogen. This hypothesis is consistent with the clinical findings that people are able to clear Salmonella infections through a CD4 cell-dependent mechanism involving IFN-γ, but are not able to eliminate M. tuberculosis once infection is established. Instead, the acquired immune response is only able to limit growth and induce a state of latent infection.
The CpG effect on human macrophages appears to be relatively specific for mycobacteria, since no growth inhibition was seen for two other intracellular pathogens, Listeria and Salmonella. However, both of these bacteria are controlled by treatment with IFN-γ. These findings suggest that CpG sequences and IFN-γ may play fundamentally different roles in the host response to Listeria and Salmonella compared to M. tuberculosis. The main role of CpG motifs released by Listeria and Salmonella may be as an adjuvant to promote a Th1 acquired immune response, and the IFN-γ produced by lymphocytes can directly activate macrophages to inhibit or kill the bacteria. In the case of tuberculosis, release of CpG motifs early in the infection could directly activate macrophages to slow M. tuberculosis proliferation, as well as produce an adjuvant effect to induce a Th1 response. However, the success of M. tuberculosis as a pathogen may depend, in part, on its ability to subvert the subsequent IFN-γ-mediated macrophage activation. Not only is the CpG antimycobacterial effect abolished, but M. tuberculosis actually grows better in IFN-γ treated macrophages. Control of the infection appears to be due to the concerted effects of a variety of cell types acting together with Th1-type cytokines.
References



