-
PDF
- Split View
-
Views
-
Cite
Cite
Lorena Butinar, Polona Zalar, Jens C. Frisvad, Nina Gunde-Cimerman, The genus Eurotium– members of indigenous fungal community in hypersaline waters of salterns, FEMS Microbiology Ecology, Volume 51, Issue 2, January 2005, Pages 155–166, https://doi.org/10.1016/j.femsec.2004.08.002
Close - Share Icon Share
Abstract
Six different species of the known teleomorphic food-borne xerophilic genus Eurotium were repeatedly isolated in a mycodiversity study of hypersaline waters. At salinities above 17% NaCl, E. amstelodami was detected most consistently, followed by E. repens and E. herbariorum, while E. rubrum, E. chevalieri and a potentially new species, “Eurotium halotolerans”, were detected only occasionally at lower salinities. The qualitative secondary metabolite profiles produced by Eurotium spp. from salterns were not different from those of Eurotium spp. from foods and other habitats. Spatiotemporal frequency of occurrence and in vitro determined adaptive ability of propagules to survive prolonged exposure to hypersaline conditions indicate that E. amstelodami, E. herbariorum, and E. repens contribute to the indigenous fungal community in hypersaline water environments, while E. rubrum, E. chevalieri and “E. halotolerans” are only temporal inhabitants of brine at lower salinities.
1 Introduction
Halophilic microorganisms are native inhabitants of highly saline environments and are thus considered extremophiles. So far, it was believed that eukaryotic organisms, with few exceptions, are not able to adapt to these extreme conditions and that such environments are populated almost exclusively by prokaryotes [1–4]. It was assumed that food-borne filamentous fungi, capable of growth at low water activities (aw under 0.85) in the presence of high solute concentrations, reflect a general xerophilic phenotype [5,6] and therefore do not populate natural hypersaline environments[7]. Fungal species that were isolated from natural moderately saline environments [8–10] were mainly categorized as ubiquitous or halotolerant flush [8,9,11]. Recently, however, a considerable diversity of eukaryotic microorganisms was discovered in hypersaline environments [12–14]. Different filamentous fungi have been isolated from hypersaline water of salterns[15] and from the Dead Sea[16], while halophilic melanized yeast-like fungi were described as part of indigenous microbiota of salterns[13].
The genus Eurotium contains several species that all grow exceptionally well at low water activities. They are therefore common in foods and feeds preserved with high concentrations of NaCl or sugar[17]. The genus Eurotium is also an important mycotoxin-producer[6]. Nevertheless, there are no systematic studies on its species biodiversity in natural niches with low water activity. So far it has been reported that different Eurotium spp. have been occasionally isolated from moderately saline soils and waters [18–20] and recently E. amstelodami, E. herbariorum and E. rubrum were isolated from the Dead Sea[16].
The aim of our study was to investigate the spatiotemporal occurrence of Eurotium spp. in hypersaline waters of different oligotrophic and eutrophic salterns around the world. Viability of propagules (mycelia and spores) of the most consistently and frequently isolated Eurotium spp. was determined in vitro after a prolonged exposure to hypersaline conditions. The size variations of basic morphological structures, growth and sporulation of the Eurotium species in a wide salinity range have been studied. The secondary metabolite profiles of strains isolated from the hypersaline environment were compared to the profiles of species isolated from common localities.
2 Materials and methods
2.1 Sampling sites and sample collection
Water samples were taken from the active Slovenian solar salterns Sečovlje at the northern Adriatic coast, at the border between Slovenia and Croatia. Due to the sub-Mediterranean climate, salt is collected only during the salt production season (May–September). The evaporating seawater is lead to ponds, where first calcite precipitates, followed by gypsum and at saturation with NaCl halite[21]. Outside the season of salt production the salinity of the water mainly remains below 5% (w/v) NaCl. Four successive evaporitic ponds (1–4) were selected for fungal isolations, covering salinities from 3% to 32% NaCl. To investigate the spatiotemporal diversity in the fungal community of the Adriatic salterns, water samples were collected from ponds containing water of different salinities every 3 weeks during the crystallization period in 1997 and once per month over a one-year period in 1999.
Hypersaline water samples were also taken from other geographic locations around the world: Dead Sea (Ein Bokek, Ein Gedi) and the Eilat saltern at the Red Sea coast of Israel, Ebro River Delta and Santa Pola salterns in Spain, Camargue salterns in France and salterns on the Atlantic coast of Portugal (Samouco), Namibia and Dominican Republic. In Spain, the samples were collected during one crystallization season in 1997, while in the other sites samples from the crystallizers were collected once or twice.
2.2 Determination of environmental parameters
The following physicochemical parameters were determined in all water samples: pH, temperature, dissolved O2 concentration and O2 saturation level, salinity (areometer) and water activity (aw) (CX-1 system, Campbell Scientific Ltd., Loughborough, UK). In the samples from the Adriatic salterns, total phosphorus, organic nitrogen and ammonia were measured, as well as Cl−, SO2−4, K+, Na+, Mg2+ concentrations, biological- (BOD) and chemical oxygen demand (COD) according to Gunde-Cimerman et al.[13].
2.3 Isolation and preservation of strains
The fungal population dynamics was followed as described elsewhere[13]. Different selective media with increased concentrations of sugar and salt were used for isolation of halo- and xerophilic mycobiota.
Agar baits in dialysis tubings and in glass tubes[22] were incubated in the Adriatic salterns crystallization pond in 1997. After five months, agar blocs were removed, cut and placed on low water activity media[13]. In 1997, biofilms from the crystallization pond were also spread on low water activity selective media[13].
The isolated and identified strains are maintained in the Culture Collection of the National Institute of Chemistry (MZKI), Slovenia and in the fungal collection (IBT) at BioCentrum-DTU, Technical University of Denmark.
2.4 Taxonomic identification and study of morphological structures
Isolates of the genus Eurotium were identified to the species level according to morphological characteristics based on phase-contrast microscopy and scanning electron microscopy (SEM). Strains were three point inoculated on the following identification media: Czapek yeast autolysate agar (CYA) with 20% and 40% (w/v) sucrose (CY20S, CY40S, respectively) [6,23], Czapek agar (CzA), Czapek agar with 20% sucrose (Cz20S), malt-yeast sucrose 40% agar (MY40S)[24], and dichloran glycerol 18% agar (DG-18)[25]. Inoculated media were incubated at 25, 30 and 37 °C in the dark for 2 weeks and examined after 1 and 2 weeks.
Cultural characteristics and micromorphology were observed after 2 weeks. Only Cz20S was used for micromorphology [24,26]. For the strains listed in Table 1, 20 measurements (N= 20) per structure were performed, except for conidia and cleistothecia, where N was 30. Mean value and 95% confidence interval were calculated for each structure. For phase-contrast microscopy, mounts were made in lactic acid. For measurements of cleistothecia no cover glass was used for making slides[27].
List of strains isolated from hypersaline water in the Adriatic salterns, which were used for different analyses: morphological measurements (1), SCAN micrography (2), in vitro viability determination (3), growth and sporulation on solid media (4), and secondary metabolite profile (5)
| Species | Strain designation in culture collections MZKI and IBT | Analyses performed |
| E. amstelodami | MZKI A-570, IBT 22853 | 3, 5 |
| E. amstelodami | MZKI A-561, IBT 25488 | 1, 2, 4, 5 |
| E. chevalieri | MZKI A-562, IBT 24508 | 1, 2, 3, 4, 5 |
| E. herbariorum | MZKI A-338, IBT 25489 | 3, 5 |
| E. herbariorum | MZKI A-427, IBT 21808 | 1, 2, 4, 5 |
| E. repens | MZKI A-408, IBT 21810 | 1, 3, 4, 5 |
| E. repens | MZKI A-567, IBT 25490 | 3, 5 |
| E. repens | IBT 22851 | 1, 5 |
| E. repens | MZKI-566, IBT 25491 | 2, 5 |
| E. rubrum | MZKI A-563, IBT 22849 | 1, 2, 3, 4, 5 |
| E. rubrum | MZKI A-564, IBT 25494 | 3, 5 |
| Eurotium sp. | MZKI A-560, IBT 24810, IBT 24504, IBT 24505; | 2, 3, 4, 5 |
| Species | Strain designation in culture collections MZKI and IBT | Analyses performed |
| E. amstelodami | MZKI A-570, IBT 22853 | 3, 5 |
| E. amstelodami | MZKI A-561, IBT 25488 | 1, 2, 4, 5 |
| E. chevalieri | MZKI A-562, IBT 24508 | 1, 2, 3, 4, 5 |
| E. herbariorum | MZKI A-338, IBT 25489 | 3, 5 |
| E. herbariorum | MZKI A-427, IBT 21808 | 1, 2, 4, 5 |
| E. repens | MZKI A-408, IBT 21810 | 1, 3, 4, 5 |
| E. repens | MZKI A-567, IBT 25490 | 3, 5 |
| E. repens | IBT 22851 | 1, 5 |
| E. repens | MZKI-566, IBT 25491 | 2, 5 |
| E. rubrum | MZKI A-563, IBT 22849 | 1, 2, 3, 4, 5 |
| E. rubrum | MZKI A-564, IBT 25494 | 3, 5 |
| Eurotium sp. | MZKI A-560, IBT 24810, IBT 24504, IBT 24505; | 2, 3, 4, 5 |
List of strains isolated from hypersaline water in the Adriatic salterns, which were used for different analyses: morphological measurements (1), SCAN micrography (2), in vitro viability determination (3), growth and sporulation on solid media (4), and secondary metabolite profile (5)
| Species | Strain designation in culture collections MZKI and IBT | Analyses performed |
| E. amstelodami | MZKI A-570, IBT 22853 | 3, 5 |
| E. amstelodami | MZKI A-561, IBT 25488 | 1, 2, 4, 5 |
| E. chevalieri | MZKI A-562, IBT 24508 | 1, 2, 3, 4, 5 |
| E. herbariorum | MZKI A-338, IBT 25489 | 3, 5 |
| E. herbariorum | MZKI A-427, IBT 21808 | 1, 2, 4, 5 |
| E. repens | MZKI A-408, IBT 21810 | 1, 3, 4, 5 |
| E. repens | MZKI A-567, IBT 25490 | 3, 5 |
| E. repens | IBT 22851 | 1, 5 |
| E. repens | MZKI-566, IBT 25491 | 2, 5 |
| E. rubrum | MZKI A-563, IBT 22849 | 1, 2, 3, 4, 5 |
| E. rubrum | MZKI A-564, IBT 25494 | 3, 5 |
| Eurotium sp. | MZKI A-560, IBT 24810, IBT 24504, IBT 24505; | 2, 3, 4, 5 |
| Species | Strain designation in culture collections MZKI and IBT | Analyses performed |
| E. amstelodami | MZKI A-570, IBT 22853 | 3, 5 |
| E. amstelodami | MZKI A-561, IBT 25488 | 1, 2, 4, 5 |
| E. chevalieri | MZKI A-562, IBT 24508 | 1, 2, 3, 4, 5 |
| E. herbariorum | MZKI A-338, IBT 25489 | 3, 5 |
| E. herbariorum | MZKI A-427, IBT 21808 | 1, 2, 4, 5 |
| E. repens | MZKI A-408, IBT 21810 | 1, 3, 4, 5 |
| E. repens | MZKI A-567, IBT 25490 | 3, 5 |
| E. repens | IBT 22851 | 1, 5 |
| E. repens | MZKI-566, IBT 25491 | 2, 5 |
| E. rubrum | MZKI A-563, IBT 22849 | 1, 2, 3, 4, 5 |
| E. rubrum | MZKI A-564, IBT 25494 | 3, 5 |
| Eurotium sp. | MZKI A-560, IBT 24810, IBT 24504, IBT 24505; | 2, 3, 4, 5 |
For scanning electron microscopy, mature cleistothecia from 14-day-old cultures on Cz20S media[26] were fixed in 0.1 M cacodylate buffer (pH 7.2) with 1% glutaraldehyde and 0.4% formaldehyde and then chemically dehydrated in alcohol series, prior to critical point drying.
2.5 Data analysis
Fungal quantity was expressed as colony forming units (CFU) per liter of sampled water[13]. Index of similarity was determined according to Krebs[28] as follows Sj=a/a + b + c (Jaccard coefficient), where a is the number of the species present in both sampling years, b the number of the species present in 1997 but not in 1999, and c is the number of species present in 1999 but not in 1997.
Spatial frequency of occurrence (FO) was calculated as the percentage of ponds (out of four ponds from the Adriatic salterns) in which a particular species was registered.
2.6 Detection of secondary metabolites profiles
The cultures listed in Table 1 and additional isolates from food, leather, soil and textiles were analysed according to the HPLC-diode array detection method of Frisvad and Thrane [29,30], as modified by Smedsgaard[31]. After seven days of inoculation at 25 °C, the isolates were analyzed on CYA with 20% sucrose using three agar plugs[31].
2.7 In vitro survival of mycelium and spores
To determine viability of E. amstelodami, E. herbariorum, E. repens, E. rubrum, E. chevalieri and Eurotium sp. in hypersaline environments, the mycelium and spores (both ascospores and conidia) of strains listed in Table 1 were in vitro suspended in water with NaCl concentrations from 0% to 30% in steps of 5% for up to three months. The method used, described by Kis-Papo et al.[32], was partly modified as indicated.
For the determination of mycelium viability fungal spores were harvested from 14-day-old cultures on agar slants with malt extract (MEA + 5% NaCl). Spores were suspended in water with 0.1% Tween 80 and filtered through sterile glass wool to minimize the amount of mycelium in the filtrate. One ml of spore suspension (105–106 spores ml−1) was added to 100 ml liquid MEA + 5% NaCl medium[6]. The spores were left to germinate for four days on a rotary shaker (100 rpm) to prevent renewed sporulation. Mycelium pellets (∼5 mm in diameter) were gradually transferred to a series of NaCl solutions (5%, 15%, 25%) to prevent osmotic shock. The stepwise incubation was continued for one day in each dilution. Finally the pellets were transferred to the test solution. Two replicates were prepared for each combination of mycelium and salt concentration. All solutions were supplemented with 1 g l−1 glucose and 1 g l−1 yeast extract. The medium was replaced every 2 weeks. Incubation was performed on a rotary shaker (100 rpm). Samples were withdrawn at the start of the experiment and after 1, 2, 3, 4, 6, 8, 10 and 12 weeks of incubation at 25 °C. Pellets (2–5) were inoculated on plates with MEA + 5% NaCl and MEA + X% NaCl, X being the same NaCl concentration as in the solution from which the sample was withdrawn. The plates were incubated at 25 °C for 1 week in the dark and growth and sporulation were recorded.
For the determination of spore viability, spores (conidia and ascospores) were harvested from 14-day-old cultures on agar slants (CzA). Spores were suspended in water with 0.1% Tween 80 added and filtered through sterile glass wool. One ml of spore suspension (104–105 spores ml−1) was introduced in tubes containing 35 ml sterile distilled water and solutions with different NaCl concentrations (5%, 10%, 15%, 20%, 25%, 30%) and 50% glucose solution. Incubation was performed at room temperature in the dark. Portions of 0.1 ml of sample were withdrawn at the start of the experiment and after 1, 2, 3, 4, 5, 6, 7, 8 and 14 weeks. Samples were inoculated as described for mycelial pellets.
2.8 Growth and sporulation rates on media with different salinities
The salinity ranges of isolated Eurotium spp. were determined on solid media (MEA) with the addition of NaCl in steps of 2.5% from 0% up to 32.5% at 25 °C by measurements of colony diameter over the 6-weeks long period of growth. The effect of salinity on sporulation was also recorded by a visual estimate according to Blaser[27].
3 Results
3.1 Physicochemical conditions in the Adriatic salterns and other studied hypersaline environments
The physicochemical parameters of water sampled from the Adriatic saltern ponds are presented in Table 2. The pH in all ponds was ∼7.2 from the beginning of December to mid-February. It then increased and remained between 7.4 and 8.2. The temperature of the brine was around 0 °C in winter and increased up to a maximum of 26 °C in July.
Physicochemical characteristics with their seasonal ranges in hypersaline water of selected ponds in the Adriatic salterns
| First pond | Second pond | Third pond | Fourth pond | |
| Salinity (%) | 3–13 | 5–21 | 4–24 | 10–30 |
| Water activity (aw) | 0.89–0.96 | 0.79–0.90 | 0.77–0.90 | 0.72–0.93 |
| O2 (mg l−1) | 4.9–10.8 | 3.4–7.0 | 2.1–6.1 | 0.5–9.3 |
| N (mg l−1) | <0.1–5.7 | <0.1–7.5 | <0.1–9.2 | <0.1–13.2 |
| P (mg l−1) | 0.01–0.09 | 0.05–0.14 | 0.01–0.08 | 0.04–0.3 |
| BOD | 11–328 | 5–730 | <1–24 | <1–73 |
| COD | 255–1250 | 680–1630 | 600–1570 | 560–1890 |
| Cl−1 (g l−1) | 9.1–104 | 12.7–128 | 19.5–151 | 9.4–184 |
| K+ (g l−1) | 0.38–3 | 0.55–2.5 | 0.43–2.9 | 0.46–4.8 |
| Na+ (g l−1) | 9.7–82 | 14.5–72.4 | 11.3–82.4 | 12.1–95 |
| SO2−4 (g l−1) | 0.39–13.6 | 0.79–13.5 | 2.7–18.2 | 0.47–26.1 |
| First pond | Second pond | Third pond | Fourth pond | |
| Salinity (%) | 3–13 | 5–21 | 4–24 | 10–30 |
| Water activity (aw) | 0.89–0.96 | 0.79–0.90 | 0.77–0.90 | 0.72–0.93 |
| O2 (mg l−1) | 4.9–10.8 | 3.4–7.0 | 2.1–6.1 | 0.5–9.3 |
| N (mg l−1) | <0.1–5.7 | <0.1–7.5 | <0.1–9.2 | <0.1–13.2 |
| P (mg l−1) | 0.01–0.09 | 0.05–0.14 | 0.01–0.08 | 0.04–0.3 |
| BOD | 11–328 | 5–730 | <1–24 | <1–73 |
| COD | 255–1250 | 680–1630 | 600–1570 | 560–1890 |
| Cl−1 (g l−1) | 9.1–104 | 12.7–128 | 19.5–151 | 9.4–184 |
| K+ (g l−1) | 0.38–3 | 0.55–2.5 | 0.43–2.9 | 0.46–4.8 |
| Na+ (g l−1) | 9.7–82 | 14.5–72.4 | 11.3–82.4 | 12.1–95 |
| SO2−4 (g l−1) | 0.39–13.6 | 0.79–13.5 | 2.7–18.2 | 0.47–26.1 |
Physicochemical characteristics with their seasonal ranges in hypersaline water of selected ponds in the Adriatic salterns
| First pond | Second pond | Third pond | Fourth pond | |
| Salinity (%) | 3–13 | 5–21 | 4–24 | 10–30 |
| Water activity (aw) | 0.89–0.96 | 0.79–0.90 | 0.77–0.90 | 0.72–0.93 |
| O2 (mg l−1) | 4.9–10.8 | 3.4–7.0 | 2.1–6.1 | 0.5–9.3 |
| N (mg l−1) | <0.1–5.7 | <0.1–7.5 | <0.1–9.2 | <0.1–13.2 |
| P (mg l−1) | 0.01–0.09 | 0.05–0.14 | 0.01–0.08 | 0.04–0.3 |
| BOD | 11–328 | 5–730 | <1–24 | <1–73 |
| COD | 255–1250 | 680–1630 | 600–1570 | 560–1890 |
| Cl−1 (g l−1) | 9.1–104 | 12.7–128 | 19.5–151 | 9.4–184 |
| K+ (g l−1) | 0.38–3 | 0.55–2.5 | 0.43–2.9 | 0.46–4.8 |
| Na+ (g l−1) | 9.7–82 | 14.5–72.4 | 11.3–82.4 | 12.1–95 |
| SO2−4 (g l−1) | 0.39–13.6 | 0.79–13.5 | 2.7–18.2 | 0.47–26.1 |
| First pond | Second pond | Third pond | Fourth pond | |
| Salinity (%) | 3–13 | 5–21 | 4–24 | 10–30 |
| Water activity (aw) | 0.89–0.96 | 0.79–0.90 | 0.77–0.90 | 0.72–0.93 |
| O2 (mg l−1) | 4.9–10.8 | 3.4–7.0 | 2.1–6.1 | 0.5–9.3 |
| N (mg l−1) | <0.1–5.7 | <0.1–7.5 | <0.1–9.2 | <0.1–13.2 |
| P (mg l−1) | 0.01–0.09 | 0.05–0.14 | 0.01–0.08 | 0.04–0.3 |
| BOD | 11–328 | 5–730 | <1–24 | <1–73 |
| COD | 255–1250 | 680–1630 | 600–1570 | 560–1890 |
| Cl−1 (g l−1) | 9.1–104 | 12.7–128 | 19.5–151 | 9.4–184 |
| K+ (g l−1) | 0.38–3 | 0.55–2.5 | 0.43–2.9 | 0.46–4.8 |
| Na+ (g l−1) | 9.7–82 | 14.5–72.4 | 11.3–82.4 | 12.1–95 |
| SO2−4 (g l−1) | 0.39–13.6 | 0.79–13.5 | 2.7–18.2 | 0.47–26.1 |
Conditions in the environment were most extreme during the peak of salt production by the end of August. In the crystallization pond (Table 2, fourth pond) the NaCl concentration increased to 30%, water activity decreased to 0.72, while in the other tested ponds (Table 2, first to third pond) salinities ranged from 13% to 24% and water activities from 0.89 to 0.93. As salinity increased, oxygen decreased from values as high as 10.3 mg l−1 down to 0.5 mg l−1 in August. Outside the salt-production season, oxygen concentrations in all ponds ranged from 8.7 to 16.0 mg l−1. Due to occasional high values of nutrients during the season, the chemical oxygen demand was between 255 and 1890 mg l−1.
Although concentrations of nitrogen and phosphorus were generally below 0.1 mg l−1, two peaks of nitrogen were observed: the first one at the beginning of the season, at salinity 8–10% (0.3–1.7 mg l−1), and the second one at salinity 21–25% (8.2–13.2 mg l−1). Simultaneous with the nitrogen peak in August, phosphorus concentrations reached a peak of 0.3 mg l−1.
Water samples from other sampled salterns were in the salinity range from 8% to 32% NaCl (aw 0.67–0.95) and oxygen concentrations from 0.5 to 6 mg l−1. The aw in the Dead Sea was 0.67, in the Eilat saltern it ranged from 0.73 to 0.95, in the Ebro River Delta from 0.73 to 0.83 and in Santa Pola from 0.73 to 0.94, in the Camargue salterns and Samouco salterns it was 0.73, in Namibian salterns it was 0.78 and in the Dominican Republic salterns it ranged from 0.73 to 0.95.
3.2 Composition of Eurotium complex and morphological characteristics of species
In the course of the mycodiversity study of the salterns, 208 Eurotium spp. isolates were investigated. For selected strains listed in Table 1, scanning electron microscopy of ascospores are presented (Fig. 1), as well as measurements of structures diagnostic for species description (Table 3). In total, six different Eurotium species were identified: E. amstelodami, E. chevalieri, E. herbariorum, E. rubrum, E. repens and Eurotium sp. The majority of the isolates belonged to E. amstelodami (74%), followed by E. repens (10%) and E. herbariorum (10%), respectively. The characteristics of the strains did not differ significantly from known species described in the literature, although they were in the upper size range (Table 3 and Fig. 1) [23,24,26,27]. The only exception represented Eurotium sp., which is probably a new species, tentatively named “E. halotolerans”.
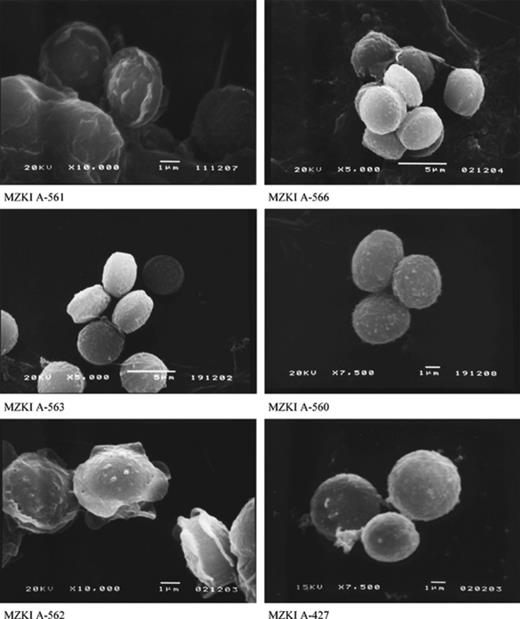
Scanning electron micrographs of E. amstelodami (MZKI A-561), E. repens (MZKI A-566), E. rubrum (MZKI A-563), “E. halotolerans” (MZKI A-560), E. chevalieri (MZKI A-562) and E. herbariorum (MZKI A-427) ascospores.
Measurements (μm) of selected structures of the Eurotium species isolated from Adriatic salterns
| Species (strain designation) | Conidiophores | Phialides | Vesicles | Conidia | Cleistothecia | Asci | Ascospores |
| E. amstelodami (MZKI A-561) | 263 ± 41 | 7.2 ± 0.8 × 4.1 ± 0.5 | 11.9 ± 3.4 | 4.5 ± 0.2 × 3.9 ± 0.1 | 80 ± 5 × 74 ± 4 | 13.0 ± 0.4 × 10.6 ± 0.2 | 5.8 ± 0.2 × 4.8 ± 0.2 |
| E. chevalieri (MZKI A-562) | 207 ± 83 | 7.1 ± 0.4 × 4.5 ± 0.2 | 18.5 ± 2.6 | 6.4 ± 0.3 × 5.4 ± 0.1 | 110 ± 11 × 96 ± 9 | 11.3 ± 0.3 × 10.1 ± 0.3 | 6.7 ± 0.2 × 4.6 ± 0.2 |
| E. repens (MZKI A-567) | 326 ± 61 | 6.7 ± 0.5 × 4.3 ± 0.2 | 16 ± 2.2 | 6.3 ± 0.2 × 5.8 ± 0.2 | 113 ± 8 × 101 ± 7 | 12.1 ± 0.4 × 11 ± 0.6 | 5.6 ± 0.2 × 5.0 ± 0.2 |
| E. repens (MZKI A-408) | 497 ± 41 | 8.8 ± 0.5 × 4.8 ± 0.2 | 29.5 ± 1.6 | 6.6 ± 0.2 × 6.0 ± 0.2 | 97 ± 7 × 86 ± 6 | 11.9 ± 0.3 × 10.4 ± 0.3 | 5.5 ± 0.2 × 4.6 ± 0.2 |
| E. rubrum (MZKI A-564) | 454 ± 60 | 7.3 ± 0.6 × 5.0 ± 0.3 | 33.3 ± 7.6 | 6.3 ± 0.2 × 5.8 ± 0.2 | 117 ± 7 × 102 ± 7 | 13.1 ± 1.8 × 10.4 ± 0.4 | 5.2 ± 0.2 × 4.2 ± 0.1 |
| E. rubrum (MZKI A-563) | 120 ± 8 × 104 ± 7 | 13.6 ± 0.5 × 11.7 ± 0.3 | 6.2 ± 0.1 × 5.0 ± 0.1 | ||||
| E. herbariorum (MZKI A-427) | 282 ± 54 | 9.0 ± 0.7 × 4.6 ± 0.3 | 18.4 ± 1.3 | 6.8 ± 0.4 × 6.7 ± 0.3 | 104 ± 9 × 95 ± 8 | 14 ± 0.5 × 11.8 ± 0.3 | 6.7 ± 0.2 × 5.2 ± 0.2 |
| Species (strain designation) | Conidiophores | Phialides | Vesicles | Conidia | Cleistothecia | Asci | Ascospores |
| E. amstelodami (MZKI A-561) | 263 ± 41 | 7.2 ± 0.8 × 4.1 ± 0.5 | 11.9 ± 3.4 | 4.5 ± 0.2 × 3.9 ± 0.1 | 80 ± 5 × 74 ± 4 | 13.0 ± 0.4 × 10.6 ± 0.2 | 5.8 ± 0.2 × 4.8 ± 0.2 |
| E. chevalieri (MZKI A-562) | 207 ± 83 | 7.1 ± 0.4 × 4.5 ± 0.2 | 18.5 ± 2.6 | 6.4 ± 0.3 × 5.4 ± 0.1 | 110 ± 11 × 96 ± 9 | 11.3 ± 0.3 × 10.1 ± 0.3 | 6.7 ± 0.2 × 4.6 ± 0.2 |
| E. repens (MZKI A-567) | 326 ± 61 | 6.7 ± 0.5 × 4.3 ± 0.2 | 16 ± 2.2 | 6.3 ± 0.2 × 5.8 ± 0.2 | 113 ± 8 × 101 ± 7 | 12.1 ± 0.4 × 11 ± 0.6 | 5.6 ± 0.2 × 5.0 ± 0.2 |
| E. repens (MZKI A-408) | 497 ± 41 | 8.8 ± 0.5 × 4.8 ± 0.2 | 29.5 ± 1.6 | 6.6 ± 0.2 × 6.0 ± 0.2 | 97 ± 7 × 86 ± 6 | 11.9 ± 0.3 × 10.4 ± 0.3 | 5.5 ± 0.2 × 4.6 ± 0.2 |
| E. rubrum (MZKI A-564) | 454 ± 60 | 7.3 ± 0.6 × 5.0 ± 0.3 | 33.3 ± 7.6 | 6.3 ± 0.2 × 5.8 ± 0.2 | 117 ± 7 × 102 ± 7 | 13.1 ± 1.8 × 10.4 ± 0.4 | 5.2 ± 0.2 × 4.2 ± 0.1 |
| E. rubrum (MZKI A-563) | 120 ± 8 × 104 ± 7 | 13.6 ± 0.5 × 11.7 ± 0.3 | 6.2 ± 0.1 × 5.0 ± 0.1 | ||||
| E. herbariorum (MZKI A-427) | 282 ± 54 | 9.0 ± 0.7 × 4.6 ± 0.3 | 18.4 ± 1.3 | 6.8 ± 0.4 × 6.7 ± 0.3 | 104 ± 9 × 95 ± 8 | 14 ± 0.5 × 11.8 ± 0.3 | 6.7 ± 0.2 × 5.2 ± 0.2 |
Measurements (μm) of selected structures of the Eurotium species isolated from Adriatic salterns
| Species (strain designation) | Conidiophores | Phialides | Vesicles | Conidia | Cleistothecia | Asci | Ascospores |
| E. amstelodami (MZKI A-561) | 263 ± 41 | 7.2 ± 0.8 × 4.1 ± 0.5 | 11.9 ± 3.4 | 4.5 ± 0.2 × 3.9 ± 0.1 | 80 ± 5 × 74 ± 4 | 13.0 ± 0.4 × 10.6 ± 0.2 | 5.8 ± 0.2 × 4.8 ± 0.2 |
| E. chevalieri (MZKI A-562) | 207 ± 83 | 7.1 ± 0.4 × 4.5 ± 0.2 | 18.5 ± 2.6 | 6.4 ± 0.3 × 5.4 ± 0.1 | 110 ± 11 × 96 ± 9 | 11.3 ± 0.3 × 10.1 ± 0.3 | 6.7 ± 0.2 × 4.6 ± 0.2 |
| E. repens (MZKI A-567) | 326 ± 61 | 6.7 ± 0.5 × 4.3 ± 0.2 | 16 ± 2.2 | 6.3 ± 0.2 × 5.8 ± 0.2 | 113 ± 8 × 101 ± 7 | 12.1 ± 0.4 × 11 ± 0.6 | 5.6 ± 0.2 × 5.0 ± 0.2 |
| E. repens (MZKI A-408) | 497 ± 41 | 8.8 ± 0.5 × 4.8 ± 0.2 | 29.5 ± 1.6 | 6.6 ± 0.2 × 6.0 ± 0.2 | 97 ± 7 × 86 ± 6 | 11.9 ± 0.3 × 10.4 ± 0.3 | 5.5 ± 0.2 × 4.6 ± 0.2 |
| E. rubrum (MZKI A-564) | 454 ± 60 | 7.3 ± 0.6 × 5.0 ± 0.3 | 33.3 ± 7.6 | 6.3 ± 0.2 × 5.8 ± 0.2 | 117 ± 7 × 102 ± 7 | 13.1 ± 1.8 × 10.4 ± 0.4 | 5.2 ± 0.2 × 4.2 ± 0.1 |
| E. rubrum (MZKI A-563) | 120 ± 8 × 104 ± 7 | 13.6 ± 0.5 × 11.7 ± 0.3 | 6.2 ± 0.1 × 5.0 ± 0.1 | ||||
| E. herbariorum (MZKI A-427) | 282 ± 54 | 9.0 ± 0.7 × 4.6 ± 0.3 | 18.4 ± 1.3 | 6.8 ± 0.4 × 6.7 ± 0.3 | 104 ± 9 × 95 ± 8 | 14 ± 0.5 × 11.8 ± 0.3 | 6.7 ± 0.2 × 5.2 ± 0.2 |
| Species (strain designation) | Conidiophores | Phialides | Vesicles | Conidia | Cleistothecia | Asci | Ascospores |
| E. amstelodami (MZKI A-561) | 263 ± 41 | 7.2 ± 0.8 × 4.1 ± 0.5 | 11.9 ± 3.4 | 4.5 ± 0.2 × 3.9 ± 0.1 | 80 ± 5 × 74 ± 4 | 13.0 ± 0.4 × 10.6 ± 0.2 | 5.8 ± 0.2 × 4.8 ± 0.2 |
| E. chevalieri (MZKI A-562) | 207 ± 83 | 7.1 ± 0.4 × 4.5 ± 0.2 | 18.5 ± 2.6 | 6.4 ± 0.3 × 5.4 ± 0.1 | 110 ± 11 × 96 ± 9 | 11.3 ± 0.3 × 10.1 ± 0.3 | 6.7 ± 0.2 × 4.6 ± 0.2 |
| E. repens (MZKI A-567) | 326 ± 61 | 6.7 ± 0.5 × 4.3 ± 0.2 | 16 ± 2.2 | 6.3 ± 0.2 × 5.8 ± 0.2 | 113 ± 8 × 101 ± 7 | 12.1 ± 0.4 × 11 ± 0.6 | 5.6 ± 0.2 × 5.0 ± 0.2 |
| E. repens (MZKI A-408) | 497 ± 41 | 8.8 ± 0.5 × 4.8 ± 0.2 | 29.5 ± 1.6 | 6.6 ± 0.2 × 6.0 ± 0.2 | 97 ± 7 × 86 ± 6 | 11.9 ± 0.3 × 10.4 ± 0.3 | 5.5 ± 0.2 × 4.6 ± 0.2 |
| E. rubrum (MZKI A-564) | 454 ± 60 | 7.3 ± 0.6 × 5.0 ± 0.3 | 33.3 ± 7.6 | 6.3 ± 0.2 × 5.8 ± 0.2 | 117 ± 7 × 102 ± 7 | 13.1 ± 1.8 × 10.4 ± 0.4 | 5.2 ± 0.2 × 4.2 ± 0.1 |
| E. rubrum (MZKI A-563) | 120 ± 8 × 104 ± 7 | 13.6 ± 0.5 × 11.7 ± 0.3 | 6.2 ± 0.1 × 5.0 ± 0.1 | ||||
| E. herbariorum (MZKI A-427) | 282 ± 54 | 9.0 ± 0.7 × 4.6 ± 0.3 | 18.4 ± 1.3 | 6.8 ± 0.4 × 6.7 ± 0.3 | 104 ± 9 × 95 ± 8 | 14 ± 0.5 × 11.8 ± 0.3 | 6.7 ± 0.2 × 5.2 ± 0.2 |
3.3 Detection of secondary metabolites profiles
The results of the HPLC analysis are presented in Table 4. All species produced echinulin, neoechinulin A, flavoglaucin, physcion, auroglaucin, dihydroauroglaucin, and tetrahydroauroglaucin, while only E. repens produced asperentin. This is in agreement with our previous analyses of the same species from low moisture foods as well as literature data [33–35]. All species produced species specific, but unknown secondary metabolites in addition to well-known compounds.
Production of secondary metabolites by Eurotium species isolated from hypersaline waters compared to isolates from foods, leather, textiles and soil
| Eurotium sp. | Asperentin | Echinulin | Neoechinulin A, B, C, D | Physcion | Flavoglaucin | Auroglaucin, dihydroauroglaucin, tetrahydroauroglaucin | Red anthraquinones | Met R |
| E. amstelodami | – | + | + | + | + | + | – | + |
| E. chevalieri | – | + | + | + | + | + | – | + |
| E. herbariorum | – | + | + | + | + | + | – | – |
| E. rubrum | – | + | + | + | + | + | + | – |
| E. repens | + | + | + | + | + | + | – | – |
| “E. halotolerans” | – | + | + | + | + | + | + | – |
| Eurotium sp. | Asperentin | Echinulin | Neoechinulin A, B, C, D | Physcion | Flavoglaucin | Auroglaucin, dihydroauroglaucin, tetrahydroauroglaucin | Red anthraquinones | Met R |
| E. amstelodami | – | + | + | + | + | + | – | + |
| E. chevalieri | – | + | + | + | + | + | – | + |
| E. herbariorum | – | + | + | + | + | + | – | – |
| E. rubrum | – | + | + | + | + | + | + | – |
| E. repens | + | + | + | + | + | + | – | – |
| “E. halotolerans” | – | + | + | + | + | + | + | – |
Isolates of Eurotium spp. used for the HPLC analysis: E. amstelodami: CBS 112.48, CBS 518.65 (NTa), CBS 519.65, IBT 13453, IBT 13454, IBT 13455, IBT 22853, IBT 23137, IBT 25488; E. chevalieri: CBS 113.34, CBS 129.54, CBS 522.65 (NTa), IBT 10587, IBT 13456, IBT 13457, IBT 24508, IBT 24837; E. herbariorum: CBS 114.30, IBT 10588, IBT 21808, IBT 25489; E. rubrum: CBS 530.65 (NTa), NRRL 75, IBT 10171, IBT 22290, IBT 22849, IBT 23139, IBT 25494; E. repens: CBS 114.30, CBS 126.55, CBS 127.55, CBS 529.65 (NTa), IBT 10581, IBT 10582, IBT 10584, IBT 21683, IBT 21684, IBT 21685, IBT 21686, IBT 21810, IBT 22287, IBT 22288, IBT 22851, IBT 23136, IBT 23141, IBT 25490, IBT 25491; “E. halotolerans”: IBT 24504, IBT 24505, IBT 24810. a Ex neotype culture.
Production of secondary metabolites by Eurotium species isolated from hypersaline waters compared to isolates from foods, leather, textiles and soil
| Eurotium sp. | Asperentin | Echinulin | Neoechinulin A, B, C, D | Physcion | Flavoglaucin | Auroglaucin, dihydroauroglaucin, tetrahydroauroglaucin | Red anthraquinones | Met R |
| E. amstelodami | – | + | + | + | + | + | – | + |
| E. chevalieri | – | + | + | + | + | + | – | + |
| E. herbariorum | – | + | + | + | + | + | – | – |
| E. rubrum | – | + | + | + | + | + | + | – |
| E. repens | + | + | + | + | + | + | – | – |
| “E. halotolerans” | – | + | + | + | + | + | + | – |
| Eurotium sp. | Asperentin | Echinulin | Neoechinulin A, B, C, D | Physcion | Flavoglaucin | Auroglaucin, dihydroauroglaucin, tetrahydroauroglaucin | Red anthraquinones | Met R |
| E. amstelodami | – | + | + | + | + | + | – | + |
| E. chevalieri | – | + | + | + | + | + | – | + |
| E. herbariorum | – | + | + | + | + | + | – | – |
| E. rubrum | – | + | + | + | + | + | + | – |
| E. repens | + | + | + | + | + | + | – | – |
| “E. halotolerans” | – | + | + | + | + | + | + | – |
Isolates of Eurotium spp. used for the HPLC analysis: E. amstelodami: CBS 112.48, CBS 518.65 (NTa), CBS 519.65, IBT 13453, IBT 13454, IBT 13455, IBT 22853, IBT 23137, IBT 25488; E. chevalieri: CBS 113.34, CBS 129.54, CBS 522.65 (NTa), IBT 10587, IBT 13456, IBT 13457, IBT 24508, IBT 24837; E. herbariorum: CBS 114.30, IBT 10588, IBT 21808, IBT 25489; E. rubrum: CBS 530.65 (NTa), NRRL 75, IBT 10171, IBT 22290, IBT 22849, IBT 23139, IBT 25494; E. repens: CBS 114.30, CBS 126.55, CBS 127.55, CBS 529.65 (NTa), IBT 10581, IBT 10582, IBT 10584, IBT 21683, IBT 21684, IBT 21685, IBT 21686, IBT 21810, IBT 22287, IBT 22288, IBT 22851, IBT 23136, IBT 23141, IBT 25490, IBT 25491; “E. halotolerans”: IBT 24504, IBT 24505, IBT 24810. a Ex neotype culture.
3.4 Spatiotemporal distribution in the Adriatic salterns
From the Adriatic salterns all six different species of the genus Eurotium were isolated. E. amstelodami, E. herbariorum and E. repens were repeatedly recovered in both years, while E. chevalieri, E. rubrum and “E. halotolerans” were isolated only in 1999. Therefore, the Jaccard coefficient (Sj) of species composition between both years is quite high (Sj = 0.5). Comparison of species composition similarity between different ponds did not reveal any apparent differences in the precrystallization (Sj = 0) and the crystallization period (Sj = 1). Thus, salinity and water activity had the major impact on distribution and occurrence of these species.
Outside the season of salt production counts for all Eurotium species remained below 100 CFU l−1. A slight increase (up to 500 CFU l−1) was detected in parallel with increased salinity (3–5% NaCl) at the beginning of the season. During the salt production season two pronounced peaks appeared (Fig. 2A), which correlated with increased nitrogen levels (data not shown). The first appeared at the NaCl range 10–15% with counts up to 5000 CFU l−1, and the second and highest within 18–25% with counts up to 30,000 CFU l−1. Between the peaks fungal counts remained below 250 CFU l−1. All Eurotium species were primarily isolated on saline media, although sugar-based media were also used. The highest CFU values were obtained on media with 17% NaCl, followed by 50% glucose on medium MY50G. The most frequently encountered species were detected on media with up to 70% glucose/fructose and 32% NaCl as well. A similar pattern of fungal dynamics was observed both years.
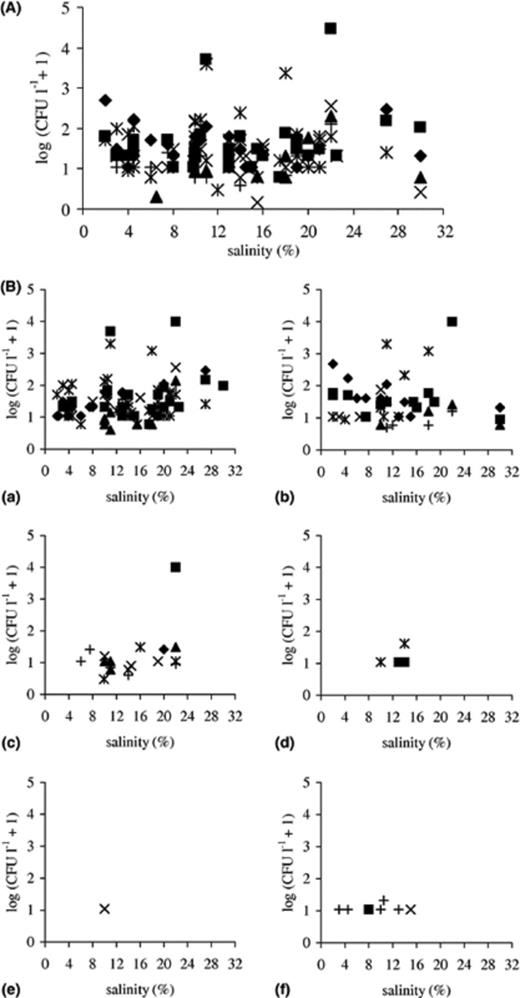
(A) Distribution of genus Eurotium along the salinity gradient on different selective media (♦ MY10-12, ◻ MY50G, δ MY70G, ∗ MEA + 17% NaCl, × MEA + 24% NaCl, + MEA + 32% NaCl). Species data were transformed by taking logarithm log(CFU l−1+ 1). (B) Distribution of (a) E. amstelodami, (b) E. repens, (c) E. herbariorum, (d) E. rubrum, (e) E. chevalieri and (f) “E. halotolerans” along the salinity gradient. Isolates were obtained using different selective low water activity media (♦ MY10-12, ◻ MY50G, δ MY70G, ∗ MEA + 17% NaCl, × MEA + 24% NaCl, +MEA + 32% NaCl). Species data were transformed by taking logarithm log(CFU l−1+ 1).
Isolated Eurotium species were divided into two groups according to their spatial frequency of occurrence (FO). The first group was represented by E. amstelodami, E. repens and E. herbariorum, which had the highest FO (50–100%) within the season of salt production, while outside the season FO lowered to 20–40%. Within this group, E. amstelodami was detected most frequently throughout both sampling years and within a broad salinity range, from 3% to 32% NaCl, followed by E. herbariorum. Lower FO values were obtained for E. repens due to lower counts in one salt production season and only occasional detection in the precrystallization period. The dynamic pattern of these species followed the pattern described for genus Eurotium. During the first peak, the highest CFU were detected for E. repens (480 CFU l−1). The second peak was more pronounced, with ∼2000 CFU l−1 for E. amstelodami and E. repens, while all three species were represented with approximately equal numbers (10,000 CFU l−1) in the highest, third peak (Fig. 2B).
The second group of Eurotium species, represented by E. rubrum, E. chevalieri and “E. halotolerans”, was detected with low counts (below 50 CFU l−1) and low FO (20–60%), primarily before extreme environmental conditions became most extreme with salinities in the range 4–14% NaCl. E. rubrum and E. chevalieri were detected only once in the crystallization period with FO 60% and 20%, respectively (Fig. 2B).
Analyses of Eurotium spp. biodiversity from eutrophic salterns revealed a richer species composition in comparison with oligotrophic hypersaline waters, represented in this study by the Dead Sea and Eilat salterns [16,36], from which almost exclusively E. amstelodami was isolated. In all sampled salterns E. amstelodami was the most frequent, followed by E. repens and E. herbariorum. These three species were also repeatedly isolated by baiting and recovered from biofilms in the Adriatic salterns. Species from the second group, E. rubrum and E. chevalieri were detected more rarely, while “E. halotolerans” was isolated only from the Adriatic salterns.
3.5 In vitro survival of mycelium and spores in hypersaline water
Isolation of the six recovered Eurotium spp. from the salterns could be due to the adaptive ability of spores or mycelia to survive in hypersaline water. Therefore, both types of propagules were tested in vitro for viability at a broad range of salinities. Results of spore survival are presented in Fig. 3.
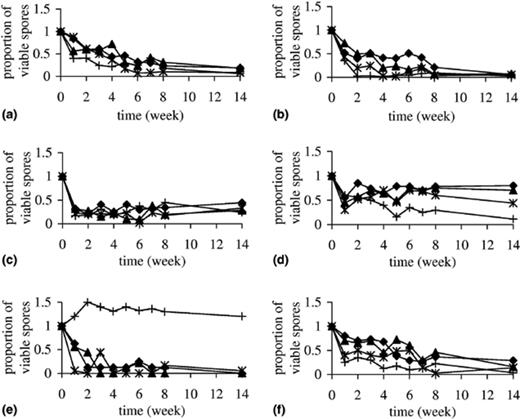
In vitro survival of spores of the Eurotium species over the 14-week long period of exposure to 20% (♦), 25% (δ), 30% NaCl (∗) and 50% glucose (+) solutions.
At the highest salinity (30% NaCl), spore viability of most species decreased drastically within the first week and then with a slower pace. Viability of E. herbariorum spores remained unchanged after the initial decrease of 65%, while only up to 15% spores of “E. halotolerans”, E. amstelodami and E. repens remained viable until the end of the incubation period (Fig. 3). Spores of E. amstelodami were initially almost unaffected, but in the end only 8% survived. The highest adaptive ability of survival in hypersaline water was shown for E. rubrum spores, since almost half of them survived. At lower tested salinities, viable spores were recovered for five out of six tested species. E. chevalieri spores were the most sensitive and remained viable only at salinities up to 20% NaCl. When spores of all species were exposed to 50% glucose, five showed a considerably higher loss of viability in comparison with corresponding aw obtained with 20% NaCl. Again, E. chevalieri represented the only exception, since its spores survived glucose exposure unaffected (Fig. 3).
Surprisingly mycelia of all tested species survived 10 weeks of incubation even at the highest tested salinities. After this time, the mycelia of “E. halotolerans” and of E. rubrum lost viability at 30% and 25% NaCl concentrations, respectively, but survived without any viability loss at all other tested salinities. Mycelia of E. amstelodami, E. herbariorum, E. repens and E. chevalieri remained viable throughout the test period of 12 weeks. Mycelial growth rate and sporulation ability, observed on the medium with 5% NaCl after exposure of mycelium in hypersaline water, were unaffected (data not shown).
3.6 Determination of growth rate and sporulation on media with different salinities
Optimal conditions for growth and sporulation were determined for all isolated Eurotium species. The growth on solid media with added NaCl was stimulated up to 10% NaCl for E. rubrum, E. chevalieri and E. amstelodami and up to 12.5% NaCl for “E. halotolerans”, E. repens and E. herbariorum (Fig. 4). The salinity growth range was different for individual species. E. amstelodami and “E. halotolerans” had the broadest salinity range, growing up to 27.5% NaCl, followed by E. repens and E. herbariorum (up to 25%), E. chevalieri (22.5%) and E. rubrum (20%). Germination, although not succeeded by growth, occurred at the highest NaCl concentration for “E. halotolerans” (32.5%), followed by E. herbariorum and E. amstelodami (30%), E. repens (27.5%), E. rubrum and E. chevalieri (25%).
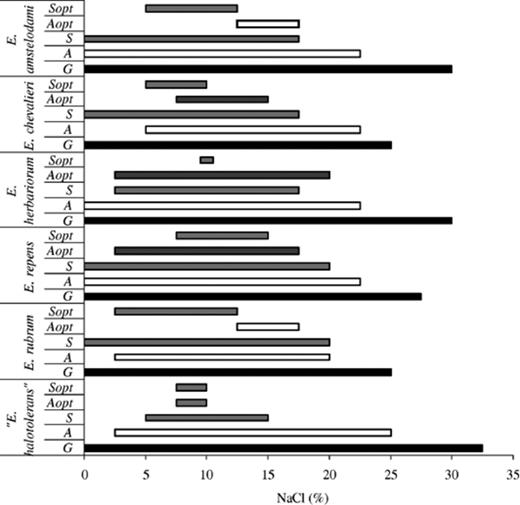
Salinity ranges for growth (G), cleistothecium formation (S), optimal production of cleistothecia (Sopt), conidia production (A) and optimal conidia production (Aopt).
Cleistothecium formation, with slight differences between species, occurred within the range 0–20% NaCl, with optimal production between 2.5% and 15% NaCl (Fig. 4). Conidia were produced in a broader salinity range (0–22.5%), with more abundant sporulation between 2.5% and 20% NaCl (Fig. 4).
4 Discussion
In our previous studies we have shown that black yeasts, as a rare example of eukaryotic extremophiles, are adapted to survival and growth in hypersaline environments[13]. Few genera of food-borne ascomycetes and conidial filamentous fungi of ascomycetous origin, such as Eurotium, Wallemia, Chrysosporium, Polypaecilum, Eremascus, Xeromyces and Basipetospora[17,37], are able to grow and sporulate in habitats characterized by low water activity. In this study we described the occurrence of Eurotium, the only genus besides Wallemia[38] isolated from natural hypersaline environments[7].
In the past, the different species of the genus Eurotium and the phylogenetically closely related Aspergilli in section Restricti[39] have been isolated both from arid and saline soil and salt marshes, particularly in Israel, Syria and Kuwait. The most frequently isolated species were: E. amstelodami, E. chevalieri, E. echinulatum, E. halophilicum, E. herbariorum, E. intermedium, E. repens, A. restrictus, E. rubrum, E. spiculosum and E. umbrosum[9,11,16,19,20,34,40–43]. During our study of Eurotium isolates from hypersaline waters of eight salterns in four continents, more than 200 strains were analysed but only six different species were isolated. These could be divided into two ecological groups. The first was represented by E. amstelodami, E. herbariorum and E. repens, that were consistently detected in all eight salterns. The highest frequency of occurrence was in samples with 18–25% NaCl. E. rubrum, E. chevalieri and “E. halotolerans”, representing the second group, were recorded in the salterns only occasionally and at lower salinities (5–15% NaCl).
The six species isolated from salterns, (except “E. halotolerans”), were the most common Eurotium species on low aw food, textiles, leather and in saline soil [34,42]. Unexpectedly, many known xerophilic food-borne species were not isolated, as for example E. halophilicum that despite of its name so far has only been detected in food[44]. E. amstelodami was the most frequently isolated species in all salterns, together with E. herbariorum and E. repens. In vitro studies showed that the spores and mycelium of these species are able to survive long-term exposure in solutions within a broad range of salt concentrations (0–30%). They sporulate at up to 22% NaCl, as previously reported only for E. herbariorum, isolated from the Dead Sea[32]. E. rubrum and “E. halotolerans” were encountered less frequently in the salterns, but they nevertheless demonstrated similar in vitro adaptive abilities for survival in hypersaline water. The probable reason is a narrower optimal salinity range for sporulation.
Results from the literature repeatedly indicate that E. amstelodami and E. rubrum prefer media containing sucrose and glucose to those with NaCl or glycerol with the same aw[5,45,46]. Nevertheless it was found that E. herbariorum (sensu lato), E. amstelodami and E. chevalieri isolated from soil could grow at NaCl concentrations up to 30%[47,48]. During our study all six Eurotium species were primarily isolated on saline media and with the exception of E. chevalieri, all tested spores survived much better in brine.
Comparisons of isolates from hypersaline and common environments showed that the size of structures, indicative for taxonomic analyses, was in most cases within the upper size range characteristic for the individual species (Table 3). It seems that slightly larger ascospore size is characteristic for isolates originating from environments with high salinity [32,49]. This may lead to difficulties in identification, since ascospore size is emphasized in taxonomic classifications.
Secondary metabolites were consistently produced by the six species of Eurotium. Most species produced the echinulins, neoechinulins, auroglaucins, flavoglaucins, and different anthraquinones such as physcion, catenarin, questin and questinol. Echinuline and flavoglaucin were only produced in the ascomata and ascospores of E. amstelodami cultures[50]. Some of these metabolites are antioxidants, e.g. flavoglaucin[51], which may help protect the spores of these species.
E. amstelodami and E. chevalieri grew well at 37 °C, while E. repens, E. rubrum and E. herbariorum grew poorly, if at all [23,27]. This subgrouping is supported by secondary metabolite differences and ascospore ornamentations. Thus the grouping of E. amstelodami, E. repens and E. herbariorum, showing the most efficiently combined adaptations to high salinities, does not correspond to the taxonomic/temperature subgrouping.
In hypersaline environments, only the most adapted species can survive. Combined use of different approaches and media, which exert a strong selective pressure, gives a selective advantage to halotolerant and halophilic organisms. According to our results, the mycobiota present in the saline environments are represented on one hand by flush through air-borne contaminants, not able to survive in brines for prolonged times. On the other hand, by a distinct halotolerant indigenous community, adapted to long-term survival and vegetative growth. Our study indicates that E. chevalieri is an opportunistic air-borne contaminant, while E. rubrum and “E. halotolerans” are only temporal inhabitants of brine at lower salinities. E. herbariorum, E. repens and E. amstelodami probably represent part of the indigenous fungal community in the salterns from all over the world. These species can be considered halophilic and are as such good model organisms for further investigations of the genetics, physiological properties and ecological roles of extremophilic eukaryotic species that, according to Pitt and Hocking[5], may belong to the most highly evolved microorganisms on earth.
Acknowledgements
This study was supported in part by the Federation of European Microbiological Societies (FEMS), the Slovenian Ministry of Education, Science and Sport and in part from the Technical Danish Research Council (Program for Predictive Biotechnology), and the Moshe Shilo Minerva centre for Marine Biogeochemistry, The Hebrew University of Jerusalem, Israel. We thank Prof. A. Oren for his help in collecting the samples in Israel and for careful reading of the manuscript.
References



