-
PDF
- Split View
-
Views
-
Cite
Cite
Václav Krištůfek, Sabine Fischer, Jens Bührmann, Andris Zeltins, Hildgund Schrempf, In situ monitoring of chitin degradation by Streptomyces lividans pCHIO12 within Enchytraeus crypticus (Oligochaeta) feeding on Aspergillus proliferans, FEMS Microbiology Ecology, Volume 28, Issue 1, January 1999, Pages 41–48, https://doi.org/10.1111/j.1574-6941.1999.tb00559.x
Close - Share Icon Share
Abstract
Enchytraeus crypticus (Oligochaeta) subsists on the fungus Aspergillus proliferans. The proliferation can be considerably enhanced by co-feeding the Gram-positive bacterium Streptomyces lividans pCHIO12 overproducing an exochitinase. In situ hybridizations with 23S rRNA-targeting, fluorescein-labelled oligonucleotides revealed that S. lividans pCHIO12 survives well within the gut of E. crypticus. Immunological analyses using a rhodamine-labelled α-chitin-binding protein (CHB1) allowed the conclusion that the bacterial enzyme degrades chitin within hyphae of A. proliferans. Our data show that the established in situ monitoring methods are valuable tools to analyze interactions between Enchytraeidae and microorganisms.
1 Introduction
The potworms, Enchytraeidae, are Oligochaeta which are highly abundant (up to 3×105 m−2) in the upper layers of acid organic soil with a relatively high moisture content. Within moder humus woodland sites, the number of enchytraeids may represent 5–10% of the total biomass. Enchytraeids mainly consume decaying organic material and therefore it is assumed that they play an important part in the turnover of nutrients in soil [1,2].
Enchytraeus crypticus [3] belongs to the family Enchytraeidae and lives in composts. Diet (quantity and quality) has been considered to be one of the most important ecological factors in determining population dynamics of invertebrates. Earlier studies [4] had indicated that enchytraeids are capable of digesting proteins, disaccharides, and various polysaccharides [5–7]. It was also shown that starch, xylan and laminarin were degraded most efficiently [7]. In addition, the enzymatic activities varied among different enchytraeid species [4,7,8]. However, more complex plant substances are thought to become accessible to enchytraeids through microbiological intervention [4,9]. Studies of the occurrence of fungi in enchytraeid intestines determined that fungi accounted for a high percentage of the gut content [10,11]. Nováková and Chalupský[12] isolated higher numbers of fungal species from enchytraeid excrements than from the surrounding soil. Recently, we showed that E. crypticus is attracted to various Streptomyces species by metabolites adhering to mycelia and (or) diffusing into the medium [13]. We suggested that it will be of special interest to test whether streptomycetes which are taken up by enchytraeids employ their enzymatic repertoire to enhance the efficiency of degradation in the guts of potworms.
Streptomycetes are Gram-positive bacteria which grow as substrate hyphae and, upon depletion of nutrients, differentiate to aerial mycelia and spores. Amendment of the soil with pure chitin or fungal mycelia led to a significantly enhanced growth of the endogenous Streptomyces strains [14,15]. Fungi are estimated to be the main contributors to soil biomass; they constitute about 70% of the total weight of the mass [16]. Therefore it has been assumed that streptomycetes play a major part in the decomposition of fungal and other chitins within soil [15,17].
Several chitinolytic enzymes were identified from various streptomycetes, including S. antibioticus[18], S. griseus[19], S. plicatus[20], and S. lividans[21]. With the help of a screening programme, Streptomyces olivaceoviridis was identified as the most efficient degrader of crystalline chitin [22], and five of its chitinases have been purified [23]. Previously we had succeeded in identifying an exochitinase gene (exo-chiO1) from S. olivaceoviridis, and after cloning, the corresponding gene was overexpressed in Streptomyces lividans[24]. The exochitinase (59 kDa) comprises a catalytic domain, an FnIII-module and a chitin-binding domain (12 kDa). The presence of the binding domain is a prerequisite for the efficient linking of the enzyme to insoluble substrates (i.e. crab shell, native chitin-containing fungal hyphae) which are then hydrolyzed by the catalytic domain [25,26]. An additional component of the chitinolytic system of streptomycetes is the presence of a chitin-binding protein, CHB1, which was shown to specifically interact with α-chitin [27,28].
In this paper we report that S. lividans carrying the plasmid pCHIO12 survives well in the gut of E. crypticus and degrades chitin within the cell wall of co-fed hyphae from the fungus A. proliferans.
2 Materials and methods
2.1 Organisms and culture conditions
E. crypticus individuals [3], provided by W. Westheide, University of Osnabrück, Germany, were propagated in the dark at 21°C on Petri dishes containing 1.2% agar in tap water, pH 7.2, and 2.0% oat flakes. For feeding experiments, the synchronized culture (young adults originating from cocoons of the same age, eight days old, and of about the same size) of E. crypticus individuals was washed three times in sterile tap water.
The chitinase overproducer S. lividans carrying the plasmid pCHIO12 [24] or the vector pIJ702 [29] were pre-cultured as shaking cultures (100 rpm) in liquid complete medium [27], supplemented with thiostrepton (10 μg ml−1), for one day at 30°C. Overnight cultures (20 ml) were diluted with 150 ml of fresh medium and then cultivated as standing cultures in 2-l Erlenmeyer flasks for 24 h. Mycelia were centrifuged (10 min, 4000×g) and washed three times in 5 mM K2HPO4/KH2PO4 buffer (pH 7.0). Simultaneously, 100 ml of yeast extract-malt extract medium (YEME, [29]) were inoculated with spores (to a concentration of 105 ml−1) of A. proliferans Tü134 (provided by H. Zähner, Tübingen) and cultivated as standing cultures at 30°C overnight in 2-l flasks. The cultures were subsequently diluted (30 ml of overnight culture plus 170 ml YEME in 2-l flasks) and incubated again for 24 h at 30°C. Fungal mycelia were centrifuged as described above, and washed two times in buffer (5 mM K2HPO4/KH2PO4, pH 7.0). For some experiments, A. proliferans mycelia were mixed at given ratios (see Section 3) with mycelia from S. lividans pIJ702 (control) or S. lividans pCHIO12 and incubated for 6 h at 30°C, prior to feeding.
2.2 Feeding experiments
Portions of each of the mycelia (0.2 g wet weight) from A. proliferans, S. lividans pCHIO12, or of a mixture of A. proliferans and S. lividans strains (see above) were put on a separate Petri dish (9 cm) which contained only 1.2% agar in tap water (pH 7.2). Ten E. crypticus young adults (synchronized culture) were placed on each plate and cultivated in the dark at a temperature of 21°C. The E. crypticus individuals (juveniles and adults) were counted over a period of six weeks (feeding experiment No. 1) or five weeks (feeding experiment No. 2) of incubation on four plates which had been arranged in parallel. Mean values were calculated from 3–4 replicates, and treatment comparisons were made using ANOVA, Scheffe test (Table 1).
| Feeding experiment no. | Fed microorganisms | Number of E. crypticus individualsa |
| 1 | A. proliferans | 682±75b |
| 2 | A. proliferans/S. lividans pIJ702 (20:1) | 627±75b |
| 3 | A. proliferans/S. lividans pCHIO12 (20:1) | 856±67c |
| Feeding experiment no. | Fed microorganisms | Number of E. crypticus individualsa |
| 1 | A. proliferans | 682±75b |
| 2 | A. proliferans/S. lividans pIJ702 (20:1) | 627±75b |
| 3 | A. proliferans/S. lividans pCHIO12 (20:1) | 856±67c |
Over a period of five weeks of incubation of E. crypticus, 0.2 g of mycelia were newly added as food every 5–7 days. The values represent the ±standard deviation (n= 3–4). The numbers followed by different letters significantly differ (P≤0.05, determined by ANOVA, Scheffe test).
| Feeding experiment no. | Fed microorganisms | Number of E. crypticus individualsa |
| 1 | A. proliferans | 682±75b |
| 2 | A. proliferans/S. lividans pIJ702 (20:1) | 627±75b |
| 3 | A. proliferans/S. lividans pCHIO12 (20:1) | 856±67c |
| Feeding experiment no. | Fed microorganisms | Number of E. crypticus individualsa |
| 1 | A. proliferans | 682±75b |
| 2 | A. proliferans/S. lividans pIJ702 (20:1) | 627±75b |
| 3 | A. proliferans/S. lividans pCHIO12 (20:1) | 856±67c |
Over a period of five weeks of incubation of E. crypticus, 0.2 g of mycelia were newly added as food every 5–7 days. The values represent the ±standard deviation (n= 3–4). The numbers followed by different letters significantly differ (P≤0.05, determined by ANOVA, Scheffe test).
2.3 In situ hybridizations
E. crypticus individuals were washed in PBS-buffer (140 mM NaCl, 2.7 mM KCl, 10.1 mM Na2HPO4, and 1.8 mM KH2PO4), and immediately fixed in 4% paraformaldehyde solution in buffer (130 mM NaCl, 10 mM sodium phosphate) for 24 h at 4°C. Afterwards, each potworm was washed in PBS-buffer and divided into three parts (region of pharynx plus esophagus, midgut or hindgut) with a scalpel. Further thin sectioning (up to 35 μm) was performed with a Cryo-cut 1800 device (Leica). The cryo-cuts (samples) were washed in PBS-buffer and then collected on gelatin-coated slides (0.1% gelatin, 0.01% KCr(SO4)2), washed in PBS and fixed in 50% ethanol for 1 h. The samples were stored in PBS/96% ethanol (1:1, v/v) at −20°C. Fixed material was immobilized by drying for 15 min at 46°C. A series of immersions in 50%, 80%, and 96% (v/v) ethanol (3 min each) completed the fixation.
Hybridizations with an oligonucleotide were carried out as described by Roller et al. [30]. The oligonucleotide 5′-TATAGTTACCTCCGCCGT-3′ corresponds to a region conserved within the 23S rRNA gene from Gram-positive bacteria (including streptomycetes), the DNA of which contains a high amount of G+C [30]. The oligonucleotide probe was commercially synthesized with a C6-TFA aminolinker (6-(trifluoracylamino)hexyl-(2-cyanoethyl)-(N,N-diisopropyl)phosphoramidite) at the 5′-end, and labelled with 5(6)-carboxyfluorescein-N-hydroxysuccinimide ester (MWG Biotech).
The inspection of the samples was performed under UV light with a Zeiss-Axiovert microscope. Photographs were taken with Kodak EPY64T film.
2.4 In situ localization of chitin
The samples were placed on gelatin-coated glass slides, washed three times for 10 min in PBS buffer (1.36 M NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, pH 7.2), stored and immobilized, as described in the previous section. The dried samples were kept at room temperature for 10 min, then washed three times in PBS and once in labelling buffer (0.4% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate), 0.05 M KH2PO4, pH 7, 0.05 M K2HPO4, pH 7, 1 M NaCl) for 5 min. CHB1 was labelled as described [28], except that fluorescein isothiocyanate was replaced by rhodamine isothiocyanate to attain a labelling degree of 1:1. The rhodamine-labelled CHB1 was suspended in labelling buffer to a final concentration of 100 μg ml−1, and 10 μl were dropped onto each sample. After storage at 4°C in the dark overnight, the sample was washed twice in PBS, covered with 10 μl of H2O, and sealed with a cover slip. The samples were inspected under UV light with a Zeiss-Axiovert microscope connected with a CCD-camera (Sensys type, Photometrics) or a Zeiss confocal laser microscope.
3 Results and discussion
3.1 Feeding experiments
Ten Enchytraeus crypticus individuals were placed on an agar plate lacking nutrients and fed every 5–7 days with 0.2-g portions of mycelia from Aspergillus proliferans, and 10 other individuals were fed with corresponding quantities of Streptomyces lividans pCHIO12 mycelia. The numbers of the progeny were counted. When fed with S. lividans pCHIO12 or A. proliferans, the numbers of E. crypticus increased by 200- or 160-fold, respectively, in the course of six weeks. From these results, it was concluded that the cell wall of the bacterium S. lividans which consists mainly of peptidoglycan (murein) and teichoic acid was degraded by enzymes residing in the gut of E. crypticus. The cell wall of the fungus A. proliferans contains, in addition to mannans and glucans, a high percentage of chitin. Therefore we assumed that the enzymatic repertoire of E. crypticus is not sufficient to degrade A. proliferans mycelia to the same extent it hydrolyzes Streptomyces mycelia. The possible contribution of a defined Streptomyces chitinase activity was tested in co-feeding experiments.
The Streptomyces olivaceoviridis exochitinase gene (exo-chiO1) had previously been cloned into the vector pIJ702 and the resulting hybrid plasmid pCHIO12 was transformed into S. lividans[24]. The transformant S. lividans pCHIO12 secretes high quantities of a 59-kDa exochitinase which hydrolyzes the chitin within A. proliferans mycelia [25,26], in contrast to the S. lividans pIJ702 containing only the cloning vector and not the cloned exo-chiO1 gene. Using identical quantities of mycelia from A. proliferans or a mixture (20:1) of A. proliferans and the control strain S. lividans pIJ702, the numbers of E. crypticus individuals (initially 10) increased by about 68- and 62-fold, respectively, in the course of five weeks. This indicated that the nutrional value of A. proliferans mycelia alone corresponded to that of the mixture. To test whether the nutrional value of A. proliferans mycelia could be enhanced by the strain secreting the 59-kDa chitinase, A. proliferans was mixed with S. lividans pCHIO12 (20:1). Under these conditions, the numbers of E. crypticus individuals (initially 10) increased by 85-fold. The sizes of the offspring were equal in all experiments. We therefore concluded that the exochitinase of S. lividans pCHIO12 causes the degradation of the chitinous layer of A. proliferans, and that, as a consequence, the fungal mycelia is more easily digested by E. crypticus.
3.2 In situ detection of microorganisms
Thin sections (cryo-cuts) of E. crypticus were analyzed by phase contrast microscopy and inspected for microorganisms (Fig. 1). Intact as well as partially degraded mycelia from A. proliferans were easily identified in the gut. In contrast, hyphae from S. lividans pCHIO12 (whose diameter is only about 1/10 of that of hyphae from A. proliferans) were barely identifiable among the fungal hyphae. Hybridization techniques using fluorescent probes have been developed [30] to rapidly detect bacteria in situ [31]. Thus a labelled oligonucleotide [30] complementary to a part within the 23S rRNA of streptomycetes was utilized for in situ hybridizations. S. lividans pCHIO12 hyphae could then unambiguously be identified, displaying green fluorescence under UV light. As expected, a control hybridization with an unlabelled oligonucleotide did not lead to fluorescent hyphae (Fig. 1).
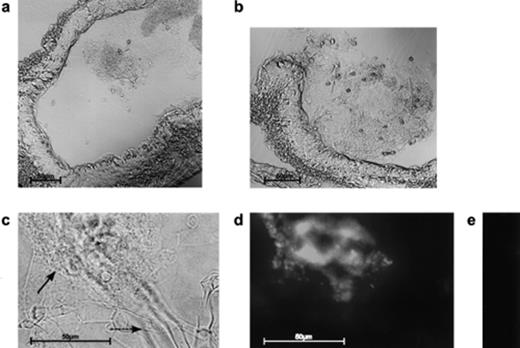
Detection of microorganisms. In cryo-cuts of the mid-gut of E. crypticus, the mixture of fungal and Streptomyces hyphae can be identified under visual light (a, b, c). A higher magnification (c) allowed the identification of the A. proliferans hyphae (—→), which are partly surrounded by a dense network of S. lividans pCHIO12 hyphae (→). Treatment of the hyphae with fluorescein-labelled (d) or unlabelled (control) (e) oligonucleotides characteristic for the 23S rRNA of G+C-rich bacteria (see Section 2) enabled us to additionally identify the Streptomyces hyphae and to inspect them under UV light (d, e).
3.3 Degradation of α-chitin
To assess whether a degradation of chitin took place, we used the chitin-binding protein CHB1 (18.7 kDa) [27,28], which interacts highly specifically with α-chitin, consisting of antiparallelly arranged polymer chains of N-acetylglucosamine, but not with β-chitin having parallelly arranged polymer chains [27]. Thus fluorescein- or rhodamine-labelled CHB1 is a valuable tool to localize crystalline α-chitin in natural biological samples, including hyphae of fungi like A. proliferans[27,28]. When E. crypticus had been fed with a mixture (20:1) of A. proliferans and the exochitinase overproducer S. lividans pCHIO12, a large portion of the Aspergillus hyphae did no longer bind CHB1. In contrast, in the control experiment prior to feeding, CHB1 interacted with Aspergillus hyphae. When S. lividans pCHIO12 was, however, replaced by the control strain (S. lividans containing only the vector pIJ702), CHB1 interacted strongly with the A. proliferans hyphae (Fig. 2, bottom). It can therefore be concluded that S. lividans pCHIO12 degraded most chitin in the fungal hyphae within the gut of E. crypticus (Fig. 2, middle). The control S. lividans pIJ702, in contrast, did not degrade a significant level of chitin within the A. proliferans hyphae. Using antibodies raised against the exochitinase, it could also be verified that the enzyme acts in the gut of E. crypticus fed with S. lividans pCHIO12, but not in E. crypticus fed with S. lividans pIJ702 (data not shown). The studies revealed that the nutrional value of A. proliferans for E. crypticus was enhanced by S. lividans pCHIO12 overproducing the exochitinase.
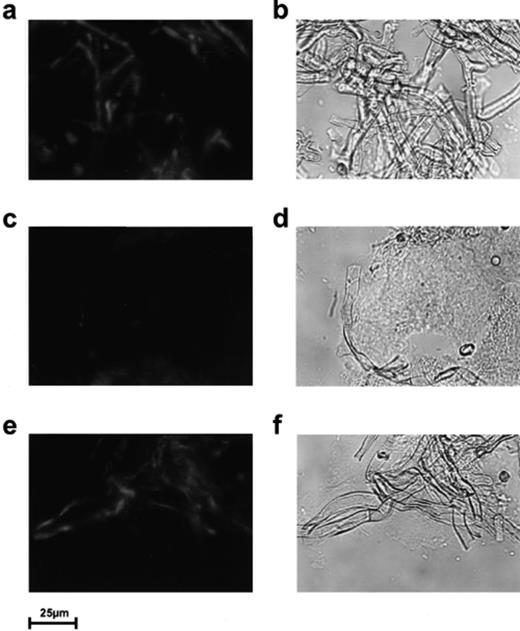
Localization of chitin. As control experiment, intact A. proliferans hyphae were inspected prior to feeding (a, b). Cryo-cuts of the gut of E. crypticus (which had been fed with a mixture of A. proliferans and S. lividans pCHIO12 (c, d) or with A. proliferans and S. lividans pIJ702 (control) (e, f)) were treated with rhodamine-labelled CHB1, as described in Section 2. Samples were inspected by confocal laser microscopy under visual light (b, d, f) (right) or under UV light (a, c, e) (left).
3.4 Conclusions
Our data show that the established in situ monitoring methods are valuable tools to analyze interactions between Enchytraeidae and microorganisms. Previous studies [10,11] had revealed that Enchytraeidae are abundant in soil and that under natural conditions their gut contains also fungal mycelia. Contrary to many other worms, E. crypticus can easily be cultivated under laboratory conditions, its generation time is relatively short. Although streptomycetes are ubiquitous in any soil type (they may make up up to 50% of its total bacterial population) [32] and degrade, in addition to chitin, lignocellulose, xylan, starch, proteins and many other substances, little is known about their interaction with other organisms. Interestingly, streptomycetes were isolated from the gut of earthworms like Lumbricus rubellus, Octolasion montanum or Aporrectodea rosea[13,33,34]. Moreover, Streptomyces strains multiply in the gut of arthropods [35], and some have been isolated from pellets produced by millipedes and woodlice [36]. However, it has not yet been investigated whether these strains are merely passive survivors or interact specifically with the hosts.
In addition to many other bacteria, cellulolytic [37] and lignocellulolytic [38]Streptomyces strains were isolated from termites. Using the methods presented in this paper, experiments to investigate the in situ degradation of cellulose and other macromolecules could be designed. Our preliminary studies [25] have shown that a soil microcosm system can easily be set up for streptomycetes. In combination with the presented methods, essential tools are thus available to investigate and understand the multiple interactions occurring in the complex natural ecosystem.
Acknowledgements
We are grateful to W. Westheide and M. Müller for E. crypticus cultures and for having placed a Cryo-cut device at our disposal. M. Lemme supported the writing of the manuscript. The work was financed by funds of the Deutsche Forschungsgemeinschaft, Germany (Schr 203/6-2), and by a grant (No. 204/93/0254) awarded to V. Krištůfek by the Czech Grant Agency, Czech Republic.
References



