-
PDF
- Split View
-
Views
-
Cite
Cite
Paula Jorge, Andreia Patrícia Magalhães, Tânia Grainha, Diana Alves, Ana Margarida Sousa, Susana Patrícia Lopes, Maria Olívia Pereira, Antimicrobial resistance three ways: healthcare crisis, major concepts and the relevance of biofilms, FEMS Microbiology Ecology, Volume 95, Issue 8, August 2019, fiz115, https://doi.org/10.1093/femsec/fiz115
Close - Share Icon Share
ABSTRACT
Worldwide, infections are resuming their role as highly effective killing diseases, as current treatments are failing to respond to the growing problem of antimicrobial resistance (AMR). The social and economic burden of AMR seems ever rising, with health- and research-related organizations rushing to collaborate on a worldwide scale to find effective solutions. Resistant bacteria are spreading even in first-world nations, being found not only in healthcare-related settings, but also in food and in the environment. In this minireview, the impact of AMR in healthcare systems and the major bacteria behind it are highlighted. Ecological aspects of AMR evolution and the complexity of its molecular mechanisms are explained. Major concepts, such as intrinsic, acquired and adaptive resistance, as well as tolerance and heteroresistance, are also clarified. More importantly, the problematic of biofilms and their role in AMR, namely their main resistance and tolerance mechanisms, are elucidated. Finally, some of the most promising anti-biofilm strategies being investigated are reviewed. Much is still to be done regarding the study of AMR and the discovery of new anti-biofilm strategies. Gladly, considerable research on this topic is generated every day and increasingly concerted actions are being engaged globally to try and tackle this problem.
INTRODUCTION
Antibiotic discovery had an unprecedented role in medical advances, saving countless lives by mitigating infectious diseases, but the rapid global emergence of resistant bacteria over the last few decades has been imperiling their worth (Martens and Demain 2017; WHO 2017a). Humankind is witnessing as antimicrobial resistance (AMR) becomes one of the biggest threats to medicine today, killing around 700 000 people worldwide each year (O'Neill 2014; Aslam et al. 2018). The etiology of AMR is multifaceted, embracing (i) overconsumption of antibiotics due to overprescription, self-medication or over-the-counter accessible antibiotics, (ii) absence of standardized guidelines for antibiotic usage, (iii) lack of sanitation/hygiene practices and (iv) access to counterfeit drugs (Morgan et al. 2011; Laxminarayan and Heymann 2012; CDC 2013; Nature Editorial 2013; Luyt et al. 2014; Read and Woods 2014; Ventola 2015). Food is also an important source of AMR (Marshall and Levy 2011), due to the widespread use of antibiotics in animals, while the increased international human traveling and animal transportation aid in AMR spreading (EFSA 2018).
In healthcare settings, the concurrence of factors such as high antibiotic consumption, vulnerable patients, invasive practices and inflow of pathogenic species has contributed to the substantial health and economic burden of AMR (Golkar, Bagasra and Pace 2014; Roca et al. 2015). To mitigate the increasing rate of AMR, main stakeholders (i.e. policy makers, public health authorities, regulatory agencies, pharmaceutical companies and the scientific community) were prompted to take a concerted action. Therefore, measures such as (i) rational/prudent use of antibiotics (Lushniak 2014), (ii) effective infection control measures, (iii) mitigation of environmental exposure, (iv) better diagnostic tools (Michael, Dominey-Howes and Labbate 2014), (v) prevention/surveillance research and (vi) development of new therapies (Roca et al. 2015) were proposed.
Initiatives and programs raising awareness and promoting strategies to improve knowledge and reflections regarding AMR are key to fight its dissemination. Noteworthy, initiatives include the B-Debate (https://www.bdebate.org), which fosters the dialogue among world-renowned multidisciplinary scientists on the growing threat of AMR at all health, animal and environmental levels. In addition, the Joint Programming Initiative on Antimicrobial Resistance (https://www.jpiamr.eu) has been defining a strategic research agenda under the assumption that only collaborative effort by an interdisciplinary team will afford the necessary critical mass and the scientific expertise to tackle AMR. Likewise, different agencies across the globe are engaged to make all efforts to control AMR. These agencies include the Global Antimicrobial Resistance Surveillance System (https://www.who.int/glass), the Centers for Disease Control and Prevention (https://www.cdc.gov), the Food and Agriculture Organization (http://www.fao.org), the European Centre for Disease Prevention and Control (https://ecdc.europa.eu/), the European Medicines Agency (https://www.ema.europa.eu/), the World Alliance Against Antibiotic Resistance (https://www.waaar.org/), the Global Health Security Agenda (https://www.ghsagenda.org/) and many others. Despite the proposed recommendations and resolutions, little progress has been made so far, and AMR shows no signs of decline.
MULTIDRUG-RESISTANT ORGANISMS: THE ‘SUPERBUGS’
AMR evolution is nothing but Darwinian selection. Microorganisms evolved to develop mechanisms to escape lethal effects of antimicrobials (Forsberg et al. 2014; Aslam et al. 2018). Notably, the aberrant use of antibiotics exerted a significant selective pressure for the development of multidrug-resistant (MDR) organisms. These ‘superbugs’ are able to resist multiple classes of antibiotics, evading the majority of current therapies (Stokes and Gillings 2011; Munita and Arias 2016) and spreading at an alarming rate, leading to abnormal rates of morbidity/mortality (Khameneh et al. 2016). A recent 2018 study shows that ‘superbug’ infections accounted for 33 000 annual deaths in Europe in 2015, a burden that has been compared to that of other diseases combined (e.g. tuberculosis, HIV, flu) (Cassini et al. 2018).
MDR infections may be triggered by Gram-negative or Gram-positive bacteria or even by fungal species. Staphylococcus aureus and Enterococcus species are among the most notorious ‘superbugs’, currently posing a pandemic threat (Watkins, David and Salata 2012; CDC 2013; Rossolini et al. 2014; Aslam et al. 2018). The most worrisome is the methicillin-resistant S. aureus (MRSA), whose ability to evolve and adapt to multiple settings (e.g. healthcare, community, livestock) has caused its rapid dispersal over the globe (Monaco et al. 2016). The spread of vancomycin-resistant enterococci (Golkar, Bagasra and Pace 2014) and the global epidemic of resistant Streptococcus pneumoniae and Mycobacterium tuberculosis (common respiratory pathogens) (Rossolini et al. 2014) also represent serious threats. Similarly, the emergence of MDR Gram-negative pathogens, typically thriving in healthcare facilities, namely Enterobacteriaceae (mostly Klebsiella pneumoniae), Pseudomonas aeruginosa, Acinetobacter spp., extended-spectrum β-lactamase (ESBL)-producing Escherichia coli and Neisseria gonorrhoeae (Rossolini et al. 2014), is particularly worrying (CDC 2013; Golkar, Bagasra and Pace 2014). In 2017, the WHO issued a global priority pathogen list of antibiotic-resistant bacteria (WHO 2017a) to help in prioritizing the research and development of new and effective antibiotic treatments, updating the previous priority pathogen list issued by the CDC in 2013 (CDC 2013). Accordingly, the WHO has stratified the resistant pathogens in three priority tiers: ‘critical’, ‘high’ and ‘medium’. Table 1 summarizes the key features regarding these top bacterial threats.
| WHO categorization . | Bacterial pathogens . | Key features . | References . |
|---|---|---|---|
| Critical | Acinetobacter baumannii, carbapenem-resistant | Most associated with HAIs worldwide, accounting for up to 20% of ICU infections worldwide. Causes pneumonia and bloodstream and wound infections, particularly in mechanically ventilated patients. Around 45% isolates are MDR, including resistance to last-resort carbapenems most often linked to the production of carbapenemases. | Potron, Poirel and Nordmann 2015; Harding, Hennon and Feldman 2017; Lee et al. 2017 |
| P. aeruginosa, carbapenem-resistant | Common cause of HAIs, including pneumonia, bloodstream, urinary tract and surgical site infections. Carbapenem resistance mostly related to porin (OprD) deficiency. Invasive isolates resistant to carbapenems were 17.8% in Europe (2015) and 19.2% in the USA (2014). | Potron, Poirel and Nordmann 2015; WHO 2017b | |
| Enterobacteriaceae, carbapenem-resistant, third-generation cephalosporin-resistant | Enterobacteriaceae include K. pneumonia, E. coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp. and Morganella spp. K. pneumoniae invasive isolates resistant to carbapenems were reported from all WHO regions, with some countries reporting up to 50%. Human isolates resistant to colistin, a last-resort antimicrobial against carbapenem-resistant Enterobacteriaceae, were already reported. 10–20% of Enterobacteriaceae isolated in the USA are resistant to ceftazidime. | Arizpe et al. 2016; Castanheira et al. 2016; WHO 2017b | |
| M. tuberculosis | M. tuberculosis infection is the precursor to tuberculosis disease, responsible for 1.5 million deaths/year. Aerial dissemination, with infection typically occurring in the lungs. Sometimes treatable with first-line drugs (isoniazid, rifampicin) but mostly resistant to several antibiotics (fluoroquinolones) and to second-line injectable drugs (amikacin, capreomycin and kanamycin). | WHO 2018a | |
| High | Enterococcus faecium, vancomycin-resistant | Most commonly isolated Gram-positive nosocomial pathogen worldwide with highly flexible genome that enables rapid adaption. Vancomycin-resistant isolates rose from 0% to more than 80% from 1980 to 2007, in the USA. Vancomycin resistance arises from reduced vancomycin-binding affinity, involving alterations in the peptidoglycan synthesis pathway. | Arias and Murray 2012; Gao, Howden and Stinear 2018 |
| S. aureus, methicillin-resistant | Among the most frequent of all antibiotic-resistant threats and leading cause of bacteremia. Outstanding versatility in adapting to different epidemiological settings (healthcare, community, animal). Characteristically MDR, with infections spreading across the globe. Infections commonly involve the skin, soft tissue, bone, joints and indwelling catheters or prosthetic devices. | Monaco et al. 2016; Hassoun, Linden and Friedman 2017 | |
| S. aureus, vancomycin- intermediate (VISA) and -resistant (VRSA) | VISA (MIC = 4–8 µg/mL) appeared in MRSA infected patients due to mutations during prolonged vancomycin therapy. VISA are associated with persistent infections, vancomycin treatment failure and poor clinical outcome. VRSA (MIC ≥ 16 µg/mL) appeared by acquisition of plasmid-borne copies of the transposon Tn1546, from vancomycin-resistant Enterococcus faecalis. VRSA infection numbers are still limited to date (14 in the USA). | Gardete and Tomasz 2014 | |
| Helicobacter pylori, clarithromycin-resistant | Most successful human gastric pathogen able to resist stomach acids, colonizing over 50% of the population. Related to gastritis, peptic ulcers, gastric adenocarcinoma, iron deficiency anemia, idiopathic thrombocytopenic purpura and vitamin B12 deficiency. Sequential, bismuth quadruple and non-bismuth quadruple therapies seam effective in high clarithromycin-resistance countries. | Alba, Blanco and Alarcón 2017; Goderska, Agudo Pena and Alarcon 2018 | |
| Campylobacter spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses, majorly gastroenteritis, primarily caused by Campylobacter jejuni. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. Macrolides (e.g. erythromycin and azithromycin) are considered as fluoroquinolone alternatives. | Bolinger and Kathariou 2017 | |
| Salmonella spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses/diarrheal diseases, namely gastroenteritis. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. | Kim et al. 2016; WHO 2018b | |
| N. gonorrhoeae, third-generation cephalosporin-resistant, fluoroquinolone-resistant | Causes gonorrhea, an obligate human infection, usually transmitted during sexual activity, often resulting in urethritis in men and cervicitis in women. Gonorrhea is rising, with 18.6% increase during 2016–17 and 75.2% increase since 2009 in the USA. Asymptomatic men (two-thirds of infected men) constitute the principal source of dissemination. | CDC 2017; Rice et al. 2017 | |
| Medium | S. pneumoniae, penicillin nonsusceptible | Encapsulated bacteria causes meningitis, septicemia and pneumonia, but also milder infections, such as sinusitis and otitis media. Major cause of morbidity and mortality worldwide, mainly in poor countries and in children under the age of 2. There are two available vaccines that target the most prevalent serotypes. | WHO 2014 |
| Haemophilus influenzae, ampicillin-resistant | Serotype b, an obligate human pathogen, is the most pathogenic, responsible for respiratory infections, ocular infection, sepsis and meningitis. Leading worldwide cause of meningitis morbidity and mortality in unimmunized populations. Highly related to chronic obstructive pulmonary disease, a leading cause of morbidity and mortality worldwide. Third-generation cephalosporins are the empiric treatment of choice. | ECDC 2017a; Sriram et al. 2018 | |
| Shigella spp., fluoroquinolone-resistant | Causes shigellosis, a major cause of diarrhea affecting mainly children under the age of 5. Between 80 and 165 million cases of shigellosis occur annually worldwide, majorly in developing countries. | ECDC 2017b; CDC 2018 |
| WHO categorization . | Bacterial pathogens . | Key features . | References . |
|---|---|---|---|
| Critical | Acinetobacter baumannii, carbapenem-resistant | Most associated with HAIs worldwide, accounting for up to 20% of ICU infections worldwide. Causes pneumonia and bloodstream and wound infections, particularly in mechanically ventilated patients. Around 45% isolates are MDR, including resistance to last-resort carbapenems most often linked to the production of carbapenemases. | Potron, Poirel and Nordmann 2015; Harding, Hennon and Feldman 2017; Lee et al. 2017 |
| P. aeruginosa, carbapenem-resistant | Common cause of HAIs, including pneumonia, bloodstream, urinary tract and surgical site infections. Carbapenem resistance mostly related to porin (OprD) deficiency. Invasive isolates resistant to carbapenems were 17.8% in Europe (2015) and 19.2% in the USA (2014). | Potron, Poirel and Nordmann 2015; WHO 2017b | |
| Enterobacteriaceae, carbapenem-resistant, third-generation cephalosporin-resistant | Enterobacteriaceae include K. pneumonia, E. coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp. and Morganella spp. K. pneumoniae invasive isolates resistant to carbapenems were reported from all WHO regions, with some countries reporting up to 50%. Human isolates resistant to colistin, a last-resort antimicrobial against carbapenem-resistant Enterobacteriaceae, were already reported. 10–20% of Enterobacteriaceae isolated in the USA are resistant to ceftazidime. | Arizpe et al. 2016; Castanheira et al. 2016; WHO 2017b | |
| M. tuberculosis | M. tuberculosis infection is the precursor to tuberculosis disease, responsible for 1.5 million deaths/year. Aerial dissemination, with infection typically occurring in the lungs. Sometimes treatable with first-line drugs (isoniazid, rifampicin) but mostly resistant to several antibiotics (fluoroquinolones) and to second-line injectable drugs (amikacin, capreomycin and kanamycin). | WHO 2018a | |
| High | Enterococcus faecium, vancomycin-resistant | Most commonly isolated Gram-positive nosocomial pathogen worldwide with highly flexible genome that enables rapid adaption. Vancomycin-resistant isolates rose from 0% to more than 80% from 1980 to 2007, in the USA. Vancomycin resistance arises from reduced vancomycin-binding affinity, involving alterations in the peptidoglycan synthesis pathway. | Arias and Murray 2012; Gao, Howden and Stinear 2018 |
| S. aureus, methicillin-resistant | Among the most frequent of all antibiotic-resistant threats and leading cause of bacteremia. Outstanding versatility in adapting to different epidemiological settings (healthcare, community, animal). Characteristically MDR, with infections spreading across the globe. Infections commonly involve the skin, soft tissue, bone, joints and indwelling catheters or prosthetic devices. | Monaco et al. 2016; Hassoun, Linden and Friedman 2017 | |
| S. aureus, vancomycin- intermediate (VISA) and -resistant (VRSA) | VISA (MIC = 4–8 µg/mL) appeared in MRSA infected patients due to mutations during prolonged vancomycin therapy. VISA are associated with persistent infections, vancomycin treatment failure and poor clinical outcome. VRSA (MIC ≥ 16 µg/mL) appeared by acquisition of plasmid-borne copies of the transposon Tn1546, from vancomycin-resistant Enterococcus faecalis. VRSA infection numbers are still limited to date (14 in the USA). | Gardete and Tomasz 2014 | |
| Helicobacter pylori, clarithromycin-resistant | Most successful human gastric pathogen able to resist stomach acids, colonizing over 50% of the population. Related to gastritis, peptic ulcers, gastric adenocarcinoma, iron deficiency anemia, idiopathic thrombocytopenic purpura and vitamin B12 deficiency. Sequential, bismuth quadruple and non-bismuth quadruple therapies seam effective in high clarithromycin-resistance countries. | Alba, Blanco and Alarcón 2017; Goderska, Agudo Pena and Alarcon 2018 | |
| Campylobacter spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses, majorly gastroenteritis, primarily caused by Campylobacter jejuni. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. Macrolides (e.g. erythromycin and azithromycin) are considered as fluoroquinolone alternatives. | Bolinger and Kathariou 2017 | |
| Salmonella spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses/diarrheal diseases, namely gastroenteritis. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. | Kim et al. 2016; WHO 2018b | |
| N. gonorrhoeae, third-generation cephalosporin-resistant, fluoroquinolone-resistant | Causes gonorrhea, an obligate human infection, usually transmitted during sexual activity, often resulting in urethritis in men and cervicitis in women. Gonorrhea is rising, with 18.6% increase during 2016–17 and 75.2% increase since 2009 in the USA. Asymptomatic men (two-thirds of infected men) constitute the principal source of dissemination. | CDC 2017; Rice et al. 2017 | |
| Medium | S. pneumoniae, penicillin nonsusceptible | Encapsulated bacteria causes meningitis, septicemia and pneumonia, but also milder infections, such as sinusitis and otitis media. Major cause of morbidity and mortality worldwide, mainly in poor countries and in children under the age of 2. There are two available vaccines that target the most prevalent serotypes. | WHO 2014 |
| Haemophilus influenzae, ampicillin-resistant | Serotype b, an obligate human pathogen, is the most pathogenic, responsible for respiratory infections, ocular infection, sepsis and meningitis. Leading worldwide cause of meningitis morbidity and mortality in unimmunized populations. Highly related to chronic obstructive pulmonary disease, a leading cause of morbidity and mortality worldwide. Third-generation cephalosporins are the empiric treatment of choice. | ECDC 2017a; Sriram et al. 2018 | |
| Shigella spp., fluoroquinolone-resistant | Causes shigellosis, a major cause of diarrhea affecting mainly children under the age of 5. Between 80 and 165 million cases of shigellosis occur annually worldwide, majorly in developing countries. | ECDC 2017b; CDC 2018 |
Abbreviations: HAIs, hospital acquired infections; ICU, intensive care unit; MIC, minimum inhibitory concentration.
| WHO categorization . | Bacterial pathogens . | Key features . | References . |
|---|---|---|---|
| Critical | Acinetobacter baumannii, carbapenem-resistant | Most associated with HAIs worldwide, accounting for up to 20% of ICU infections worldwide. Causes pneumonia and bloodstream and wound infections, particularly in mechanically ventilated patients. Around 45% isolates are MDR, including resistance to last-resort carbapenems most often linked to the production of carbapenemases. | Potron, Poirel and Nordmann 2015; Harding, Hennon and Feldman 2017; Lee et al. 2017 |
| P. aeruginosa, carbapenem-resistant | Common cause of HAIs, including pneumonia, bloodstream, urinary tract and surgical site infections. Carbapenem resistance mostly related to porin (OprD) deficiency. Invasive isolates resistant to carbapenems were 17.8% in Europe (2015) and 19.2% in the USA (2014). | Potron, Poirel and Nordmann 2015; WHO 2017b | |
| Enterobacteriaceae, carbapenem-resistant, third-generation cephalosporin-resistant | Enterobacteriaceae include K. pneumonia, E. coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp. and Morganella spp. K. pneumoniae invasive isolates resistant to carbapenems were reported from all WHO regions, with some countries reporting up to 50%. Human isolates resistant to colistin, a last-resort antimicrobial against carbapenem-resistant Enterobacteriaceae, were already reported. 10–20% of Enterobacteriaceae isolated in the USA are resistant to ceftazidime. | Arizpe et al. 2016; Castanheira et al. 2016; WHO 2017b | |
| M. tuberculosis | M. tuberculosis infection is the precursor to tuberculosis disease, responsible for 1.5 million deaths/year. Aerial dissemination, with infection typically occurring in the lungs. Sometimes treatable with first-line drugs (isoniazid, rifampicin) but mostly resistant to several antibiotics (fluoroquinolones) and to second-line injectable drugs (amikacin, capreomycin and kanamycin). | WHO 2018a | |
| High | Enterococcus faecium, vancomycin-resistant | Most commonly isolated Gram-positive nosocomial pathogen worldwide with highly flexible genome that enables rapid adaption. Vancomycin-resistant isolates rose from 0% to more than 80% from 1980 to 2007, in the USA. Vancomycin resistance arises from reduced vancomycin-binding affinity, involving alterations in the peptidoglycan synthesis pathway. | Arias and Murray 2012; Gao, Howden and Stinear 2018 |
| S. aureus, methicillin-resistant | Among the most frequent of all antibiotic-resistant threats and leading cause of bacteremia. Outstanding versatility in adapting to different epidemiological settings (healthcare, community, animal). Characteristically MDR, with infections spreading across the globe. Infections commonly involve the skin, soft tissue, bone, joints and indwelling catheters or prosthetic devices. | Monaco et al. 2016; Hassoun, Linden and Friedman 2017 | |
| S. aureus, vancomycin- intermediate (VISA) and -resistant (VRSA) | VISA (MIC = 4–8 µg/mL) appeared in MRSA infected patients due to mutations during prolonged vancomycin therapy. VISA are associated with persistent infections, vancomycin treatment failure and poor clinical outcome. VRSA (MIC ≥ 16 µg/mL) appeared by acquisition of plasmid-borne copies of the transposon Tn1546, from vancomycin-resistant Enterococcus faecalis. VRSA infection numbers are still limited to date (14 in the USA). | Gardete and Tomasz 2014 | |
| Helicobacter pylori, clarithromycin-resistant | Most successful human gastric pathogen able to resist stomach acids, colonizing over 50% of the population. Related to gastritis, peptic ulcers, gastric adenocarcinoma, iron deficiency anemia, idiopathic thrombocytopenic purpura and vitamin B12 deficiency. Sequential, bismuth quadruple and non-bismuth quadruple therapies seam effective in high clarithromycin-resistance countries. | Alba, Blanco and Alarcón 2017; Goderska, Agudo Pena and Alarcon 2018 | |
| Campylobacter spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses, majorly gastroenteritis, primarily caused by Campylobacter jejuni. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. Macrolides (e.g. erythromycin and azithromycin) are considered as fluoroquinolone alternatives. | Bolinger and Kathariou 2017 | |
| Salmonella spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses/diarrheal diseases, namely gastroenteritis. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. | Kim et al. 2016; WHO 2018b | |
| N. gonorrhoeae, third-generation cephalosporin-resistant, fluoroquinolone-resistant | Causes gonorrhea, an obligate human infection, usually transmitted during sexual activity, often resulting in urethritis in men and cervicitis in women. Gonorrhea is rising, with 18.6% increase during 2016–17 and 75.2% increase since 2009 in the USA. Asymptomatic men (two-thirds of infected men) constitute the principal source of dissemination. | CDC 2017; Rice et al. 2017 | |
| Medium | S. pneumoniae, penicillin nonsusceptible | Encapsulated bacteria causes meningitis, septicemia and pneumonia, but also milder infections, such as sinusitis and otitis media. Major cause of morbidity and mortality worldwide, mainly in poor countries and in children under the age of 2. There are two available vaccines that target the most prevalent serotypes. | WHO 2014 |
| Haemophilus influenzae, ampicillin-resistant | Serotype b, an obligate human pathogen, is the most pathogenic, responsible for respiratory infections, ocular infection, sepsis and meningitis. Leading worldwide cause of meningitis morbidity and mortality in unimmunized populations. Highly related to chronic obstructive pulmonary disease, a leading cause of morbidity and mortality worldwide. Third-generation cephalosporins are the empiric treatment of choice. | ECDC 2017a; Sriram et al. 2018 | |
| Shigella spp., fluoroquinolone-resistant | Causes shigellosis, a major cause of diarrhea affecting mainly children under the age of 5. Between 80 and 165 million cases of shigellosis occur annually worldwide, majorly in developing countries. | ECDC 2017b; CDC 2018 |
| WHO categorization . | Bacterial pathogens . | Key features . | References . |
|---|---|---|---|
| Critical | Acinetobacter baumannii, carbapenem-resistant | Most associated with HAIs worldwide, accounting for up to 20% of ICU infections worldwide. Causes pneumonia and bloodstream and wound infections, particularly in mechanically ventilated patients. Around 45% isolates are MDR, including resistance to last-resort carbapenems most often linked to the production of carbapenemases. | Potron, Poirel and Nordmann 2015; Harding, Hennon and Feldman 2017; Lee et al. 2017 |
| P. aeruginosa, carbapenem-resistant | Common cause of HAIs, including pneumonia, bloodstream, urinary tract and surgical site infections. Carbapenem resistance mostly related to porin (OprD) deficiency. Invasive isolates resistant to carbapenems were 17.8% in Europe (2015) and 19.2% in the USA (2014). | Potron, Poirel and Nordmann 2015; WHO 2017b | |
| Enterobacteriaceae, carbapenem-resistant, third-generation cephalosporin-resistant | Enterobacteriaceae include K. pneumonia, E. coli, Enterobacter spp., Serratia spp., Proteus spp., Providencia spp. and Morganella spp. K. pneumoniae invasive isolates resistant to carbapenems were reported from all WHO regions, with some countries reporting up to 50%. Human isolates resistant to colistin, a last-resort antimicrobial against carbapenem-resistant Enterobacteriaceae, were already reported. 10–20% of Enterobacteriaceae isolated in the USA are resistant to ceftazidime. | Arizpe et al. 2016; Castanheira et al. 2016; WHO 2017b | |
| M. tuberculosis | M. tuberculosis infection is the precursor to tuberculosis disease, responsible for 1.5 million deaths/year. Aerial dissemination, with infection typically occurring in the lungs. Sometimes treatable with first-line drugs (isoniazid, rifampicin) but mostly resistant to several antibiotics (fluoroquinolones) and to second-line injectable drugs (amikacin, capreomycin and kanamycin). | WHO 2018a | |
| High | Enterococcus faecium, vancomycin-resistant | Most commonly isolated Gram-positive nosocomial pathogen worldwide with highly flexible genome that enables rapid adaption. Vancomycin-resistant isolates rose from 0% to more than 80% from 1980 to 2007, in the USA. Vancomycin resistance arises from reduced vancomycin-binding affinity, involving alterations in the peptidoglycan synthesis pathway. | Arias and Murray 2012; Gao, Howden and Stinear 2018 |
| S. aureus, methicillin-resistant | Among the most frequent of all antibiotic-resistant threats and leading cause of bacteremia. Outstanding versatility in adapting to different epidemiological settings (healthcare, community, animal). Characteristically MDR, with infections spreading across the globe. Infections commonly involve the skin, soft tissue, bone, joints and indwelling catheters or prosthetic devices. | Monaco et al. 2016; Hassoun, Linden and Friedman 2017 | |
| S. aureus, vancomycin- intermediate (VISA) and -resistant (VRSA) | VISA (MIC = 4–8 µg/mL) appeared in MRSA infected patients due to mutations during prolonged vancomycin therapy. VISA are associated with persistent infections, vancomycin treatment failure and poor clinical outcome. VRSA (MIC ≥ 16 µg/mL) appeared by acquisition of plasmid-borne copies of the transposon Tn1546, from vancomycin-resistant Enterococcus faecalis. VRSA infection numbers are still limited to date (14 in the USA). | Gardete and Tomasz 2014 | |
| Helicobacter pylori, clarithromycin-resistant | Most successful human gastric pathogen able to resist stomach acids, colonizing over 50% of the population. Related to gastritis, peptic ulcers, gastric adenocarcinoma, iron deficiency anemia, idiopathic thrombocytopenic purpura and vitamin B12 deficiency. Sequential, bismuth quadruple and non-bismuth quadruple therapies seam effective in high clarithromycin-resistance countries. | Alba, Blanco and Alarcón 2017; Goderska, Agudo Pena and Alarcon 2018 | |
| Campylobacter spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses, majorly gastroenteritis, primarily caused by Campylobacter jejuni. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. Macrolides (e.g. erythromycin and azithromycin) are considered as fluoroquinolone alternatives. | Bolinger and Kathariou 2017 | |
| Salmonella spp., fluoroquinolone-resistant | Leading cause of foodborne illnesses/diarrheal diseases, namely gastroenteritis. Antibiotic treatment is only recommended in vulnerable patients, such as the young, the elderly and patients with weakened immunity. | Kim et al. 2016; WHO 2018b | |
| N. gonorrhoeae, third-generation cephalosporin-resistant, fluoroquinolone-resistant | Causes gonorrhea, an obligate human infection, usually transmitted during sexual activity, often resulting in urethritis in men and cervicitis in women. Gonorrhea is rising, with 18.6% increase during 2016–17 and 75.2% increase since 2009 in the USA. Asymptomatic men (two-thirds of infected men) constitute the principal source of dissemination. | CDC 2017; Rice et al. 2017 | |
| Medium | S. pneumoniae, penicillin nonsusceptible | Encapsulated bacteria causes meningitis, septicemia and pneumonia, but also milder infections, such as sinusitis and otitis media. Major cause of morbidity and mortality worldwide, mainly in poor countries and in children under the age of 2. There are two available vaccines that target the most prevalent serotypes. | WHO 2014 |
| Haemophilus influenzae, ampicillin-resistant | Serotype b, an obligate human pathogen, is the most pathogenic, responsible for respiratory infections, ocular infection, sepsis and meningitis. Leading worldwide cause of meningitis morbidity and mortality in unimmunized populations. Highly related to chronic obstructive pulmonary disease, a leading cause of morbidity and mortality worldwide. Third-generation cephalosporins are the empiric treatment of choice. | ECDC 2017a; Sriram et al. 2018 | |
| Shigella spp., fluoroquinolone-resistant | Causes shigellosis, a major cause of diarrhea affecting mainly children under the age of 5. Between 80 and 165 million cases of shigellosis occur annually worldwide, majorly in developing countries. | ECDC 2017b; CDC 2018 |
Abbreviations: HAIs, hospital acquired infections; ICU, intensive care unit; MIC, minimum inhibitory concentration.
WHAT IS AMR?
Understanding the evolution, divergence and spread of AMR, along with the mechanisms behind it, is the main step in predicting and preventing this threat. In addition, it is important to understand the underlying concepts of AMR, such as resistance, heteroresistance and tolerance, to facilitate knowledge dissemination and integration in the development of novel strategies to defeat it. Resistance, although tolerance may fit some of the criteria, is mainly classified in three forms: intrinsic, acquired or adaptive. Frequently, microorganisms exhibit more than one form of resistance simultaneously, greatly contributing to the difficulty in finding suitable treatments. As such, all these aspects are discussed next.
Ecological evolution of AMR
Since the beginning of the antibiotic age, with the introduction of penicillin in the 1940s (Gaynes 2017), researchers and physicians have been made aware of how strongly and quickly microorganisms fight back. Indeed, in 1941, penicillin was first administered, and in 1942, penicillin-resistant bacteria were detected. Similarly, methicillin was introduced in 1960, and in 1961, methicillin resistance was reported. With resistant strains propagating in this increasingly rapid pace, antibiotic utilization quickly led a golden era of medicine to the current AMR crisis (Landecker 2016).
Regardless of its clear impact in modern medicine, however, AMR is actually an ancient and natural phenomenon, as microorganisms always had to defend themselves against naturally occurring antibiotics, with AMR evolving alongside their production (Perry, Westman and Wright 2014). In reality, several studies have revealed the existence of resistance genes in microorganisms preceding the antibiotic era. For instance, genes encoding resistance to natural antibiotics, namely β-lactams, tetracyclines and glycopeptides, were found in 5000- and 30 000-year-old permafrost sediments (D'Costa et al. 2011; Perron et al. 2015). Surprisingly, resistance genes against modern semi-synthetic antibiotics that do not occur naturally in microorganisms, namely amikacin, were also found (Perron et al. 2015). More recently, Paenibacillus sp. LC231 from a 4-million-year-old isolated cave was found to harbor conserved resistance genes to most clinically used antibiotics (Pawlowski et al. 2016). These studies demonstrate that AMR is a natural phenomenon preceding the modern selective pressure of antibiotics, which may be simply selecting for pre-existing, hence intrinsic, determinants in the resistome (i.e. the resistance genetic pool of all microorganisms).
Besides its ancient intrinsicality, the major issue of AMR is its ability to spread from one microorganism to another. Although it was first believed that AMR was only inherited vertically within a resistant population, researchers quickly realized that bacteria were able to acquire resistant determinants from other bacteria by horizontal gene transfer, as further explained later. This ability to exchange genetic material has been the great source of bacterial genetic variation over time, in which the resistome acts as a widely available and sharable resource (Landecker 2016).
Despite AMR dissemination being primordial, its frequency and distribution has suffered an anthropogenic impact and changed historically, driven and sustained by the scale of antibiotic usage in clinical, veterinary, husbandry and agricultural settings. For example, due to the large use of antibiotics, host microbiota, although harmless, nowadays carries a high content of resistance genes specific for the antibiotics used in medical and food production settings of a given country (Forslund et al. 2014). This creates a genetic pool that facilitates microbial pathogens to acquire resistance determinants when in contact with the host. The rate of bacterial release and uptake of genetic material and genetic recombination is also accelerated in the presence of an external stress such as antibiotics, but also heavy metals and disinfectants coming from industrial settings (Landecker 2016).
Resistance genetic elements originating from anthropogenic sources are spreading not only within host microbiota, but also to the environment, including remote areas with minimal antibiotic exposure (Bartoloni et al. 2004, 2009; Pallecchi et al. 2008; McCann et al. 2019), very likely due to waste streams emanating from human activity. In truth, a great portion of antibiotics used for humans and animals is excreted and released unchanged into the environment, either due to incomplete metabolization or due to disposal of unused drugs into the sewer, greatly contributing for the high load of antibiotics encountered in the environment today (Gillings and Stokes 2012).
Intrinsic AMR
Intrinsic AMR refers to the innate ability of microorganisms to resist to a specific antimicrobial due to genes in their genome encoding inherent structural or functional traits that provides them protection (Blair et al. 2015). This is evident in the disparate efficacies of most antibiotics against Gram-negative versus Gram-positive bacteria due to their inherent distinct cell wall composition acting as barrier to the entrance of antibiotics into the cells (Arzanlou, Chai and Venter 2017; Petchiappan and Chatterji 2017). For instance, vancomycin, a common antibiotic in MRSA treatment, is effective against Gram-positive bacteria because it easily reaches their peptidoglycan cell wall. Due to constraints in overcoming the outer membrane of Gram-negative bacteria, vancomycin is ineffective against these bacteria (Rice 2012). Similarly, daptomycin is active against Gram-positive bacteria, but it is unable to act against Gram-negative bacteria due to the lower proportion of anionic phospholipids in their cytoplasmic membrane (Randall et al. 2013). Several studies are tackling this issue by modifying existing compounds to make them active against Gram-negative bacteria. For example, a recent study was successful in optimizing arylomycins, a weak class of natural products, to produce a potent, broad-spectrum molecule, G0775, active against Gram-negative bacteria by inhibiting the essential bacterial type I signal peptidase (Smith et al. 2018). Another example is the conversion of the natural product deoxynybomycin, only active against Gram-positive bacteria, into an antibiotic able to accumulate inside and be active against Gram-negative bacteria, with the help of computational modeling (Richter et al. 2017).
High levels of AMR can be achieved through intrinsic restricted or selective outer membrane permeability, drug efflux pump systems and/or expression of intrinsic antibiotic resistance genes (Blair et al. 2015). Bacteria can limit the entry of broad-spectrum drugs, e.g. carbapenems and cephalosporins, by reducing or replacing nonspecific porin proteins by specific or more selective protein channels (Nikaido 2003; Fernandez and Hancock 2012). For instance, P. aeruginosa deficient in OprD porin, responsible for diffusion of small peptides, displays resistance to carbapenems (Pechère and Köhler 1999). This kind of bacterial mechanism is well studied and it has been reviewed previously (Kumar and Schweizer 2005; Langton, Henderson and Herbert 2005; Poole 2005). Drug efflux systems are protein complexes located in the cell wall of Gram-negative bacteria responsible for expelling toxic molecules such as antibiotics. Several bacteria possess genes encoding efflux pumps, greatly contributing to AMR (Blair, Richmond and Piddock 2014; Sanchez-Romero and Casadesus 2014). P. aeruginosa is a well-characterized example with clinically relevant efflux pumps such as the MexAB-OprM and MexXY/OprM(OprA) systems, which contribute to a stable and consistent resistance to a wide range of antibiotics and protection against molecules targeting the ribosomal machinery, respectively (Li et al. 1994; Li, Livermore and Nikaido 1994; Li, Nikaido and Poole 1995). Microorganisms can also be intrinsically resistant to antibiotics due to the expression of antibiotic resistance genes (Liu et al. 2010; Blake and O'Neill 2013; Xu et al. 2017; Peterson and Kaur 2018). For instance, β-lactam antibiotics have no action against M. tuberculosis because these bacteria inherently produce β-lactamases, such as BlaC, that hydrolyze the β-lactam ring inactivating this class of antibiotics (Smith, Wolff and Nguyen 2012). Another example of intrinsic resistance is the absence of a susceptible target site for an antibiotic to act on (Blair et al. 2015). For instance, the biocide triclosan is ineffective against P. aeruginosa because it carries the fabV gene encoding a triclosan-resistant enoyl-ACP reductase, the site of action of triclosan (Zhu et al. 2010).
Acquired AMR
Microorganisms can acquire or develop resistance, this being what most greatly contributes to the AMR crisis (Blair et al. 2015). Acquired resistance arises when an originally antibiotic-sensitive organism becomes resistant through the acquisition and incorporation of new genetic material (e.g. plasmids, transposons, integrons, naked DNA) from other microorganisms by horizontal gene transfer or as a result of mutations of chromosomal (intrinsic) genes (Arzanlou, Chai and Venter 2017; Pang et al. 2019). The spread of β-lactam resistance among bacteria is the major example, as several species are able to acquire plasmids encoding β-lactamase genes leading to the emergence of, for example, ESBL- and metallo-β-lactamase-producing P. aeruginosa, ESBL-producing E. coli, H. influenzae, N. gonorrhoeae, Salmonella, Shigella and Vibrio cholera (Rawat and Nair 2010).
In general, acquired resistance can be mediated by (i) reduced antibiotic uptake and (ii) increased antibiotic efflux, reducing its intracellular concentration, (iii) antibiotic modification or inactivation and (iv) antibiotic target modification by genetic mutation or post-translational modification. Often, these mechanisms are combined, contributing to the expression of high levels of AMR, as is the case of increased resistance observed against β-lactams (Arzanlou, Chai and Venter 2017). As intrinsic resistance, acquired resistance is stable and transmitted vertically (Blair et al. 2015). This vast topic is only outlined here, but is reviewed in detail in Nikaido (2009) and Blair et al. (2015).
Frequently, acquired and intrinsic mechanisms are closely related, as mutations can alter the expression of intrinsic resistance mechanisms. For instance, carbapenem resistance in Enterobacteriaceae generally involves the production of β-lactamases. Nevertheless, if mutations in porin production occur, bacteria can reduce or even end β-lactamase production (Wozniak et al. 2012; Lavigne et al. 2013; Tangden et al. 2013). Another example is that mutations can enhance P. aeruginosa intrinsic antibiotic resistance through loss of oprD porin expression, via mutation in the oprD gene or its associated regulatory proteins, and de-repression of chromosomal AmpC β-lactamase and MexAB-OprM multidrug efflux pump, conferring resistance to β-lactam antibiotics (Taylor, Yeung and Hancock 2014). Efflux pumps are one of the major contributors to intrinsic resistance that microorganisms can mobilize onto plasmids and transfer to other bacteria. For instance, IncH1 plasmid isolated from Citrobacter freundii includes genes encoding the New Delhi metallo-β-lactamase 1, but also a tripartite resistance nodulation division pump (Dolejska et al. 2013).
Adaptive AMR
Adaptive AMR is one of the most complex forms of bacterial resistance (Fernández, Breidenstein and Hancock 2011). It consists in the ability to alter gene or protein expression very rapidly in response to an antibiotic insult or environmental cues, such as pH, temperature, nutrient or oxygen limitation (Fernández, Breidenstein and Hancock 2011; Motta, Cluzel and Aldana 2015; Arzanlou, Chai and Venter 2017). Development of adaptive AMR in the presence of antibiotics is usually observed when cells are exposed first to nonlethal levels of such agents, but may escalate to where bacteria are able to survive otherwise lethal concentrations if they are consecutively exposed to increasing antibiotic doses. In fact, bacteria can increase their level of resistance gradually and across generations if the stimulus endures, indicating the existence of some type of resistance memory (Sandoval-Motta and Aldana 2016).
Unlike intrinsic and acquired resistance, adaptive resistance is unstable, transient and highly dependent on the presence of antibiotics, and it cannot be vertically transmitted. After removal of the triggering factor, microorganisms revert to their ‘original state’, regaining susceptibility, although the original level of resistance may not be restored (Fernández, Breidenstein and Hancock 2011; Arzanlou, Chai and Venter 2017; Pang et al. 2019). Because of this, adaptive AMR has been linked with the phenomenon of MIC baseline creep seen in many bacterial species, in which the average MIC increases from the moment of antibiotic introduction onward, making them more likely to achieve the resistance breakpoint over time (Fernández, Breidenstein and Hancock 2011).
Because of its transient nature, adaptive resistance represents one of the biggest challenges in designing effective antimicrobial therapies, explaining the common differences found between in vitro and in vivo antibiotic susceptibilities (Fernández, Breidenstein and Hancock 2011). Adaptive resistance represents a crucial biological advantage and an intelligent survival mechanism since microorganisms do not pay the fitness costs associated with irreversible mutations (Motta, Cluzel and Aldana 2015), reverting to their ‘original state’ when more advantageous (Andersson and Hughes 2010; Motta, Cluzel and Aldana 2015).
There are several mechanisms of adaptive resistance, including epigenetic inheritance, population heterogeneity, mutability, gene amplification, efflux pumps and biofilm formation (Sanchez-Romero and Casadesus 2014; Motta, Cluzel and Aldana 2015). The molecular mechanisms behind adaptive resistance are still poorly understood but apparently quite complex, involving intricate regulatory pathways. Moreover, adaptive resistance may interplay with intrinsic or acquired resistance (Fernández, Breidenstein and Hancock 2011) as the genetic mutations or epigenetic changes triggered by environmental conditions can influence the expression of intrinsic and acquired mechanisms of resistance (Sanchez-Romero and Casadesus 2014; Motta, Cluzel and Aldana 2015). A great example of this phenomenon is shown in a recent study, where a subpopulation of E. coli cells with increased expression of efflux pumps was found to also have a higher mutability rate due to the decreased expression of the DNA mismatch repair gene, which led to mutants with higher antibiotic resistance (El Meouche and Dunlop 2018). Biofilm formation is a ‘perfect’ example of the interplay of the three types of resistance. Bacteria undergo genetic and phenotypic alterations to adhere and produce an exopolymeric matrix to bind to a surface and to other bacteria (Stewart 2014; Donné and Dewilde 2015; Kumar et al. 2017).
Resistance, heteroresistance and tolerance
Despite very commonly encountered in AMR studies, the concepts of resistance, heteroresistance and tolerance are sometimes misused, being of importance to elucidate them. Resistance refers to the ability of microorganisms to survive and grow at increased antibiotic concentrations for long periods and it is quantifiable by assessing the MIC (Brauner et al. 2016). However, it sometimes happens that different antibiotic susceptibilities exist within the same bacterial population, which can include resistant subpopulations. This phenomenon is termed heteroresistance and, although generally disregarded in clinical settings, it is critical in foreseeing the success of a given antimicrobial therapy, since poor designed treatments may select for the resistant populations. Heteroresistance is usually detected in MBC, disc diffusion or e-test assays when discrete colonies are grown in the zone of inhibition, and can be confirmed by a population analysis profiling assay (El-Halfawy and Valvano 2015).
In turn, tolerance refers to the ability of microorganisms to survive a transient exposure to increased antibiotic concentrations, even those above the MIC. However, unlike resistance, tolerance is only temporary, as it just takes more time for the antibiotic to kill bacteria. Tolerance can be due to slow growth, which in turn can be inherent, i.e. characteristic of a given species or strain, or non-inherent, i.e. caused by poor growth conditions (e.g. biofilms), stress factors (e.g. antibiotics) or bacterial stationary growth phase. Tolerance, however, may also be due to antibiotic application to a bacterial population in the lag growth phase, in which they are transitioning from growth arrest to an exponential growth phase (Brauner et al. 2016; Balaban et al. 2019). For more detailed information about resistance and tolerance definitions, the two cited reviews are recommended. In the next section, focus will be given to biofilms and their resistance and tolerance traits.
BIOFILM RESISTANCE AND TOLERANCE
What are biofilms?
Contrary to the typical idea of single-species free-floating bacteria, microorganisms naturally reside in groups, establishing complex and dynamic consortia called biofilms. The ability of microorganisms to persist and thrive within biofilms is an important feature denoting critical concern in clinical settings. Indeed, biofilms play a significant role in microbial survival and persistence in natural ecosystems, thus being ubiquitous in Nature and considered an ancient form of microbial adaptation. Remarkably, it is speculated that the transition of microorganisms to the biofilm mode of growth established the first multicellular life form as an adaptive response to the extreme conditions encountered in early Earth (de la Fuente-Núñez et al. 2013).
Biofilms are usually characterized as well-organized structures of microorganisms attached to biotic or abiotic surfaces and whose cells are encased and protected by a self-produced polymeric matrix. Typically, the biofilm life cycle encompasses three stages, namely (i) attachment, (ii) maturation and (iii) dispersion. The first stage initiates by the reversible binding of bacteria to a surface followed by their irreversible attachment. Next, bacterial growth and matrix production take place, leading to increased biomass and maturation of the biofilm. At this stage, biofilms develop microenvironments dependent on nutrient and oxygen gradients, with cells developing different phenotypes depending on their spatial organization. Finally, biofilms eventually disperse, allowing cells to migrate and colonize other areas (Bjarnsholt et al. 2013).
Concerning the impact of biofilms in healthcare settings, it is important to realize that the vast majority of infections are actually biofilm mediated (Høiby et al. 2015). Biofilm infections can be device related (e.g. catheters, implants, contact lenses, prosthetic valves and joints) or tissue related (e.g. endocarditis, chronic otitis media, lung infections in cystic fibrosis, chronic wounds) (Lebeaux, Ghigo and Beloin 2014). In these infections, the physiological features of biofilm cells and the matrix surrounding them contribute to their higher resistance/tolerance to external stresses, including the action of antimicrobials and the immune system, allowing the establishment of persistent/chronic infections (Grant and Hung 2013; Donné and Dewilde 2015; Kumar et al. 2017). Moreover, most biofilm infections normally have a polymicrobial etiology, with phylogenetically different microorganisms coexisting (Peters et al. 2012; Giaouris et al. 2015; Costa-Orlandi et al. 2017). The polymicrobial nature of most biofilm-mediated infections can lead to the chronic scenario of infection (Stacy et al. 2016), as the interactions among the resident species may augment the severity of the infection and contribute for the recalcitrance toward conventional therapy (Wolcott et al. 2013; Schroeder, Brooks and Brooks 2017). Biofilms employ different yet concerted resistance and tolerance mechanisms illustrated in Fig. 1 and further detailed in the next sections.
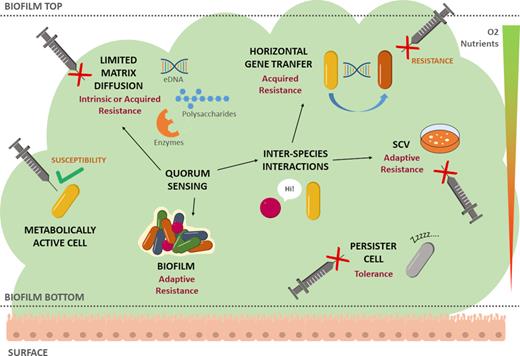
Biofilm resistance and tolerance mechanisms. Main resistance and tolerance mechanisms (in black) are characterized as intrinsic, acquired or adaptive. Different geometric forms and colors denote different bacterial species. Nutrient and oxygen gradients are illustrated as a downward triangle going from high (green top) to low (red bottom) concentration. Inability of antimicrobials to act upon the cell is represented as a red cross sign. Dormant or persister cell is depicted in gray. Abbreviation: SCV, small colony variant.
Extracellular matrix
Microorganisms living in a biofilm are surrounded and encased by a majorly self-produced matrix, which can comprise over 90% of the total mass of the biofilm (Flemming and Wingender 2010). The biofilm matrix is a complex and intricate amalgamation of different hydrated extracellular polymeric substances (EPS), including polysaccharides, proteins, nucleic acids and lipids. These molecules offer biofilms their structure and mechanical stability by forming a three-dimensional network that supports biofilm adherence and cell immobilization (Flemming and Wingender 2010). The matrix and its constituents make up the first barrier to the entry and diffusion of foreign substances into the biofilm, often impeding them from reaching the cells, thus greatly prompting biofilm AMR (Fig. 1). Yet, and despite its significance, antimicrobial penetration hindrance does not fully explain the resistance phenomena seen in biofilm scenarios, with some antibiotics rapidly reaching the biofilm cells while failing at compromising their viability (Hall and Mah 2017). As explained in the next sections, the mechanisms through which resistance and tolerance appear in biofilms are complex.
A major and important matrix component is extracellular DNA (eDNA), ubiquitous to almost all biofilms and with structural and cell-to-cell interconnecting functions (Whitchurch et al. 2002; Barken et al. 2008). eDNA has shown to confer protection from aminoglycosides to P. aeruginosa biofilms, most likely due to its electrostatic interaction with positively charged antibiotics (Chiang et al. 2013). Notably, the presence of an antimicrobial can itself promote the eDNA release by the biofilm cells to the matrix. For instance, biofilms of Staphylococcus epidermidis doubled their amount in eDNA when treated with vancomycin, thus benefiting from its affinity for the positively charged antibiotic, which was prevented from reaching the cells and exerting its activity (Doroshenko et al. 2014). eDNA has also been shown to induce the expression of resistance genes. This occurs by chelating cations such as Mg2+ and by creating an acidic microdomain around itself, two environmental signals that activate signaling pathways linked to AMR, such as the PhoPQ and PmrAB two-component systems in S. Typhimurium (Johnson et al. 2013) and P. aeruginosa (Wilton et al. 2016). Furthermore, eDNA has also been related with increased horizontal gene transfer in biofilms, serving as vehicle for resistance genes and causing the rapid spread of resistance between competent biofilm cells (Okshevsky and Meyer 2015).
Other matrix components affecting biofilm resistance are polysaccharides, crucial matrix components influencing biofilm adhesion and structure while also offering protection against antimicrobials. For example, the polysaccharide Psl from P. aeruginosa has shown to provide resistance to colistin, polymyxin B, tobramycin and ciprofloxacin probably via electrostatic interactions, and this protective effect was extended to non-Psl-producing species, such as E. coli and S. aureus (Billings et al. 2013). The biofilm matrix can also contain secreted antibiotic-modifying enzymes. For instance, secreted β-lactamases were able to degrade the antibiotic ampicillin in K. pneumoniae biofilms, impeding it from reaching the cells in the biofilm (Anderl, Franklin and Stewart 2000).
Nutritional constraints and persister cells
Biofilms are a complex architectural conglomerate, not only due to their diverse composition in terms of EPS but also for possessing heterogeneous microhabitats caused by the establishment of an oxygen and nutrient gradient. This gradient derives from the faster oxygen and nutrient consumption compared to their diffusion rates through the biofilm, causing biofilm cells to appear stratified according to oxygen and nutrient availability. Remarkably, oxygen and nutrient depletion in lower layers can cause biofilm cells to adopt a low metabolic state or even cause cell death (Flemming et al. 2016). This gradient phenomenon partially explains the physiological heterogeneity encountered in most biofilms, characterized by cells with diverse gene expressions, metabolic activities and phenotypes, which translates into different AMR and tolerance abilities.
A great example of how nutritional constraints affect biofilm tolerance to antimicrobials is the case of cells that reduce their metabolic activity and enter a slow growth or dormancy state when nutrients and oxygen are low or absent, achieving the phenotype of persister cells (Hall and Mah 2017). This type of persistence is known as time-dependent persistence or ‘tolerance by slow growth’ (Brauner et al. 2016). Typically, most antimicrobials act best on fast-growing metabolically active cells. For example, β-lactams act by preventing the reassembly of the peptidoglycan layer bonds during bacterial growth, causing cell lysis (Horne and Tomasz 1977), while fluoroquinolones inhibit DNA gyrase, causing DNA damage (Crumplin and Smith 1976). Persister cells are able to diminish the antimicrobial effectiveness of these and other antimicrobials without any genetic changes by simply stopping their metabolism and growth (Olsen 2015).
Persistence is characterized by occurring only in a subset of cells that usually comprise less than 1% of the microbial population of a biofilm, with antimicrobials only effectively killing the remainder of the biofilm cells (Brauner et al. 2016). As such, the persister phenotype is a major reason why certain antimicrobials are ineffective despite being able to reach the cells within a biofilm and is one of the main contributors to biofilm infection relapsing, as the surviving cells can regrow after antimicrobial treatment and maintain the infection (Conlon 2014). Gladly, efforts are being made to target these specific and troublesome bacterial subpopulations. For example, a recent study was successful in achieving total persister eradication by activating the ClpP protease with the acyldepsipeptide antibiotic (ADEP4). This made the protease nonspecific, leading to persister cells’ self-digestion. Furthermore, ADEP4 combination with rifampicin was able to completely eradicate an in vivo S. aureus biofilm infection (Conlon et al. 2013).
Unlike resistant bacterial populations, persistence is characterized by a biphasic killing curve, which translates the different rates that bacteria are killed within the same population. Also, persistent bacteria, unlike resistant ones, are unable to replicate in the presence of an antimicrobial, a characteristic that also differentiates this phenomenon from the one of heteroresistance (Balaban et al. 2019). Despite its importance, the molecular mechanisms behind the changes from susceptible to persister phenotypes are still being unraveled. Persister cells have also been linked to ATP depletion in S. aureus (Conlon et al. 2016), E. coli (Shan et al. 2017) and P. aeruginosa (Cameron et al. 2018). A more recent study showed that the msaABCR operon, previously linked to virulence, biofilm development and antibiotic resistance, is involved as well (Sahukhal, Pandey and Elasri 2017). Another recent finding shows that the presence of antimicrobials can induce the persister phenotype, namely through the putative de-N-acetylase DnpA after P. aeruginosa biofilm exposure to fluoroquinolones (Khandekar et al. 2018).
Interestingly, the occurrence of persister cell memory has been recently described, by which cells of E. coli, Acinetobacter radioresistens, S. Typhimurium, S. epidermidis and Bacillus subtilis retain their persister phenotype up to weeks after being removed from biofilm cultures (Miyaue et al. 2018). Persister cells are characterized by presenting temporary and thus reversible tolerance toward antimicrobial treatment, but the length in which cells remain in a persistent state due to the described memory effect gives them an extra advantage for surviving in antimicrobial-containing environments. For more detailed information about persister cells, the reviews by Conlon, Rowe and Lewis (,2015), Brauner et al. (2016), Fisher, Gollan and Helaine (2017), Van den Bergh, Fauvart and Michiels (2017) and Balaban et al. (2019) are recommended.
In addition to causing the described phenotypic changes in biofilm cells, low availability of oxygen, or hypoxia, has been also related to the expression of resistance-related genes. Specifically, the mexEF-oprN and mexCD-oprJ efflux pump genes are upregulated in P. aeruginosa in low-oxygen conditions (Schaible, Taylor and Schaffer 2012; Tata et al. 2016). Additionally, hypoxia may further protect biofilms from antimicrobials by impairing the formation of reactive oxygen species, which have been linked to cell killing by bactericidal antibiotics (Hall and Mah 2017). Low nutrient concentration, specifically amino acids, may also enhance biofilm tolerance through the stringent response, in which an accumulation of uncharged tRNAs triggers the production of the alarmone stress signal guanosine tetraphosphate (ppGpp). This response causes an induction of a lag phase (transient growth arrest), which has been showed to improve tolerance to antibiotics (Brauner et al. 2016; Hall and Mah 2017). Of notice, in a recent study, low pH, another environmental factor, has been proven to cause the latter effect (Vulin et al. 2018).
Quorum sensing
The mechanisms by which microorganisms within a biofilm regulate their activities are coordinated through a cell-to-cell communication system known as quorum sensing (QS). QS is used by bacteria (and fungi) to sense population density and regulate gene expression accordingly, serving as channel for intra- and interspecies communication, allowing the establishment of intimate relationships of competition or cooperation, but also for communication between the microorganisms and its host (Li and Tian 2012; Grandclément et al. 2016). Microorganisms regulate these activities by releasing, sensing and responding to small QS signal molecules termed autoinducers (AIs). When AI concentration reaches a threshold due to an increase in population density, these signal molecules activate receptors with the ability to alter gene expression, promoting beneficial behaviors under a particular condition, such as virulence factor expression, motility and biofilm formation (Grandclément et al. 2016; Hawver, Jung and Ng 2016; Knecht et al. 2016). Using QS, microorganisms can switch from acting as individual cells to operating in a concerted multicellular fashion, thereby switching to the biofilm mode of growth and accommodating to or escaping from antimicrobial stresses (Filkins and O'Toole 2015; Passos da Silva et al. 2017).
QS plays a key role in biofilm AMR, although the mechanisms behind it are still being unraveled. QS influences the production of EPS, which are key players in biofilm resistance, as described earlier. For example, the PqsABCDE/PqsR QS system in P. aeruginosa stimulates the production of eDNA (Pérez-Pérez et al. 2017), which is highly related with AMR in biofilms, as described previously. QS has also been linked to the upregulation of resistance genes, as is the case of oxacillinase 51, AmpC, AdeA and AdeB in A. baumannii (Dou et al. 2017). More recently, Chromobacterium violaceum was reported to use QS to increase its resistance to bactobolin, a Burkholderia thailandensis antibiotic, by increasing transcription of a putative antibiotic efflux pump (Evans et al. 2018).
As stated, QS may serve interspecies communication, with the AI from one species interfering with signaling pathways of other species present in the same biofilm, thus altering gene expression or directly affecting the physiology of the cohabitants (Schertzer, Boulette and Whiteley 2009; Elias and Banin 2012). The role of interspecies communication in biofilm resistance is further explored next.
Interspecies interactions
Most of the research on biofilm resistance has been focused on single-species biofilms. However, these simple laboratory models do not illustrate the true nature of biofilm communities, since most of biofilm-mediated infections are actually polymicrobial (Wolcott et al. 2013; Gabrilska and Rumbaugh 2015). The inclusion of the multispecies factor in AMR studies is pivotal, as it is becoming increasingly clear that interactions between different species can modulate the overall consortium behavior, resulting in enhanced AMR and infection severity (Dalton et al. 2011; Peters et al. 2012; Murray et al. 2014; Bowen et al. 2018).
By enclosing multiple species, biofilms obtain numerous ecological advantages, with established interactions, either cooperative or competitive, usually resulting in a beneficial outcome to the biofilm. A cooperative interaction is, for example, the metabolite cross-feeding between Aggregatibacter actinomycetemcomitans and Streptococcus gordonii that benefits the latter while also enhancing A. actinomycetemcomitans virulence (Ramsey, Rumbaugh and Whiteley 2011). Regarding competitive interactions, a great example is the one established between P. aeruginosa and S. aureus. P. aeruginosa produces the enzyme LasA that selectively lyses S. aureus, whose content in iron is used for P. aeruginosa growth, increasing its pathogenic potential under low-iron conditions (Mashburn et al. 2005). However, S. aureus growth is not completely inhibited by P. aeruginosa, with the latter inducing expression of virulence factors and facilitating the emergence of small colony variants in S. aureus (Mitchell et al. 2010). This phenotype allows S. aureus to survive in proximity with P. aeruginosa, being linked to infection persistence, establishment of intracellular infections and lower antimicrobial susceptibility due to their reduced metabolic state (Garcia et al. 2013; Proctor et al. 2014). These interactions raise a healthcare concern, as polymicrobial biofilm infections are typically more severe and recalcitrant to treatment (Wolcott et al. 2013). For instance, P. aeruginosa and S. aureus co-infection delayed wound healing and triggered host inflammatory responses (Pastar et al. 2013). Also, P. aeruginosa displayed higher virulence when grown with Gram-positive bacteria in vivo (Korgaonkar et al. 2013).
Regarding specifically AMR, several works have emphasized the increasing resistance to antibiotics in multispecies biofilms (Adam, Baillie and Douglas 2002; Lopes et al. 2012; Lee et al. 2014; Magalhães, Lopes and Pereira 2017) and some examples are presented in Table 2. The studies reviewed suggest that mechanisms such as interspecies signaling, biofilm matrix production and horizontal gene transfer are major contributors to the increased multispecies biofilm resistance. Since much is still unknown, it is imperative to continue the study of interspecies interactions (from a molecular standpoint) that lead to the increased AMR of biofilms.
Examples of interspecies interactions leading to increased AMR in polymicrobial biofilms for common antimicrobial agents.
| Antimicrobial . | Species . | Interaction outcome . | References . |
|---|---|---|---|
| Amoxicillin | Moraxella catarrhalis, S. pneumoniae | M. catarrhalis secreted β-lactamases protected S. pneumonia from amoxicillin treatment. | Perez et al. 2014 |
| Ampicillin | H.influenzae, M. catarrhalis | M. catarrhalis secreted β-lactamases protected H. influenzae from ampicillin treatment. | Armbruster et al. 2010 |
| Azithromycin | M. catarrhalis, S. pneumoniae | S. pneumoniae protected M. catarrhalis from azithromycin treatment by a signaling molecule AI-2 independent mechanism. | Perez et al. 2014 |
| Benzalkonium chloride | Listeria monocytogenes, Pseudomonas putida | L. monocytogenes increased P. putida resistance to benzalkonium chloride. | Giaouris et al. 2013 |
| Cefotaxime | Dolosigranulum pigrum, Inquilinus limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to cefotaxime. | Lopes et al. 2012 |
| Chloramphenicol | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to chloramphenicol. | Lopes et al. 2012 |
| Chlorine | Enterobacteriaceae cloacae, E. coli, P. aeruginosa, Stenotrophomonas maltophilia | Multispecies biofilms of the four bacteria displayed increased resistance to chlorine. | Schwering et al. 2013 |
| Ciprofloxacin | I. limosus, P. aeruginosa, S. aureus, S. maltophilia | P. aeruginosa increased S. aureus, I. limosus and S. maltophilia resistance to ciprofloxacin. | Magalhães et al. 2017 |
| Clarithromycin | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to clarithromycin. | Armbruster et al. 2010 |
| Clindamycin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to clindamycin. | Lopes et al. 2012 |
| Colistin | P. aeruginosa, S. maltophilia | S. maltophilia increased P. aeruginosa resistance to colistin. | Ryan et al. 2008 |
| Essential oils of citronella and lemon | E. coli, S. aureus | S. aureus and E. coli increased resistance to citronella and lemon essential oils when cocultured. | Millezi et al. 2012 |
| Gentamicin | E. faecalis, Finegoldia magna, P. aeruginosa, S. aureus | P. aeruginosa increased resistance to gentamicin when cocultured with S. aureus, E. faecalis and F. magna. | Dalton et al. 2011 |
| Hydrogen peroxide | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to hydrogen peroxide. | Antonic et al. 2013 |
| Ofloxacin | Candida albicans, E. coli | C. albicans β-1,3-glucan, a matrix component, increased E. coli resistance to ofloxacin by acting as a barrier to its diffusion in the biofilm. | De Brucker et al. 2015 |
| ortho-Phthalaldehyde | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of ortho-phthalaldehyde. | Sanchez-Vizuete et al. 2015 |
| Peracetic acid | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of peracetic acid. | Sanchez-Vizuete et al. 2015 |
| Polymyxin B | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to polymyxin B. | Antonic et al. 2013 |
| Rifampicin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to rifampicin. | Lopes et al. 2012 |
| Sodium dodecyl sulfate (SDS) | Pseudomonas fluorescens, K. pneumoniae, P. aeruginosa | P. fluorescens increased resistance to SDS when cocultured with K. pneumoniae and P. aeruginosa. | Lee et al. 2014 |
| Tobramycin | P. aeruginosa, S. maltophilia | P. aeruginosa alginate protected S. maltophilia from tobramycin treatment. | Pompilio et al. 2015 |
| Trimethoprim–sulfamethoxazole | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to trimethoprim–sulfamethoxazole. | Armbruster et al. 2010 |
| Vancomycin | P. aeruginosa, S. aureus | P. aeruginosa HQNO increases S. aureus resistance to vancomycin. | Orazi and O'Toole 2017 |
| Antimicrobial . | Species . | Interaction outcome . | References . |
|---|---|---|---|
| Amoxicillin | Moraxella catarrhalis, S. pneumoniae | M. catarrhalis secreted β-lactamases protected S. pneumonia from amoxicillin treatment. | Perez et al. 2014 |
| Ampicillin | H.influenzae, M. catarrhalis | M. catarrhalis secreted β-lactamases protected H. influenzae from ampicillin treatment. | Armbruster et al. 2010 |
| Azithromycin | M. catarrhalis, S. pneumoniae | S. pneumoniae protected M. catarrhalis from azithromycin treatment by a signaling molecule AI-2 independent mechanism. | Perez et al. 2014 |
| Benzalkonium chloride | Listeria monocytogenes, Pseudomonas putida | L. monocytogenes increased P. putida resistance to benzalkonium chloride. | Giaouris et al. 2013 |
| Cefotaxime | Dolosigranulum pigrum, Inquilinus limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to cefotaxime. | Lopes et al. 2012 |
| Chloramphenicol | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to chloramphenicol. | Lopes et al. 2012 |
| Chlorine | Enterobacteriaceae cloacae, E. coli, P. aeruginosa, Stenotrophomonas maltophilia | Multispecies biofilms of the four bacteria displayed increased resistance to chlorine. | Schwering et al. 2013 |
| Ciprofloxacin | I. limosus, P. aeruginosa, S. aureus, S. maltophilia | P. aeruginosa increased S. aureus, I. limosus and S. maltophilia resistance to ciprofloxacin. | Magalhães et al. 2017 |
| Clarithromycin | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to clarithromycin. | Armbruster et al. 2010 |
| Clindamycin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to clindamycin. | Lopes et al. 2012 |
| Colistin | P. aeruginosa, S. maltophilia | S. maltophilia increased P. aeruginosa resistance to colistin. | Ryan et al. 2008 |
| Essential oils of citronella and lemon | E. coli, S. aureus | S. aureus and E. coli increased resistance to citronella and lemon essential oils when cocultured. | Millezi et al. 2012 |
| Gentamicin | E. faecalis, Finegoldia magna, P. aeruginosa, S. aureus | P. aeruginosa increased resistance to gentamicin when cocultured with S. aureus, E. faecalis and F. magna. | Dalton et al. 2011 |
| Hydrogen peroxide | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to hydrogen peroxide. | Antonic et al. 2013 |
| Ofloxacin | Candida albicans, E. coli | C. albicans β-1,3-glucan, a matrix component, increased E. coli resistance to ofloxacin by acting as a barrier to its diffusion in the biofilm. | De Brucker et al. 2015 |
| ortho-Phthalaldehyde | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of ortho-phthalaldehyde. | Sanchez-Vizuete et al. 2015 |
| Peracetic acid | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of peracetic acid. | Sanchez-Vizuete et al. 2015 |
| Polymyxin B | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to polymyxin B. | Antonic et al. 2013 |
| Rifampicin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to rifampicin. | Lopes et al. 2012 |
| Sodium dodecyl sulfate (SDS) | Pseudomonas fluorescens, K. pneumoniae, P. aeruginosa | P. fluorescens increased resistance to SDS when cocultured with K. pneumoniae and P. aeruginosa. | Lee et al. 2014 |
| Tobramycin | P. aeruginosa, S. maltophilia | P. aeruginosa alginate protected S. maltophilia from tobramycin treatment. | Pompilio et al. 2015 |
| Trimethoprim–sulfamethoxazole | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to trimethoprim–sulfamethoxazole. | Armbruster et al. 2010 |
| Vancomycin | P. aeruginosa, S. aureus | P. aeruginosa HQNO increases S. aureus resistance to vancomycin. | Orazi and O'Toole 2017 |
Examples of interspecies interactions leading to increased AMR in polymicrobial biofilms for common antimicrobial agents.
| Antimicrobial . | Species . | Interaction outcome . | References . |
|---|---|---|---|
| Amoxicillin | Moraxella catarrhalis, S. pneumoniae | M. catarrhalis secreted β-lactamases protected S. pneumonia from amoxicillin treatment. | Perez et al. 2014 |
| Ampicillin | H.influenzae, M. catarrhalis | M. catarrhalis secreted β-lactamases protected H. influenzae from ampicillin treatment. | Armbruster et al. 2010 |
| Azithromycin | M. catarrhalis, S. pneumoniae | S. pneumoniae protected M. catarrhalis from azithromycin treatment by a signaling molecule AI-2 independent mechanism. | Perez et al. 2014 |
| Benzalkonium chloride | Listeria monocytogenes, Pseudomonas putida | L. monocytogenes increased P. putida resistance to benzalkonium chloride. | Giaouris et al. 2013 |
| Cefotaxime | Dolosigranulum pigrum, Inquilinus limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to cefotaxime. | Lopes et al. 2012 |
| Chloramphenicol | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to chloramphenicol. | Lopes et al. 2012 |
| Chlorine | Enterobacteriaceae cloacae, E. coli, P. aeruginosa, Stenotrophomonas maltophilia | Multispecies biofilms of the four bacteria displayed increased resistance to chlorine. | Schwering et al. 2013 |
| Ciprofloxacin | I. limosus, P. aeruginosa, S. aureus, S. maltophilia | P. aeruginosa increased S. aureus, I. limosus and S. maltophilia resistance to ciprofloxacin. | Magalhães et al. 2017 |
| Clarithromycin | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to clarithromycin. | Armbruster et al. 2010 |
| Clindamycin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to clindamycin. | Lopes et al. 2012 |
| Colistin | P. aeruginosa, S. maltophilia | S. maltophilia increased P. aeruginosa resistance to colistin. | Ryan et al. 2008 |
| Essential oils of citronella and lemon | E. coli, S. aureus | S. aureus and E. coli increased resistance to citronella and lemon essential oils when cocultured. | Millezi et al. 2012 |
| Gentamicin | E. faecalis, Finegoldia magna, P. aeruginosa, S. aureus | P. aeruginosa increased resistance to gentamicin when cocultured with S. aureus, E. faecalis and F. magna. | Dalton et al. 2011 |
| Hydrogen peroxide | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to hydrogen peroxide. | Antonic et al. 2013 |
| Ofloxacin | Candida albicans, E. coli | C. albicans β-1,3-glucan, a matrix component, increased E. coli resistance to ofloxacin by acting as a barrier to its diffusion in the biofilm. | De Brucker et al. 2015 |
| ortho-Phthalaldehyde | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of ortho-phthalaldehyde. | Sanchez-Vizuete et al. 2015 |
| Peracetic acid | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of peracetic acid. | Sanchez-Vizuete et al. 2015 |
| Polymyxin B | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to polymyxin B. | Antonic et al. 2013 |
| Rifampicin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to rifampicin. | Lopes et al. 2012 |
| Sodium dodecyl sulfate (SDS) | Pseudomonas fluorescens, K. pneumoniae, P. aeruginosa | P. fluorescens increased resistance to SDS when cocultured with K. pneumoniae and P. aeruginosa. | Lee et al. 2014 |
| Tobramycin | P. aeruginosa, S. maltophilia | P. aeruginosa alginate protected S. maltophilia from tobramycin treatment. | Pompilio et al. 2015 |
| Trimethoprim–sulfamethoxazole | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to trimethoprim–sulfamethoxazole. | Armbruster et al. 2010 |
| Vancomycin | P. aeruginosa, S. aureus | P. aeruginosa HQNO increases S. aureus resistance to vancomycin. | Orazi and O'Toole 2017 |
| Antimicrobial . | Species . | Interaction outcome . | References . |
|---|---|---|---|
| Amoxicillin | Moraxella catarrhalis, S. pneumoniae | M. catarrhalis secreted β-lactamases protected S. pneumonia from amoxicillin treatment. | Perez et al. 2014 |
| Ampicillin | H.influenzae, M. catarrhalis | M. catarrhalis secreted β-lactamases protected H. influenzae from ampicillin treatment. | Armbruster et al. 2010 |
| Azithromycin | M. catarrhalis, S. pneumoniae | S. pneumoniae protected M. catarrhalis from azithromycin treatment by a signaling molecule AI-2 independent mechanism. | Perez et al. 2014 |
| Benzalkonium chloride | Listeria monocytogenes, Pseudomonas putida | L. monocytogenes increased P. putida resistance to benzalkonium chloride. | Giaouris et al. 2013 |
| Cefotaxime | Dolosigranulum pigrum, Inquilinus limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to cefotaxime. | Lopes et al. 2012 |
| Chloramphenicol | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to chloramphenicol. | Lopes et al. 2012 |
| Chlorine | Enterobacteriaceae cloacae, E. coli, P. aeruginosa, Stenotrophomonas maltophilia | Multispecies biofilms of the four bacteria displayed increased resistance to chlorine. | Schwering et al. 2013 |
| Ciprofloxacin | I. limosus, P. aeruginosa, S. aureus, S. maltophilia | P. aeruginosa increased S. aureus, I. limosus and S. maltophilia resistance to ciprofloxacin. | Magalhães et al. 2017 |
| Clarithromycin | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to clarithromycin. | Armbruster et al. 2010 |
| Clindamycin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to clindamycin. | Lopes et al. 2012 |
| Colistin | P. aeruginosa, S. maltophilia | S. maltophilia increased P. aeruginosa resistance to colistin. | Ryan et al. 2008 |
| Essential oils of citronella and lemon | E. coli, S. aureus | S. aureus and E. coli increased resistance to citronella and lemon essential oils when cocultured. | Millezi et al. 2012 |
| Gentamicin | E. faecalis, Finegoldia magna, P. aeruginosa, S. aureus | P. aeruginosa increased resistance to gentamicin when cocultured with S. aureus, E. faecalis and F. magna. | Dalton et al. 2011 |
| Hydrogen peroxide | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to hydrogen peroxide. | Antonic et al. 2013 |
| Ofloxacin | Candida albicans, E. coli | C. albicans β-1,3-glucan, a matrix component, increased E. coli resistance to ofloxacin by acting as a barrier to its diffusion in the biofilm. | De Brucker et al. 2015 |
| ortho-Phthalaldehyde | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of ortho-phthalaldehyde. | Sanchez-Vizuete et al. 2015 |
| Peracetic acid | B. subtilis, S. aureus | B. subtilis ypqP gene protected S. aureus from biocide action of peracetic acid. | Sanchez-Vizuete et al. 2015 |
| Polymyxin B | P. aeruginosa, S. aureus | P. aeruginosa induction of pigment synthesis and catalase upregulation in S. aureus increased its resistance to polymyxin B. | Antonic et al. 2013 |
| Rifampicin | D. pigrum, I. limosus, P. aeruginosa | P. aeruginosa increased I. limosus and D. pigrum resistance to rifampicin. | Lopes et al. 2012 |
| Sodium dodecyl sulfate (SDS) | Pseudomonas fluorescens, K. pneumoniae, P. aeruginosa | P. fluorescens increased resistance to SDS when cocultured with K. pneumoniae and P. aeruginosa. | Lee et al. 2014 |
| Tobramycin | P. aeruginosa, S. maltophilia | P. aeruginosa alginate protected S. maltophilia from tobramycin treatment. | Pompilio et al. 2015 |
| Trimethoprim–sulfamethoxazole | H. influenzae, M. catarrhalis | H. influenzae signaling molecule AI-2 induced M. catarrhalis resistance to trimethoprim–sulfamethoxazole. | Armbruster et al. 2010 |
| Vancomycin | P. aeruginosa, S. aureus | P. aeruginosa HQNO increases S. aureus resistance to vancomycin. | Orazi and O'Toole 2017 |
Anti-biofilm strategies
A comprehensive knowledge of AMR mechanisms is crucial to find suitable anti-biofilm strategies. So far, these essentially belong to three different approaches, i.e. inhibition of bacterial attachment to surfaces, interference with signal molecules that modulate biofilm formation and disruption of the biofilm architecture (Parrino et al. 2019), for which examples are given in Table 3.
Examples of anti-biofilm molecules and their mechanisms of action, according to different strategies.
| Anti-biofilm strategy . | Mechanism of action . | Molecules . | References . |
|---|---|---|---|
| Inhibition of bacterial attachment to surfaces | Anti-adhesive surface properties | Hydrophilic polymers and surfaces with micro- and nanoscale topography | Huang et al. 2002; Hsu et al. 2013; Kang et al. 2016 |
| Antimicrobial surface properties | Antimicrobial peptides, essential oils, metallic nanoparticles, bacteriophages | Agnihotri, Mukherji and Mukherji 2013; Wang, Sauvageau and Elias 2016; Zaltsman et al. 2017; He et al. 2018 | |
| Interference with signal molecules modulating biofilm formation | AI degradation | Lactonases, acylases and oxidoreductases | Wang et al. 2004; Lade, Paul and Kweon 2014; Chan, Liu and Chang 2015 |
| AI synthesis inhibition | Halogenated furanone compounds, quercetin, cycloleucine, nickel and cadmium | Kim et al. 2008; Vega et al. 2014; Yadav et al. 2014; Gopu, Meena and Shetty 2015 | |
| AI antagonization | AHL analogues (cyclic sulfur compounds, phenolic compounds), AI-2 analogues (ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD), AIP analogues (cyclic peptides, RNA III) | Brackman and Coenye 2015; Hossain et al. 2017 | |
| c-Di-GMP signaling system inhibition | LP 3134, LP 3145, LP 4010, LP 1062 | Bachovchin et al. 2009; Sambanthamoorthy et al. 2014 | |
| Disruption of biofilm architecture | EPS/matrix degradation | Polysaccharide-degrading enzymes (dispersin B, endolysins), nucleases (DNase I), proteases (proteinase K, trypsin) | Kaplan et al. 2003; Sugimoto et al. 2018 |
| Biofilm dispersion | Nitric oxide, cis-2-decenoic acid, ethylenediaminetetraacetic acid, lactoferrin | Banin, Vasil and Greenberg 2005; Banin, Brady and Greenberg 2006; Barraud et al. 2009; Kumar Shukla and Rao 2013; Marques, Davies and Sauer 2015 |
| Anti-biofilm strategy . | Mechanism of action . | Molecules . | References . |
|---|---|---|---|
| Inhibition of bacterial attachment to surfaces | Anti-adhesive surface properties | Hydrophilic polymers and surfaces with micro- and nanoscale topography | Huang et al. 2002; Hsu et al. 2013; Kang et al. 2016 |
| Antimicrobial surface properties | Antimicrobial peptides, essential oils, metallic nanoparticles, bacteriophages | Agnihotri, Mukherji and Mukherji 2013; Wang, Sauvageau and Elias 2016; Zaltsman et al. 2017; He et al. 2018 | |
| Interference with signal molecules modulating biofilm formation | AI degradation | Lactonases, acylases and oxidoreductases | Wang et al. 2004; Lade, Paul and Kweon 2014; Chan, Liu and Chang 2015 |
| AI synthesis inhibition | Halogenated furanone compounds, quercetin, cycloleucine, nickel and cadmium | Kim et al. 2008; Vega et al. 2014; Yadav et al. 2014; Gopu, Meena and Shetty 2015 | |
| AI antagonization | AHL analogues (cyclic sulfur compounds, phenolic compounds), AI-2 analogues (ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD), AIP analogues (cyclic peptides, RNA III) | Brackman and Coenye 2015; Hossain et al. 2017 | |
| c-Di-GMP signaling system inhibition | LP 3134, LP 3145, LP 4010, LP 1062 | Bachovchin et al. 2009; Sambanthamoorthy et al. 2014 | |
| Disruption of biofilm architecture | EPS/matrix degradation | Polysaccharide-degrading enzymes (dispersin B, endolysins), nucleases (DNase I), proteases (proteinase K, trypsin) | Kaplan et al. 2003; Sugimoto et al. 2018 |
| Biofilm dispersion | Nitric oxide, cis-2-decenoic acid, ethylenediaminetetraacetic acid, lactoferrin | Banin, Vasil and Greenberg 2005; Banin, Brady and Greenberg 2006; Barraud et al. 2009; Kumar Shukla and Rao 2013; Marques, Davies and Sauer 2015 |
Examples of anti-biofilm molecules and their mechanisms of action, according to different strategies.
| Anti-biofilm strategy . | Mechanism of action . | Molecules . | References . |
|---|---|---|---|
| Inhibition of bacterial attachment to surfaces | Anti-adhesive surface properties | Hydrophilic polymers and surfaces with micro- and nanoscale topography | Huang et al. 2002; Hsu et al. 2013; Kang et al. 2016 |
| Antimicrobial surface properties | Antimicrobial peptides, essential oils, metallic nanoparticles, bacteriophages | Agnihotri, Mukherji and Mukherji 2013; Wang, Sauvageau and Elias 2016; Zaltsman et al. 2017; He et al. 2018 | |
| Interference with signal molecules modulating biofilm formation | AI degradation | Lactonases, acylases and oxidoreductases | Wang et al. 2004; Lade, Paul and Kweon 2014; Chan, Liu and Chang 2015 |
| AI synthesis inhibition | Halogenated furanone compounds, quercetin, cycloleucine, nickel and cadmium | Kim et al. 2008; Vega et al. 2014; Yadav et al. 2014; Gopu, Meena and Shetty 2015 | |
| AI antagonization | AHL analogues (cyclic sulfur compounds, phenolic compounds), AI-2 analogues (ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD), AIP analogues (cyclic peptides, RNA III) | Brackman and Coenye 2015; Hossain et al. 2017 | |
| c-Di-GMP signaling system inhibition | LP 3134, LP 3145, LP 4010, LP 1062 | Bachovchin et al. 2009; Sambanthamoorthy et al. 2014 | |
| Disruption of biofilm architecture | EPS/matrix degradation | Polysaccharide-degrading enzymes (dispersin B, endolysins), nucleases (DNase I), proteases (proteinase K, trypsin) | Kaplan et al. 2003; Sugimoto et al. 2018 |
| Biofilm dispersion | Nitric oxide, cis-2-decenoic acid, ethylenediaminetetraacetic acid, lactoferrin | Banin, Vasil and Greenberg 2005; Banin, Brady and Greenberg 2006; Barraud et al. 2009; Kumar Shukla and Rao 2013; Marques, Davies and Sauer 2015 |
| Anti-biofilm strategy . | Mechanism of action . | Molecules . | References . |
|---|---|---|---|
| Inhibition of bacterial attachment to surfaces | Anti-adhesive surface properties | Hydrophilic polymers and surfaces with micro- and nanoscale topography | Huang et al. 2002; Hsu et al. 2013; Kang et al. 2016 |
| Antimicrobial surface properties | Antimicrobial peptides, essential oils, metallic nanoparticles, bacteriophages | Agnihotri, Mukherji and Mukherji 2013; Wang, Sauvageau and Elias 2016; Zaltsman et al. 2017; He et al. 2018 | |
| Interference with signal molecules modulating biofilm formation | AI degradation | Lactonases, acylases and oxidoreductases | Wang et al. 2004; Lade, Paul and Kweon 2014; Chan, Liu and Chang 2015 |
| AI synthesis inhibition | Halogenated furanone compounds, quercetin, cycloleucine, nickel and cadmium | Kim et al. 2008; Vega et al. 2014; Yadav et al. 2014; Gopu, Meena and Shetty 2015 | |
| AI antagonization | AHL analogues (cyclic sulfur compounds, phenolic compounds), AI-2 analogues (ursolic acid, isobutyl-4,5-dihydroxy-2,3-pentanedione (isobutyl-DPD) and phenyl-DPD), AIP analogues (cyclic peptides, RNA III) | Brackman and Coenye 2015; Hossain et al. 2017 | |
| c-Di-GMP signaling system inhibition | LP 3134, LP 3145, LP 4010, LP 1062 | Bachovchin et al. 2009; Sambanthamoorthy et al. 2014 | |
| Disruption of biofilm architecture | EPS/matrix degradation | Polysaccharide-degrading enzymes (dispersin B, endolysins), nucleases (DNase I), proteases (proteinase K, trypsin) | Kaplan et al. 2003; Sugimoto et al. 2018 |
| Biofilm dispersion | Nitric oxide, cis-2-decenoic acid, ethylenediaminetetraacetic acid, lactoferrin | Banin, Vasil and Greenberg 2005; Banin, Brady and Greenberg 2006; Barraud et al. 2009; Kumar Shukla and Rao 2013; Marques, Davies and Sauer 2015 |
As bacterial adhesion is the first step in biofilm formation, a number of surface modifications have been developed to prevent bacterial attachment and/or kill adhered bacteria through the immobilization of antimicrobials (Desrousseaux et al. 2013; Hasan, Crawford and Ivanova 2013; Alves et al. 2016). Among the antimicrobial agents explored in surface functionalization, antimicrobial peptides, enzymes, bacteriophages and essential oils stand out as promising alternatives to antibiotics. These natural bactericidal compounds are considered to have a low propensity for the development of AMR due to their mechanisms of action (Glinel et al. 2012). For instance, antimicrobial peptides target the bacterial membrane, so their activity does not require cells to be metabolically active (Kumar, Kizhakkedathu and Straus 2018), allowing them to effectively kill cells that are dormant or nongrowing, such as persister cells (Batoni, Maisetta and Esin 2016; Grassi et al. 2017). In turn, bacteriophages are natural bacterial predators, comprising a promising option to overcome biofilm barriers when used in combined therapies or after being genetically engineered with new functions to overcome biofilm obstacles (Pires et al. 2017).
Another anti-biofilm strategy is the interference with signal molecules that modulate biofilm development, where QS along with intracellular signaling by bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP) has been the subject of great attention (LaSarre and Federle 2013; Parrino et al. 2019). QS interference can be achieved by degrading AI or inhibiting their production, limiting the activity of QS signal receptors or mimicking AI using structurally synthetic compounds. A number of compounds targeting QS using these approaches have been identified, such as quorum sensing inhibitors that block the action of AI and quorum quenching enzymes that degrade signaling molecules (Hirakawa and Tomita 2013; Rémy et al. 2018; Kalia et al. 2019). It is postulated that resistance to these anti-QS strategies would develop slowly, making them promising alternatives to traditional antibiotics (Turkina and Vikström 2018). In turn, c-di-GMP has been described as a key mediator of biofilm formation, especially in Gram-negative bacteria, by stimulating the biosynthesis of adhesins, adhesive pili and EPS, and by inhibiting bacterial motility. Since c-di-GMP is not essential for bacterial growth, its inhibition should not promote resistance, therefore being a good target for the development of anti-biofilm compounds (Valentini and Filloux 2016).
After bacterial attachment to a surface, large amounts of EPS are produced. Given its role in biofilm resistance, as described earlier, a promising strategy to dismantle established biofilms relies on the use of enzymes targeting EPS (Alves and Pereira 2014). Matrix disruptive enzymes, such as alginate lyase (Ramsey and Wozniak 2005), DNase I (Sugimoto et al. 2018), lysozyme (Ragland and Criss 2017), dispersin B (Kaplan et al. 2003) and lysostaphin (Bastos, Coutinho and Coelho 2010), have been extensively investigated with this aim, many times in combination with other antimicrobials (Jorge, Alves and Pereira 2019). Another strategy to target established biofilms relies on the stimulation of the natural stage of biofilm dispersal. Although this comprises a survival strategy of biofilms to colonize new areas, the dispersed and free cells are technically more susceptible to antimicrobials and host defenses (Kostakioti, Hadjifrangiskou and Hultgren 2013). For instance, it has been demonstrated that a low concentration of c-di-GMP leads to biofilm self-destruction. As such, c-di-GMP should be considered as a good target for a biofilm dispersion strategy (O'Toole and Ha 2015). Despite promising results, most of these strategies fail to be tested and validated using in vivo models, so the development of strategies to fight biofilms is still urgently needed (Magana et al. 2018).
CONCLUSION
The solution for the hitches caused by one of the smallest life forms on this planet, i.e. bacteria, remains an unsolved riddle, as these microorganisms do not cease to amaze with their ability to circumvent every ‘curve ball’ thrown their way. Their capability to evolve and adapt has led to a modern healthcare crisis as they become resistant to most, and sometimes all, available antibiotics. The complex issue of AMR, it seems, is a ‘many-fronts battle’, with biofilm formation being a substantial portion of the problem. This ancient form of bacterial adaptation is itself a form of AMR that escalates when resistant bacteria are its originators, making antibiotics forced to surpass not only bacterial resistance mechanisms (e.g. efflux pumps, cell wall modifications) but also biofilm specific constraints (e.g. EPS matrix, persister cells).
The engagement from health- and research-related organizations worldwide is being put to the test, with many believing that only a concerted global action will result in AMR mitigation. In order to do so, unraveling the molecular mechanisms behind this phenomenon is pivotal in order to elaborate new effective antimicrobial strategies. Although much has yet to be done, substantial research is created every day to tackle this problem. Innovative solutions, such as surface functionalization to prevent biofilm formation, discovery of compounds that interfere with bacterial communication and enzyme application to disperse grown biofilms, are just a few examples. Slowly but surely we will come to a solution; let us hope it is not too late!
FUNDING
This work was supported by the Portuguese Foundation for Science and Technology (FCT) under the scope of the strategic funding of UID/BIO/04469/2019 unit and COMPETE 2020 (POCI-01-0145-FEDER-006684) and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020–Programa Operacional Regional do Norte. The authors also acknowledge COMPETE2020 and FCT for the project POCI-01-0145-FEDER-029841 and FCT for the Ph.D. grants of Andreia Magalhães (grant number SFRH/BD/132165/2017) and Tânia Grainha (grant number SFRH/BD/136544/2018).
Conflict of interest. None declared.
REFERENCES
Author notes
Paula Jorge and Andreia Patrícia Magalhães should be considered joint first authors.



