-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Lucia Specchia, Flavia Beccia, Maria Gabriella Cacciuttolo, Diego Maria Tona, Matteo Di Pumpo, Martina Porcelli, Alberto Lontano, Valerio Flavio Corona, Patrizia Laurenti, Stefania Boccia, Roberta Pastorino, An integrated pathway for influenza vaccination across primary and secondary care using a clinical decision support system: model definition and predictive impact analysis, European Journal of Public Health, Volume 34, Issue 6, December 2024, Pages 1117–1124, https://doi.org/10.1093/eurpub/ckae137
Close - Share Icon Share
Abstract
Influenza is an important public health issue given its significant burden of disease. In Italy, the unsatisfactory coverage rate in people ≥65 years underlines the need to improve the current vaccination pathway. This study aims to define an integrated pathway across primary and secondary care, facilitated by a digital clinical decision support system (CDSS), to enhance vaccination coverage in people ≥65 years by actively recruiting patients in hospitals and administering vaccination. Moreover, the study seeks to gauge the potential epidemiological and economic impact of this approach. The methodology consisted of two main phases: definition of the integrated pathway and CDSS and estimation of the potential epidemiological and economic impact resulting from the implementation of the pathway in the whole Lazio region. Assuming an increase of influenza vaccination coverage from the current rate of 60% to 65% in ≥65 years old population in the Lazio region thanks to the pathway implementation, an increase of 8% in avoided influenza cases, avoided influenza- or pneumonia-related hospitalizations and avoided influenza-related outpatient visits was estimated with a relative increase in savings for hospitalizations and outpatient visits of up to 11.85%. Setting the vaccination coverage at 70%, the impact is doubled. Alongside offering a predictive estimate of the pathway’s potential impact, both epidemiological and economic, this project, with its robust methodology, may serve as a scalable and transferable model for enhancing vaccination coverage at national and international level.
Creating an integrated pathway across primary and secondary care would effectively enhance influenza vaccination coverage in people aged ≥65 years in Lazio region in Italy, from a predictive impact analysis results.
Using a clinical decision support system for in-hospital recruitment could benefit the in-hospital recruitment of eligible patients.
An increase of influenza vaccination coverage from 60% to 65%, among people aged ≥65 years, would resulting in 8% decrease of influenza cases, influenza- or pneumonia-related hospitalizations, and influenza-related outpatient visits.
We estimate up to 11.85% relative increase in savings for hospitalizations and outpatient visits with the implementation of integrated pathway, highlighting the potential for doubling the impact with a vaccination coverage rate of 70%.
The proposed model of an integrated pathway is a scalable and transferable model for enhancing vaccination coverage at national and international level.
Introduction
Influenza is an important public health issue given its significant epidemiological and economic burden of disease. Within Europe, the European Centre for Disease Prevention and Control (ECDC) estimates 50 million symptomatic cases annually, with a variable mortality ranging from 15 000 to 70 000 individuals [1]. In Italy, a country of 60 million inhabitants, between 4% and 9% contract influenza each year with approximately 8000 fatal cases [2]. Excess death rates for the elderly have been estimated to be over six times higher than in the general population, with the majority of influenza-related deaths (65–96%) occurring in individuals aged ≥65 years [3].
According to a cost-benefit analysis on the economic and fiscal implications of influenza, pneumococcal, and herpes zoster vaccines in Italy, investing in influenza vaccination results in benefits that are 1.8 times the initial investment in terms of fiscal impact and 11.1 times in terms of productivity loss [4].
Given these considerations and in alignment with the guidelines of the World Health Organization Immunization Agenda 2030 [5], the Italian Vaccine Prevention Plan (PNPV) [6] recognizes as a public health priority the reduction of the burden of vaccine-preventable infectious diseases, through the identification of effective and homogeneous strategies to be implemented at national level.
Concerning influenza, yearly the Italian Health Ministry provides recommendations for seasonal disease prevention and control identifying people categories for which flu vaccination is highly recommended [7]. People aged ≥65 years as well as people of all ages with underlying pathologies increasing the risk of complications following the infection are the main recipients of the flu vaccine offer. Currently, influenza vaccination coverage for ≥65 population stands at 56.70% in Italy and at 60.20% in the Lazio region, with even lower rates in other regions, which fail to reach the 40% threshold [8]. These statistics highlight the urgent need for strategies and actions aimed at promoting new vaccination pathways, closer to healthcare places including Hospital, to improve vaccination adherence and coverage. However, initiatives focused on significantly improving vaccination coverage through a genuinely patient-centered approach are still lacking. Furthermore, Mission 6 of the National Recovery and Resilience Plan (PNRR) aims to leverage opportunities for improving health service through technological innovation, including the integration of clinical and administrative data [9]. This need stems from Italy’s lag in digitizing both public and private healthcare compared with other European countries.
Nonetheless, experiences of integrating new technologies both at Hospital and community level to enhance vaccination coverage remain few, fragmented and uncoordinated. While virtuous examples exist, such as the Digital Vaccine Record available in selected regions through the patient’s Public Digital Identity System (SPID), the absence of systems enabling the automatic and continuous identification of patients needing vaccination based on specific risk conditions remains a notable gap.
In this context, the Italian Health Ministry, as complementary funding to the PNRR, funded the DigitAl lifelong pRevEntion (DARE) project aimed at: promoting technologically advanced models and solutions for surveillance, prevention, health promotion, and health safety; applying innovative digital tools, both in the general population and at-risk categories, helping to bridge social and territorial disparities in the offer of integrated social-health services [10].
This study, which is part of the DARE project, aims to define an integrated pathway across primary and secondary care enhanced by a digital clinical decision support system (CDSS) tool to increase influenza vaccination coverage among patients aged ≥65 years in the Lazio region, through the active patient in-Hospital recruitment and the subsequent vaccination. Moreover, a predictive estimate of the pathway potential impact, both in terms of epidemiological and economic influenza burden of disease, is provided.
Methods
The methodology adopted comprised two phases. The first phase involved defining the integrated pathway and the digital CDSS that it is enhanced by. The second phase entailed assessing the epidemiological and economic potential impact resulting from the pathway implementation throughout the whole Lazio region and the consequent increased regional influenza vaccination coverage.
For the purposes of this study, the terms ‘primary care’ and ‘secondary care’ are defined as follows:
The term ‘primary care’ is used to describe a coordinated and integrated complex of health and social-health services, delivered in care settings that are situated as close as possible to the places where the patient and their family reside. This is often referred to as ‘first level care’. The objective is to safeguard the overall health of the population through the promotion and prevention of illness, as well as the treatment of prevalent pathologies, both chronic and acute, through the implementation of appropriate mechanisms to ensure continuity of care. The primary care sector is responsible for a number of key areas, including the provision of care by family physicians, paediatricians and continuity of care physicians; the delivery of home care; and the management of patients with complex needs, including the elderly, disabled and dependent individuals [11, 12].
Secondary care, on the other hand, is specialist care provided on an ambulatory or inpatient basis, typically following a referral from a primary care physician. It requires a higher level of specialisation than primary care can provide, including more sophisticated knowledge, skills and equipment [13].
Definition of the integrated pathway
The definition of the integrated pathway involved the following steps:
Creation of a multidisciplinary working group (WG) featuring cross-sectoral expertise, including public health experts and health services researchers, health managers, healthcare professionals from both hospitals and local health authorities (LHAs), general practitioners, as well as IT engineers and technicians;
WG periodic meetings focused on:
The assessment of the feasibility of the whole integrated pathway across primary and secondary care;
the development of an algorithm to identify eligible patients among those accessing hospital;
the definition of data feeding the algorithm and the identification of data sources/flows;
the definition of the patient journey and its flow-chart representation.
Predictive estimate of the potential impact of the integrated pathway
The estimate was obtained through the development of a model to assess the epidemiological and economic impact of influenza vaccination in people ≥65 years in the Italian region of Lazio.
For the purposes of analysis, the following indicators were considered:
burden of influenza disease among adults ≥65 years, expressed in terms of laboratory confirmed influenza cases [14], influenza- or pneumonia-related hospitalizations [15] and influenza-related outpatient visits (Supplementary Table S1) [16];
vaccine effectiveness of the seasonal influenza vaccine among adults ≥65 years, expressed in terms of avoided cases of: influenza [17], influenza- or pneumonia-related hospitalizations [17] and influenza-related outpatient visits (Supplementary Table S2) [18];
unit costs of outpatient visits and hospitalizations for influenza (Supplementary Table S3) [19].
For each indicator, data were retrieved and extracted from institutional databases [14, 15] and scientific literature [16–18].
The starting point was the influenza burden of disease, as reported by institutional bodies/scientific evidence (Supplementary Tables S1 and S3) [14–16, 19], in the Lazio region population aged ≥65 years (1 308 040 people in 2023) [20].
The predictive estimate of the epidemiological and economic potential impact of the integrated pathway was based on a scenario analysis. Three scenarios were explored, differing only in the vaccination coverage rate, with all other considered data remaining unchanged.
In scenario 1, the impact on vaccination among the ≥65 years Lazio region population was assessed based on the current vaccination coverage rate (60.20%) [8]. Scenarios 2 and 3 analyses were based on the intermediate (65%) and final (70%) desired coverage rates according to PNPV [21]. The difference between the three scenarios served as an estimate of the potential impact of the implementation of the integrated pathway supported by the digital CDSS and the consequent increased vaccination coverage rate.
a. Epidemiological impact analysis
Based on vaccine effectiveness values reported by scientific evidence (Supplementary Table S2) [17, 18], the impact of influenza vaccination was assessed among the Lazio region population ≥65 in terms of number of avoided influenza cases [17], hospitalizations for influenza and/or influenza-related pneumonia [17], and influenza-related outpatient visits [18].
b. Economic impact analysis
The estimated avoided costs were calculated by multiplying the number of avoided influenza- or pneumonia-related hospitalizations and outpatient visits applying by their respective unit costs (Supplementary Table S3) [19].
The analyses were performed on Microsoft Excel (Microsoft Office Excel 365), and a sheet with the formulae adopted is provided as Supplementary File S1.
Results
The integrated pathway: model definition
The model is depicted in the flow diagram shown in Figure 1.
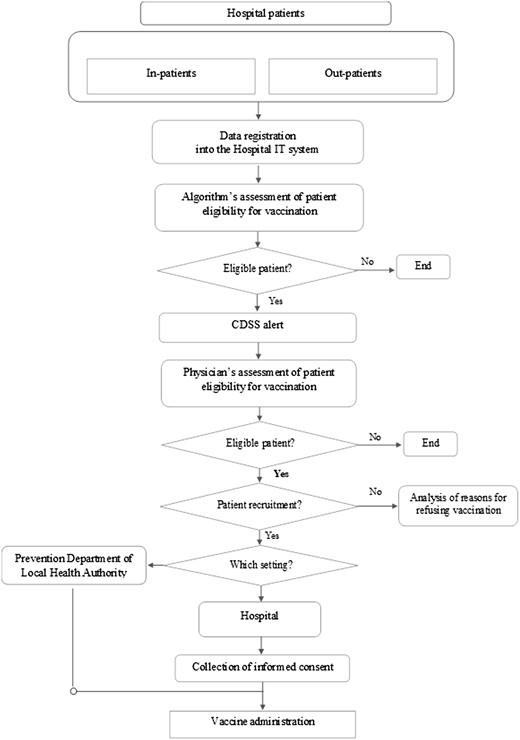
Flow-chart of the CDSS-based integrated pathway for influenza vaccination.
The patient journey starts with the identification of eligible patients (age ≥65 years) for influenza vaccination among those accessing the hospital. This identification is based on a CDSS generating alerts to remind clinicians automatically about the need for influenza vaccination in patients aged ≥65 years. The CDSS relies on a digital algorithm, which (i) retrieves age data from the Hospital Information System and (ii) cross-references these data with those from the Regional Vaccine Registry to obtain information on vaccination history and exclude already vaccinated patients from the alert generation.
The CDSS tool is integrated into the front-end of the Hospital Information System in such a way as to provide clinicians with an alert signal becoming visible in the patient’s electronic health record (EHR) once patient eligibility has been verified by the algorithm.
The algorithm’s assessment of patient eligibility for vaccination is subsequently complemented by the in-person assessment carried out by the clinician, who confirms the patient’s suitability (excluding clinical contraindications) and obtains vaccination consent. Reasons for refusing vaccination are investigated in line with PNPV recommendations [6].
Upon completing the eligibility assessment, in-patients are offered vaccination within the hospital, integrated into the treatment plan. Out-patients have a dual option: vaccination in the hospital or at the prevention department of the LHA, traditionally responsible for vaccination activities. In the latter case, selected patients are contacted for appointment booking, reminders and useful information.
The choice of vaccination setting is at the physician’s discretion, considering factors like physical impairment or logistical challenges. Before vaccination, in the chosen setting, the physician administers and collects the patient’s informed consent.
The potential impact of the integrated pathway
This section reports the results of the scenario analyses carried out based on the 60.20% current vaccination coverage rate (scenario 1) and the intermediate 65% (scenario 2) and final 70% (scenario 3) desired coverages (Table 1).
| . | Scenario 1 current vaccination coverage rate (60.2%) . | Scenario 2 vaccination coverage rate at 65% . | Delta scenario 2 vs scenario 1 . | Delta % scenario 2 vs scenario 1 . | Scenario 3 vaccination coverage rate at 70% . | Delta scenario 3 vs scenario 1 . | Delta % scenario 3 vs scenario 1 . |
|---|---|---|---|---|---|---|---|
| Avoided cases | 1517.76 (724.39–2069.68) | 1638.78 (782.15–2234.70) | 121.02 (57.76–165.02) | 7.97% (3.80–10.87%) | 1764.84 (842.31–2406.60) | 247.08 (117.92–336.92) | 16.27% (7.77–22.20%) |
| Avoided hospitalizations | 269.41 (153.95–352.80) | 290.89 (166.22–380.93) | 21.48 (12.27–28.13) | 7.97% (4.56–10.44%) | 313.26 (179.01–410.23) | 43.86 (25.06–57.43) | 16.27% (9.30–21.32%) |
| Avoided costs for hospitalizations | 808 225.35–1 616 450.70 (461 844.00–2 116 785.00) | 872 668.56–1 745 337.13 (498 667.75–2 285 560.53) | 64 443.22–128 886.40 (36 823.75–168 775.53) | 939 796.92–1 879 593.83 (537 026.81–2 461 372.88) | 131 571.60–263 143.10 (75 182.81–344 587.88) | ||
| Avoided outpatient visits | 123 708.41 (59 770.95–183 902.38) | 133 572.20 (64 536.74–198 565.69) | 9863.79 (4765.79–14 663.31) | 7.97% (3.85–11.85%) | 143 846.99 (69 501.11–213 839.98) | 20 138.58 (9730.16–29 937.60) | 16.27% (7.86–24.20%) |
| Avoided costs for outpatient visits | 2 474 168.23–29 690 018.75 (1 195 419.08–44 136 570.84) | 2 671 444.1–32 057 329.21 (1 290 734.89–47 655 765.86) | 197 275.90–2 367 310.00 (95 315.81–3 519 195.02) | 2 876 939.80–34 523 277.61 (1 390 022.19–51 321 594.00) | 402 771.60–4 833 259.00 (194 603.11–7 185 023.16) |
| . | Scenario 1 current vaccination coverage rate (60.2%) . | Scenario 2 vaccination coverage rate at 65% . | Delta scenario 2 vs scenario 1 . | Delta % scenario 2 vs scenario 1 . | Scenario 3 vaccination coverage rate at 70% . | Delta scenario 3 vs scenario 1 . | Delta % scenario 3 vs scenario 1 . |
|---|---|---|---|---|---|---|---|
| Avoided cases | 1517.76 (724.39–2069.68) | 1638.78 (782.15–2234.70) | 121.02 (57.76–165.02) | 7.97% (3.80–10.87%) | 1764.84 (842.31–2406.60) | 247.08 (117.92–336.92) | 16.27% (7.77–22.20%) |
| Avoided hospitalizations | 269.41 (153.95–352.80) | 290.89 (166.22–380.93) | 21.48 (12.27–28.13) | 7.97% (4.56–10.44%) | 313.26 (179.01–410.23) | 43.86 (25.06–57.43) | 16.27% (9.30–21.32%) |
| Avoided costs for hospitalizations | 808 225.35–1 616 450.70 (461 844.00–2 116 785.00) | 872 668.56–1 745 337.13 (498 667.75–2 285 560.53) | 64 443.22–128 886.40 (36 823.75–168 775.53) | 939 796.92–1 879 593.83 (537 026.81–2 461 372.88) | 131 571.60–263 143.10 (75 182.81–344 587.88) | ||
| Avoided outpatient visits | 123 708.41 (59 770.95–183 902.38) | 133 572.20 (64 536.74–198 565.69) | 9863.79 (4765.79–14 663.31) | 7.97% (3.85–11.85%) | 143 846.99 (69 501.11–213 839.98) | 20 138.58 (9730.16–29 937.60) | 16.27% (7.86–24.20%) |
| Avoided costs for outpatient visits | 2 474 168.23–29 690 018.75 (1 195 419.08–44 136 570.84) | 2 671 444.1–32 057 329.21 (1 290 734.89–47 655 765.86) | 197 275.90–2 367 310.00 (95 315.81–3 519 195.02) | 2 876 939.80–34 523 277.61 (1 390 022.19–51 321 594.00) | 402 771.60–4 833 259.00 (194 603.11–7 185 023.16) |
| . | Scenario 1 current vaccination coverage rate (60.2%) . | Scenario 2 vaccination coverage rate at 65% . | Delta scenario 2 vs scenario 1 . | Delta % scenario 2 vs scenario 1 . | Scenario 3 vaccination coverage rate at 70% . | Delta scenario 3 vs scenario 1 . | Delta % scenario 3 vs scenario 1 . |
|---|---|---|---|---|---|---|---|
| Avoided cases | 1517.76 (724.39–2069.68) | 1638.78 (782.15–2234.70) | 121.02 (57.76–165.02) | 7.97% (3.80–10.87%) | 1764.84 (842.31–2406.60) | 247.08 (117.92–336.92) | 16.27% (7.77–22.20%) |
| Avoided hospitalizations | 269.41 (153.95–352.80) | 290.89 (166.22–380.93) | 21.48 (12.27–28.13) | 7.97% (4.56–10.44%) | 313.26 (179.01–410.23) | 43.86 (25.06–57.43) | 16.27% (9.30–21.32%) |
| Avoided costs for hospitalizations | 808 225.35–1 616 450.70 (461 844.00–2 116 785.00) | 872 668.56–1 745 337.13 (498 667.75–2 285 560.53) | 64 443.22–128 886.40 (36 823.75–168 775.53) | 939 796.92–1 879 593.83 (537 026.81–2 461 372.88) | 131 571.60–263 143.10 (75 182.81–344 587.88) | ||
| Avoided outpatient visits | 123 708.41 (59 770.95–183 902.38) | 133 572.20 (64 536.74–198 565.69) | 9863.79 (4765.79–14 663.31) | 7.97% (3.85–11.85%) | 143 846.99 (69 501.11–213 839.98) | 20 138.58 (9730.16–29 937.60) | 16.27% (7.86–24.20%) |
| Avoided costs for outpatient visits | 2 474 168.23–29 690 018.75 (1 195 419.08–44 136 570.84) | 2 671 444.1–32 057 329.21 (1 290 734.89–47 655 765.86) | 197 275.90–2 367 310.00 (95 315.81–3 519 195.02) | 2 876 939.80–34 523 277.61 (1 390 022.19–51 321 594.00) | 402 771.60–4 833 259.00 (194 603.11–7 185 023.16) |
| . | Scenario 1 current vaccination coverage rate (60.2%) . | Scenario 2 vaccination coverage rate at 65% . | Delta scenario 2 vs scenario 1 . | Delta % scenario 2 vs scenario 1 . | Scenario 3 vaccination coverage rate at 70% . | Delta scenario 3 vs scenario 1 . | Delta % scenario 3 vs scenario 1 . |
|---|---|---|---|---|---|---|---|
| Avoided cases | 1517.76 (724.39–2069.68) | 1638.78 (782.15–2234.70) | 121.02 (57.76–165.02) | 7.97% (3.80–10.87%) | 1764.84 (842.31–2406.60) | 247.08 (117.92–336.92) | 16.27% (7.77–22.20%) |
| Avoided hospitalizations | 269.41 (153.95–352.80) | 290.89 (166.22–380.93) | 21.48 (12.27–28.13) | 7.97% (4.56–10.44%) | 313.26 (179.01–410.23) | 43.86 (25.06–57.43) | 16.27% (9.30–21.32%) |
| Avoided costs for hospitalizations | 808 225.35–1 616 450.70 (461 844.00–2 116 785.00) | 872 668.56–1 745 337.13 (498 667.75–2 285 560.53) | 64 443.22–128 886.40 (36 823.75–168 775.53) | 939 796.92–1 879 593.83 (537 026.81–2 461 372.88) | 131 571.60–263 143.10 (75 182.81–344 587.88) | ||
| Avoided outpatient visits | 123 708.41 (59 770.95–183 902.38) | 133 572.20 (64 536.74–198 565.69) | 9863.79 (4765.79–14 663.31) | 7.97% (3.85–11.85%) | 143 846.99 (69 501.11–213 839.98) | 20 138.58 (9730.16–29 937.60) | 16.27% (7.86–24.20%) |
| Avoided costs for outpatient visits | 2 474 168.23–29 690 018.75 (1 195 419.08–44 136 570.84) | 2 671 444.1–32 057 329.21 (1 290 734.89–47 655 765.86) | 197 275.90–2 367 310.00 (95 315.81–3 519 195.02) | 2 876 939.80–34 523 277.61 (1 390 022.19–51 321 594.00) | 402 771.60–4 833 259.00 (194 603.11–7 185 023.16) |
Scenario 1—Current vaccination coverage rate at 60.2%
Considering data on the number of inhabitants ≥65 years in the Lazio region [20], the regional incidence of influenza cases [16] and current vaccination coverage rate [8], and the vaccine effectiveness in preventing influenza cases in the target population [17], 1517.76 (95% CI: 724.39–2069.69) flu cases prevented in the Lazio region population ≥65 years were estimated.
Taking into account the rate of influenza- or pneumonia-related hospitalizations [17] and the vaccine effectiveness in preventing them in the target population [19], 269.41 (95% CI: 153.95–352.80) hospitalizations were estimated as avoided in the population ≥65 years in the Lazio region. Considering hospitalizations’ unit costs [21], average savings of up to 808 225.35–1 616 450.70 (95% CI: 461 844.00–2 116 785.00) euros were estimated.
Based on the rate of influenza-related outpatient visits [18] and the vaccine effectiveness in preventing them in the target population [20], 123 708.41 (95% CI: 59 770.95–183 902.38) avoided outpatient visits were calculated. Considering the large variability in the relative costs [21], the estimated savings range from 2 474 168.23 to 29 690 018.75 (95% CI: 1 195 419.08–44 136 570.84) euros.
Scenario 2—Vaccination coverage rate at 65%
Considering a 65% vaccination coverage rate in the Lazio region population ≥65 years, 1638.78 (95% CI: 782.15–2234.70) preventable flu cases and 290.89 (95% CI: 166.22–380.93) preventable influenza- or pneumonia-related hospitalizations were estimated, with relative savings of up to 872 668.56–1 745 337.13 (95% CI: 498 667.75–2 285 560.53) euros.
Moreover, at this coverage rate, flu vaccination could prevent 133 572.20 (95% CI: 64 536.74–198 565.69) outpatient visits with estimated savings ranging from 2 671 444.10 to 32 057 329.21 (95% CI: 1 290 734.89–47 655 765.86) euros.
Scenario 3—Vaccination coverage rate at 70%
Setting the vaccination coverage at 70% in the Lazio region population ≥65 years, 1764.84 (95% CI: 842.31–2406.60) cases of influenza and 313.26 (95% CI: 179.01–410.23) influenza- or pneumonia-related hospitalizations were estimated as potentially preventable with consequent savings ranging from 939 796.92–1 879 593.83 (95% CI: 537 026.81–2 461 372.88) euros.
In addition, at this coverage rate, flu immunization could prevent 143 846.99 (95% CI: 69 501.11–213 839.98) outpatient visits with relative predicted cost savings from 2 876 939.80 to 34 523 277.61 (95% CI: 1 390 022.19–51 321 594.00) euros.
As for the predictive estimate of the integrated pathway potential impact, assuming an increase of influenza vaccination coverage from the current rate of 60.20% (scenario 1) to 65% (scenario 2) in the population ≥65 throughout the Lazio region following the pathway implementation, an increase of 8% in avoided influenza cases, avoided influenza- or pneumonia-related hospitalizations and avoided influenza-related outpatient visits were estimated with a relative increase in savings for hospitalizations and outpatients visits from 3.85% to 11.85%. Setting the vaccination coverage at 70% in the target population, an increase of 16.30% in avoided influenza cases, avoided hospitalizations and avoided outpatient visits were estimated with a relative increase in savings for hospitalizations and outpatients visits from 7.86% to 24.20%.
Discussion
The innovative pathway proposed by this study is aimed at promoting the integration of primary and secondary care, optimizing the use of resources and enhancing vaccination coverage among patients aged ≥65 years. Starting from the definition of the digital algorithm for the identification of eligible patients and the generation of CDSS alerts to clinicians, the model incorporates artificial intelligence (AI) into current hospital health statistics. Furthermore, it provides a patient pathway with the option of receiving flu vaccination within the hospital, thereby boosting vaccination compliance within the target population.
Pathways are a key tool for clinical governance, meeting healthcare needs across various settings while pursuing the best or most effective use of resources [22]. The implementation of an integrated pathway across primary and secondary care could ensure appropriate and safe continuity of care, establishing a functional link between the hospital’s highly complex level of care and that of the local health services.
The pathway proposed in this study mainly deals with influenza vaccination coverage, addressing people aged ≥65 years. Italian policies identify this specific population subgroup as an at-risk category for which to implement vaccination coverage, that consistently falls below the target [8]. Indeed, vaccine hesitancy and misconceptions cause concerns in older people that can challenge both vaccination adherence and coverage [23]. According to Stefanizzi et al. [24], age and comorbidities, particularly cardiovascular disease, diabetes and pulmonary disease, are factors that influence vaccine uptake, profiling themselves as contributors to vaccine hesitancy. On the other hand, according to Roller-Wirnsberger et al. [25] multiple chronic diseases and frequent access to routine healthcare may positively influence vaccine uptake, but lack of personalized information and relationships with healthcare providers are hindering factors. The doctor-patient relationship fostered by the innovative integrated pathway proposed could be instrumental in providing information on influenza, vaccination, vaccine eligibility criteria and side effects [26], addressing key vaccination hesitancy factors and promoting vaccination adherence. Moreover, beyond supporting continuity of care, the model also represents an example of personalization of a primary prevention program. This approach is not only functional for vaccination adherence but, more generally, it promotes the therapeutic alliance, yielding several beneficial outcomes [27].
In addition to the multiple highlighted opportunities associated with the integrated pathway and approach, patient engagement should also be considered [28]. In an Italian study on adherence to COVID-19 vaccination, health engagement has been found to be positively related to the intention to vaccinate, with this aspect partially mediated by the general attitude towards vaccines [29].
In the proposed model, the hospital-territory dualism is recomposed in the paradigm shift of primary prevention pathways in secondary care settings. It effectively captures about 20% of chronic patients requiring acute care, adding this percentage to the audience of traditional public health programs (territorial pathway, prevention departments) [30].
Bringing the initiative to the patient/citizen, through engagement and active offer of vaccination, has been proven to be effective in many contexts, such as in-hospital vaccinations for healthcare workers and in-company vaccinations for workers [31, 32].
Another relevant aspect of the integrated pathway is the adoption of an AI tool to identify suitable patients and improve their recruitment and adherence to vaccination. A study by Srinivasan et al. [33] proved that an American initiative towards hospitalized children based on EHR triggers, education provider and peer comparison increased the percentage of patients discharged with at least 1 dose of the vaccine by a factor of 5. According to another study by Chapman et al. [34], an EHR reminder of influenza vaccine uptake among eligible elderly hospitalized inpatients is associated with almost a 50% higher likelihood of vaccination. Furthermore, as reported by Stephens et al. [35], an EHR-based immunization reminder of 15 000 patients improved captured immunization opportunities in patients with chronic medical conditions. Other Authors report that EHR best practice alerts significantly increase vaccination rates in patients with chronic conditions receiving immunosuppressive medications, both in a hospital-based academic practice and in a community-based practice [36]. Moreover, according to Chen et al. [37], adding a machine learning algorithm to the EHR-based alerts could effectively increase the signal-to-noise ratio and positively impact the clinician’s interaction with these alerts, as well as the effectiveness of the tool. Digital tools hold great promise for fostering prevention programs [38], and the improvements in data collection, harmonization and sharing, and the widespread adoption of AI make this field even more promising [39]. Integrating hospitals into community-based prevention programs benefits from multiprofessional integration and cooperative effort with considerable consequences on vaccination rates [40]. Therefore, AI tools could add to the positive impact of an in-hospital pathway for vaccinations for elderly patients.
This article should be considered with some limitations and strengths.
To date, the proposed model is exclusively focused on flu vaccination and targeted to the elderly population accessing Hospitals in the Lazio region, Italy. However, it is easily scalable by incorporating additional vaccinations and considering other at-risk population subgroups. On patient consent, the co-administration mode could be proposed in order to simplify the vaccination schedule, by administering a maximum of two vaccines in the same vaccination session. Additionally, subsequent vaccination sessions could be scheduled with the patient, considering the opportunity offered by the pathway to flexibly change the setting based on the patient’s evolving conditions or needs. The implementation of the model is currently affected by the lack of full integration of information systems (hospital and LHA); however, it is expected that a higher level of integration will be achieved in the near future, thanks to national and international institutional efforts towards health data interoperability and systems integration [39]. Furthermore, the attempt to intertwine primary and secondary care activity flows for primary prevention for elderly patients is the first step in overcoming the historical dichotomy between the two settings.
As for the estimate of the integrated pathway’s potential impact, factors other than vaccination were not considered when explaining the avoided flu cases. In the hypothetical scenarios with vaccine coverage rates at 65% and 70%, the vaccine effectiveness data, the population ≥65 residing in Lazio, the hospitalization rates for influenza and pneumonia, and the prevalence of doctor visits were considered fixed as well as the ranges for costs. Additionally, data on other factors such as the virulence of the disease, the presence of factors limiting or promoting the spread of disease in the target population, and specific characteristics of the virus, vaccine or population were not taken into account.
These considerations may imply a potential overestimation of benefits of the integrated pathway implementation; however, it should be noted that the effects of herd immunity were not considered.
Additionally, data regarding hospitalization rates, outpatient visits and costs were extracted from institutional databases and scientific literature and referred to the study population, introducing an inherent error rate that cannot be precisely estimated. Nevertheless, any available error measures have been disclosed in the methods and considered in the calculations for the reported results. Regardless, it is crucial to recognize that this study provides an estimate of the potential benefits associated with the enhanced influenza vaccination coverage following the implementation of the integrated pathway in the Lazio region. The study does not aim to conduct a pre-post comparison or predictive analysis based on the actual trends in vaccination coverage.
To evaluate the real impact on the AI-driven integrated pathway implementation, a retrospective analysis of the project results will be needed. This analysis should consider the actual changes in vaccination coverage and burden of disease in terms of influenza cases, hospitalizations, outpatient visits, and associated direct and indirect costs.
Conclusion
Flu vaccination coverage is an extremely important public health issue, given the substantial influenza burden of disease. Despite numerous recommendations, Italy struggles with suboptimal coverage. Incentives for digital health and digital prevention under the PNRR can help to increase coverage through the development of specific projects and tools. This study introduces a model based on an integrated pathway across primary and secondary care supported by a digital tool, designed to boost influenza vaccination coverage rates among patients aged ≥65 in the Lazio Region.
Alongside offering a predictive estimate of the pathway’s potential impact, both epidemiological and economic, the project results, relying on a robust methodology, may serve as a scalable and transferable model for enhancing vaccination adherence and coverage nationally and internationally.
Supplementary data
Supplementary data are available at EURPUB online.
Conflict of interest: None declared.
Funding
This research was co-funded by the Complementary National Plan PNC-I.1 ‘Research initiatives for innovative technologies and pathways in the health and welfare sector’ D.D. 931 of 06/06/2022, DARE—DigitAl lifelong pRevEntion initiative, code PNC0000002, CUP: (B53C22006400001).
Data availability
The data underlying this article are available in the article and in its online Supplementary Material S1.
References
WHO. Immunization Agenda 2030, 2020. https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 (5 February 2024, date last accessed).
WHO. Primary Health Care, 2024. https://www.who.int/health-topics/primary-health-care (9 August 2024, date last accessed).
NHS England. The Healthcare Ecosystem. NHS Engl Digit 2022. https://digital.nhs.uk/developer/guides-and-documentation/introduction-to-healthcare-technology/the-healthcare-ecosystem (9 August 2024, date last accessed).
ISTAT. Health for All—Italia, 2023. https://www.istat.it/it/archivio/14562 (30 December 2023, date last accessed).
The European House Ambrosetti. Il Valore Della Vaccinazione Antinfluenzale,
Crump N. Integrated care pathways – re-engineering the NHS for clinical governance. Lancaster University: The Department of Organisation, Work and Technology. 2000. (Organisation, Work and Technology Working Paper Series).




Comments