-
PDF
- Split View
-
Views
-
Cite
Cite
Virginia de Garcia, Polona Zalar, Silvia Brizzio, Nina Gunde-Cimerman, María van Broock, Cryptococcus species (Tremellales) from glacial biomes in the southern (Patagonia) and northern (Svalbard) hemispheres, FEMS Microbiology Ecology, Volume 82, Issue 2, November 2012, Pages 523–539, https://doi.org/10.1111/j.1574-6941.2012.01465.x
Close - Share Icon Share
Abstract
Cryptococcus species (Basidiomycota) were isolated as the predominant yeast from glacial biomes of both Patagonia (Argentina) and the Svalbard archipelago (Norway). For a selected group of Cryptococcus belonging to Tremellales, assimilative profile, production of extracellular hydrolytic enzymes and ribosomal DNA internal transcribed spacer and large subunit (D1/D2) sequences were analysed. Cryptococcus victoriae, which was originally described from Antarctica, was the most frequently found species at both locations. High variability within the species was observed and described at the genotypic and phenotypic levels, two newly described species were found in both Patagonia and Svalbard: Cryptococcus fonsecae and Cryptococcus psychrotolerans. Two other new species were found only in Patagonia: Cryptococcus frias and Cryptococcus tronadorensis. Three additional new taxa were found, but they are not named as they were only represented by single isolates.
Introduction
Ice in nature has long been considered as only containing microorganisms that have been randomly deposited on its surface. However, it is now known that different types of ice provide biomes that can support active microbial growth and reproduction (Gostincar et al., 2010). Initially, the microbial presence was investigated only at the prokaryotic level. However, recent studies have shown that fungi, as primarily as basidiomycetous yeast, also represent an important part of glacial microbial communities in both polar and mountainous glacial environments around the world (Margesin et al., 2003; Bergauer et al., 2005; Buzzini et al., 2005; Butinar et al., 2007; de Garcia et al., 2007; Turchetti et al., 2008, 2011; Branda et al., 2010). Basidiomycetous yeast also predominates in permafrost soils of the Arctic and Antarctica (Vishniac, 2006). Species of the genus Cryptococcus (Connell et al., 2008; Turchetti et al., 2011) are among the most frequently isolated from such environments.
Cryptococcus species are distributed into four orders: Tremellales, Trichosporonales, Filobasidiales and Cystofilobasidiales (Kurtzman et al., 2011). Species isolated from cold environments belong to all these orders. This is also the case for the Cryptococcus sensu stricto group (Tremellales), which includes the type species and also the most medically important species, Cryptococcus neoformans (Cooper, 2011). Agricultural importance has also been shown for this group. For example, Cryptococcus laurentii has been reported to inhibit growth of phytopathogenic fungi (Robiglio et al., 2011), and it can be used together with Cryptococcus albidus for control of postharvest diseases in stored fruit (Abadias et al., 2003; Qin et al., 2004; Schisler et al., 2011). Cryptococcus species can also be used for degradation of phenolic and polycyclic aromatic hydrocarbons (Johnson & Echavarri-Erasun, 2011). Consequently, the interest in these microorganisms is not limited to their biodiversity and ecological role, but includes potential industrial uses of economic value (Margesin et al., 2007; Shivaji & Prasad, 2009).
Identification and phylogenetic placement of the basidiomycetous yeast can be difficult because of intraspecific variability (Fell et al., 2000; Scorzetti et al., 2002; Fonseca et al., 2011). Particularly within Tremellales, Cryptococcus is highly heterogeneous and includes numerous species that are yet to be described (Fell et al., 2000; Sampaio et al., 2002; Scorzetti et al., 2002; Inácio et al., 2005; de Garcia et al., 2010).
The aim of the present study was to present and identify the predominant groups of basidiomycetous Cryptococcus yeast species, obtained in two independent studies in Norway and Argentina. The focus was on the species from the order Tremellales isolated from rarely explored glacial biomes in the northern and southern hemispheres, and to compare their biodiversity. Cryptococcus species predominated over the other yeast in both locations. Comparisons of physiology and phylogeny of isolates has revealed unexpected diversity, which has resulted in the description of new varieties and species, some of them found in both geographically distant locations.
Materials and methods
Description of sampling sites and isolation methods
Sampling in Patagonia (Argentina) was performed in February and March in 2004, 2008 and 2010. The samples were taken from: (1) meltwater from the Rio Manso, Castaño Overo and Frias glaciers of Mount Tronador (71°50′W, 41°11′S), in the Nahuel Huapi National Park; (2) ice from the Perito Moreno glacier (73°51′W, 49°15′S) in the ‘Los Glaciares’ National Park; and (3) sea water from the meridian of Cape Horn, Argentinian Sea (66°34′W, 57°25′S). Sampling in Svalbard (Norway) was performed in May 2001, August 2003 and July 2008. The samples were taken in Kongsfjorden, on the western coast of Spitsbergen (79°N, 12°E), from: (1) glacial meltwater; (2) superficial sea water; and (3) subglacial ice. The surface of the ice samples was melted and discarded, and the remaining ice was superficially rinsed with sterile distilled water and melted. The resulting water was filtered through Millipore membrane filters (0.45 μm pore diameter). The yeast from Patagonia were isolated after filtration and incubation at 10 °C, as described by de Garcia et al. (2007); those from Svalbard were isolated following the protocol of Gunde-Cimerman et al. (2003), and were incubated at 25 °C. The precise origins of all of the strains studied here are listed in Table 1.
Strains of the genus Cryptococcus studied, and their origin and the GenBank accession number of their sequences
| Species | Strain numbers | Substrate | Locality, Country | Isolated by | GenBank accession numbers | |
| D1/D2 | ITS | |||||
| C. carnescens | EXF-1549 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norways | N. Gunde-Cimerman | JN193440 | – |
| EXF-1551 | JN193441 | JN193461 | ||||
| EXF-1591 | JN193442 | JN193462 | ||||
| EXF-1621 | JN193443 | JN193463 | ||||
| C. foliicola | CRUB 1267 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Río Negro, Argentina | V. de Garcia | GU560001 | GU997160 |
| C. frias sp nov. | CRUB 1303 | Frias river, Río Negro, Argentina | GU560005 | GU997163 | ||
| CRUB 1250 | GU560004 | GU997162 | ||||
| C. fonsecae sp. nov | EXF-3792 | Hypersaline saltern water | Sečovlje, Slovenia | N. Gunde-Cimerman | JN193446 | JN193466 |
| EXF-4087 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193447 | JN193468 | ||
| CRUB 1765 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193448 | JN193467 | |
| CRUB 1766 | JN193451 | – | ||||
| CRUB 1767 | JN193449 | – | ||||
| CRUB 1768 | JN193450 | – | ||||
| SJ008 | – | San Juan Islands, Vancouver | Fraser et al. | AY953961 | – | |
| A57 | Sea water | Portugal | M. Gadanho & J.P. Sampaio | AF485974 | – | |
| C. psychrotolerans sp. nov | EXF-1583 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | JN193464 |
| EXF-1528 | Subglacial ice | Austre Lovénbreen Glaciers, Svalbard, Norway | JN193444 | JN193465 | ||
| CRUB 1769 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193445 | – | |
| CBS 6578 | Sea water | – | J.W. Fell | AB035053 | AB035049 | |
| C. aff. tephrensis | CBS 9799 | Arctic dwarf shrub Dryas octopetala | Norway | J. Pryce Miller | CBS database | CBS database |
| CBS 9023 | Flowering plant of Pulmonaria stiriaca | Hessen, Marburg, Germany | M. Herzberg | AF406896 | – | |
| EXF-3999 | Stream water | Kongsvegen, Svalbard, Norway | N. Gunde-Cimerman | GU586202 | GU997158 | |
| EXF-3749 | Stream water | GU586203 | GU997157 | |||
| EXF-3875 | GU997140 | GU997161 | ||||
| EXF-6553 | Surface ice | DQ640490 | – | |||
| C. tronadorensis sp. nov. | CRUB 1258 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Rio negro, Argentina | V. de Garcia | GU560002 | GU997164 |
| CRUB 1299 | GU560003 | GU997165 | ||||
| C. victoriae type group | CBS 8685T | Soil | Antarctica | Montes et al. | AF363647 | AF444469 |
| CBS 8920 | S. Thomas-Hall | AY040650 | AY040656 | |||
| CBS 8915 | AY040652 | AY040655 | ||||
| CBS 9267 | Aggregated grey- brown silty soil with coarse organic remains | Alaska, Nome | H.S. Vishniac | CBS database | CBS database | |
| CBS 9565 | ||||||
| CRUB 1399 | Acidic river | Rio Agrio-Lake Caviahue | G. Russo | EF585176 | – | |
| DBVPG 4835 | Glacial water | Italian Alps | E. Branda | EU287884 | – | |
| DBVPG 4830 | EU287882 | – | ||||
| TP-Snow-Y33 | Glacier surface snow | Tibetan Plateau China | S. Shao | JN400774 | JN400815 | |
| ESAB12 | Food | Portugal: Tras-os-Montes | R. Calhelha | AJ749830 | – | |
| I1-4 | Processed meat products | Denmark | Nielsen et al. | EU194455 | – | |
| CBS 9000 | Flowering plant of Helleborus foetidus | Germany | M. Herzberg | AF406899 | – | |
| CBS 6550 | Forced rhubarb | UK | R. Buhagiar | AF444711 | AF444447 | |
| KCTC 17059 | Rhizosphere soil of Platycodon grandiflorum | Korea | S.G. Hong & K.S. Bae | AF459674 | – | |
| AY-99 | Deschampsia cespitosa green leaves | Russia | A. Yurkov | FN357207 | – | |
| AY-92 | Equisetum sylvaticum green leaves | FN357206 | – | |||
| CRUB 1262 | Glacial meltwater | Rio Manso (Garganta del diablo waterfall), Río Negro, Argentina | V. de Garcia | GU559996 | GU997143 | |
| CRUB 1254 | GU559995 | GU997147 | ||||
| CRUB 1260 | Frias river, Río Negro, Argentina | GU559997 | GU997145 | |||
| CRUB 1264 | GU559994 | GU997148 | ||||
| CRUB 1263 | GU559998 | GU997149 | ||||
| CRUB 1266 | GU559999 | GU997150 | ||||
| EXF-3832 | Subglacial ice | Kongsfjorden, Svalbard, Norway | N. Gunde-Cimerman | GU586196 | GU997144 | |
| EXF-1582 | Darkly pigmented ice | Austre Brøggerbreen glacier, Kongsvegen, Norway | JN193432 | JN193452 | ||
| EXF-1588 | Sea water | Svalbard, Norway | JN193433 | JN193453 | ||
| EXF-1623 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norway | JN193434 | JN193454 | ||
| EXF-3831 | Melt water | Kongsfjorden, Svalbard, Norway | GU586193 | GU997141 | ||
| EXF-3748 | GU586192 | GU997142 | ||||
| EXF-3721 | GU586200 | GU997154 | ||||
| EXF-4012 | Sea water | GU586195 | GU997146 | |||
| EXF-3912 | GU586194 | GU997152 | ||||
| EXF-4085 | GU586198 | GU997159 | ||||
| EXF-3827 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193435 | JN193455 | ||
| EXF-6542 | Subglacial ice | Kongsvegen Glacier, Svalbard, Norway | JN193436 | JN193456 | ||
| EXF-6550 | Austre Lovénbreen Glacier, Svalbard, Norway | JN193437 | JN193457 | |||
| EXF-6549 | JN193460 | |||||
| EXF-6535 | JN193438 | JN193458 | ||||
| EXF- 6534 | Columnar ice crystals at the outflow of glacier melt water | Kongsfjorden, Svalbard, Norway | JN193439 | JN193459 | ||
| EXF-4020 | Sea water | GU586201 | GU997156 | |||
| EXF-3923 | GU586204 | GU997155 | ||||
| EXF-4011 | Stream water | GU586197 | GU997153 | |||
| C. victoriae non-type group | CRUB 1757 | Ice | Perito Moreno, Santa Cruz, Argentina | V. de Garcia | GU560000 | GU997151 |
| CBS 8908 | Soil | Antarctica | S. Thomas-Hall | AY040653 | AY040654 | |
| CBS 8884 | Seawater (reef) | Bahamas | A. Statzell-Tallman | AF444741 | AF444645 | |
| VTT C-04542 | Industrial malting ecosystem | Finland | Laitila et al. | DQ377664 | – | |
| LU9-1 | Grape | Denmark | Lederer et al. | HM146911 | – | |
| A114 | Sea water | Portugal | M. Gadanho & J. P. Sampaio | AF485971 | – | |
| Cryptococcus sp. 1 | EXF-1596 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | – |
| Cryptococcus. sp. 2 | EXF-3926 | Subglacial ice | Kongsvegen, Svalbard,Norway | GU586199 | GU997166 | |
| Species | Strain numbers | Substrate | Locality, Country | Isolated by | GenBank accession numbers | |
| D1/D2 | ITS | |||||
| C. carnescens | EXF-1549 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norways | N. Gunde-Cimerman | JN193440 | – |
| EXF-1551 | JN193441 | JN193461 | ||||
| EXF-1591 | JN193442 | JN193462 | ||||
| EXF-1621 | JN193443 | JN193463 | ||||
| C. foliicola | CRUB 1267 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Río Negro, Argentina | V. de Garcia | GU560001 | GU997160 |
| C. frias sp nov. | CRUB 1303 | Frias river, Río Negro, Argentina | GU560005 | GU997163 | ||
| CRUB 1250 | GU560004 | GU997162 | ||||
| C. fonsecae sp. nov | EXF-3792 | Hypersaline saltern water | Sečovlje, Slovenia | N. Gunde-Cimerman | JN193446 | JN193466 |
| EXF-4087 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193447 | JN193468 | ||
| CRUB 1765 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193448 | JN193467 | |
| CRUB 1766 | JN193451 | – | ||||
| CRUB 1767 | JN193449 | – | ||||
| CRUB 1768 | JN193450 | – | ||||
| SJ008 | – | San Juan Islands, Vancouver | Fraser et al. | AY953961 | – | |
| A57 | Sea water | Portugal | M. Gadanho & J.P. Sampaio | AF485974 | – | |
| C. psychrotolerans sp. nov | EXF-1583 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | JN193464 |
| EXF-1528 | Subglacial ice | Austre Lovénbreen Glaciers, Svalbard, Norway | JN193444 | JN193465 | ||
| CRUB 1769 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193445 | – | |
| CBS 6578 | Sea water | – | J.W. Fell | AB035053 | AB035049 | |
| C. aff. tephrensis | CBS 9799 | Arctic dwarf shrub Dryas octopetala | Norway | J. Pryce Miller | CBS database | CBS database |
| CBS 9023 | Flowering plant of Pulmonaria stiriaca | Hessen, Marburg, Germany | M. Herzberg | AF406896 | – | |
| EXF-3999 | Stream water | Kongsvegen, Svalbard, Norway | N. Gunde-Cimerman | GU586202 | GU997158 | |
| EXF-3749 | Stream water | GU586203 | GU997157 | |||
| EXF-3875 | GU997140 | GU997161 | ||||
| EXF-6553 | Surface ice | DQ640490 | – | |||
| C. tronadorensis sp. nov. | CRUB 1258 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Rio negro, Argentina | V. de Garcia | GU560002 | GU997164 |
| CRUB 1299 | GU560003 | GU997165 | ||||
| C. victoriae type group | CBS 8685T | Soil | Antarctica | Montes et al. | AF363647 | AF444469 |
| CBS 8920 | S. Thomas-Hall | AY040650 | AY040656 | |||
| CBS 8915 | AY040652 | AY040655 | ||||
| CBS 9267 | Aggregated grey- brown silty soil with coarse organic remains | Alaska, Nome | H.S. Vishniac | CBS database | CBS database | |
| CBS 9565 | ||||||
| CRUB 1399 | Acidic river | Rio Agrio-Lake Caviahue | G. Russo | EF585176 | – | |
| DBVPG 4835 | Glacial water | Italian Alps | E. Branda | EU287884 | – | |
| DBVPG 4830 | EU287882 | – | ||||
| TP-Snow-Y33 | Glacier surface snow | Tibetan Plateau China | S. Shao | JN400774 | JN400815 | |
| ESAB12 | Food | Portugal: Tras-os-Montes | R. Calhelha | AJ749830 | – | |
| I1-4 | Processed meat products | Denmark | Nielsen et al. | EU194455 | – | |
| CBS 9000 | Flowering plant of Helleborus foetidus | Germany | M. Herzberg | AF406899 | – | |
| CBS 6550 | Forced rhubarb | UK | R. Buhagiar | AF444711 | AF444447 | |
| KCTC 17059 | Rhizosphere soil of Platycodon grandiflorum | Korea | S.G. Hong & K.S. Bae | AF459674 | – | |
| AY-99 | Deschampsia cespitosa green leaves | Russia | A. Yurkov | FN357207 | – | |
| AY-92 | Equisetum sylvaticum green leaves | FN357206 | – | |||
| CRUB 1262 | Glacial meltwater | Rio Manso (Garganta del diablo waterfall), Río Negro, Argentina | V. de Garcia | GU559996 | GU997143 | |
| CRUB 1254 | GU559995 | GU997147 | ||||
| CRUB 1260 | Frias river, Río Negro, Argentina | GU559997 | GU997145 | |||
| CRUB 1264 | GU559994 | GU997148 | ||||
| CRUB 1263 | GU559998 | GU997149 | ||||
| CRUB 1266 | GU559999 | GU997150 | ||||
| EXF-3832 | Subglacial ice | Kongsfjorden, Svalbard, Norway | N. Gunde-Cimerman | GU586196 | GU997144 | |
| EXF-1582 | Darkly pigmented ice | Austre Brøggerbreen glacier, Kongsvegen, Norway | JN193432 | JN193452 | ||
| EXF-1588 | Sea water | Svalbard, Norway | JN193433 | JN193453 | ||
| EXF-1623 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norway | JN193434 | JN193454 | ||
| EXF-3831 | Melt water | Kongsfjorden, Svalbard, Norway | GU586193 | GU997141 | ||
| EXF-3748 | GU586192 | GU997142 | ||||
| EXF-3721 | GU586200 | GU997154 | ||||
| EXF-4012 | Sea water | GU586195 | GU997146 | |||
| EXF-3912 | GU586194 | GU997152 | ||||
| EXF-4085 | GU586198 | GU997159 | ||||
| EXF-3827 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193435 | JN193455 | ||
| EXF-6542 | Subglacial ice | Kongsvegen Glacier, Svalbard, Norway | JN193436 | JN193456 | ||
| EXF-6550 | Austre Lovénbreen Glacier, Svalbard, Norway | JN193437 | JN193457 | |||
| EXF-6549 | JN193460 | |||||
| EXF-6535 | JN193438 | JN193458 | ||||
| EXF- 6534 | Columnar ice crystals at the outflow of glacier melt water | Kongsfjorden, Svalbard, Norway | JN193439 | JN193459 | ||
| EXF-4020 | Sea water | GU586201 | GU997156 | |||
| EXF-3923 | GU586204 | GU997155 | ||||
| EXF-4011 | Stream water | GU586197 | GU997153 | |||
| C. victoriae non-type group | CRUB 1757 | Ice | Perito Moreno, Santa Cruz, Argentina | V. de Garcia | GU560000 | GU997151 |
| CBS 8908 | Soil | Antarctica | S. Thomas-Hall | AY040653 | AY040654 | |
| CBS 8884 | Seawater (reef) | Bahamas | A. Statzell-Tallman | AF444741 | AF444645 | |
| VTT C-04542 | Industrial malting ecosystem | Finland | Laitila et al. | DQ377664 | – | |
| LU9-1 | Grape | Denmark | Lederer et al. | HM146911 | – | |
| A114 | Sea water | Portugal | M. Gadanho & J. P. Sampaio | AF485971 | – | |
| Cryptococcus sp. 1 | EXF-1596 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | – |
| Cryptococcus. sp. 2 | EXF-3926 | Subglacial ice | Kongsvegen, Svalbard,Norway | GU586199 | GU997166 | |
Numbers in bold are isolates from this study.
CRUB, Regional University Center of Bariloche (Centro Regional Universitario Bariloche); EXF, Culture Collection of Extremophilic Fungi, Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia; CBS, Centraalbureau voor Schimmelcultures; DBVPG, Industrial Yeasts Collection of Dipartimento di Biologia Vegetale, Università di Perugia, Italy; KCTC, Korean Collection for Type Cultures; VTT, Technical Research Centre of Finland.
Strains of the genus Cryptococcus studied, and their origin and the GenBank accession number of their sequences
| Species | Strain numbers | Substrate | Locality, Country | Isolated by | GenBank accession numbers | |
| D1/D2 | ITS | |||||
| C. carnescens | EXF-1549 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norways | N. Gunde-Cimerman | JN193440 | – |
| EXF-1551 | JN193441 | JN193461 | ||||
| EXF-1591 | JN193442 | JN193462 | ||||
| EXF-1621 | JN193443 | JN193463 | ||||
| C. foliicola | CRUB 1267 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Río Negro, Argentina | V. de Garcia | GU560001 | GU997160 |
| C. frias sp nov. | CRUB 1303 | Frias river, Río Negro, Argentina | GU560005 | GU997163 | ||
| CRUB 1250 | GU560004 | GU997162 | ||||
| C. fonsecae sp. nov | EXF-3792 | Hypersaline saltern water | Sečovlje, Slovenia | N. Gunde-Cimerman | JN193446 | JN193466 |
| EXF-4087 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193447 | JN193468 | ||
| CRUB 1765 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193448 | JN193467 | |
| CRUB 1766 | JN193451 | – | ||||
| CRUB 1767 | JN193449 | – | ||||
| CRUB 1768 | JN193450 | – | ||||
| SJ008 | – | San Juan Islands, Vancouver | Fraser et al. | AY953961 | – | |
| A57 | Sea water | Portugal | M. Gadanho & J.P. Sampaio | AF485974 | – | |
| C. psychrotolerans sp. nov | EXF-1583 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | JN193464 |
| EXF-1528 | Subglacial ice | Austre Lovénbreen Glaciers, Svalbard, Norway | JN193444 | JN193465 | ||
| CRUB 1769 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193445 | – | |
| CBS 6578 | Sea water | – | J.W. Fell | AB035053 | AB035049 | |
| C. aff. tephrensis | CBS 9799 | Arctic dwarf shrub Dryas octopetala | Norway | J. Pryce Miller | CBS database | CBS database |
| CBS 9023 | Flowering plant of Pulmonaria stiriaca | Hessen, Marburg, Germany | M. Herzberg | AF406896 | – | |
| EXF-3999 | Stream water | Kongsvegen, Svalbard, Norway | N. Gunde-Cimerman | GU586202 | GU997158 | |
| EXF-3749 | Stream water | GU586203 | GU997157 | |||
| EXF-3875 | GU997140 | GU997161 | ||||
| EXF-6553 | Surface ice | DQ640490 | – | |||
| C. tronadorensis sp. nov. | CRUB 1258 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Rio negro, Argentina | V. de Garcia | GU560002 | GU997164 |
| CRUB 1299 | GU560003 | GU997165 | ||||
| C. victoriae type group | CBS 8685T | Soil | Antarctica | Montes et al. | AF363647 | AF444469 |
| CBS 8920 | S. Thomas-Hall | AY040650 | AY040656 | |||
| CBS 8915 | AY040652 | AY040655 | ||||
| CBS 9267 | Aggregated grey- brown silty soil with coarse organic remains | Alaska, Nome | H.S. Vishniac | CBS database | CBS database | |
| CBS 9565 | ||||||
| CRUB 1399 | Acidic river | Rio Agrio-Lake Caviahue | G. Russo | EF585176 | – | |
| DBVPG 4835 | Glacial water | Italian Alps | E. Branda | EU287884 | – | |
| DBVPG 4830 | EU287882 | – | ||||
| TP-Snow-Y33 | Glacier surface snow | Tibetan Plateau China | S. Shao | JN400774 | JN400815 | |
| ESAB12 | Food | Portugal: Tras-os-Montes | R. Calhelha | AJ749830 | – | |
| I1-4 | Processed meat products | Denmark | Nielsen et al. | EU194455 | – | |
| CBS 9000 | Flowering plant of Helleborus foetidus | Germany | M. Herzberg | AF406899 | – | |
| CBS 6550 | Forced rhubarb | UK | R. Buhagiar | AF444711 | AF444447 | |
| KCTC 17059 | Rhizosphere soil of Platycodon grandiflorum | Korea | S.G. Hong & K.S. Bae | AF459674 | – | |
| AY-99 | Deschampsia cespitosa green leaves | Russia | A. Yurkov | FN357207 | – | |
| AY-92 | Equisetum sylvaticum green leaves | FN357206 | – | |||
| CRUB 1262 | Glacial meltwater | Rio Manso (Garganta del diablo waterfall), Río Negro, Argentina | V. de Garcia | GU559996 | GU997143 | |
| CRUB 1254 | GU559995 | GU997147 | ||||
| CRUB 1260 | Frias river, Río Negro, Argentina | GU559997 | GU997145 | |||
| CRUB 1264 | GU559994 | GU997148 | ||||
| CRUB 1263 | GU559998 | GU997149 | ||||
| CRUB 1266 | GU559999 | GU997150 | ||||
| EXF-3832 | Subglacial ice | Kongsfjorden, Svalbard, Norway | N. Gunde-Cimerman | GU586196 | GU997144 | |
| EXF-1582 | Darkly pigmented ice | Austre Brøggerbreen glacier, Kongsvegen, Norway | JN193432 | JN193452 | ||
| EXF-1588 | Sea water | Svalbard, Norway | JN193433 | JN193453 | ||
| EXF-1623 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norway | JN193434 | JN193454 | ||
| EXF-3831 | Melt water | Kongsfjorden, Svalbard, Norway | GU586193 | GU997141 | ||
| EXF-3748 | GU586192 | GU997142 | ||||
| EXF-3721 | GU586200 | GU997154 | ||||
| EXF-4012 | Sea water | GU586195 | GU997146 | |||
| EXF-3912 | GU586194 | GU997152 | ||||
| EXF-4085 | GU586198 | GU997159 | ||||
| EXF-3827 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193435 | JN193455 | ||
| EXF-6542 | Subglacial ice | Kongsvegen Glacier, Svalbard, Norway | JN193436 | JN193456 | ||
| EXF-6550 | Austre Lovénbreen Glacier, Svalbard, Norway | JN193437 | JN193457 | |||
| EXF-6549 | JN193460 | |||||
| EXF-6535 | JN193438 | JN193458 | ||||
| EXF- 6534 | Columnar ice crystals at the outflow of glacier melt water | Kongsfjorden, Svalbard, Norway | JN193439 | JN193459 | ||
| EXF-4020 | Sea water | GU586201 | GU997156 | |||
| EXF-3923 | GU586204 | GU997155 | ||||
| EXF-4011 | Stream water | GU586197 | GU997153 | |||
| C. victoriae non-type group | CRUB 1757 | Ice | Perito Moreno, Santa Cruz, Argentina | V. de Garcia | GU560000 | GU997151 |
| CBS 8908 | Soil | Antarctica | S. Thomas-Hall | AY040653 | AY040654 | |
| CBS 8884 | Seawater (reef) | Bahamas | A. Statzell-Tallman | AF444741 | AF444645 | |
| VTT C-04542 | Industrial malting ecosystem | Finland | Laitila et al. | DQ377664 | – | |
| LU9-1 | Grape | Denmark | Lederer et al. | HM146911 | – | |
| A114 | Sea water | Portugal | M. Gadanho & J. P. Sampaio | AF485971 | – | |
| Cryptococcus sp. 1 | EXF-1596 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | – |
| Cryptococcus. sp. 2 | EXF-3926 | Subglacial ice | Kongsvegen, Svalbard,Norway | GU586199 | GU997166 | |
| Species | Strain numbers | Substrate | Locality, Country | Isolated by | GenBank accession numbers | |
| D1/D2 | ITS | |||||
| C. carnescens | EXF-1549 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norways | N. Gunde-Cimerman | JN193440 | – |
| EXF-1551 | JN193441 | JN193461 | ||||
| EXF-1591 | JN193442 | JN193462 | ||||
| EXF-1621 | JN193443 | JN193463 | ||||
| C. foliicola | CRUB 1267 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Río Negro, Argentina | V. de Garcia | GU560001 | GU997160 |
| C. frias sp nov. | CRUB 1303 | Frias river, Río Negro, Argentina | GU560005 | GU997163 | ||
| CRUB 1250 | GU560004 | GU997162 | ||||
| C. fonsecae sp. nov | EXF-3792 | Hypersaline saltern water | Sečovlje, Slovenia | N. Gunde-Cimerman | JN193446 | JN193466 |
| EXF-4087 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193447 | JN193468 | ||
| CRUB 1765 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193448 | JN193467 | |
| CRUB 1766 | JN193451 | – | ||||
| CRUB 1767 | JN193449 | – | ||||
| CRUB 1768 | JN193450 | – | ||||
| SJ008 | – | San Juan Islands, Vancouver | Fraser et al. | AY953961 | – | |
| A57 | Sea water | Portugal | M. Gadanho & J.P. Sampaio | AF485974 | – | |
| C. psychrotolerans sp. nov | EXF-1583 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | JN193464 |
| EXF-1528 | Subglacial ice | Austre Lovénbreen Glaciers, Svalbard, Norway | JN193444 | JN193465 | ||
| CRUB 1769 | Sea water | Cape Horn Meridian Argentinian Sea | V. de Garcia | JN193445 | – | |
| CBS 6578 | Sea water | – | J.W. Fell | AB035053 | AB035049 | |
| C. aff. tephrensis | CBS 9799 | Arctic dwarf shrub Dryas octopetala | Norway | J. Pryce Miller | CBS database | CBS database |
| CBS 9023 | Flowering plant of Pulmonaria stiriaca | Hessen, Marburg, Germany | M. Herzberg | AF406896 | – | |
| EXF-3999 | Stream water | Kongsvegen, Svalbard, Norway | N. Gunde-Cimerman | GU586202 | GU997158 | |
| EXF-3749 | Stream water | GU586203 | GU997157 | |||
| EXF-3875 | GU997140 | GU997161 | ||||
| EXF-6553 | Surface ice | DQ640490 | – | |||
| C. tronadorensis sp. nov. | CRUB 1258 | Glacial meltwater | Rio Manso (Garganta del Diablo waterfall), Rio negro, Argentina | V. de Garcia | GU560002 | GU997164 |
| CRUB 1299 | GU560003 | GU997165 | ||||
| C. victoriae type group | CBS 8685T | Soil | Antarctica | Montes et al. | AF363647 | AF444469 |
| CBS 8920 | S. Thomas-Hall | AY040650 | AY040656 | |||
| CBS 8915 | AY040652 | AY040655 | ||||
| CBS 9267 | Aggregated grey- brown silty soil with coarse organic remains | Alaska, Nome | H.S. Vishniac | CBS database | CBS database | |
| CBS 9565 | ||||||
| CRUB 1399 | Acidic river | Rio Agrio-Lake Caviahue | G. Russo | EF585176 | – | |
| DBVPG 4835 | Glacial water | Italian Alps | E. Branda | EU287884 | – | |
| DBVPG 4830 | EU287882 | – | ||||
| TP-Snow-Y33 | Glacier surface snow | Tibetan Plateau China | S. Shao | JN400774 | JN400815 | |
| ESAB12 | Food | Portugal: Tras-os-Montes | R. Calhelha | AJ749830 | – | |
| I1-4 | Processed meat products | Denmark | Nielsen et al. | EU194455 | – | |
| CBS 9000 | Flowering plant of Helleborus foetidus | Germany | M. Herzberg | AF406899 | – | |
| CBS 6550 | Forced rhubarb | UK | R. Buhagiar | AF444711 | AF444447 | |
| KCTC 17059 | Rhizosphere soil of Platycodon grandiflorum | Korea | S.G. Hong & K.S. Bae | AF459674 | – | |
| AY-99 | Deschampsia cespitosa green leaves | Russia | A. Yurkov | FN357207 | – | |
| AY-92 | Equisetum sylvaticum green leaves | FN357206 | – | |||
| CRUB 1262 | Glacial meltwater | Rio Manso (Garganta del diablo waterfall), Río Negro, Argentina | V. de Garcia | GU559996 | GU997143 | |
| CRUB 1254 | GU559995 | GU997147 | ||||
| CRUB 1260 | Frias river, Río Negro, Argentina | GU559997 | GU997145 | |||
| CRUB 1264 | GU559994 | GU997148 | ||||
| CRUB 1263 | GU559998 | GU997149 | ||||
| CRUB 1266 | GU559999 | GU997150 | ||||
| EXF-3832 | Subglacial ice | Kongsfjorden, Svalbard, Norway | N. Gunde-Cimerman | GU586196 | GU997144 | |
| EXF-1582 | Darkly pigmented ice | Austre Brøggerbreen glacier, Kongsvegen, Norway | JN193432 | JN193452 | ||
| EXF-1588 | Sea water | Svalbard, Norway | JN193433 | JN193453 | ||
| EXF-1623 | Subglacial ice | Austre Lovénbreen Glacier, Svalbard, Norway | JN193434 | JN193454 | ||
| EXF-3831 | Melt water | Kongsfjorden, Svalbard, Norway | GU586193 | GU997141 | ||
| EXF-3748 | GU586192 | GU997142 | ||||
| EXF-3721 | GU586200 | GU997154 | ||||
| EXF-4012 | Sea water | GU586195 | GU997146 | |||
| EXF-3912 | GU586194 | GU997152 | ||||
| EXF-4085 | GU586198 | GU997159 | ||||
| EXF-3827 | Subglacial ice with gypsum inclusions | Austre Lovénbreen Glacier, Norway | JN193435 | JN193455 | ||
| EXF-6542 | Subglacial ice | Kongsvegen Glacier, Svalbard, Norway | JN193436 | JN193456 | ||
| EXF-6550 | Austre Lovénbreen Glacier, Svalbard, Norway | JN193437 | JN193457 | |||
| EXF-6549 | JN193460 | |||||
| EXF-6535 | JN193438 | JN193458 | ||||
| EXF- 6534 | Columnar ice crystals at the outflow of glacier melt water | Kongsfjorden, Svalbard, Norway | JN193439 | JN193459 | ||
| EXF-4020 | Sea water | GU586201 | GU997156 | |||
| EXF-3923 | GU586204 | GU997155 | ||||
| EXF-4011 | Stream water | GU586197 | GU997153 | |||
| C. victoriae non-type group | CRUB 1757 | Ice | Perito Moreno, Santa Cruz, Argentina | V. de Garcia | GU560000 | GU997151 |
| CBS 8908 | Soil | Antarctica | S. Thomas-Hall | AY040653 | AY040654 | |
| CBS 8884 | Seawater (reef) | Bahamas | A. Statzell-Tallman | AF444741 | AF444645 | |
| VTT C-04542 | Industrial malting ecosystem | Finland | Laitila et al. | DQ377664 | – | |
| LU9-1 | Grape | Denmark | Lederer et al. | HM146911 | – | |
| A114 | Sea water | Portugal | M. Gadanho & J. P. Sampaio | AF485971 | – | |
| Cryptococcus sp. 1 | EXF-1596 | Basal ice | Conwaybreen glacier, Svalbard, Norway | N. Gunde-Cimerman | DQ644575 | – |
| Cryptococcus. sp. 2 | EXF-3926 | Subglacial ice | Kongsvegen, Svalbard,Norway | GU586199 | GU997166 | |
Numbers in bold are isolates from this study.
CRUB, Regional University Center of Bariloche (Centro Regional Universitario Bariloche); EXF, Culture Collection of Extremophilic Fungi, Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia; CBS, Centraalbureau voor Schimmelcultures; DBVPG, Industrial Yeasts Collection of Dipartimento di Biologia Vegetale, Università di Perugia, Italy; KCTC, Korean Collection for Type Cultures; VTT, Technical Research Centre of Finland.
Yeast characterisation and identification
Yeast characterisation and identification to the genus level was performed based on morphological characteristics, coupled with standard physiological tests (assimilation of carbon and nitrogen compound were performed in solid media, glucose fermentation was carry out in stationary liquid media), as described by Kurtzman et al. (2011). Mating experiments were performed on glucose yeast-extract agar (GY agar: 0.2% glucose, 0.1% yeast extract, 2% agar; Kurtzman et al., 2011), with cultures incubated for 2 months at 18 °C and checked microscopically once per week.
Cell size and morphology were determined under differential interference contrast microscopy using an Olympus BX51 microscope with an attached DP12 camera and CellB Imaging Software. The cultures used here were grown on yeast-extract, malt-extract, peptone-glucose agar (YM agar: 0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1% glucose, 2% agar; Kurtzman et al., 2011), incubated at 18 °C. One percent poly-l-lysine was used to attach the cells to the slides (Mazia et al., 1975). The presence of capsules was observed after negative staining of cultures grown on YM agar incubated at 5 and 18 °C, using Indian ink. At least 50 cells were measured, and the mean values were calculated. The cell sizes of the type strains and of our isolates were compared using Students' t-tests.
The protocols for DNA extraction and the PCR conditions followed were as described by Libkind et al. (2003). For DNA sequence analysis, internal transcribed spacer (ITS) ribosomal (r)DNA was amplified using the ITS1 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and ITS 4 (5′-TCC TCC GCT TAT TGA TAT GC-3′) primers (White et al., 1990). The D1/D2 domains of the large subunit of rDNA (LSU rDNA) were amplified using the NL1 (5′-GCA TAT CAA TAA GCG GAG GAA AAG-3′) and NL4 (5′-GGT CCG TGT TTC AAGACG G-3′) primers (Boekhout et al., 1995).
Sequencing was performed by Macrogen Sequencing Service (Korea). BigDye terminator cycle sequencing kits were used in sequence reactions (Applied Biosystems, Foster City, CA). The sequences were obtained using an ABI Prism 3700 PCR machine (Applied Biosystems). The sequences downloaded from GenBank are indicated in the gene trees by their GenBank accession numbers; newly generated sequences are indicated by their strain numbers (see also Table 1).
PCR fingerprinting and mini/microsatellite-primed PCR (MSP-PCR) with the M13 primer were performed on all of the Cryptococcus victoriae isolates included in this study, according to Libkind et al. (2003).
Phylogenetic analysis
The ITS and LSU (D1/D2) rDNA sequences were automatically aligned using ClustalX, and the alignments were adjusted manually using Molecular Evolutionary Genetics Analysis software, version 5 (mega5; Tamura et al., 2011). To estimate the phylogenetic relationships on the basis of the LSU rDNA (D1/D2 domains) and ITS sequences, neighbour-joining analysis (K2P) was performed using mega5 (Tamura et al., 2011). To test the reproducibility of the results, the Bayesian Markov chain Monte-Carlo method of phylogenetic inference was applied, as implemented in the mrbayes programme (Ronquist & Huelsenbeck, 2003). Parsimony networks were constructed from the aligned sequences with the tcs 1.21 programme (Clement et al., 2000), with gapped positions excluded from the analysis.
Extracellular enzymatic activity
The strains were tested for their ability to degrade starch, protein (casein), pectin, carboxymethyl cellulose and fatty acids, according to procedures described by Brizzio et al. (2007). Calibrated suspensions of 1.0 × 106 cells mL−1 grown for 24–48 h were inoculated onto the surface of agar plates using a multipoint inoculation device (Brizzio et al., 2007). The plates contained the substrates for above-mentioned activities, and they were incubated at 5 and 20 °C. The enzymatic activities were analysed after 5 days in the samples incubated at 20 °C, and after 21 days in those incubated at 5 °C. The enzymatic activities for the specific substrates were evaluated as described by Brizzio et al. (2007).
Results
According to the LSU rDNA analysis, psychrotolerant yeast (all the strains isolated grew at 5 °C and up to 25 °C) from the two sampling locations were grouped within three of six clades of Tremellales: Bulleromyces, Victoriae and Kwoniella (Figs 1 and 2). The results presented are also supported by Bayesian analysis (data not shown).
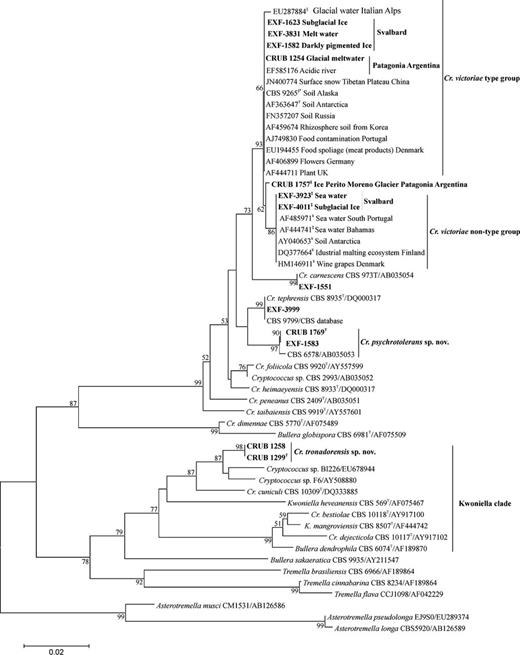
Phylogenetic placement of the species in Victoriae and Knowiella clades (Tremellales) obtained by neighbour joining (distance K2P method) of the LSU rDNA gene D1/D2 domains. Bar, substitutions accumulated every 100 nucleotides. Nucleotide differences (nd) of Cryptococcus victoriae strains with type strain are represented as §: 1 nd; ¥: 2 nd and ‡: 3 nd. *The sequence was obtained in CBS databank, no GeneBank number was available. Strain numbers in bold represent isolates from the present study. Bootstrap values higher than 50% are shown (1000 replicates). TType strain. Asterotremella music, Asterotremella pseudolonga and Asterotremella longa were designated as the outgroup species for this analysis.
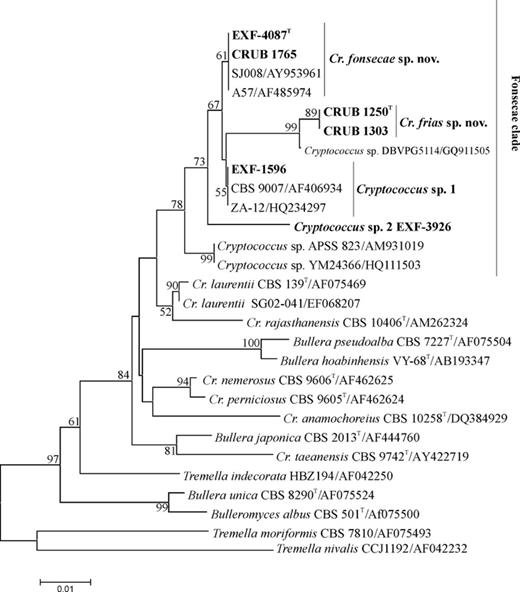
Phylogenetic placement of species in Bulleromyces clade (Tremellales) obtained by neighbour joining (distance K2P method) of the LSU rDNA gene D1/D2 domains. Bar, substitutions accumulated every 100 nucleotides. Strain numbers in bold represent isolates from the present study. Bootstrap values higher than 50% are shown (1000 replicates). TType strain. Tremella moriformis and Tremella nivalis were designated as the outgroup species for this analysis.
Cryptococcus victoriae and related species
The most frequent species isolated from these cold glacial environments of both Patagonia and Svalbard was C. victoriae, of which 27 strains were isolated and identified. The cell size of the C. victoriae type strain CBS 8586 described by Montes et al. (1999) was 3 × 2 μm, whereas Thomas-Hall et al. (2002) reported slightly larger cells of variable sizes, from 3 to 5 × 2 to 3 μm. Our measurements were in agreement with Thomas-Hall et al. (2002), with a median of 5 × 3 μm. The cell sizes of the related strains in the present study were variable, from 5 to 6 × 3 to 4 μm, and no statistical differences were found between the values obtained for the strains studied, or for the type strain. Small capsules were observed in all the strains studied, although there were no differences in their sizes when the cells were grown at low temperature (5 °C) (Fig. 3a–a(i) and b–b(i)). No sexual reproduction was observed in the mating experiments.

Micromorphology of vegetative cells after 3 days of growth on yeast extract-malt extract agar incubated at 18 °C. Column 1 and 3: cells and capsules negative stained with Indian ink; column 2 and 4: budding cells (Nomarski optics). Bar, 10 μm, valid for all pictures. (a, a(i)) Cryptococcus victoriae, CBS 8685T; (b, b(i)) C. victoriae nontype group, EXF-4020; (c, c(i)) Cryptococcus psychrotolerans sp. nov., CRUB 1769T; (d, d(i)) Cryptococcus tronadorensis sp. nov., CRUB 1299T; (e, e(i)) Cryptococcus fonsecae sp. nov., EXF-4087T; (f, f(i)) Cryptococcus frias sp nov., CRUB 1250T.
The physiological tests showed variations in the assimilation of sorbitol, glucosamine, soluble starch, citrate, nitrate and creatinine, in comparison with the type strain CBS 8586 (see Table 2), as also observed for the MSP-PCR fingerprinting analysis (data not shown) and the sequence analysis.
Assimilation profiles of the Cryptococcus victoriae strains
1 – Group of strains with no LSU (D1/D2 domain) rDNA nucleotide differences in comparison to the type strain.
2 – Group of strains with one nucleotide difference in comparison to the type strain.
Growth tests: C-sources: Sor, l-sorbose; Glm, glucosamine; Ino, myo-inositol; Suc, succinate; Sta, soluble starch; Cit, citrate; N-sources: Nit, nitrate; Cre, creatinine; SF: starch formation. −, negative; +, positive; s, slow; v, variable; w, weak.
Results obtained from Montes et al. (1999).
Assimilation profiles of the Cryptococcus victoriae strains
1 – Group of strains with no LSU (D1/D2 domain) rDNA nucleotide differences in comparison to the type strain.
2 – Group of strains with one nucleotide difference in comparison to the type strain.
Growth tests: C-sources: Sor, l-sorbose; Glm, glucosamine; Ino, myo-inositol; Suc, succinate; Sta, soluble starch; Cit, citrate; N-sources: Nit, nitrate; Cre, creatinine; SF: starch formation. −, negative; +, positive; s, slow; v, variable; w, weak.
Results obtained from Montes et al. (1999).
Analyses of the LSU and ITS rDNA sequences revealed polymorphism in strains clustering together with C. victoriae type strain CBS 8586, which suggested a species complex (Fig. 4). The above-mentioned parsimony network analysis based on concatenated sequences showed that 16 out of 27 strains studied were identical to C. victoriae and were considered as an ancestral group, seven strains showed one nucleotide substitution in comparison to the type strain, and the remaining eight strains showed from 3 to 5 nucleotide substitutions. Based on these data, two groups were identified C. victoriae type group (identical to type strain) and C. victoriae nontype group, which showed more than one nucleotide difference in comparison to the type strain (Fig. 4).
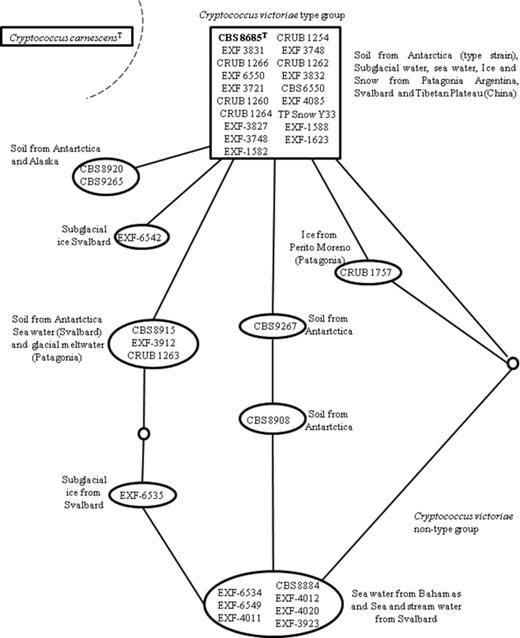
Parsimony network analysis of the combined ITS and LSU rDNA gene D1/D2 domains of strains of Cryptococcus victoriae and its relatives. Each connecting line represents one substitution and each small circle represents a missing intermediate sequence. A rectangle identifies the sequence identified as ancestral by the analysis. The shaded area shows a subset of strains that differ from the type by three or fewer substitutions. The dashed line shows that Cryptococcus carnescensT was excluded from the network.
The strains described by Thomas-Hall et al. (2002) from Lichen Valley (Antarctica) (CBS 8915, CBS 8920, CBS 8908) and 18 strains isolated from different regions around the world (Fig. 1) were conspecific with the C. victoriae type group reported here. Six strains showed one nucleotide difference in comparison to the type strain whereas five strains showed two or three differences; thus we include them in the C. victoriae nontype group, with this also supported by parsimony network analysis (Fig. 4).
The data for the six extracellular enzymatic activities tested showed that C. victoriae and related strains can produce cellulases and esterases at both of the temperatures tested (5 and 20 °C; Table 4).
Among the strains studied from the subglacial ice of the Austre Lovénbreen glacier (Svalbard), four strains of Cryptococcus carnescens were isolated that are identical to the type strain CBS 973. One Cryptococcus strain was isolated from glacial meltwater from Patagonia (CRUB 1267) that clustered together with the type strain of Cryptococcus foliicola and is related to the at present nonaccommodated Cryptococcus CBS 2339, which was isolated from human, and to Cryptococcus heimaeyensis (Sugita et al., 2000; Takashima et al., 2003).
Four strains that are closely related to Cryptococcus tephrensis were isolated from the stream water from Kongsfjorden (Svalbard). In the ITS region, two of these strains (EXF-3999, EXF-3875) showed six nucleotide differences to the type strain; these are related to CBS 9799, which was isolated from the dwarf shrub Dryas octopetala in the High Arctic, Svalbard, Norway, and to CBS 9023, which was isolated from the flowering plant Pulmonaria stiriaca in Germany (Fig. 1); these are currently identified as C. victoriae.
An undescribed species related to C. tephrensis was recognised based on three isolates in the present study. One strain originated from the water of the Austral Argentinean Sea (CRUB 1769), and two from the ice of the Svalbard glacier in Norway (EXF-1528, EXF-1583). The strain CBS 6578 was isolated from sea water in the Pacific Ocean (Fell & Jones, 1976), it was originally identified as C. laurentii, and is currently identified as C. tephrensis (Takashima et al., 2003); according to results of this study it is related to the newly proposed species Cryptococcus psychrotolerans.
Within this group, there were 7 (1.5%) nucleotide differences in the LSU region and 28 (6.9%) nucleotide differences in the ITS region, in comparison with the sequences of C. tephrensis CBS 8935T; this justifies the decision to describe a new species, named here as C. psychrotolerans. In addition, this species differs from the closely related C. tephrensis by the absence of cellulolytic activity (Table 4).
Kwoniella clade
In the second group of Cryptococcus (Tremellales), strains related to Cryptococcus heveanensis (Kwoniella heveanensis) were also isolated, and they are recognised and proposed here as a new species, named Cryptococcus tronadorensis. These isolates originated from the glacial meltwater from Patagonia Argentina (Mount Tronador). Compared with the closest sequences, their LSU sequences showed six nucleotide differences to Cryptococcus sp. F6 (Wang & Yang, genebank), and 14 to Cryptococcus sp. BI226 (Landell et al., GeneBank). The ITS sequences were not available in genebank for comparison.
Sexual genus Kwoniella, and species Kwoniella mangroviensis closely related to C. heveanensis was discovered by Statzell-Tallman et al. (2008); recently Metin et al. (2010) described sexual state of this species (K. heveanensis). Cryptococcus tronadorensis, belongs to Kwoniella clade (Fig. 1), however sexual state was not observed, to perform a deeper analysis in this regard, higher number of isolates are needed to define if this species belongs to this clade or a new one.
Bulleromyces clade
A new clade was discovered that is composed of four so-far-undescribed taxa related to C. laurentii (Fig. 2). Two of these are described in the present study as Cryptococcus frias and Cryptococcus fonsecae. One of the remaining potentially new species is represented by a single isolate (EXF-3926) from subglacial ice from Kongsfjorden glacier (Norway), and the second is represented by four isolates: Two endophytic yeasts from Populus euphractica (China), one isolate from the flowers of Helleborus foetidus (Germany) and one from the Svalbard glacial environment (EXF-1596) (Fig. 2).
The newly proposed species C. frias is described based on two isolates from the glacial environments of Patagonia, Argentina. The most closely related strain was isolated from the glacial environments of the Italian Alps, with five nucleotide differences in the LSU. This species shows 7 and 12 differences in the LSU to C. fonsecae and Cryptococcus sp. 1, respectively (Fig. 2).
Cryptococcus fonsecae sp. nov. is described based on eight isolates: four from the Austral Sea (Patagonia), one from subglacial ice with gypsum inclusions (Svalbard) and a single strain from geographically diverse sea-water habitats: hypersaline saltern water on the Mediterranean coast in Slovenia (the present study), sea water in south Portugal (Gadanho et al., 2003) and the San Juan Islands, Vancouver Island (Fraser et al., 2006).
Table 3 summarises the differential phenotypic characteristics selected (assimilation tests) for the proposed new species and the related species described.
Selected phenotypic characteristics of the newly described Cryptococcus and related known species
| Species | Mel | Sor | Eth | Ery | Glu | Glm | Sta | Man | Cre | Growth at 5 °C |
| C. cuniculi | − | s | + | − | nd | − | + | + | − | nd |
| C. fonsecaesp. nov. | + | − | − | − | − | w | − | − | + | + |
| C. friassp. nov. | + | − | − | − | + | + | − | + | + | + |
| C. heveanensis | − | + | + | + | + | − | − | + | − | nd |
| C. laurentii | + | −/s | +/s | + | + | + | + | + | + | nd |
| C. nemorosus | + | + | + | + | + | + | + | + | − | nd |
| C. perniciosus | + | + | − | + | − | + | + | + | − | nd |
| C. psychrotoleranssp. nov. | + | − | − | + | + | w | − | + | + | + |
| C. tephrensis | + | + | − | + | + | + | +/s | + | − | nd |
| C. tronadorensissp. nov. | w | + | + | − | + | w | − | + | − | + |
| Cryptococcussp. 1 EXF-1596 | w | w | − | − | − | w | w | + | − | + |
| Cryptococcussp. 2 EXF-3926 | + | w | − | − | − | − | − | − | − | + |
| Species | Mel | Sor | Eth | Ery | Glu | Glm | Sta | Man | Cre | Growth at 5 °C |
| C. cuniculi | − | s | + | − | nd | − | + | + | − | nd |
| C. fonsecaesp. nov. | + | − | − | − | − | w | − | − | + | + |
| C. friassp. nov. | + | − | − | − | + | + | − | + | + | + |
| C. heveanensis | − | + | + | + | + | − | − | + | − | nd |
| C. laurentii | + | −/s | +/s | + | + | + | + | + | + | nd |
| C. nemorosus | + | + | + | + | + | + | + | + | − | nd |
| C. perniciosus | + | + | − | + | − | + | + | + | − | nd |
| C. psychrotoleranssp. nov. | + | − | − | + | + | w | − | + | + | + |
| C. tephrensis | + | + | − | + | + | + | +/s | + | − | nd |
| C. tronadorensissp. nov. | w | + | + | − | + | w | − | + | − | + |
| Cryptococcussp. 1 EXF-1596 | w | w | − | − | − | w | w | + | − | + |
| Cryptococcussp. 2 EXF-3926 | + | w | − | − | − | − | − | − | − | + |
Species in bold are isolates from this study.
Growth tests: C-sources: Mel, melibiose; Sor, l-sorbose; Eth, Ethanol; Ery, erythritol; Glu, glucitol; Glm, glucosamine; Sta, soluble starch; Man, mannitol; N-source: Cre, creatinine.
−, negative; +, positive; s, slow; w, weak; nd, not determined.
Selected phenotypic characteristics of the newly described Cryptococcus and related known species
| Species | Mel | Sor | Eth | Ery | Glu | Glm | Sta | Man | Cre | Growth at 5 °C |
| C. cuniculi | − | s | + | − | nd | − | + | + | − | nd |
| C. fonsecaesp. nov. | + | − | − | − | − | w | − | − | + | + |
| C. friassp. nov. | + | − | − | − | + | + | − | + | + | + |
| C. heveanensis | − | + | + | + | + | − | − | + | − | nd |
| C. laurentii | + | −/s | +/s | + | + | + | + | + | + | nd |
| C. nemorosus | + | + | + | + | + | + | + | + | − | nd |
| C. perniciosus | + | + | − | + | − | + | + | + | − | nd |
| C. psychrotoleranssp. nov. | + | − | − | + | + | w | − | + | + | + |
| C. tephrensis | + | + | − | + | + | + | +/s | + | − | nd |
| C. tronadorensissp. nov. | w | + | + | − | + | w | − | + | − | + |
| Cryptococcussp. 1 EXF-1596 | w | w | − | − | − | w | w | + | − | + |
| Cryptococcussp. 2 EXF-3926 | + | w | − | − | − | − | − | − | − | + |
| Species | Mel | Sor | Eth | Ery | Glu | Glm | Sta | Man | Cre | Growth at 5 °C |
| C. cuniculi | − | s | + | − | nd | − | + | + | − | nd |
| C. fonsecaesp. nov. | + | − | − | − | − | w | − | − | + | + |
| C. friassp. nov. | + | − | − | − | + | + | − | + | + | + |
| C. heveanensis | − | + | + | + | + | − | − | + | − | nd |
| C. laurentii | + | −/s | +/s | + | + | + | + | + | + | nd |
| C. nemorosus | + | + | + | + | + | + | + | + | − | nd |
| C. perniciosus | + | + | − | + | − | + | + | + | − | nd |
| C. psychrotoleranssp. nov. | + | − | − | + | + | w | − | + | + | + |
| C. tephrensis | + | + | − | + | + | + | +/s | + | − | nd |
| C. tronadorensissp. nov. | w | + | + | − | + | w | − | + | − | + |
| Cryptococcussp. 1 EXF-1596 | w | w | − | − | − | w | w | + | − | + |
| Cryptococcussp. 2 EXF-3926 | + | w | − | − | − | − | − | − | − | + |
Species in bold are isolates from this study.
Growth tests: C-sources: Mel, melibiose; Sor, l-sorbose; Eth, Ethanol; Ery, erythritol; Glu, glucitol; Glm, glucosamine; Sta, soluble starch; Man, mannitol; N-source: Cre, creatinine.
−, negative; +, positive; s, slow; w, weak; nd, not determined.
All the Cryptococcus strains isolated from cold environments showed the production of extracellular enzymes at low (5 °C) and moderate (20 °C) temperatures. Extracellular esterase and cellulase activities were more frequent (Table 4). Similar assimilation patterns were seen within each species, and some differences were seen among the different species. The most active species were C. tronadorensis sp. nov., C. fonsecae sp. nov. and Cryptococcus sp. 2, where all the enzyme activities tested were seen at both of the temperatures tested (Table 4).
Enzymatic profiles of the Cryptococcus species two different temperatures
| Species | Esterase | Pectinase pH5 | Pectinase pH7 | Cellulase | ||||
| 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | |
| C. victoriae type group | + | +/− | − | − | − | − | +/− | +/− |
| C. victoriae nontype group | + | +/− | − | − | − | − | + | + |
| C. aff. tephrensis | + | + | − | − | − | − | + | + |
| C. carnescens | + | + | − | − | − | − | + | + |
| C. foliiacea | + | + | − | − | − | − | − | + |
| C. psychrotolerans sp. nov | + | + | − | − | − | − | − | − |
| C. tronadorensis sp. nov. | + | +/− | + | + | + | +/− | + | + |
| C. fonsecae sp. nov | + | + | + | + | +/− | − | + | + |
| Cryptococcus sp. 1 | + | + | − | − | − | − | + | + |
| Cryptococcus sp. 2 | + | + | + | + | + | + | + | + |
| C. frias sp. nov. | +/− | +/− | − | − | − | − | + | + |
| Species | Esterase | Pectinase pH5 | Pectinase pH7 | Cellulase | ||||
| 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | |
| C. victoriae type group | + | +/− | − | − | − | − | +/− | +/− |
| C. victoriae nontype group | + | +/− | − | − | − | − | + | + |
| C. aff. tephrensis | + | + | − | − | − | − | + | + |
| C. carnescens | + | + | − | − | − | − | + | + |
| C. foliiacea | + | + | − | − | − | − | − | + |
| C. psychrotolerans sp. nov | + | + | − | − | − | − | − | − |
| C. tronadorensis sp. nov. | + | +/− | + | + | + | +/− | + | + |
| C. fonsecae sp. nov | + | + | + | + | +/− | − | + | + |
| Cryptococcus sp. 1 | + | + | − | − | − | − | + | + |
| Cryptococcus sp. 2 | + | + | + | + | + | + | + | + |
| C. frias sp. nov. | +/− | +/− | − | − | − | − | + | + |
Amylase and protease activities were negative for all the strains tested, at both temperatures. Values for 5 °C were obtained after 20 days of incubation, and values at 20 °C after 5 days of incubation.
−, negative; +, positive; w, weak.
Enzymatic profiles of the Cryptococcus species two different temperatures
| Species | Esterase | Pectinase pH5 | Pectinase pH7 | Cellulase | ||||
| 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | |
| C. victoriae type group | + | +/− | − | − | − | − | +/− | +/− |
| C. victoriae nontype group | + | +/− | − | − | − | − | + | + |
| C. aff. tephrensis | + | + | − | − | − | − | + | + |
| C. carnescens | + | + | − | − | − | − | + | + |
| C. foliiacea | + | + | − | − | − | − | − | + |
| C. psychrotolerans sp. nov | + | + | − | − | − | − | − | − |
| C. tronadorensis sp. nov. | + | +/− | + | + | + | +/− | + | + |
| C. fonsecae sp. nov | + | + | + | + | +/− | − | + | + |
| Cryptococcus sp. 1 | + | + | − | − | − | − | + | + |
| Cryptococcus sp. 2 | + | + | + | + | + | + | + | + |
| C. frias sp. nov. | +/− | +/− | − | − | − | − | + | + |
| Species | Esterase | Pectinase pH5 | Pectinase pH7 | Cellulase | ||||
| 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | 5 °C | 20 °C | |
| C. victoriae type group | + | +/− | − | − | − | − | +/− | +/− |
| C. victoriae nontype group | + | +/− | − | − | − | − | + | + |
| C. aff. tephrensis | + | + | − | − | − | − | + | + |
| C. carnescens | + | + | − | − | − | − | + | + |
| C. foliiacea | + | + | − | − | − | − | − | + |
| C. psychrotolerans sp. nov | + | + | − | − | − | − | − | − |
| C. tronadorensis sp. nov. | + | +/− | + | + | + | +/− | + | + |
| C. fonsecae sp. nov | + | + | + | + | +/− | − | + | + |
| Cryptococcus sp. 1 | + | + | − | − | − | − | + | + |
| Cryptococcus sp. 2 | + | + | + | + | + | + | + | + |
| C. frias sp. nov. | +/− | +/− | − | − | − | − | + | + |
Amylase and protease activities were negative for all the strains tested, at both temperatures. Values for 5 °C were obtained after 20 days of incubation, and values at 20 °C after 5 days of incubation.
−, negative; +, positive; w, weak.
Discussion
Cryptocccus victoriae was first described by Montes et al. (1999) in association with soil from Southern Victoria Land in Antarctica. Soon after the publication of the original description, Thomas-Hall et al. (2002) described new isolates that differed morphologically and phylogenetically from the Antarctic type strain. The truly cosmopolitan distribution of this species in cold areas of the world became more apparent with successive isolations from extremely cold water-related environments, such as glacial ice from the Arctic (Butinar et al., 2007) and from the Italian Alps (Turchetti et al., 2008; Branda et al., 2010). Its poly-extremotolerant character became further evident with its isolation from the acidic volcanic waters of the Río Agrio in Patagonia (Russo et al., 2008). Although mainly found in cold terrestrial habitats, isolates of C. victoriae have also been obtained from a range of different habitats in temperate regions: soil and rhizosphere soil in Korea (Hong et al., 2002), sea water in Portugal (Gadanho et al., 2003), roots, rhizosphere and seeds of different plants in Germany and Austria (Renker et al., 2004; Wuczkowski & Prillinger, 2004), the gut of the insect Chrysoperla rufilabris (Neuroptera: Chrysopidae) in the USA (Woolfolk & Inglis, 2004), an industrial malting area and indoor air in Finland (Laitila et al., 2006; Pitkäranta et al., 2008) and a dry meat processing factory in Norway (Asefa et al., 2009).
The presence of C. victoriae in aquatic environments outside the polar areas has been less frequently documented. In summary, C. victoriae is a species that inhabits very diverse environments and climatic zones, and that has the ability to adapt to a variety of environmental conditions. Thus, C. victoriae can be considered as a generalist species, which are typically characterised by the ability to tolerate a variety of stressful environments, but not the most extreme conditions.
These generalist species can adapt because of their so-called ‘robust genotypes’, which allow their persistence across varied environments without obligate adaptation to local conditions. This is achieved by structuring their populations into groups, and thus initiating their specialisation into specialist species (Gostincar et al., 2010).
Parsimony network analysis provides a statistical, theory-laden approach to the delineation of phylogenetic yeast species from sequence data. This method aims to distinguish within sequence space, variations that can be regarded as polymorphisms (within species) from differences that are the consequences of speciation (Lachance et al., 2010). The parsimony network analyses of the ITS, and the LSU rDNA sequences of the C. victoriae isolates occupying different ecological niches reinforces the concept that they are members of a single evolutionary lineage. The variability observed in the C. victoriae strains can be considered either an intrinsic characteristic of the species, or a possible initiation of speciation. The data presented in the present study support this statement, through showing the high plasticity of C. victoriae in terms of its physiology, morphology and molecular characteristics.
According to Fonseca et al. (2011), minor differences in the nucleotide sequences of LSU rDNA in comparison to the C. victoriae type strain do not suffice for the description of new species; therefore, other loci and phenotypic characteristics also have to be studied. Based on the dataset used in multilocus analysis in C. neoformans (Tremellales) (Findley et al., 2009), analyses of the largest subunit of RNA polymerase II (RPB1), the second largest subunit of RNA polymerase II (RPB2) (degenerate primers were used), and elongation factor 1 alpha (EF1) were also planned for these C. victoriae isolates. However, no PCR products with published primers were obtained for the C. victoriae strains. In our experience, deeper analysis and the construction of new primer sets are necessary for multilocus analysis of this species.
Cryptococcus tephrensis was initially isolated from soil in Iceland and described by Vishniac (2002), and later it was also isolated from soil in the Moscow region (Yurkov, 2006), and recently from glacial Alpine environments (Branda et al., 2010). Cryptococcus tephrensis is closely related to C. victoriae, C. foliicola and Cryptococcus peneanus (Montes et al., 1999; Takashima et al., 2003). In the present study, four strains that deviate from C. tephrensis were isolated from the Arctic glacial environments. To date, all the reports of this species have originated exclusively from cold environments, indicating its psychrotolerant nature. Our data indicate that C. tephrensis and the related strains obtained in the present study (EXF-3749, EXF-3875, EXF-3999, EXF-6553) showed variability. To determine a potential complex of yet-unidentified species, additional studies of different molecular markers will be necessary, as well as additional information on their life cycle (e.g. sexual state); both these will be facilitated by the isolation of related species.
A new species C. psychrotolerans (Victoriae clade) that is related to C. tephrensis is described based on four isolates, two of which originated from glacier ice (EXF-1528, EXF-1583), and two from sea water (CRUB 1769, CBS 6578). Strain CBS 6578 was previously identified as C. laurentii, and it is included in the description of this new species. All the relevant information was obtained from the online CBS page (http://www.cbs.knaw.nl/collections/BioloMICS.aspx) and from Takashima et al. (2003).
The description of the newly proposed species, C. tronadorensis (Kwoniella clade) from the glacial environments is based on the D1/D2 LSU rDNA sequences, which are closely related to other two strains: one isolated from Taiwan (Cryptococcus sp. F6; Wang and Yang, genebank, unpublished) and one of unknown provenance (Cryptococcus sp. BI226; Landell M, Ramos J, Leoncini O and Valente P GeneBank, unpublished data). Unfortunately, there is no information available on the origins of these strains; additional information and other isolates of this species are needed for a better understanding of its ecology and life cycle.
Based on the good bootstrap support of a new clade related to C. laurentii (Bulleromyces clade), the description of two new species isolated from the glacial and saline environments is performed. The new clade is composed of yet-undescribed taxa, the majority of which originate from cold and marine environments in the northern and southern hemispheres (C. frias sp. nov., Cryptococcus sp. DBVPG 5114, C. fonsecae sp. nov., Cryptococcus sp. 2 EXF-1569, Cryptococcus sp. 3 EXF-3926). Isolates phylogenetically related to Cryptococcus sp. 2, included in C. laurentii or C. aff. laurentii, were isolated from nectar of H. foetidus in Germany (CBS 9007; Herzberg et al., 2002) and from P. euphractica along the Tarim River in China (ZA-12 and ZA-3, Abdurehim Z., GeneBank).
The state of the genus Cryptococcus represents probably one of the most complex taxonomic problems in yeast systematics (Wuczkowski et al., 2011). New species descriptions within this taxonomic group are evidently needed, as exemplified by the new species related to C. laurentii described in the present study. As Shivaji & Prasad (2009) showed, Cryptococcus species have a ubiquitous presence in polar areas. Although Cryptococcus species have been reported in most yeast studies from Antarctica, the delineation of these species based on phenotypic characteristics might have been incorrect. Sequence analyses have resulted in the description of several additional new Cryptococcus species that were previously considered to be C. laurentii. Several reports have also indicated misidentification of Cryptococcus flavescens and C. victoriae (Fonseca et al., 2011). As Fonseca et al. (2011) pointed out recently, in the last edition of ‘The Yeasts’, some species are not as common as previously considered and new ones can be identified, as included in the present study.
Cryptococcus strains isolated show heterotrophic metabolism and the ability to degrade organic macromolecules through the secretion of extracellular hydrolytic enzymes. This indicates high plasticity and their potential auxiliary role as biogeochemical nutrient recyclers in these environments. They have relatively broad amplitude of ecological tolerance, as they can survive acidic conditions, low temperatures and low nutrient concentrations. This ‘phenotypic plasticity’ is also characterised by the presence of a capsule, which confers stress tolerance in glacial biomes, as well as in the human body (Gostincar et al., 2010).
The present study shows that not only extremely cold terrestrial environments but also extremely cold aquatic environments provide sources for new fungal taxa with distinct metabolic and potentially interesting biotechnological properties. Glacial biomes represent unique habitats and are generally unexplored reservoirs of unknown microbial species. The microbial biodiversity of these biomes is much higher than previously expected, at the level of both bacteria and eukaryotic species. As global warming is resulting in the melting of glaciers throughout the regions of the world, these cold ecosystems are in danger of disappearing (Thomas-Hall et al., 2010). The isolation of these yeasts will allow the collection, discovery and description of new species before they are being released into the soil, rivers and oceans of the world.
Description of four new Cryptococcus species
These new species are anamorphic yeasts that are related to the subphylum Agaromycotina, class Tremellomycetes, order Tremellales, family Tremalaceae.
Cryptococcus psychrotolerans sp. nov. de Garcia, Zalar, Brizzio, Gunde-Cimerman & van Broock.
Etymology: C. psychrotolerans (psy.chro.tol′er.ans. Gr. adj. psychros cold; L. pres. part. tolerans tolerating; N.L. part. adj. psychrotolerans cold-tolerating).
MycoBank: MB 800033
After 7 days on malt extract/yeast extract agar at 18 °C, the colonies are cream coloured, smooth and opaque, with an entire margin. The cells are ovoidal to ellipsoidal, 5.9–7.0 × 4.2–5.4 μm, and they multiply by multilateral budding. In Dalmau plates, after 2 weeks on cornmeal agar, pseudohyphae and true hyphae are not formed. No positive mating reactions are observed among the three strains isolated.
Glucose is not fermented. Glucose, d-galactose, d-glucosamine (weak), d-xylose, l-arabinose, d-arabinose, l-rhamnose, maltose, trehalose, cellobiose, arbutin, salicin, melibiose, lactose, raffinose, melezitose, inulin, meso-erytritol, xylitol, d-glucitol, d-mannitol, myo-inositol, glucono-δ-lactose, d-gluconate, d-glucuronate, d-galacturonate, succinate and lactate (weak) are assimilated. No growth occurs on l-sorbose, d-ribose, soluble starch, glycerol, ribitol, galactitol, citrate, methanol, ethanol, hexadecane and isopropanol. Assimilation of nitrogen compounds: positive for nitrite, l-lysine and creatinine. No growth is observed on nitrate. Growth in vitamin-free medium is positive. Growth in amino-acid-free medium is positive. Growth observed at 5–25 °C; no growth at 30 °C. No growth on YM agar with 10% sodium chloride. No growth observed in 50% glucose/yeast extract (0.5%). No growth in 100 μg mL−1 cycloheximide. Urease activity is positive. Diazonium Blue B reaction is positive.
Holotype: The type strain of C. psychrotolerans sp. nov. is CRUB 1769, which was recovered from sea water at the Cape Horn Meridian in the Argentinian Sea. The strain has been deposited in the Culture Collection of Extremophilic Fungi (EX), Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia, as EXF-7039T, and in the collection of the Yeast Division of the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, as CBS XX.
Cryptococcus tronadorensis sp. nov. de Garcia, Zalar, Brizzio, Gunde-Cimerman & van Broock.
Etymology: C. tronadorensis (N.L. masc. adj., tronadorensis, referring to Mount Tronador, the name of the mountain with the glacial meltwaters from which the strains of this species originated).
MycoBank: MB 800034
After 7 days on malt extract/yeast extract agar at 18 °C the colonies are cream coloured, smooth and opaque, with an entire margin. The cells are subglobose to elipsoid, 6.2–4.7 × 3.4–3.1 μm, with a capsule, and they multiply by multilateral budding. In Dalmau plates after 2 weeks on cornmeal agar, pseudohyphae and true hyphae are not formed. No positive mating reactions are observed among the two strains of C. tronadorensis.
Glucose is not fermented. Glucose, l-sorbose, sucrose, d-galactose, d-glucosamine (weak), d-ribose, d-xylose, l-arabinose, d-arabinose, l-rhamnose, glucono-δ-lactose, salicin, maltose, trehalose, cellobiose, arbutin, melibiose (weak), lactose, raffinose (weak), melezitose, ribitol, glycerol (slow), xylitol, d-glucitol, d-mannitol, galactitol (weak), myo-inositol, d-glucuronate, succinate, ethanol are assimilated. No growth occurs on meso-erythritol, soluble starch, citrate, methanol, hexadecane and isopropanol. Assimilation of nitrogen compounds: positive for l-lysine, d-glucosamine and cadaverine. No growth is observed on creatine, creatinine, nitrite and nitrate. Growth in vitamin-free medium is positive. Growth in amino-acid-free medium is positive. Growth observed at 5 °C and is weak at 25 °C; no growth at 30 °C. Growth on YM agar with 10% sodium chloride is absent. Growth in 50% glucose/yeast extract (0.5%) is negative. Starch-like compounds are produced. In 100 μg mL−1 cycloheximide growth is absent. Urease activity is positive. Diazonium Blue B reaction is positive.
Holotype: the type strain of C. tronadorensis sp. nov. is CRUB 1299T, which was isolated from the Rio Manso (Garganta del Diablo waterfall), which originates from the Manso glacier of Mount Tronador, Nahuel Huapi National Park, San Carlos de Bariloche, Río Negro, Argentina. The strain has been deposited in the Culture Collection of Extremophilic Fungi (EX), Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia, as strain EXF-6801T, and in the collection of the Yeast Division of the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, as CBS XX.
Cryptococcus fonsecae sp. nov. de Garcia, Zalar, Brizzio, Gunde-Cimerman & van Broock.
Etymology: C. fonsecae (fon.se′cae. L. gen. sing. m. adj. fonsecae of Fonseca, in honour of the Portuguese yeast researcher Alvaro Fonseca for his contributions to yeast systematics and ecology).
MycoBank: MB 800035
After 7 days on malt extract/ yeast extract agar at 18 °C, the colonies are light pink, smooth and opaque, with an entire margin. The cells are mainly globose, 5.7–4.9 × 5.6–4.5 μm, with capsule, and they multiply by multilateral budding. In Dalmau plates after 2 weeks on cornmeal agar, pseudohyphae and true hyphae are not formed. No positive mating reactions are observed among the strains of C. fonsecae.
Glucose is not fermented. Glucose, sucrose, d-galactose, d-glucosamine (weak), d-xylose, l-rhamnose (weak), d-mannitol (slow), myo-inositol, salicin (weak), arbutin (weak), lactose, maltose, melibiose (weak), l-arabinose, trehalose, cellobiose, raffinose, melezitose, glycerol (slow), d-glucuronate (weak), xylitol are assimilated. No growth occurs on l-sorbose, d-ribose, d-arabinose, meso-erythritol, ribitol, d-glucitol, galactitol, glucono-δ-lactose, galacturonate, succinate, citrate, ethanol, soluble starch, methanol, hexadecane and isopropanol. Assimilation of nitrogen compounds: positive for nitrite, l-lysine, d-glucosamine, creatine (weak) and cadaverine. No growth is observed on nitrate and creatinine. Growth in vitamin-free medium is positive. Growth in amino-acid-free medium is positive. Growth observed at 5 °C, and is weak at 25 °C; no growth at 30 °C. Growth on YM agar with 10% sodium chloride is absent. Growth in 50% glucose/yeast extract (0.5%) is negative. Starch-like compounds are produced. In 100 μg mL−1 cycloheximide, growth is absent. Urease activity is positive. Diazonium Blue B reaction is positive.
Holotype: the type strain of C. fonsecae sp. nov. is EXF-4087T, which was isolated from subglacial ice from Svalbard, from the Austre Lovénbreen glacier in Kongsfjorden, on the western coast of Spitsbergen.
Cryptococcus frias sp. nov. de Garcia, Zalar, Brizzio, Gunde-Cimerman & van Broock.
Etymology: C. frias (N.L. masc. adj., frias, referring to Frias glacier, name of the glacier where the meltwater originated, as the source of this species).
MycoBank: MB 800036
After 7 days on malt extract/yeast extract agar at 18 °C, the colonies are yellow, smooth and opaque, with an entire margin. The cells are ovoidal to ellipsoidal, 6.9–5.6 × 3.8–3.4 μm, with a capsule, and they multiply by multilateral budding. In Dalmau plates after 2 weeks on cornmeal agar, pseudohyphae and true hyphae are not formed. No positive mating reactions are observed among the two strains of C. tronadorensis.
Glucose is not fermented. Glucose, sucrose, d-galactose, d-glucosamine, d-ribose, d-xylose, l-arabinose, d-arabinose, l-rhamnose, glucono-δ-lactose (weak), salicin (weak), maltose, trehalose, cellobiose, arbutin, melibiose, lactose, raffinose, melezitose, ribitol, glycerol (slow), d-glucuronate (weak), xylitol, d-glucitol, d-mannitol, galactitol (weak), myo-inositol, succinate are assimilated. No growth occurs on l-sorbose, meso-erythritol, soluble starch, citrate, ethanol, methanol, hexadecane and isopropanol. Assimilation of nitrogen compounds: positive for l-lysine, d-glucosamine, creatinine, creatine (weak) and cadaverine. No growth is observed on nitrite and nitrate. Growth in vitamin-free medium is positive. Growth in amino-acid-free medium is positive. Growth observed at 5 °C and is weak at 25 °C; no growth at 30 °C. Growth on YM agar with 10% sodium chloride is absent. Growth in 50% glucose/yeast extract (0.5%) is negative. Starch-like compounds are produced. In 100 μg mL−1 cycloheximide growth is absent. Urease activity is positive. Diazonium Blue B reaction is positive.
Holotype: The type strain of C. frias sp. nov. is CRUB 1250T, which was isolated from the Frias River meltwaters that originate from the Frias glacier of Mount Tronador, Nahuel Huapi National Park, San Carlos de Bariloche, Río Negro, Argentina. The strain has been deposited in the Culture Collection of Extremophilic Fungi (EX), Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia as EXF-5992T, and in the collection of the Yeast Division of the Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands, as CBS XX.
Acknowledgements
This study was accomplished with financial aid from the Ministerio de Ciencia y Tecnología (International Bilateral Cooperation MINCYT–MHEST SLO08/11), Universidad Nacional del Comahue (Project B143), Consejo Nacional de Investigaciones Científicas y Tecnológicas, CONICET (PhD Fellowship to Virginia de Garcia, Buque Puerto Deseado campaign 2010, Project PIP424), ANPCyT (PICT06-1176). We thank the authorities of Parques Nacionales (Argentina) for permission for water sample collection within the National Parks of Argentina, and Dr. Sonia Fontenla for providing the Perito Moreno samples. A part of this study was supported by the European ARCFAC-026129-2008-10 project.
References
Supporting Information
Additional Supporting Information may be found in the online version of the article:
Author notes
Editor: Dirk Wagner



