-
PDF
- Split View
-
Views
-
Cite
Cite
Csaba Herczku, Csaba Kun, Istvan Edes, Zoltan Csanadi, Radiofrequency catheter ablation of premature ventricular complexes improved left ventricular function in a non-responder to cardiac resynchronization therapy, EP Europace, Volume 9, Issue 5, May 2007, Pages 285–288, https://doi.org/10.1093/europace/eum005
Close - Share Icon Share
Abstract
Frequent premature ventricular complexes (PVCs) have been demonstrated to cause tachycardiomyopathy in some individuals with a structurally normal heart. We report a patient with severe congestive cardiomyopathy which did not respond to cardiac resynchronization therapy (CRT). Ambulatory monitoring and interrogation of the device memory revealed frequent monomorphic PVCs that were considered a potential cause of the failure of CRT. Radiofrequency ablation of the focus at the postero-inferior left ventricle eliminated the arrhythmia, with a resultant rapid improvement in the clinical status and echo parameters. As PVCs are often associated with severe heart failure, the presence of frequent extrasystoles may be an underrecognized cause of a non-response to resynchronization therapy.
Introduction
Cardiac resynchronization therapy (CRT) has evolved as a highly successful treatment of congestive heart failure associated with an intra- and/or inter-ventricular conduction delay. Large-scale clinical trials have revealed improvements in quality of life and functional capacity, and more recently a mortality benefit. However, a subgroup of patients exhibits only a minimal or no response after a technically successful implant. This phenomenon has been recognized since the very beginning of the CRT era; several explanations have been proposed, including suboptimal patient selection or positioning of the left- and right ventricular electrodes, and difficulties in post-implantation device programming to maximize the haemodynamic benefit.
Isolated premature ventricular complexes (PVCs) have recently been implicated as a cause of impaired left ventricular function in case reports and retrospective analyses. 1–7 Frequent isolated ectopic beats, mostly originating from the right ventricular outflow tract have been reported as a cause of tachycardiomyopathy, a reversible form of congestive heart disease that resolves after elimination of the culprit arrhythmia. Most of the patients featuring in these reports were individuals with no organic heart disease and the ventricular dysfunction was attributed exclusively to the provoking arrhythmia. Premature ventricular complexes also occur in patients with organic heart disease, and especially those with poor left ventricular function. All patients who are candidates for CRT belong to this cohort. Somewhat surprisingly, we have found no publication in which the underlying mechanism of a non-response to resynchronization therapy was attributed to frequent PVCs. We now report a case in which we ascribe the lack of a response to CRT to frequent PVCs, and where a marked improvement was achieved by transcatheter elimination of the arrhythmia.
Case report
A 57-year-old male with non-ischaemic dilated cardiomyopathy was referred for CRT. He was in New York Heart Association (NYHA) III heart failure and was on optimal medical therapy, including carvedilol, an ACE inhibitor, digitalis, diuretics, and acenocoumarol. The 12-lead ECG showed left bundle branch block with a QRS duration of 140 ms. Transthoracic echocardiography demonstrated a dilated left ventricle with impaired systolic function and moderate mitral regurgitation ( Table 1 ). Tissue Doppler imaging (TDI) revealed an intraventricular wall motion delay, with the latest activation recorded at the posterior wall. Frequent ventricular ectopy (15% of all beats) and runs of non-sustained ventricular tachycardia were documented on 24-h ambulatory monitoring.
| . | Before implantation . | 6 weeks post-implantation . | 6 months post-implantation . | 6 months post-ablation . |
|---|---|---|---|---|
| Ejection fraction (%) | 20 | 18 | 16 | 28 |
| End diastolic diameter of the left ventricle (mm) | 85 | 82 | 80 | 79 |
| End systolic diameter of the left ventricle (mm) | 66 | 74 | 73 | 66 |
| Mitral regurgitation (grade) | II° | III° | III° | I° |
| . | Before implantation . | 6 weeks post-implantation . | 6 months post-implantation . | 6 months post-ablation . |
|---|---|---|---|---|
| Ejection fraction (%) | 20 | 18 | 16 | 28 |
| End diastolic diameter of the left ventricle (mm) | 85 | 82 | 80 | 79 |
| End systolic diameter of the left ventricle (mm) | 66 | 74 | 73 | 66 |
| Mitral regurgitation (grade) | II° | III° | III° | I° |
| . | Before implantation . | 6 weeks post-implantation . | 6 months post-implantation . | 6 months post-ablation . |
|---|---|---|---|---|
| Ejection fraction (%) | 20 | 18 | 16 | 28 |
| End diastolic diameter of the left ventricle (mm) | 85 | 82 | 80 | 79 |
| End systolic diameter of the left ventricle (mm) | 66 | 74 | 73 | 66 |
| Mitral regurgitation (grade) | II° | III° | III° | I° |
| . | Before implantation . | 6 weeks post-implantation . | 6 months post-implantation . | 6 months post-ablation . |
|---|---|---|---|---|
| Ejection fraction (%) | 20 | 18 | 16 | 28 |
| End diastolic diameter of the left ventricle (mm) | 85 | 82 | 80 | 79 |
| End systolic diameter of the left ventricle (mm) | 66 | 74 | 73 | 66 |
| Mitral regurgitation (grade) | II° | III° | III° | I° |
Resynchronization therapy was offered for the patient and an Insync III pacemaker (Medtronic Inc., Minneapolis, MN, USA) was implanted, with the left ventricular lead (Attain OTW, Medtronic Inc.) positioned in a posterior branch of the coronary sinus. The QRS duration was reduced to 120 ms ( Figure 1 ) after the implantation, and no significant intraventricular delay was observed on TDI echocardiography. Device was programmed to DDD at lower rate of 50 bpm, however, device optimization (V–V and AV delay) could not be performed properly before his discharge or at 6 weeks post-implantation, due to frequent PVCs. The AV delay (sensed) was programmed to 110 ms and the V–V delay to 0 (equal). During the following 6 months, the clinical status of the patient did not improve; in fact, he was hospitalized for acute heart failure requiring intravenous diuretics on five occasions. Frequent monomorphic PVCs were still present in the 12-lead ECG recordings confirmed by 20% of ventricular sensed beats in the device log. The echocardiographic parameters indicated no significant change, except for a worsening of the mitral regurgitation ( Table 1 ). In order to avoid disturbance of the echo measurements by the arrhythmia, they were obtained during a period of stable biventricular capture in at least six consecutive cycles. The pacemaker was otherwise functioning well, mostly in tracking mode at a sinus rate of 50–60 bpm with excellent sensing and pacing parameters in all three leads. A decision was made to attempt the radiofrequency catheter ablation (RFCA) of the monomorphic PVCs, guided by CARTO electroanatomical mapping (CARTO™, Biosense Webster, Diamond Bar, CA, USA). Both ventricles were mapped during monomorphic premature beats, using a 4-mm tip deflectable catheter (NAVISTAR™, Biosense Webster). The focus of the PVCs was localized to the postero-inferior left ventricle ( Figure 2 ), where the fifth RF energy application permanently abolished all premature beats. We delivered two more applications to ensure the long-term result. The overall application time was 347 ms, the average temperature 57 C°, the maximum delivered power 37 W, the average 22 W. Proper device optimization became possible after successful RFCA; the AV delay was programmed to 120 ms and the V–V delay to 20 ms, with the left ventricle paced first. A marked clinical improvement was observed within a few weeks post-RFCA: the functional class decreased to NYHA I, with no need for hospitalization during the following 6 months, and the dose of oral diuretics was reduced. Interrogation of the device log revealed that the sensed ventricular event rate had fallen to < 4%. Improvements were also evident on echocardiography ( Figure 3 ). It was noteworthy that a similar intraventricular delay could still be measured, 6 months post-RFCA with the ventricular pacing temporarily switched off as that measured before device implantation.
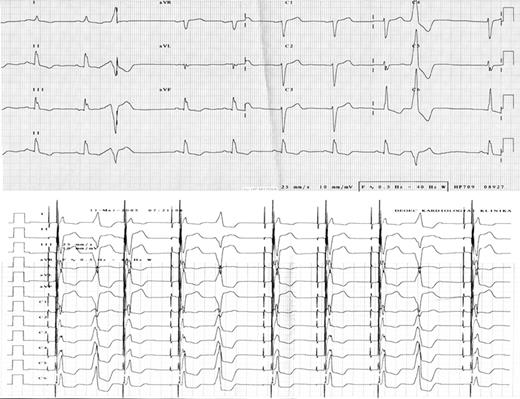
12-Lead ECG before (upper panel) and after (lower panel) CRT implantation. Frequent ventricular ectopies can be observed in both recordings.
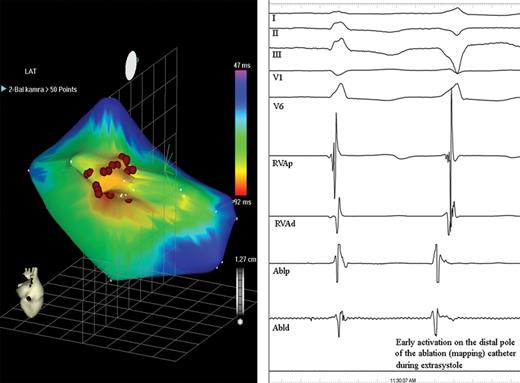
Electroanatomical activation map of the left ventricle from a posterior view, obtained during monomorphic ventricular premature beats (left). The site of the earliest activation in the left ventricle (red area) was covered by several RF applications (red dots) to ensure a long-term result. We recorded more than one point on the CARTO map at each application. Right: Intracardiac recordings show early local activation on the distal ablation pole (Abld) at the site of successful RF application preceding the QRS onset by 44 ms (I-II-III-V1-V6: surface ECG leads; RVAp, RVAd: proximal and distal poles of the catheter in the right ventricular apex; Ablp: proximal ablation electrodes).
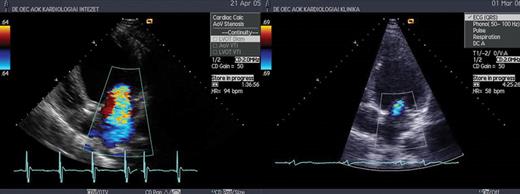
Severe mitral regurgitation 6 months after CRT implantation (left), and minimal mitral regurgitation 6 months after ablation (right).
Discussion
In all reports of PVC-induced tachycardiomyopathy, the extrasystoles originated from the right ventricular outflow tract, supporting the concept that the LV dysfunction in these patients is caused by asynchronous ventricular activation, similar to that often due to left bundle branch block. In our patient, however, the PVCs originated from the left ventricle. One explanation for this phenomenon could be the truncation of diastole by the premature beats, leading to insufficient ventricular filling. Another possibility is that the frequent PVCs eliminated the beneficial effect of the CRT. Inaccuracy of echo measurements before RFCA, because of the frequent PVCs, cannot be excluded entirely, despite the significant efforts made to ensure reliability. However, the marked improvement in clinical status in this patient makes it unlikely that the improvement in the echo-derived systolic function was simply a measurement artefact. On the other hand, proper echo-guided fine tuning of the AV and V–V delays was feasible only after the arrhythmia was controlled. Accordingly, the resolution of the tachycardia-mediated cardiomyopathy, the more effective resynchronization therapy and the increase in the proportion of paced beats might all contribute to the positive changes observed after effective arrhythmia control had been achieved.
As mentioned above, we have not been able to find even a single literature report similar to our case, in which frequent PVCs were implicated as responsible for a non-response to resynchronization. This is rather surprising, as frequent PVCs should not be a rare phenomenon in a patient population with moderate to severe heart failure and poor LV function. In a recent report, 4 tachycardiomyopathy was observed in patients with a PVC frequency of > 20% of all ventricular beats in a 24-h Holter recording. However, these were individuals with structurally normal hearts and their LV parameters normalized once the arrhythmia was eliminated. The frequency of extrasystoles required to cause a significant deterioration in patients with structural heart disease is unknown. Frequent PVCs may be an underrecognized phenomenon in non-responder patients after CRT. This possibility should be considered when device interrogation reveals a high rate of ventricular sensed beats in the device memory.
References
- ambulatory monitoring
- cardiac arrhythmia
- ventricular function, left
- cardiomyopathy, dilated
- premature ventricular contractions
- echocardiography
- radiofrequency catheter ablation
- heart failure
- left ventricle
- heart ventricle
- heart
- memory
- cardiac resynchronization therapy
- radiofrequency ablation
- medical devices
- complex



