-
PDF
- Split View
-
Views
-
Cite
Cite
Philip Spurrell, Kayvan Kamalvand, Mike Higson, Neil Sulke, Temporal changes in atrial refractoriness following DC cardioversion of persistent atrial fibrillation in man, EP Europace, Volume 6, Issue 3, 2004, Pages 229–235, https://doi.org/10.1016/j.eupc.2004.01.006
Close - Share Icon Share
Abstract
Studies have demonstrated shortening of the atrial effective refractory period (ERP) after episodes of atrial fibrillation (AF). This is termed atrial remodelling. It is unclear whether restoration of SR after persistent AF in patients with a clinical substrate results in reversal of this shortening and whether this is maintained long term.
The ERP was determined at mid-lateral right atrial wall (MLRA) and right atrial appendage (RAA) at 600 ms and 400 ms drive cycle lengths and at basic sinus cycle length in 81 patients with persistent AF immediately, 24 h and 2 weeks following external DC cardioversion. All atrially active drugs were stopped for at least 5 half lives. (1) Prolongation of the ERP was observed at both atrial sites and all cycle lengths up to 24 h post cardioversion (p<0.0001). (2) However, between 24 h and 2 weeks a subsequent shortening occurred in the ERP returning it to near post cardioversion levels. (3) The ERP was significantly longer at 24 h post cardioversion in patients who remained in SR for 2 weeks or longer compared with those who reverted to AF.
Prolongation of the atrial ERP occurred following restoration of SR in persistent AF patients but was not maintained and displayed a biphasic pattern such that by 2 weeks the ERP had returned to baseline values. Despite this finding, a longer ERP at 24 h post cardioversion was associated with maintenance of SR in the medium-term.
Introduction
Atrial fibrillation (AF) is the commonest arrhythmia encountered in clinical practice and is responsible for considerable morbidity and mortality [1,,2]. Epidemiological and electrophysiological studies support the concept that AF is a self-perpetuating and progressive arrhythmia; paroxysmal AF progresses to sustained AF despite therapeutic intervention in up to 18% of patients even in the absence of underlying structural heart disease [3]. Increased duration of AF preceding cardioversion is associated with a lower rate of success and maintenance of sinus rhythm (SR) [4,,5]. Studies in animals and man have demonstrated that shortening of the atrial effective refractory period (ERP) with attenuation of physiological rate-related shortening occurs following episodes of AF [6–,9]. These changes have been termed ‘atrial remodelling’ and favour the perpetuation and maintenance of the arrhythmia. Studies have shown that restoration of SR results in reversal of these electrophysiological changes [10–,12].
This study aimed to document the electrophysiological changes that occur following restoration of SR in patients with long-standing persistent AF up to 2 weeks' post cardioversion.
Methods
Patients
Study subjects were recruited from consecutive patients referred from General Practitioners or medical outpatients for cardioversion of AF and were therefore representative of the local AF population. The study had ethical committee approval and all patients gave written informed consent before enrolment. Patients with an implanted pacemaker/defibrillator and patients on amiodarone were excluded. Eighty-one patients (mean age, 69±9 years, 64% male) with persistent AF for greater than 1 month (mean duration 12±22 months) were studied. Mean left atrial diameter on M-mode echo was 45±7 mm. Left ventricular end-diastolic diameter was 50±10 mm and percentage fractional shortening was 32±9%. Patients were anticoagulated with warfarin for a minimum of 4 weeks before cardioversion. Serum potassium was 4.6±0.4 mmol/L, serum magnesium was 0.9±0.2 mmol/L and serum corrected calcium was 2.5±0.1 mmol/L. All patients were biochemically euthyroid. Demographics are displayed in Table 1.
| All patients | AF group | SR group | |
| Number | 81 | 50 | 28 |
| Age (years) | 69 ± 9 | 69 ± 8 | 69 ± 11 |
| Sex (%male) | 64 | 62 | 64 |
| Prior cardioversions | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.3 ± 0.5 |
| Aetiology* (%) | MVD 23, BP 44, IHD 21, ALC 6, DCM 1, LONE 36 | MVD 28, BP 42, IHD 18, ALC 8, DCM 2, LONE 34 | MVD 18, BP 46, IHD 25, ALC 4, LONE 39 |
| AF duration (months) | 12 ± 22 | 14 ± 25 | 9 ± 18 |
| LA size (mm) | 45 ± 7 | 44 ± 6 | 45 ± 8 |
| FS (%) | 32 ± 9 | 31 ± 10 | 32 ± 9 |
| SSS | 10 | 6 | 4 |
| All patients | AF group | SR group | |
| Number | 81 | 50 | 28 |
| Age (years) | 69 ± 9 | 69 ± 8 | 69 ± 11 |
| Sex (%male) | 64 | 62 | 64 |
| Prior cardioversions | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.3 ± 0.5 |
| Aetiology* (%) | MVD 23, BP 44, IHD 21, ALC 6, DCM 1, LONE 36 | MVD 28, BP 42, IHD 18, ALC 8, DCM 2, LONE 34 | MVD 18, BP 46, IHD 25, ALC 4, LONE 39 |
| AF duration (months) | 12 ± 22 | 14 ± 25 | 9 ± 18 |
| LA size (mm) | 45 ± 7 | 44 ± 6 | 45 ± 8 |
| FS (%) | 32 ± 9 | 31 ± 10 | 32 ± 9 |
| SSS | 10 | 6 | 4 |
MVD, mitral valve disease; BP, hypertension; IHD, ischaemic heart disease; ALC, alcohol; DCM, dilated cardiomyopathy; SSS, sick sinus syndrome; LA, left atrium; FS, fractional shortening. NB: 3 patients lost to follow-up and therefore not classified.
| All patients | AF group | SR group | |
| Number | 81 | 50 | 28 |
| Age (years) | 69 ± 9 | 69 ± 8 | 69 ± 11 |
| Sex (%male) | 64 | 62 | 64 |
| Prior cardioversions | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.3 ± 0.5 |
| Aetiology* (%) | MVD 23, BP 44, IHD 21, ALC 6, DCM 1, LONE 36 | MVD 28, BP 42, IHD 18, ALC 8, DCM 2, LONE 34 | MVD 18, BP 46, IHD 25, ALC 4, LONE 39 |
| AF duration (months) | 12 ± 22 | 14 ± 25 | 9 ± 18 |
| LA size (mm) | 45 ± 7 | 44 ± 6 | 45 ± 8 |
| FS (%) | 32 ± 9 | 31 ± 10 | 32 ± 9 |
| SSS | 10 | 6 | 4 |
| All patients | AF group | SR group | |
| Number | 81 | 50 | 28 |
| Age (years) | 69 ± 9 | 69 ± 8 | 69 ± 11 |
| Sex (%male) | 64 | 62 | 64 |
| Prior cardioversions | 0.2 ± 0.5 | 0.2 ± 0.5 | 0.3 ± 0.5 |
| Aetiology* (%) | MVD 23, BP 44, IHD 21, ALC 6, DCM 1, LONE 36 | MVD 28, BP 42, IHD 18, ALC 8, DCM 2, LONE 34 | MVD 18, BP 46, IHD 25, ALC 4, LONE 39 |
| AF duration (months) | 12 ± 22 | 14 ± 25 | 9 ± 18 |
| LA size (mm) | 45 ± 7 | 44 ± 6 | 45 ± 8 |
| FS (%) | 32 ± 9 | 31 ± 10 | 32 ± 9 |
| SSS | 10 | 6 | 4 |
MVD, mitral valve disease; BP, hypertension; IHD, ischaemic heart disease; ALC, alcohol; DCM, dilated cardiomyopathy; SSS, sick sinus syndrome; LA, left atrium; FS, fractional shortening. NB: 3 patients lost to follow-up and therefore not classified.
Electrical cardioversion
All atrially active drugs were stopped for at least 5 half lives before participation in the study. A 6 French 11 cm Super Arrow-Flex sheath (Arrow International Inc., Reading, PA, USA) was inserted under local anaesthesia (1% lidocaine) into the right femoral vein. The sheath was removed after the 24-h post cardioversion assessment. Patients were sedated for the cardioversion procedure only using 5 mL aliquots of intravenous diazepam. When adequate sedation had been achieved DC cardioversion was performed externally using a Physio-Control Lifepak 9 external defibrillator delivering a synchronised 5 ms monophasic pulse delivered via 82 cm2 paddles and conductive gel pads. Defibrillation shocks (360 J) were delivered antero-posteriorly alternating with antero-laterally up to a total of 4 shocks as required.
Atrial electrophysiological measurements
A Quadripolar catheter (Daig Corp, Minnetonka, MN, USA) with 5 mm electrode spacing was inserted via the sheath to the right atrium. The electrophysiological (EP) study was performed using the Cardiolab system (Prucka Engineering Inc., USA). Pacing was performed at twice the diastolic threshold. The ERP was assessed at 2 sites in random order, the mid-lateral right atrial wall (MLRA) and right atrial appendage (RAA). A fluoroscopic image grab was taken of the catheter at each atrial site to record the anatomical position. This was reviewed at later EP assessments to ensure that the anatomical position was the same. The ERP was determined by the extrastimulus technique. An extrastimulus (S2) was introduced after 8 sensed/paced beats (S1) at 600 ms and 400 ms drive cycle lengths. The coupling interval (S1S2) was increased in steps of 10 ms until atrial capture was seen. The ERP was defined as the longest S1S2 that failed to capture the atrium. In 53 patients the ERP was also determined for basic sinus cycle length (BCL). The EP assessment was performed between 5 and 15 min post cardioversion and repeated, using the same method, at 24 h and again at 2 weeks' post cardioversion. The assessment at 2 weeks' post cardioversion required insertion of a femoral sheath as previously described.
Statistical analysis
Data are presented as mean ± standard deviation. Comparison of independent variables was analysed by the Mann–Whitney U test. Comparison of paired variables was analysed by the Wilcoxon signed rank test. The Bonferroni correction was applied to all multiple comparisons. A p value of less than 0.05 was considered significant.
Results
Eighty-one of a total of 99 (82%) patients were successfully cardioverted to SR. Sixty-eight of those cardioverted (84%) were still in SR at 24 h and 28 (35%) at 2 weeks. Ten (12%) patients spontaneously reverted to AF within 24 h of cardioversion. Thirty-seven (46%) patients reverted to AF between 24 h and 2 weeks' post cardioversion. Nine patients reverted to AF during the EP protocol (3 patients reverted to AF during the immediate post cardioversion assessment and 6 patients reverted to AF during the 2 weeks' post cardioversion assessment but none reverted at the 24 h post cardioversion assessment). Three patients were lost to follow-up after the 24 h post cardioversion assessment. There was no significant difference in baseline and 2 week post cardioversion biochemistry. There was no significant difference in pacing thresholds at baseline, 24 h or 2 weeks for the MLRA (1.5±0.4 vs 1.5±0.5 vs 1.6±0.4 (p=NS)) or RAA (1.5±0.4 vs 1.4±0.4 vs 1.6±0.5 (p=NS)).
Change in the atrial effective refractory period up to 2 weeks' post cardioversion
Following restoration of SR a significant prolongation in the ERP (p<0.0001) was observed at 24 h post cardioversion (Table 2). This occurred at both atrial sites and all assessed cycle lengths at 24 h post cardioversion (Fig. 1).
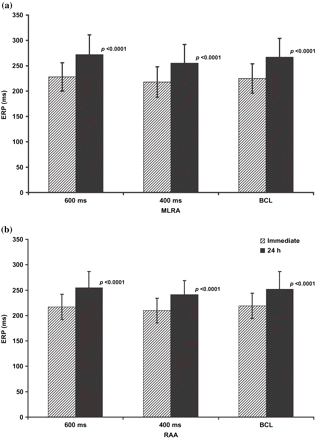
Figure displays the ERP at the right atrial appendage (RAA) and the mid-lateral right atrial wall (MLRA) at basic sinus cycle length (BCL), 600 ms (600) and 400 ms (400) drive cycle lengths ‘immediately’ and 24 h post cardioversion for the 68 patients still in SR at 24 h.
Effective refractory period for all patients in SR at 24 h and for all patients in SR at 2 weeks' post cardioversion
| Variable | Patients in SR at 24 h | Patients in SR at 2 weeks | |||
| 0 h | 24 h | 0 h | 24 h | 2 weeks | |
| MLRA 600 ms | 228 (28) | 272 (39) | 237 (35) | 288 (42) | 249 (40) |
| MLRA 400 ms | 218 (30) | 255 (37) | 232 (38) | 270 (40) | 246 (34) |
| MLRA BCL | 225 (29) | 267 (37) | 242 (26) | 291 (33) | 257 (42) |
| RAA 600 ms | 217 (25) | 255 (32) | 224 (29) | 267 (38) | 239 (41) |
| RAA 400 ms | 210 (24) | 241 (28) | 220 (27) | 252 (36) | 230 (32) |
| RAA BCL | 219 (25) | 252 (35) | 225 (26) | 273 (33) | 252 (38) |
| Variable | Patients in SR at 24 h | Patients in SR at 2 weeks | |||
| 0 h | 24 h | 0 h | 24 h | 2 weeks | |
| MLRA 600 ms | 228 (28) | 272 (39) | 237 (35) | 288 (42) | 249 (40) |
| MLRA 400 ms | 218 (30) | 255 (37) | 232 (38) | 270 (40) | 246 (34) |
| MLRA BCL | 225 (29) | 267 (37) | 242 (26) | 291 (33) | 257 (42) |
| RAA 600 ms | 217 (25) | 255 (32) | 224 (29) | 267 (38) | 239 (41) |
| RAA 400 ms | 210 (24) | 241 (28) | 220 (27) | 252 (36) | 230 (32) |
| RAA BCL | 219 (25) | 252 (35) | 225 (26) | 273 (33) | 252 (38) |
Effective refractory period for all patients in SR at 24 h and for all patients in SR at 2 weeks' post cardioversion
| Variable | Patients in SR at 24 h | Patients in SR at 2 weeks | |||
| 0 h | 24 h | 0 h | 24 h | 2 weeks | |
| MLRA 600 ms | 228 (28) | 272 (39) | 237 (35) | 288 (42) | 249 (40) |
| MLRA 400 ms | 218 (30) | 255 (37) | 232 (38) | 270 (40) | 246 (34) |
| MLRA BCL | 225 (29) | 267 (37) | 242 (26) | 291 (33) | 257 (42) |
| RAA 600 ms | 217 (25) | 255 (32) | 224 (29) | 267 (38) | 239 (41) |
| RAA 400 ms | 210 (24) | 241 (28) | 220 (27) | 252 (36) | 230 (32) |
| RAA BCL | 219 (25) | 252 (35) | 225 (26) | 273 (33) | 252 (38) |
| Variable | Patients in SR at 24 h | Patients in SR at 2 weeks | |||
| 0 h | 24 h | 0 h | 24 h | 2 weeks | |
| MLRA 600 ms | 228 (28) | 272 (39) | 237 (35) | 288 (42) | 249 (40) |
| MLRA 400 ms | 218 (30) | 255 (37) | 232 (38) | 270 (40) | 246 (34) |
| MLRA BCL | 225 (29) | 267 (37) | 242 (26) | 291 (33) | 257 (42) |
| RAA 600 ms | 217 (25) | 255 (32) | 224 (29) | 267 (38) | 239 (41) |
| RAA 400 ms | 210 (24) | 241 (28) | 220 (27) | 252 (36) | 230 (32) |
| RAA BCL | 219 (25) | 252 (35) | 225 (26) | 273 (33) | 252 (38) |
In 28 patients still in SR 2 weeks' post cardioversion significant prolongation of the ERP was also observed from baseline to 24 h post cardioversion at both atrial sites and all cycle lengths. However, following this initial prolongation, a subsequent shortening of the ERP occurred between 24 h and 2 weeks' post cardioversion demonstrating a biphasic pattern (Fig. 2). At the MLRA the ERP 24 h and 2 weeks' post cardioversion at a cycle length of 600 ms was 288±42 and 249±40 ms (p<0.001) and at 400 ms was 270±40 and 246±34 ms (p=NS), respectively. At the RAA the ERP 24 h and 2 weeks' post cardioversion at a cycle length of 600 ms was 267±38 and 239±41 ms (p<0.01) and at 400 ms was 252±36 and 230±32 ms (p<0.05), respectively. The ERPs at 2 weeks' and immediately post cardioversion were not significantly different.
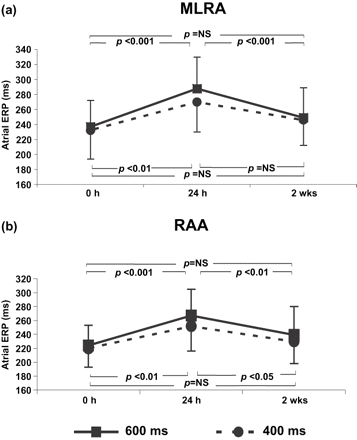
Comparison of the atrial effective refractory period (ERP) immediately (0 h), 24 h and 2 weeks' post cardioversion at (a) mid-lateral right atrial wall (MLRA), (b) right atrial appendage (RAA) at 600 ms and 400 ms drive cycle lengths for the 28 patients still in SR at 2 weeks.
Comparison of AF and SR groups
Patients were divided into 2 groups: those who maintained SR for greater or less than 2 weeks' post cardioversion termed ‘SR’ or ‘AF’, respectively. Clinical characteristics for each group are shown in Table 1. There were no significant differences although there was a trend towards a shorter preceding AF duration in the SR group. Immediately following cardioversion all mean ERPs were longer in the SR group than the AF group (Table 3) reaching statistical significance for the MLRA at 400 ms cycle length (SR 230±37 ms, AF 210±21 ms, p<0.01) and basic sinus cycle length (SR 239±27 ms, AF 218±26 ms, p<0.05).
| Variable | 0 h | 24 h | ||
| AF | SR | AF | SR | |
| MLRA 600 ms | 223 (22) | 236 (34) | 263 (36) | 286 (41)† |
| MLRA 400 ms | 210 (21) | 230 (37)* | 247 (34) | 267 (39)* |
| MLRA BCL | 218 (26) | 239 (27)† | 255 (34) | 288 (33)* |
| RAA 600 ms | 213 (25) | 222 (28) | 246 (26) | 265 (37)† |
| RAA 400 ms | 206 (22) | 218 (26) | 234 (20) | 251 (34)† |
| RAA BCL | 216 (25) | 224 (25) | 243 (32) | 269 (35)† |
| Variable | 0 h | 24 h | ||
| AF | SR | AF | SR | |
| MLRA 600 ms | 223 (22) | 236 (34) | 263 (36) | 286 (41)† |
| MLRA 400 ms | 210 (21) | 230 (37)* | 247 (34) | 267 (39)* |
| MLRA BCL | 218 (26) | 239 (27)† | 255 (34) | 288 (33)* |
| RAA 600 ms | 213 (25) | 222 (28) | 246 (26) | 265 (37)† |
| RAA 400 ms | 206 (22) | 218 (26) | 234 (20) | 251 (34)† |
| RAA BCL | 216 (25) | 224 (25) | 243 (32) | 269 (35)† |
*p<0.01
†p<0.05.
| Variable | 0 h | 24 h | ||
| AF | SR | AF | SR | |
| MLRA 600 ms | 223 (22) | 236 (34) | 263 (36) | 286 (41)† |
| MLRA 400 ms | 210 (21) | 230 (37)* | 247 (34) | 267 (39)* |
| MLRA BCL | 218 (26) | 239 (27)† | 255 (34) | 288 (33)* |
| RAA 600 ms | 213 (25) | 222 (28) | 246 (26) | 265 (37)† |
| RAA 400 ms | 206 (22) | 218 (26) | 234 (20) | 251 (34)† |
| RAA BCL | 216 (25) | 224 (25) | 243 (32) | 269 (35)† |
| Variable | 0 h | 24 h | ||
| AF | SR | AF | SR | |
| MLRA 600 ms | 223 (22) | 236 (34) | 263 (36) | 286 (41)† |
| MLRA 400 ms | 210 (21) | 230 (37)* | 247 (34) | 267 (39)* |
| MLRA BCL | 218 (26) | 239 (27)† | 255 (34) | 288 (33)* |
| RAA 600 ms | 213 (25) | 222 (28) | 246 (26) | 265 (37)† |
| RAA 400 ms | 206 (22) | 218 (26) | 234 (20) | 251 (34)† |
| RAA BCL | 216 (25) | 224 (25) | 243 (32) | 269 (35)† |
*p<0.01
†p<0.05.
At 24 h post cardioversion the ERP at the RAA was longer in the SR group at 600 ms cycle length (SR 265±37 ms, AF 246±26 ms, p<0.05); at 400 ms cycle length (SR 251±34 ms, AF 234±20 ms, p<0.05); at basic sinus cycle length (SR 269±35 ms, AF 243±32 ms, p<0.05). The ERP at the MLRA was also longer in the SR group at 600 ms cycle length (SR 286±41 ms, AF 263±36 ms, p<0.05); at 400 ms cycle length (SR 267±39 ms, AF 247±34 ms, p<0.01); at basic sinus cycle length (SR 288±33 ms, AF 255±34 ms, p<0.01).
Discussion
The main findings of this study were as follows:
Following successful DC cardioversion of patients with persistent AF, a biphasic pattern of change in atrial refractoriness was observed. A significant prolongation in atrial refractoriness was seen at 24 h compared with baseline and a significant shortening followed this from 24 h to 2 weeks. Atrial refractoriness at 2 weeks was comparable with baseline values.
Most recurrences of AF were observed between 24 h and 2 weeks' post cardioversion.
In those patients still in SR at 2 weeks, a trend towards increased atrial refractoriness was observed immediately post cardioversion compared with those patients who reverted and was significantly longer at 24 h post cardioversion at both atrial sites and all cycle lengths assessed.
Changes in atrial refractoriness
Following episodes of AF, shortening of the atrial ERP has been demonstrated in animal and human studies [7,,8,,13]. In chronically instrumented goats, 2 weeks of rapid atrial pacing resulted in significant shortening of the ERP [6]. Once SR was restored there was a gradual prolongation in the ERP and after 1 week it returned to normal values. This has been termed ‘reversed remodelling’. Following 7 h of rapid atrial pacing in a canine model a similar recovery of refractoriness was observed but over a shorter time period of 30 min [14]. In a human model, Daoud et al. found that less than 10 min of SR was sufficient to reverse the electrophysiological changes induced by 5 min of rapid atrial pacing [7]. These studies suggest that the electrophysiological changes that occur as a consequence of AF might also resolve in patients with clinical rather than induced AF once SR is restored. In addition the duration of the arrhythmia prior to restoration of SR may also have an influence on this process of ‘reversed’ remodelling.
In patients with persistent AF we have shown a significant prolongation in the ERP in the 24 h following cardioversion. However, by 2 weeks the ERP had shortened again to near acute values suggesting that ‘reversed’ remodelling is a transient phenomenon in man. It is unclear why this ‘late’ shortening in the ERP should occur.
Many studies have shown that AF results in shortening in the atrial effective refractory period. However, it has also been demonstrated that heart failure induced by chronic rapid ventricular pacing results in a prolongation in atrial refractoriness [15–,17]. AF is known to be associated with a tachycardiomyopathy and is probably present to some degree in most patients with AF [18]. Thus the ERP seen immediately post cardioversion may be a product of tachycardia-related shortening of the ERP competing with heart-failure related prolongation. The subsequent changes seen in atrial refractoriness following restoration of SR may occur as a result of resolution of these 2 processes. Yu et al. demonstrated that reversal of electrical remodelling was a rapid process completing within a few days of restoring SR [10]. The tachycardiomyopathy and heart-failure related prolongation of the ERP may resolve more slowly and this may explain the shortening in the ERP between 24 h and 2 weeks seen in this study. This biphasic pattern of ERP change has been demonstrated by Schoonderwoerd et al. in a goat model [19]. With rapid atrial pacing a significant shortening in atrial refractoriness was seen but in the animals developing a tachycardiomyopathy a later prolongation in the ERP was observed such that it approached baseline values. Changes in atrial wall distension have been shown to affect atrial refractoriness and therefore may have influenced our findings [20].
Other studies have assessed the temporal changes in the ERP following restoration of SR in patients with clinical AF. The first study assessed atrial electrophysiology following internal cardioversion but also re-evaluated the ERP at 4 weeks' post cardioversion in 8 patients demonstrating a significant prolongation of ERP at this time [21]. However, half of these subjects were taking amiodarone which affects the ERP and remodelling process. Yu et al. documented the temporal change in the ERP at RAA and distal coronary sinus following external cardioversion in patients with persistent AF [10]. Initial evaluation was undertaken 30 min after cardioversion and then daily up to 5 days. Prolongation of the ERP occurred following restoration of SR and reached a steady state on the third day after cardioversion. No further evaluations were made of atrial electrophysiology after the fifth day. These data are entirely consistent with our findings suggesting that ‘reversed’ remodelling is maximal at between 24 and 72 h post cessation of AF. Sparks et al. assessed atrial electrophysiology at 15 min, 30 min and 3 weeks following ablation in 10 patients with chronic atrial flutter [11]. In contrast to our study the ERP at 3 weeks was significantly longer than at baseline. However, this was in a small number of patients with a different arrhythmic basis.
Manios et al. studied the changes in the ERP (500 ms) in patients with persistent spontaneous AF following successful internal atrioversion [12]. Assessment was made immediately, 24 h and 4 weeks' post internal cardioversion of AF. All 3 EP assessments were completed in 16 patients. They found that there was a significant prolongation in the ERP from immediate values to 24 h but in contrast to this study that there was no further significant change in the ERP between 24 h and 4 weeks.
Comparison of AF and SR groups
Patients who remained in SR for 2 weeks or more following cardioversion were observed to have significantly longer ERPs at 24 h post cardioversion than those who reverted to AF. It has previously been observed that shorter refractory periods are associated with an increased vulnerability to AF [6,,13,,22]. In addition Olsson et al. reported an association between short monophasic action potential duration post cardioversion and early recurrence of AF [23]. Prolongation in atrial refractoriness does not favour the maintenance of AF and causes a reduction in the number of AF wavelets that can be supported by the same atrial mass [24,,25]. A reduction in the number of sustainable AF wavelets increases the likelihood that the arrhythmia will terminate.
Study limitations
Catheters used for assessment of atrial electrophysiology were repositioned in the atrium for each EP assessment. Image grabs were taken of each catheter position at the baseline study to ensure that the same anatomical site was assessed at later studies. However, it would be impossible to guarantee that the catheter was in the same position microscopically.
Electrophysiological assessment at multiple atrial sites, including the left atrium, would provide further information on regional differences in atrial refractoriness and also changes in refractoriness over time following restoration of SR. However, this would increase the risk of reinitiation of AF in vulnerable patients. The evaluation of a greater number of cycle lengths would also provide greater information on the response of atrial refractoriness to changes in cycle length but likewise would significantly increase the risk of reinitiation of AF.
Assessment of the atrial monophasic action potential (MAP) duration would provide further information on single myocardial cell action potential duration [26]. Concurrent evaluation of ERP and MAP may provide further clues to the mechanisms behind changes seen in this study. Echocardiographic assessment was only performed at baseline. Serial assessments of left and right atrial dimensions would have allowed comparison of atrial size changes and electrophysiological findings. However, transthoracic echocardiography is a poor modality for demonstrating small changes in atrial size, particularly the right atrium.
Conclusion
AF leads to electrical remodelling of the atria that favours maintenance and persistence of the arrhythmia. Despite successful restoration of SR reinitiation of AF is common. This phenomenon may be explained by failure of atrial refractoriness to return and remain at ‘normal’ levels following cardioversion. Despite this finding, a longer ERP at 24 h post cardioversion was associated with maintenance of SR in the medium-term.



