-
PDF
- Split View
-
Views
-
Cite
Cite
Bogdan Nuta, Ian Lines, Inga MacIntyre, Guy A. Haywood, Biventricular ICD implant using endocardial LV lead placement from the left subclavian vein approach and transseptal puncture via the transfemoral route, EP Europace, Volume 9, Issue 11, November 2007, Pages 1038–1040, https://doi.org/10.1093/europace/eum176
Close - Share Icon Share
Abstract
We present the case of a 72 years old diabetic male patient with severe dilated ischaemic cardiomyopathy and New York Heart Association functional class III symptoms and previous unsuccessful attempts to cardiac resynchronization therapy using the conventional epicardial left ventricular (LV) pacing through the coronary sinus. He also had an indication for ICD implantation. We successfully implanted a biventricular ICD system from the standard left subclavian vein approach using endocardial placement of the LV lead via a transfemorally performed transeptal puncture. This technique offered him a suitable alternative to either a thoracoscopic LV lead placement (not routinely performed in our centre) or a high-risk thoracotomy procedure and multisite pacing using epicardial leads.
Introduction
Cardiac resynchronization therapy has established benefits for patients with severe symptomatic heart failure. However, in a small minority of patients, conventional epicardial left ventricular (LV) lead placement via the coronary sinus is not feasible principally due to anatomic considerations. Biventricular pacing (BiV) using endocardial LV lead placement via the transeptal (TS) approach has been described in cases where epicardial pacing was not possible.1,2 The technique employed was either a combination of transfemoral and internal jugular approaches or performing the whole implant using the latter route, but this was complicated by the need to snare the LV lead if implanted transfemorally or tunnel it subcutaneously from the neck to the device in the right subclavian area.3 We implanted the system from the left subclavian approach and placed the LV lead endocardially through the TS puncture which was performed from the usual transfemoral route.
Case report
A 72 year old male diabetic patient with severe dilated ischaemic cardiomyopathy following a large myocardial infarction in 1987 was found to have intermittent heart block and in view of his high burden of atrial tachyarrhythmia needing amiodarone treatment was implanted with a back up single chamber pacemaker in 1991. He was subsequently admitted to hospital several times between 1995 and 1999 for exacerbations of his heart failure symptoms and in 1999 underwent left heart catheterization which showed angiographic evidence of significant LV systolic dysfunction with an ejection fraction of less than 30% and a blocked left anterior descending artery supplying an anterior wall aneurysm; therefore, no revascularization was considered necessary. A more recent echocardiogram demonstrated evidence of severe LV dysfunction with an ejection fraction below 30% with anterior wall akinesia and a 24-h ECG revealed a brief episode of non-sustained monomorphic ventricular tachycardia. The 12 lead ECG demonstrated sinus rhythm and evidence of significant electrical dyssynchrony with a right ventricular (RV) paced QRS duration of 260 ms. There was additional evidence of mechanical dyssynchrony in the form of interventricular dyssynchrony with VV delay over 60 ms. When eventually an upgrade of his pacing system was considered necessary, he had two attempts at resynchronization therapy using the conventional epicardial LV pacing in 1999 and 2003, but it was not possible to enter the posterolateral branch of the coronary sinus in view of its acutely angulated origin, despite employing an Amplatz left (AL2) diagnostic catheter using a telescopic approach, in an attempt to improve guiding catheter support. Although a stable position of the LV lead was achieved in the great cardiac vein, the pacing threshold was unacceptably high in view of extensively scarred tissue.
In May 2006, he was hospitalized with worsening symptoms and signs of heart failure.
He was initially treated for pulmonary oedema with intravenous diuretics, his angiotensin-converting enzyme inhibitors were optimized and spironolactone was added. He was then offered a BiV ICD implanted using the technique described below.
Using aseptic technique, the old pacemaker pocket was opened and the existing RV pacing lead was capped off and buried deeply in to the pocket. Three separate left subclavian vein punctures were performed and the guidewires secured in place. First, a new RV bipolar active fixation dual coil shock lead was implanted at the RV apex. Next, the active fixation bipolar LV pacing lead was positioned close to the interatrial septum (IAS) using the stylet provided.
The transeptal puncture (TSP) was performed from the right femoral vein as usual with a Daig SL1 sheath, dilator, and a Brockenbrough needle (Figure 1). A standard 0.035 inch J tip guidewire was introduced in the left atrium (LA) and the sheath pulled back into the right atrium (RA). Few attempts at crossing the IAS with the LV lead using the inner stylet were unsuccessful. We then used a deflectable tip Select Secure sheath from the left subclavian vein, but it was not possible to cross with the sheath tip. At this stage, a 6F modified Amplatz right (AR mod) diagnostic angiography catheter was used inside the deflectable tip Select Secure sheath in a telescopic manner, but again proved unhelpful. However, exchanging the AR mod catheter for a 6F left internal mammary artery facilitated the crossing of the second 0.035 inch J tip guidewire in the LA. Yet, the deflectable tip Select Secure sheath would still not cross into the LA to allow the passage of the LV lead. Therefore, we proceeded to predilating the IAS using a 6 × 40-mm Optiplast XT peripheral angioplasty balloon (CR Bard, Murray Hill, NJ, USA) inflated at 10 atmospheres. This finally allowed the crossing of the deflectable sheath over the peripheral angioplasty balloon from the LA into the LV (Figure 2) which facilitated fixation of the LV lead in the mid-posterior free wall with the stylet placed at the tip. The lead stability was excellent and the pacing threshold was below 1 V at 0.4 ms pulse width and an R wave sensed at 18 mV
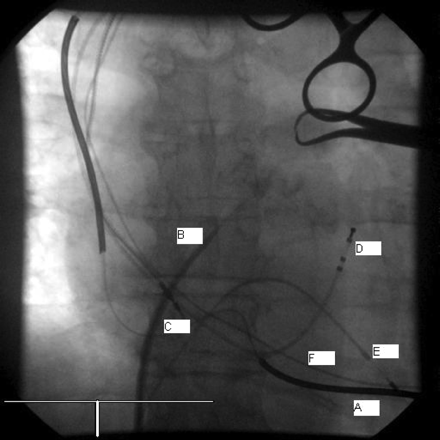
Transeptal puncture using the Daig SL1 System (AP view); A, Active fixation dual shock coil DF and pacing lead; B, Daig SL1 puncture kit; C, LV bipolar lead still in RA; D, CS lead (temporary); E, RVA temporary Electrode (for orientation); F, old RVA lead. RAO, right anterior oblique, DF, defibrillation, LV, left ventricle, CS, coronary sinus, RVA, right ventricular apex.
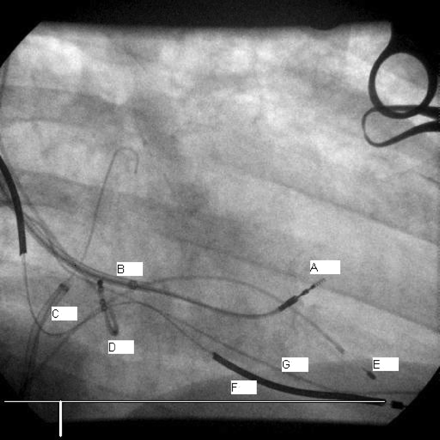
LV lead placement (RAO view). A, Endo-cardial LV lead in position; B, deflectable tip select secure sheath; C, Daig SL1 transeptal sheath in RA with 0.35 inch J tip guidewire in LA; D, temporary CS electrode; E, temporary RVA electrode; F, Active fixation DF and pacing RVA lead; G, old RVA lead (see Figure 1 for abbreviations).
Finally, we implanted a bipolar active fixation RA lead at the RA appendage with good electrophysiological parameters. The leads were connected to an Insync III Marquis BiV ICD device (Medtronic Inc., MI, USA) which was implanted prepectorally. The activated clotting time at the end of the procedure was 278.
Formal anticoagulation with subcutaneous heparin was commenced the same day with target for the partial thromboplastin time of two to three times the normal values. Warfarin was also started the same day with a 10 mg loading dose and a target INR of 2.5–3.5 was aimed for. In view of a relatively lengthy procedure, we elected not to perform the defibrillation threshold testing at the same sitting, but set empirical therapies instead and arranged the induction at a later date on an outpatient basis. A post-procedure bedside echocardiogram was performed and demonstrated no evidence of a pericardial effusion.
His symptoms of heart failure have objectively improved over the subsequent few days and his diuretics were reduced. His chest X-Ray and ICD check the day after the implant were both satisfactory. The ECG post-implant showed evidence of atrial and BiV pacing with a paced AV delay of 130 ms and in fact the QRS duration has decreased from 260 ms pre-implant to 168 ms after the implant.
However, 4 days after the procedure, he became suddenly unwell, sweaty, complaining of pleuritic sounding chest pain and became hypotensive and tachycardic. Emergency bedside echocardiography revealed a significant pericardial effusion which was drained immediately in the catheter laboratory without complications.
Ten days later, he was re-established on warfarin with the same INR target of 2.5–3.5 and was discharged home with planned follow-up in the ICD clinic. He continued to improve clinically at 3, 6, and 9 months follow-up visits with New York Heart Association class II symptoms currently and remains free of any thrombo-embolic complications. We acknowledge that restoration of AV synchrony may have contributed to the clinical improvement, but continuous RV pacing in the context of severe LV dysfunction would have likely led to overall deterioration of his cardiac output. Yet, despite the clinical improvement (objectively over 380 m on 6 min walk test), there was only marginal improvement of the LV ejection fraction on echocardiography at 6 months; it is expected that this will show further improvement at subsequent follow-up. Also, there has been no evidence of ICD therapies being delivered at all following the implant.
Conflict of interest: none declared.



