-
PDF
- Split View
-
Views
-
Cite
Cite
Henry Müller, Christian Westendorf, Erich Leitner, Leonid Chernin, Kathrin Riedel, Silvia Schmidt, Leo Eberl, Gabriele Berg, Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48, FEMS Microbiology Ecology, Volume 67, Issue 3, May 2009, Pages 468–478, https://doi.org/10.1111/j.1574-6941.2008.00635.x
Close - Share Icon Share
Abstract
The rhizosphere-associated bacterium Serratia plymuthica HRO-C48 is not only able to suppress symptoms caused by soil-borne pathogens but is also able to stimulate growth of plants. Detailed knowledge about the underlying mechanisms and regulation are crucial for the application in biocontrol strategies. To analyse the influence of N-acyl homoserine lactone (AHL)-mediated communication on the biocontrol activity, the AHL-degrading lactonase AiiA was heterologously expressed in the strain, resulting in abolished AHL production. The comparative analysis of the wild type and AHL negative mutants led to the identification of new AHL-regulated phenotypes. In the pathosystem Verticillium dahliae–oilseed rape, the essential role of AHL-mediated signaling for disease suppression was demonstrated. In vitro, the regulatory function of AHLs in the synthesis of the plant growth hormone indole-3-acetic acid is shown for the first time. Additionally, swimming motility was found to be negatively AHL regulated. In contrast, production of extracellular hydrolytic enzymes is shown to be positively AHL-regulated. HRO-C48 emits a broad spectrum of volatile organic compounds that are involved in antifungal activity and, interestingly, whose relative abundances are influenced by quorum sensing (QS). This study shows that QS is crucial for biocontrol activity of S. plymuthica and discusses the impact for the application of the strain as a biocontrol agent.
Introduction
Soil-borne fungal pathogens like Rhizoctonia solani Kühn and Verticillium dahliae Kleb. cause extremely high yield losses worldwide (Sneh et al., 1998; Tjamos et al., 2000). Because of the ecological behaviour of the pathogens, for example their broad host range, saprophytic life style and long survival as microsclerotia in soil, they area difficult to suppress. Methyl bromide, which was used to destroy microsclerotia in soil, is toxic for many soil organisms. It is also classified as a class I ozone-depleting substance and was therefore taken off the market as agreed in the Montreal Protocol (1987). The use of naturally occurring microorganisms with antagonistic activity against soil-borne pathogens to control them is a promising and ecologically friendly alternative (Whipps, 2001; Weller et al., 2002; Haas & Défago, 2005).
Serratia plymuthica is a ubiquitous inhabitant of the rhizosphere of different plant species and includes strains antagonistic to soil-borne pathogens (Berg, 2000; Berg et al., 2002). The strain S. plymuthica HRO-C48 was selected because of its antagonistic activity and plant growth-promoting effects (Kalbe et al., 1996; Berg et al., 1999). Serratia plymuthica HRO-C48 is rhizosphere competent and capable of suppressing V. dahliae in strawberry, oilseed rape and olive, and R. solani on lettuce (Kurze et al., 2001; Grosch et al., 2005; Müller & Berg, 2008). The strain is commercially used as a biocontrol agent (RhizoStar®, E-nema GmbH Raisdorf, Germany). In spite of the fact that the strain is able to colonize the rhizosphere for extended periods of time and, in principle, exhibits excellent biocontrol and plant growth promotion activities, inconsistencies concerning these beneficial effects were observed in field trails. To improve the performance of this biocontrol agent, a fundamental understanding of the regulatory mechanisms involved in the expression of biocontrol-related traits is required.
Mechanisms responsible for antagonistic activity include a wide range of properties, for example antibiosis, competition for colonization sites, nutrients and minerals, parasitism and lysis (Whipps, 2001). The interaction with the host plant plays an important role and includes mechanisms such as the production of phytohormones, rhizosphere competence, enhancement of the availability of nutrients and induction of systemic resistance (Lugtenberg et al., 2002). Recently it was shown that volatile organic compounds (VOCs) produced by rhizobacteria are involved in the interaction with pathogenic fungi as well as with host plants (Kai et al., 2007; Vespermann et al., 2007). However, previous work has shown that especially the production of antagonistic compounds by rhizobacteria is often regulated by N-acyl homoserine lactone (AHL)-dependent quorum-sensing (QS) systems (Steidle et al., 2002; Coulthurst et al., 2004; Eberl, 2006; Liu et al., 2007a). Interestingly, for Serratia marcescens MG1 (Eberl et al., 1999; Riedel et al., 2001) evidence was presented that the AHL molecules induce components of the innate immune system in tomato (Schuhegger et al., 2006). Although several QS-regulated traits have been identified in Serratia (for a review see van Houdt et al., 2007), there is no study analysing its role in biocontrol of plant pathogens, as has been demonstrated for Pseudomonas (Wood et al., 1997; Wei & Zhang, 2006). Furthermore, little is known about the involvement of AHLs in VOC synthesis in rhizobacteria.
For strain S. plymuthica HRO-C48 we observed a cell density-dependent effect on plant growth promotion (Kurze et al., 2001) and reduced biocontrol effects at low cell densities under field conditions, suggesting that QS may play a role in the regulation of biocontrol activity in situ. In a recent report it was shown that in S. plymuthica HRO-C48 the production of the antifungal agent pyrrolnitrin in vitro is regulated by the SplI/SplR QS system that utilizes N-3-oxo-hexanoyl homoserine lactone as the major signal molecule (Liu et al., 2007b). The objective of the present study was to analyse the influence of AHL production on biocontrol activities and relevant phenotypes of the strain. However, the splI mutant AHL-4 still produced residual amounts of AHLs (Liu et al., 2007b). Therefore, we constructed an AHL-negative derivative of S. plymuthica HRO-C48 by introducing a plasmid encoding the AiiA lactonase (Dong et al., 2001), which hydrolyses AHL molecules. This approach is commonly referred to as quorum quenching. The quenched strain as well as the splI mutant AHL-4 were phenotypically characterized and compared with the wild-type strain.
Materials and methods
Bacterial strains and culture conditions
Serratia plymuthica HRO-C48 (DZMZ 12502), the spontaneous rifampicin-resistant mutant HRO-C48 Rifr, the miniTn5Km2lacZ mutant HRO-C48 AHL-4 (Liu et al., 2007b) and the AHL-lactonase-expressing derivate S. plymuthica HRO-C48 pME6863 were routinely grown at 30 °C in standard II nutrient broth (SIFIN, Berlin, Germany), on standard II nutrient agar (SIFIN) and AB minimal media (Clark & Maaloe, 1967) containing 0.5% (w/v) glucose (ABG medium) and 0.5% (w/v) citrate (ABC medium), respectively. Where required, growth media were supplemented with antibiotics purchased from Sigma (St. Louis, MO) at the following concentrations: rifampicin at 100 μg mL−1, kanamycin at 50 μg mL−1 and tetracycline at 40 μg mL−1.
Heterologous expression of the AiiA lactones
Plasmids pME6863 (aiiA) and pME6000 (vector control) (Molina et al., 2003) conferring tetracycline resistance were transformed to the helper strain Escherichia coli S17-1 by electroporation. The transformant served as donor strain for conjugation to transfer the plasmids to S. plymuthica HRO-C48. Tetracycline-resistant clones were verified for the presence of the aiiA-bearing plasmid pME6863 by PCR analysis.
Extraction and detection of AHLs
Analyses of AHL profiles were performed using thin-layer chromatography (TLC) according to Geisenberger (2000). AHLs were extracted twice from the cell-free supernatant with dichloromethane (250 : 100, supernatant: dichloromethane). Before evaporation to dryness and resolving in ethyl acetate, residual water in the extract was removed by anhydrous sodium sulphate. The extracts were separated on a C 18RP TLC plate (Merck, Darmstadt, Germany) using methanol (60%, v/v) as mobile phase. The presence of AHLs was detected by overlaying the TLC plate with an exponentially grown culture of AHL biosensor strains. The bioluminescent monitor strain E. coli MT102 (pSB403) exhibits the highest sensitivity for 3-oxo-C6-HSL and related molecules. Light emission was visualised using a luminometer. Short-chain AHLs were detected with the aid of the indicator strain Chromobacterium violaceum CV026 (McClean et al., 1997), which produces the pigment violacein in presence of C4- and C6-HSLs.
Biocontrol of Verticillium wilt under greenhouse conditions
Two independent greenhouse experiments were performed to investigate the involvement of AHL signalling molecules in the biocontrol activity of S. plymuthica HRO-C48 with respect to disease suppression and rhizosphere colonization. Seeds from oilseed rape (Brassica napus var. oleifera cv. TALENT; NPZ Hohenlieth, Germany) were inoculated with either S. plymuthica HRO-C48 WT, HRO-C48 pME6863 or HRO-C48 AHL-4 by means of biopriming (Müller & Berg, 2008). Briefly, bacteria were grown for 24 h, sedimented by centrifugation (5000 g, 10 min) and resuspended to adjust the cell number to log10 10.0 CFU mL−1 using sterile 0.9% NaCl solution. For treatment, seeds were immersed in the cell suspension for 5 h at 20 °C under agitation. Infiltrated seed were dried for 24 h at 20 °C. For determination of cell counts, 20 seeds were transferred into 1.0-mL sterile 0.9% sodium chloride solution. Primed seeds were grounded for 1 min using an autoclaved mortar and pestle. Suspensions were serially diluted and plated onto NA medium. Plates were incubated for 24 h at 30 °C and CFU were counted to calculate the means of CFU per seed. Overall, 16 seeds for each strain and 16 noninoculated seeds (control) were sown in pots (one per pot) with a volume of 250.0 mL containing nonsterile propagation compost (Einheitserdewerk, Uetersen, Germany) mixed with vermiculite (4 : 1, v/v) (Vermiculit Dämmstoffe, Spröckhövel, Germany). To evaluate the effect on the development of Verticillium wilt, propagation compost/vermiculite mixture was supplemented with fungal inoculum (19 : 1, v/v), which consisted of microsclerotia of V. dahliae var. longisporum strain ELV25 (Messner et al., 1996). Microsclerotia were produced in Czapek–Dox–vermiculite medium (1 : 20, v/v) inoculated with a 2-week-old culture of V. dahliae (Czapek–Dox broth, 20 °C at 120 r.p.m.) and incubated at room temperature for 4 weeks. The final pathogen density was 50–60 microsclerotia g−1 soil. Plants were grown over a period of 9 weeks under greenhouse conditions at 25±10 °C and assimilation light (14 h light/10 h dark period). They were watered every second day. After appearance of the first symptoms the disease reaction of plants was assessed by severity of symptoms on a 1–9 scale (1=no symptoms, 2=few dark-coloured wires, 3=oldest leaf with strong symptoms, 4=loss of the oldest leaf, 5=about 50% of leaves with strong symptoms, 6=loss of about 50% of the leaves, 7=loss of over 50% of the leaves, 8=only the vegetation conus left, 9=dead plant) at weekly intervals. Data on disease severity was used to calculate area under disease process curve (AUDPC) determined as AUDPC=Σ((Si+Sti+1)/2) × (ti+1−ti) where Si is the symptoms severity and ti is the date of assessment of symptom severity (Zeise, 1992).
For enumeration of root colonizing S. plymuthica cells, the experiment was performed as described above except that plants were grown in noninfested soil. In contrast to the biocontrol experiments, for root colonization studies strain HRO-C48 WT was replaced by the spontaneous rifampicin-resistant mutant HRO-C48 Rifr, allowing selective reisolation from the rhizosphere. Roots with adhering soil from six plants of each variant and sampling time (10, 20 and 30 days after sawing) were sampled into sterile bags. Material of two plants were combined (5.0 g) to extract rhizosphere-associated Serratia cells by adding 50 mL of distilled water and homogenizing in a Stomacher laboratory blender for 180 s (BagMixer, Interscience, St. Nom, France). Samples were serially diluted with sterile 0.9% NaCl and plated onto NA medium amended with the appropriate antibiotic. Plates were incubated for 24 h at 30 °C and CFU were counted to calculate the means of colonies (log10 CFU) based on root fresh weight (RFW).
Chitinases and protease activity assays
Chitin-degrading abilities of S. plymuthica HRO-C48 strains were tested using quantitative chromogenic enzyme assay (Frankowski et al., 2001). For quantitative detection of extracellular chitinases, strains to be tested were cultivated for 2 days at 30 °C and 150 r.p.m. in ABG medium supplemented with colloidal chitin solution (10.0 g L−1). Cell-free supernatant was prepared by centrifugation (15 000 g, 30 min), sterile filtration (Miniart, Sartorius, pore size 0.25 μm), and addition of protease inhibitor. To 150.0 μL of culture filtrate, 450.0 μL substrate solution [0.2% carboxymethyl-chitin-remazol brilliant violet (CM-chitin-RBV)], 100.0 mM succinate buffer (1 : 2, v/v) (pH 6.0) were added, filled up with ABG medium to a final volume of 600.0 μL and incubated at 30 °C overnight. After addition of 100.0 μL 2.0 M HCl, the preparation was incubated on ice for 1 h. Precipitate was removed by centrifugation for 10 min at 15 000 g before absorption of the supernatant was measured photospectrometrically at 550 nm.
To investigate proteolytic activity of strains, a quantitative chromogenic enzyme assay was employed. Bacteria were grown for 1 day at 30 °C and 150 r.p.m. in Luria–Bertani (LB) broth. Cell-free supernatants 150.0 μL obtained by centrifugation (15 000 g, 30 min) and subsequent sterile filtration (pore size 0.25 μm), were mixed with 250.0 μL substrate solution [2.0% (w/v) azoalbumin (pH 7.2)], and filled up to 400.0 μL with corresponding media. After incubation overnight at 30 °C the preparation was admixed with 1.2 mL 10% (v/v) trichloroacetic acid, followed by 15 min incubation at room temperature and centrifugation for 10 min at 15 000 g. NaOH 1.0 M, 750.0 μL was added to the preparation before absorption was measured at 440 nm. All measurements were done in three replicates for each strain; 150.0 μL of medium served as control.
Detection of pyrrolnitrin
Excretion of the antifungal compound pyrrolnitrin by bacteria was studied by TLC as described earlier (Salcher & Lingens, 1980). Bacterial cells grown on NA supplemented with 2.0% (v/v) glycerine were scrapped from the medium after cultivation for 3 days at 30 °C and suspended in 6.0 mL distilled water. Cells were pelleted by centrifugation (10 000 g, 5 min, 4 °C) and resuspended in 6.0 mL acetone/water mixture (4 : 1, v/v). Acetone was removed using a rotating evaporator. One volume of chloroform was added, the suspension was shaken and the chloroform removed prior dissolving extract in 400.0 μL acetone and fractionating on K60 F254 plates (Merck). Synthetic pyrrolnitrin (Sigma) served as analytical standard. After separation in chloroform/acetone (9 : 1, v/v) a TLC plate was coated with a thin layer of 40 °C Waksman agar (WA) containing 5.0 g tryptone, 5.0 g NaCl, 3.0 g meat extract, 10.0 g glucose, 20.0 g agar–agar, and distilled water to a final volume of 1.0 L and inoculated with some agar plugs of a R. solani culture (WA, 20 °C, 7 days). Inhibition zones caused by bacterial-borne antibiotics were analysed after an incubation period of 7 days at 20 °C in the absence of light.
Detection of indole-3-acetic acid (IAA)
IAA excretion by S. plymuthica HRO-C48 strains was determined using colorimetric analysis developed by Sawar & Kremer (1995) and modified by Berg (2002). Growth medium consisting of glucose (5.0 g), yeast extract (25.0 mg), l-tryptophan (0.2 g) and distilled water (to 1.0 L) was inoculated with cell material from the preculture (0.5 × TSA, 30 °C, 24 h) to an OD600 nm of 0.5. After cultivation at 20 °C and 150 r.p.m. for 72 h in the absence of light, cell-free supernatant was mixed with Salkowski reagent [50.0 mM FeCl3, 35.0% (v/v) perchloric acid] at the ratio of 3 : 2 and incubated for 30 min in the absence of light. IAA concentration was measured photospectrometrically using the microplate reader Spectramax-250 (Molecular devices, Union City, CA) at 530 nm and quantified using a standard curve.
Detection of siderophores
Detection of siderophores was performed under iron-limited conditions using universal siderophore assay developed by Schwyn & Neilands (1987). Iron (III) ions form a complex with chrome-azurol-S dye. For testing, cell material was transferred onto chrome azurol-S medium and incubated for 3–5 days at 30 °C. The diameter of the discoloured zone, which is indicative of siderophore activity, was measured.
Biofilm formation on polystyrene surface
Biofilm formation capacity of S. plymuthica HRO-C48 strain WT and pME6863 was analysed in polystyrene microtiter plates under static conditions using different media: ABC, ABG and LB medium (Pratt & Kolter, 1998). Before the experiments, the strains were precultured for 24 h (LB, 30 °C, 150 r.p.m.). Then 200.0 μL of the preculture (adjusted to OD600 nm 0.1) was added to 20.0 mL test medium and regrown for 1 h (30 °C, 150 r.p.m.). Cavities of a 96-well microtiter plate were filled with 100.0 μL of sterile test medium and inoculated with cells from 1-h-culture using a toothpick. Altogether, five multiwell plates, one per sampling (3, 6, 24, 48 and 72 h), were prepared, each contained four replicates per isolate and medium. The plates were sealed and incubated at 30 °C. To detect biofilm formation, medium was removed from the cavities, thoroughly rinsed with distilled H2O, dried and covered with 100.0 μL crystal violet solution. After 30 min of incubation at room temperature, crystal violet solution was discarded. After an additional washing step, cavities were dried and adsorbed colour dye was dissolved in 120.0 μL DSMO. Solution was mixed with 800.0 μL absolute ethanol to a final volume of 920 μL and subjected to spectrophotometrical measurement at 570 nm.
Analysis of VOCs
The inhibitory effect of bacterial volatile compounds on the fungi R. solani and V. dahliae was investigated using dual culture assay. For this purpose half-divided Petri dishes containing fungal (WA) and bacterial (NA) growth medium, respectively, were utilized. As fungal inoculum served 5-mm-diameter agar plugs from either R. solani or V. dahliae colonies. Serratia plymuthica HRO-C48 isolates were applied simultaneously by seeding 200.0 μL preculture (NB, 30 °C, 24 h, 150 r.p.m.) on the nutrient agar-containing side of the Petri dishes. Plates were double sealed with parafilm and incubated at 20 °C in the absence of light. The experiment was repeated three times with five replicates for each fungus and bacterial strain. Fungi grown in the presence of noninoculated NA served as control. After 5 days for R. solani and after 22 days for V. dahliae the suppression effect was determined by measuring the average diameter and subsequent calculation of the area of the fungal colony. For the analytical examination of VOC patterns, Serratia isolates were cultivated at 30 °C in 20-mL glass vials (Chromtech, Idstein, Germany) containing 6 mL inclined nutrient agar. After 24 h, vials were closed hermetically by a magnetic crimp cap with a PTFE-lined silicone rubber septum (LaPhaPack, Langerwehe, Germany) and analysed by headspace-solid phase microextraction-gas chromatography mass spectrometry (HS-SPME-GC-MS). The experiments were repeated twice with seven technical replicates.
HS-SPME-GC-MS
The volatile compounds in the headspace of HRO-C48 WT and HRO-C48 pME6863 cultures were sampled in alternating order by enriching on a 2-cm stable flex 50/30 μm divinylbenzene/carboxen/polydimethyl-siloxane SPME fibre (Supelco, Bellafonte, PA) for 60 min at 30 °C on an automated SPME sampler (CTC, Chromtech). Desorption of the volatiles was performed directly into the hot injection system of a GCD Series instrument (Hewlett-Packard) with an electroionization mass selective detector. The split/splitless injector, which was operated in the splitless mode during desorption for 2 min, was operated at 270 °C and an SPME liner with 0.75-mm inner diameter was installed. The analytical column was a 30 m HP-5 with 0.25-mm inner diameter and 1-μm film thickness (Agilent) with helium as carrier gas. The temperature programme started at 30 °C for 1 min followed by a ramp of 5 °C min−1 to 290 °C, held for 1 min. The flow was 0.8 mL min−1 (32 cm s−1 linear velocity) in a constant flow mode. The column was coupled to the mass selective detector via a direct interface, which was operated at 280 °C. The mass range for data acquisition was 20–300 amu with a scan rate of 2.67 scans s−1. Electron multiplier voltage was set by the automated tune parameters. For statistical analysis the GC-MS data were directly imported into software package msstat (ANALYT-MTC, Muehlheim/Ruhr, Germany) for principal component analysis (PCA).
Results
Quorum quenching in S. plymuthica HRO-C48
As reported by Liu (2007b), S. plymuthica HRO-C48 produces a set of AHLs, including N-butanoyl-HSL (BHL), N-hexanoyl-HSL (HHL) and, predominantly, 3-oxo-hexonoyl (OHHL). Importantly, spent culture extracts of the transconjungant HRO-C48 pME6863 contained no detectable AHL molecules when the AHL biosensors C. violaceum CV026 and Escherichia coli MT102 (pSB403) were used for AHL detection (Fig. 1). These results indicate that the aiiA lactonase gene on the introduced plasmid pME6863 was expressed, and efficiently inactivated all AHLs produced by the strain. In contrast, the transposon mutant AHL-4 releases residual amounts of AHLs molecules, which showed migration patterns similar to those of the parental strain. Serratia plymuthica HRO-C48 bearing the control vector pME6000 exhibited a AHL profile similar to that of the wild-type strain.
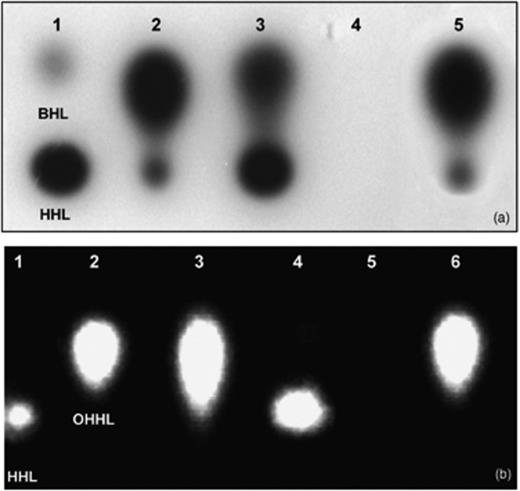
AHL profiles of Serratia plymuthica HRO-C48 WT and its mutants. Cell-free culture supernatants were analysed by TLC overlaid with the reporter strains Chromobacterium violaceum CV026 (a) and Escherichia coli MT102 (pSB403) (b). (a) lane 1: BHL (200.0 ng) and HHL (20.0 ng); lane 2: 1.0 μL crude extract from HRO-C48 WT; lane 3: 10.0 μL crude extract from HRO-C48 AHL-4; lane 4: 10.0 μL crude extract from HRO-C48 pME6863; lane 5: 1.0 μL crude extract from HRO-C48 pME6000. (b) lane 1: HHL (200.0 ng); lane 2: OHHL (20.0 ng); lane 3: 1.0 μL crude extract from HRO-C48 WT; lane 4: 10.0 μL crude extract from HRO-C48 AHL-4; lane 5: 10.0 μL crude extract from HRO-C48 pME6863; lane 6: 1.0 μL crude extract from HRO-C48 pME6000.
Suppression of Verticillium wilt in oilseed rape is AHL dependent
To investigate the role of AHLs on biocontrol activity of S. plymuthica HRO-C48 ad planta, greenhouse experiments were performed using the pathosystem oilseed rape, V. dahliae. Seeds from winter oilseed rape cv. Talent were inoculated with S. plymuthica HRO-C48 by biopriming and sown in V. dahliae V25-infested soil. The AUDPC, which is an indicator of disease severity, was recorded at weekly intervals for 5 weeks starting from the appearance of the first symptoms. Symptoms caused by Verticillium were observed in all plants included in this experiment. Plants grown from seeds treated with S. plymuthica HRO-C48 wild-type strain showed a statistically significantly reduced development of Verticillium wilt when compared with the noninoculated control. The AUDPC was reduced by 14.9±7.1% (experiment I) and 17.5±9.7% (experiment II), respectively (Fig. 2). The level of the disease in plants inoculated with AHL mutants HRO-C48 pME6863 and HRO-C48 AHL-4 was virtually indistinguishable that of the noninoculated control. The control effect against V. dahliae in oilseed rape usually exhibited by the wild-type strain was completely lost (Table 1). These results demonstrate that the biocontrol activity of S. plymuthica HRO-C48 under greenhouse conditions is dependent on a functional QS system. Given that for the majority of biocontrol agents several mechanisms are involved in biocontrol activity, we next analysed the role of AHLs in regulating factors required for the interaction with the host plant as well as with the fungal pathogen.
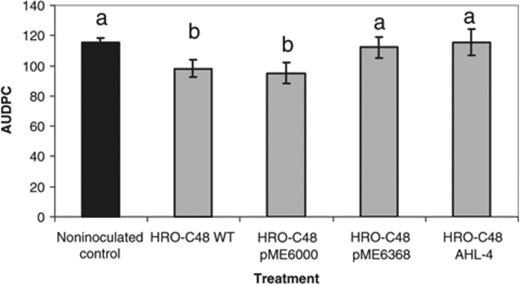
Effect of Serratia plymuthica HRO-C48 WT and its mutants on the development of Verticillium wilt in oilseed rape expressed by AUDPC in comparison with the noninoculated control. Seeds treated with S. plymuthica HRO-C48 cells at concentrations of log10 6.0 using biopriming were grown in Verticillium dahliae-infested soil (50–60 microsclerotia g−1soil) for 9 weeks under greenhouse conditions. Values represent the average of two independent experiments each consisting of 16 plants. The same letters above the bars symbolize no significant differences according to Fisher's protected LSD (P≤0.05). Error bars indicate SDs.
Phenotypic characterization of Serratia plymuthica HRO-C48 pME6863 and HRO-C48 AHL-4 compared with the wild-type strain
Phenotypic characterization of Serratia plymuthica HRO-C48 pME6863 and HRO-C48 AHL-4 compared with the wild-type strain
Analysis of factors influencing the interaction with the plant: root colonization, biofilm formation, IAA production and motility
The wild-type and the AHL-quenched derivatives of S. plymuthica HRO-C48 were analysed with respect to functions assumed to be important for bacteria–plant interaction. Root colonizing experiments were conducted using plants grown from bioprimed seeds inoculated with log10 6.0 CFU per seed. No differences in the colonization behaviour of the three strains were observed over a period of 31 days after sowing. Ten days after sowing, the population sizes of the wild-type S. plymuthica HRO-C48 (5.4±0.9 CFU g−1 RFW), the quenched derivative HRO-C48 pME6863 (5.5±0.7 CFU g−1 RFW), and the splI mutant HRO-C48 AHL-4 (5.5±0.3 CFU g−1 RFW) were virtually indistinguishable. In the following 10 days the cell density decreased to 3.9±0.7 CFU g−1 RFW (HRO-C48 WT), 3.1±0.6 CFU g−1 RFW (HRO-C48 pME6863) and 3.3±0.8 CFU g−1 RFW (HRO-C48 WT) and remained stable until 31 days postinoculation. These results imply that the ability to colonize the plant rhizosphere is not influenced by AHLs. Biofilm formation on an abiotic surface was tested in a microtitre-plate assay. Depending on the growth media, S. plymuthica exerted different degrees of biofilm formation. In the presence of glucose (ABG medium), HRO-C48 formed denser biofilms than in LB and ABC medium. However, under given conditions, no difference between the wild-type strain and the quenched derivative was observed, indicating that QS is not essential for surface colonization. We also tested the wild type and AHL-negative derivatives for swimming motility by inoculating the strains on LB plates containing 0.3% agar. Both strains exhibited swimming motility. However, the swimming zones of the quenched strain (68.0±4.55 mm) and the splI mutant (75.3±4.7 mm) were increased by 29.5% and 43.3%, respectively, compared with the diameter of the swimming zone of the parental strain (52.5±9.0 mm). This result indicates that swimming motility is repressed by the QS system. Serratia plymuthica HRO-C48 was previously shown to synthesize the phytohormone IAA (Kalbe et al., 1996). The IAA concentrations in the supernatants of the wild-type strain and the AHL-deficient strain were determined as described in Materials and methods. Inactivation of AHLs via introduction of the AiiA lactonase resulted in a significant increase in IAA concentration in the culture supernatant (15.9±6.36 vs. 48.2±18.56 μg mL−1) (Table 1), suggesting that the QS systems operating in S. plymuthica HRO-C48 suppress IAA production.
Analysis of antifungal traits: proteolytic and chitinolytic activity, and production of pyrrolnitrin and siderophores
Serratia plymuthica HRO-C48 synthesizes a set of factors, including chitinases, proteases, siderophores and the antibiotic agent pyrrolnitrin, which are responsible for its antagonistic activity. Measurements of proteolytic and chitinolytic activities in the supernatants of the wild type and the AHL-negative derivative revealed that the production of these hydrolytic enzymes was severely impaired in the quenched strain (Table 1). No effect on the production of siderophores, as assessed on CAS indicator plates, was observed. The production of pyrrolnitrin was determined by a TLC-based method. To this end, cultures of the wild type and the quenched strain were extracted with acetone and the constituents were separated by TLC. In the case of the wild-type strain an inhibition zone was observed at a position similar to the one of synthetic PRN standard (Rf=0.89). A much smaller inhibition zone was observed with the quenched derivative, indicating that production of pyrrolnitrin in S. plymuthica HRO-C48 is AHL-dependent.
AHL-dependent production of VOCs
Using divided plates the effect of headspace volatiles emitted by S. plymuthica HRO-C48 on the growth of V. dahliae and R. solani was analysed. Both the wild type and the transconjugant HRO-C48 pME6863 were able to suppress the growth of the fungi, albeit to different degrees. When exposed to VOCs of S. plymuthica HRO-C48 for 5 days, growth of R. solani was reduced by 34.9±9.5% and an even stronger growth inhibition effect was observed with VOCs produced by the quenched strain (50.1±4.8%) and miniTn5 mutant (48.9±3.8%). The volatiles also influenced the capacity of the fungus to form microsclerotia. After 30 days the number and the size of the resting structures were diminished. Again, the effects of the AHL-negative derivative were more pronounced than the one observed with the wild type (data not shown). Likewise, growth of V. dahliae was inhibited more strongly by VOCs of the derivates with impaired AHL synthesis (pME6863: 86.4±1.3%; AHL-4: 89.0±4.4%) than by those produced by the wild type (80.4±5.1%).
GS-MS analyses of HRO-C48 WT and HRO-C48 pME6863 revealed that both derivates emit distinct patterns of volatile organic substances. Overall, 20 compounds could be detected in the headspace of S. plymuthica cultures (c.f. Kai et al., 2007). In spite of the fact that identical compounds were detected for both derivates of HRO-C48, the VOC profiles of the investigated strains could be clearly differentiated by PCA of the obtained chromatograms (Fig. 3a). In Fig. 3b characteristic chromatograms of each of the two strains are depicted. The chromatograms show five dominant volatiles, which were identified by MS as 2-butanone, 3-methylbutanal, 2-pentanone, 1-methylbutanol and dimethyl disulphide. With the exception of 2-butanone (retention time 7.07 min), these substances were produced by the two strains in considerably different amounts: the concentrations of 3-ethylbutanal (7.07 min), 2-pentanone (8.08 min) and 1-methylbutanol (9.70 min) were increased in the AHL-deficient strain relative to the wild type by 29.4±21.93%, 66.1±6.94% and 20.0±3.76%, respectively. In contrast, the amount of dimethyl disulphide (10.27 min) in the headspace of cultures of the quenched strain was decreased by 56.1±36.05% relative to the amount produced by the wild type. In conclusion, these data suggest that AHLs are involved in the regulation of processes that lead to the emission of organic compounds, which, in turn, have inhibitory effects on the growth of R. solani and V. dahliae.
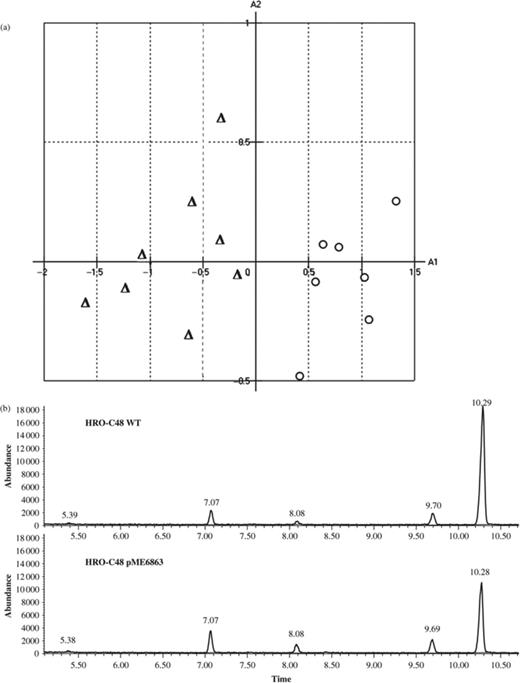
(a) PCA of volatile patterns deduced from GC profiles of headspace volatiles emitted by Serratia plymuthica HRO-C48 WT (Δ) and HRO-C48 pME6863 (+). Bacteria were cultivated for 24 h on nutrient agar prior enrichment and sampling of VOCs. (b) Representative GC profiles of VOCs emitted by S. plymuthica HRO-C48 WT and HRO-C48 pME6863. MS analysis and subsequent database search of dominant peaks resulted in the identification of the following compounds: 2-butanone (retention time: 5.38–5.39), 3-methylbutanal (retention time: 7.07), 2-pentanone (retention time: 8.08), 3-methyl-1-butanol (retention time: 9.69–9.70), dimethyl-disulphide (retention time: 10.28–10.29).
Discussion
In this study we have demonstrated that AHL-mediated QS is crucial for biocontrol activity of S. plymuthica HRO-C48. Moreover, we have identified new factors that may be responsible for biocontrol activity. Production of the plant growth hormone IAA and swimming motility were found to be negatively AHL regulated, whereas production of extracellular proteolytic and chitinolytic enzymes was positively regulated. The broad spectrum of VOCs produced by HRO-C48 are involved in antifungal activity and are, at least in part, regulated by QS. The AHL-regulated synthesis of pyrrolnitrin that was already described by Liu (2007b) was confirmed. In spite of fact that the splI mutant AHL-4 still produced residual amounts of AHLs (Liu et al., 2007b), suggesting the presence of a second AHL synthase in this organism, the mutant showed the same phenotypes as the quorum-quenched derivative.
Inactivation of the AHL signal molecules by heterologous expression of the AiiA lactonase completely abolished the ability of the strain to protect oilseed rape plants against V. dahliae in greenhouse experiments. All greenhouse studies were carried out under high pathogen pressure. In general, the pathogen V. dahliae is difficult to handle because the fungus grows very slowly. For our objective, a rapid greenhouse assay to investigate all parameters was required. For these reasons, in our experiments we applied the pathogen at inoculum densities, which are much higher than those found in natural soils (Tjamos et al., 2000). Consequentially, the results of these greenhouse trials were used to study the effect of QS and do not reflect the efficiency of S. plymuthica under real conditions, which is much higher (Berg et al., 1999; Kurze et al., 2001).
Mechanisms involved in microorganism–plant interaction, such as production of phytohormones, rhizosphere competence, enhancement of nutrient availability and induction of systemic resistance in the host plants, can have an indirect influence on biocontrol activity. IAA is a phytohormone, produced by plant-associated bacteria as well as by the plants themselves, which is responsible for the stimulation of plant growth. In our experiments we found that IAA is upregulated in the AHL-deficient derivative of S. plymuthica HRO-C48. To our knowledge this is the first report showing AHL-dependent regulation of IAA biosynthesis. We hypothesize that at low population density, the bacteria excrete higher amounts of IAA and thus stimulate plant growth, which in turn provides the bacterial population with additional nutrients to reach a critical size at which the QS system is triggered. At high population density, IAA production of the bacteria is reduced because high levels of IAA may cause damage and promote disease. However, at the same time the increased concentrations of AHL signal molecules will stimulate IAA production of the plant (A. Hartmann, pers. commun.). Other factors, which are important for plant–microorganism interaction, are the ability to produce biofilms and to colonize the rhizosphere (Wei & Zhang, 2006). QS-dependent biofilm formation has been shown for various bacteria including plant-associated members of the genera Pseudomonas, Burkholderia and Serratia (Fuqua et al., 1999), whereas the influence on the colonization of the rhizosphere has only been shown for Pseudomonas fluorescens 224 (Wei & Zhang, 2006). In S. plymuthica HRO-C48 neither biofilm formation on an abiotic surface nor colonization behaviour of oilseed rape was found to be influenced by QS. Serratia plymuthica HRO-C48 neither produces biosurfactants nor exhibits swimming motility (data not shown). However, the swarming motility of the strain was negatively controlled by QS. This is reminiscent of work in Yersinia pseudotuberculosis and Yersinia enterocolitica, for which an involvement of AHL-dependent QS in the control of control of swimming has been demonstrated (Atkinson et al., 2006).
Pathogen–microorganism interactions are directly linked to biocontrol activity (Whipps, 2001). In this study we have shown that in S. plymuthica HRO-C48, expression of several antagonistic traits were QS-regulated. Chitinases and proteases are important antifungal factors (Whipps, 2001). Serratia plymuthica HRO-C48 produces three chitinases and an unknown number of proteases (Frankowski et al., 2001). Both chitinolytic and proteolytic activity was found to be AHL-regulated in HRO-C48. This finding is in agreement with previous results obtained for another strain of S. plymuthica (Van Houdt et al., 2007). The finding that heterologous expression of the AiiA lactonase in S. plymuthica HRO-C48 greatly reduced pyrrolnitrin production is in full agreement with a previous study that showed that the SplI/SplR QS system is controlling pyrrolnitrin production in S. plymuthica (Liu et al., 2007b). Additionally, the production of VOCs was analysed. Their involvement in antifungal activity was clearly shown and found to be differentially regulated by AHLs. Although a several compounds could be identified and differences in the amount of production were shown, the importance of single substances is not clear. On the other hand, the results open a window for new questions in research and many biotechnological applications.
Serratia plymuthica HRO-C48 is a commercially available biocontrol agent. What impact does the results of the present study have on practical applications? From the data presented it is obvious that it would be beneficial for biocontrol purposes if the QS system of the strain were triggered when the strain colonizes the plant rhizosphere. Scher (1994) estimated that the crucial population density for successful biocontrol is log10 4.0 CFU g−1 RFW. Populations of HRO-C48 in the rhizosphere of diseased oilseed rape plants (this study) and strawberry plants (Kurze et al., 2001) reach at least this critical threshold concentration, which appears to suffice for activating the QS system of the organism. On the other hand, natural occurring quorum-quenching organisms (bacteria, fungi, plants, animals) may significantly influence the activity of biological control agents (Dong et al., 2001).
In conclusion, QS in S. plymuthica HRO-C48 is involved in the regulation of functions that affect the host plant and other factors that inhibit growth of pathogenic fungi. Anthropogenic intervention with bacterial QS systems with the aim to manipulate bacterial communities and their functions for the benefit of repressing plant pathogens and stimulating plant growth is promising. However, a stable and high population density of the biocontrol strain has to be considered as the key factor for promoting biocontrol activities. This may be realized using highly competitive root colonizers in combination with efficient inoculation techniques.
Acknowledgements
This study was supported by a grant of the Deutsche Forschungsgemeinschaft to G.B., the Deutsche Bundesstiftung Umwelt to H.M. and of the DAAD to C.W.
References
Author notes
Editor: Christoph Tebbe



