-
PDF
- Split View
-
Views
-
Cite
Cite
Gianfranco Mazzocca, Tiziana Giovannini, Fabio Frascarelli, Alessandro Fabiani, Antonio Burali, Giampiero Giappichini, Giuseppe Bidi, Daniele Bernabò, Enrico Manfredini, Giorgio Corbucci, Heart rate regularisation in patients with permanent atrial fibrillation implanted with a VVI(R) pacemaker, EP Europace, Volume 6, Issue 3, 2004, Pages 236–242, https://doi.org/10.1016/j.eupc.2004.02.002
Close - Share Icon Share
Abstract
Irregularity of ventricular cycles is a cause of haemodynamic impairment and symptoms in patients with atrial fibrillation (AF).
Aim of the study was to determine the optimal pacing rate to stabilise ventricular cycle length at rest in patients with chronic AF, bradycardiac symptoms and VVI pacing.
The compensatory pause (CP) in AF, as defined by Langendorf, was used as a reference value in pacing the heart. The spontaneous mean heart rate (MHR) was assessed with the PM OFF. The CP was then calculated with the pacing rate programmed at 40 bpm. Four pacing rates were tested: rate of the CP (RCP), RCP+5 bpm, RCP−5 bpm and RCP−10 bpm.
RCP provided a good estimate of the MHR (r=0.92). Pacing percentage (P%) was 24±15% at the pacing rate of RCP−10 bpm, 39±19% at RCP−5 bpm, 63±17% at RCP, and 79±19% at RCP+5 bpm (p<0.001). The corresponding HR modestly increased from 65±13 bpm to 66±13 bpm (p=NS), 68±13 bpm (p<0.001) and 71±13 bpm (p<0.001), respectively.
The RCP estimates, during pacing, what the spontaneous MHR would be. Ventricular stimulation at the RCP causes a high P%, stabilising cardiac cycles with a modest increase in HR.
Introduction
In patients with atrial fibrillation (AF) haemodynamic disturbance is caused by the loss of atrial systole and by an inappropriately rapid and irregular ventricular rate. Patients with permanent AF and an indication for VVI(R) stimulation account for about 16% of the total number of antibradycardia devices implanted per year in Italy [1]. Some of these patients have an acceptable spontaneous heart rate (HR) with symptomatic pauses requiring pacing, and an irregular ventricular rhythm. In this clinical condition, haemodynamic impairment can be caused by the loss of atrial systole, the absence of tricuspid and mitral valve annular contractions and the irregularity of cardiac cycle length [2–,4]. An implanted pacemaker can pace the heart so as to avoid pauses and also to limit beat-to-beat variations in the cardiac cycles [5–,7]. It is easy to programme the lower rate of a pacemaker to avoid pauses, while it is difficult to determine the optimal pacing rate to stabilise the ventricular rhythm. Indeed, a relatively low pacing rate may be unsuitable for this purpose, while a relatively high pacing rate may increase the patient's HR excessively. A reference parameter specific for each patient, according to which the pacing rate could be set in a reasonable range, may be the compensatory pause in AF, which was defined by Langendorf as the spontaneous escape interval (Fig. 1) following a paced beat [8]. It has been reported [9] that the mean cycle of the compensatory pause is always longer than the mean cycle of the heart during AF. Theoretically, pacing the heart with a cycle length similar to the mean compensatory pause (CP) could stabilise the rhythm with a high pacing percentage (P%) without increasing the HR. As illustrated by Greenhut et al. [7] a P% ranging from 52% to 84% may induce a reduction of 50% in ventricular rate variability. Lee et al. [10] reported a 50% reduction in ventricular rate variability with 61% paced ventricular beats. The aim of this study was to assess the optimal range of pacing rate at rest for each patient in order to achieve stable ventricular rhythm without substantially increasing HR.
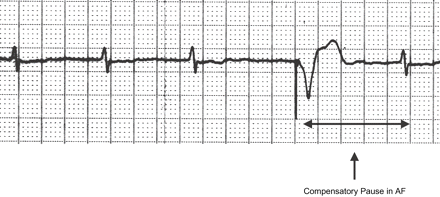
The spontaneous escape interval following the paced beat is by definition the CP in AF.
Methods
We defined a stable ventricular rhythm as a rhythm corresponding to a P%>60%. Since pacing cycles are regular by definition, we assumed that a P%>60% corresponded to a good level of stabilisation [5,,7]. We defined the maximum acceptable HR increase as an HR increase ≤10% with respect to the patient's intrinsic HR, which we called mean HR (MHR). MHR was measured with the pacemaker OFF by analysing 1 min ECG traces. We then evaluated the CP in AF by analysing the ECG trace until 10 CPs were seen at the minimum pacing rate of 40 bpm, or 50 bpm in the event of complete inhibition of the pacemaker at 40 bpm. Fusion beats were excluded from the analysis, the cycle length of each CP was measured and the corresponding mean value calculated. The CP was our parameter of reference for determining the steps of pacing rate specific for each patient. For this reason, we converted the cycle of the CP expressed in milliseconds to a rate expressed in bpm, through the formula 60,000/CP. This conversion was performed to simplify the setting of the pacing rate by the programmer. We defined this value as the rate corresponding to the CP (RCP) that represents a theoretical value obtained by averaging nonconsecutive cycles; so it is not a really measured HR. We then paced each patient at the following pacing rates: RCP−10 bpm, RCP−5 bpm, RCP bpm, RCP+5 bpm. All patients were evaluated at rest and the four different pacing rates were programmed. After 1 min at each step, we recorded the corresponding real HR, P%, and the percentage of spontaneous cycles shorter than 600 ms to estimate the incidence of short RR intervals (SRR%). This cycle length corresponds to a cut-off rate of 100 bpm. At each step data were compared with those of the previous one. Data were collected through the analysis of a 1 min ECG trace starting 1 min after the programming of the new pacing rate. A sampling interval of 1 min seems reliable to assess MHR during AF, while the use of complex mathematical methods for assessing HR regularisation would need at least 9 min [11].
Data were expressed as mean ± standard deviation, and statistical analysis was performed by paired two-tailed t-test. A p value <0.05 was regarded as significant.
Patient population
Patients with permanent AF and indication for VVI(R) pacemaker (Jade 3 SSI and Clarity SSIR; Vitatron) were evaluated at rest with the pacemaker OFF. All patients with MHR>45 bpm were eligible for the study. We excluded patients with MHR<45 bpm in the basal condition, as they would always be stimulated by the pacemaker and the resulting rhythm would be intrinsically stable even at a pacing rate of 40 bpm. We enrolled 29 patients (21 M, 8 F) with a mean age of 76±7 years and without other important cardiomyopathies.
Results
The basal MHR of the patients and the corresponding CP (ms) and RCP (bpm) are reported in Table 1. The RCP was 64±13 bpm and the MHR was 65±12 bpm, with a high correlation factor r=0.92. RCP estimates well the MHR in patients without the need to switch off pacing.
The value of the rate of the compensatory pauses (RCP) is highly correlated to the spontaneous mean heart rate (MHR) (r=0.92)
| Pts | CP (ms) | RCP (bpm) | MHR (bpm) |
| 1 | 1092 | 55 | 76 |
| 2 | 1228 | 49 | 54 |
| 3 | 808 | 74 | 70 |
| 4 | 1016 | 59 | 60 |
| 5 | 1058 | 57 | 60 |
| 6 | 1196 | 50 | 52 |
| 7 | 842 | 71 | 72 |
| 8 | 660 | 91 | 86 |
| 9 | 728 | 82 | 80 |
| 10 | 1120 | 54 | 50 |
| 11 | 1246 | 48 | 46 |
| 12 | 660 | 91 | 88 |
| 13 | 1246 | 48 | 46 |
| 14 | 1136 | 53 | 54 |
| 15 | 776 | 77 | 70 |
| 16 | 1064 | 56 | 60 |
| 17 | 741 | 81 | 78 |
| 18 | 828 | 72 | 70 |
| 19 | 1066 | 56 | 58 |
| 20 | 1012 | 59 | 54 |
| 21 | 1092 | 55 | 54 |
| 22 | 1132 | 53 | 56 |
| 23 | 1008 | 60 | 58 |
| 24 | 766 | 78 | 82 |
| 25 | 1028 | 58 | 58 |
| 26 | 786 | 76 | 74 |
| 27 | 988 | 61 | 64 |
| 28 | 872 | 69 | 72 |
| 29 | 836 | 72 | 74 |
| Average | 967 | 64 | 65 |
| SD | 181 | 13 | 12 |
| Pts | CP (ms) | RCP (bpm) | MHR (bpm) |
| 1 | 1092 | 55 | 76 |
| 2 | 1228 | 49 | 54 |
| 3 | 808 | 74 | 70 |
| 4 | 1016 | 59 | 60 |
| 5 | 1058 | 57 | 60 |
| 6 | 1196 | 50 | 52 |
| 7 | 842 | 71 | 72 |
| 8 | 660 | 91 | 86 |
| 9 | 728 | 82 | 80 |
| 10 | 1120 | 54 | 50 |
| 11 | 1246 | 48 | 46 |
| 12 | 660 | 91 | 88 |
| 13 | 1246 | 48 | 46 |
| 14 | 1136 | 53 | 54 |
| 15 | 776 | 77 | 70 |
| 16 | 1064 | 56 | 60 |
| 17 | 741 | 81 | 78 |
| 18 | 828 | 72 | 70 |
| 19 | 1066 | 56 | 58 |
| 20 | 1012 | 59 | 54 |
| 21 | 1092 | 55 | 54 |
| 22 | 1132 | 53 | 56 |
| 23 | 1008 | 60 | 58 |
| 24 | 766 | 78 | 82 |
| 25 | 1028 | 58 | 58 |
| 26 | 786 | 76 | 74 |
| 27 | 988 | 61 | 64 |
| 28 | 872 | 69 | 72 |
| 29 | 836 | 72 | 74 |
| Average | 967 | 64 | 65 |
| SD | 181 | 13 | 12 |
The reported compensatory pauses (CPs) represent the average value of 10 CPs recorded at rest in every patient. The corresponding RCP is calculated by the formula RCP (bpm)=60,000/CP.
The value of the rate of the compensatory pauses (RCP) is highly correlated to the spontaneous mean heart rate (MHR) (r=0.92)
| Pts | CP (ms) | RCP (bpm) | MHR (bpm) |
| 1 | 1092 | 55 | 76 |
| 2 | 1228 | 49 | 54 |
| 3 | 808 | 74 | 70 |
| 4 | 1016 | 59 | 60 |
| 5 | 1058 | 57 | 60 |
| 6 | 1196 | 50 | 52 |
| 7 | 842 | 71 | 72 |
| 8 | 660 | 91 | 86 |
| 9 | 728 | 82 | 80 |
| 10 | 1120 | 54 | 50 |
| 11 | 1246 | 48 | 46 |
| 12 | 660 | 91 | 88 |
| 13 | 1246 | 48 | 46 |
| 14 | 1136 | 53 | 54 |
| 15 | 776 | 77 | 70 |
| 16 | 1064 | 56 | 60 |
| 17 | 741 | 81 | 78 |
| 18 | 828 | 72 | 70 |
| 19 | 1066 | 56 | 58 |
| 20 | 1012 | 59 | 54 |
| 21 | 1092 | 55 | 54 |
| 22 | 1132 | 53 | 56 |
| 23 | 1008 | 60 | 58 |
| 24 | 766 | 78 | 82 |
| 25 | 1028 | 58 | 58 |
| 26 | 786 | 76 | 74 |
| 27 | 988 | 61 | 64 |
| 28 | 872 | 69 | 72 |
| 29 | 836 | 72 | 74 |
| Average | 967 | 64 | 65 |
| SD | 181 | 13 | 12 |
| Pts | CP (ms) | RCP (bpm) | MHR (bpm) |
| 1 | 1092 | 55 | 76 |
| 2 | 1228 | 49 | 54 |
| 3 | 808 | 74 | 70 |
| 4 | 1016 | 59 | 60 |
| 5 | 1058 | 57 | 60 |
| 6 | 1196 | 50 | 52 |
| 7 | 842 | 71 | 72 |
| 8 | 660 | 91 | 86 |
| 9 | 728 | 82 | 80 |
| 10 | 1120 | 54 | 50 |
| 11 | 1246 | 48 | 46 |
| 12 | 660 | 91 | 88 |
| 13 | 1246 | 48 | 46 |
| 14 | 1136 | 53 | 54 |
| 15 | 776 | 77 | 70 |
| 16 | 1064 | 56 | 60 |
| 17 | 741 | 81 | 78 |
| 18 | 828 | 72 | 70 |
| 19 | 1066 | 56 | 58 |
| 20 | 1012 | 59 | 54 |
| 21 | 1092 | 55 | 54 |
| 22 | 1132 | 53 | 56 |
| 23 | 1008 | 60 | 58 |
| 24 | 766 | 78 | 82 |
| 25 | 1028 | 58 | 58 |
| 26 | 786 | 76 | 74 |
| 27 | 988 | 61 | 64 |
| 28 | 872 | 69 | 72 |
| 29 | 836 | 72 | 74 |
| Average | 967 | 64 | 65 |
| SD | 181 | 13 | 12 |
The reported compensatory pauses (CPs) represent the average value of 10 CPs recorded at rest in every patient. The corresponding RCP is calculated by the formula RCP (bpm)=60,000/CP.
P% significantly increased (p<0.001) at each step with respect to the previous one. P% was 24±15% at the pacing rate of RCP−10 bpm, 39±19% at the pacing rate of RCP−5 bpm, 63±17% at the pacing rate of RCP, and 79±19% at the pacing rate of RCP+5 bpm (Fig. 2).
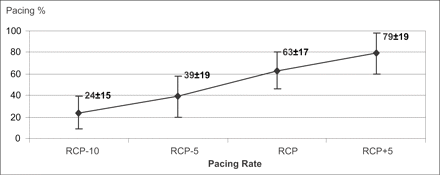
The P% significantly increases as a function of the pacing rate in the range RCP−10 bpm to RCP+5 bpm (p<0.001).
HR increased from 65±13 bpm at the first step to 66±13 bpm at the pacing rate of RCP−5 bpm (p=NS). HR was 68±13 bpm and 71±13 bpm at the pacing rates of RCP and RCP+5 bpm, respectively (p<0.001 at each step with respect to the previous one). Fig. 3 shows a modest increase in HR on increasing the pacing rate from RCP−10 bpm to RCP+5 bpm.
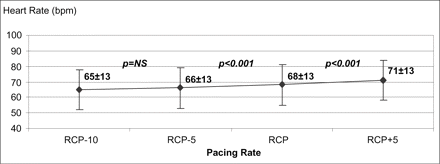
HR slightly increases by pacing the right ventricle (RV) in the range RCP−10 bpm to RCP+5 bpm.
SRR% was 9.8±8% at the pacing rate of RCP−10 bpm and 9.3±10% at the pacing rate of RCP−5 bpm, without a statistically significant difference (p=0.089). SRR% was 4.1±6% at the pacing rate of RCP, with a statistically significant difference with respect to the previous two steps (p<0.002). SRR% was not assessable at the pacing rate of RCP+5 bpm, owing to the sporadic occurrence of cardiac cycles shorter than 600 ms during a 1 min recording (Fig. 4).
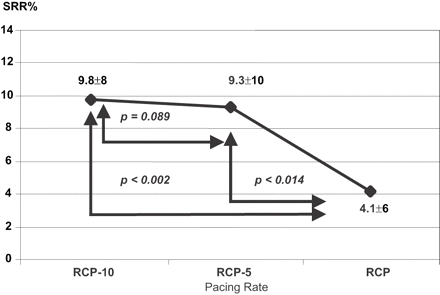
The incidence of ventricular cycles shorter than 600 ms significantly decreases at the pacing rate of RCP.
Discussion
The first result of our study is that the RCP represents a good estimate of the MHR of the patient. This means that we do not need to switch off stimulation to assess the spontaneous rate; it could be enough to analyse the average value of 10 consecutive compensatory pauses. We did not investigate whether fewer compensatory pauses were enough to yield a good estimate of the MHR, and there are no specific data in the literature. Pritchett et al. [9] collected five values of compensatory pauses following each specific coupling interval of the extrastimulus delivered for inducing them. On this unique basis we decided to collect 10 values for each patient at rest with the pacing rate programmed to 40/50 bpm, in order to achieve a more precise and reliable mean value. In this condition, the pacemaker paced the heart only sporadically during relatively long cardiac cycles, allowing an easy collection of the CPs. In patients with chronic AF and a relatively high HR, the pacemaker should pace at a higher rate in order to elicit some compensatory pauses.
Knowing the MHR in each patient enables us to establish a reference parameter when choosing the best pacing rate to programme. From a clinical point of view, when we pace the heart at a rate much lower than the MHR, we cannot exert any effect on rate regularisation. On the other hand, increasing the pacing rate can stabilise the rhythm, but can also increase the patient's HR. Ideally, we should regularise the rhythm without increasing the rate. Of course, the ablate and pace technique would achieve this goal, but our patients were symptomatic only with bradycardia, and their quality of life was not altered by their occasional episodes of relatively high rate. For this reason ablate and pace was not proposed for this patient population, while it is a valid clinical option in drug refractory and highly symptomatic patients with a relatively high spontaneous rate [12]. Previous studies on rate regularisation with special pacing algorithms showed a rate increase from 81 bpm to 88 bpm on average [13] in patients who were candidates for ablate and pace, and during an acute cath-lab procedure a significant haemodynamic improvement was reported with ventricular pacing [14] resulting in rate increases from 93 bpm to 103 bpm following the application of a stabilisation algorithm. So a rate increase of about 10% seems reasonable also from a haemodynamic point of view. In our patients, being candidates for standard antibradycardia pacing, this limit is even more acceptable since the reference rate is a mean heart rate of 65±12 bpm.
Our study shows that there is a pacing rate band around the MHR or the RCP that combines a modest increase in HR with a considerable increase in P%, meaning regularisation without altering the HR. This pacing rate band lies in the RCP–(RCP+5) bpm range. With a pacing rate programmed in this range, we can induce a P%>60% without substantially increasing HR. The goal of stabilising the rhythm without increasing the HR could therefore be achieved.
If the pacing rate exceeded the upper limit of the band (RCP+5 bpm), the P% could probably become about 100% and the HR would increase linearly with the pacing rate, but this point was not investigated. Theoretically, any increase in the pacing rate above RCP+5 bpm could cause a corresponding increase in HR. The value RCP+5 bpm may represent the upper limit of the pacing rate. On the other hand, we could argue that maintaining a P% in the 60–80% range would determine a stable rhythm, with an acceptable and modest increase in HR and a low incidence of short spontaneous cardiac cycles. There are no specific definitions of “short cardiac cycles” and we defined them as intervals shorter than 600 ms since the proposed stimulation should theoretically reduce the incidence of all cardiac intervals shorter than the pacing interval, and a cut-off value of 550 ms for a similar application was recently proposed [15]. In this case we preferred a less selective threshold of 600 ms.
In accordance with our results, the target pacing rate can be identified either from the value of RCP or from the current P%. Langendorf and Pritchett's findings are in agreement with the observation that paced beats prevent the occurrence of a subsequent short cycle, as the interval of the CP is on average longer than the mean spontaneous interval. Therefore, our findings show that the interval of the CP is not always longer than the mean escape interval in each patient as reported in Table 1, and the reason could be the different timing of assessing the CP and the MHR or the intrinsic differences between patients. However, the RCP and the MHR are on average similar and highly correlated, so the assumption of preventing short cardiac cycles after pacing seems valid and in accordance with the results.
Thus, the best pacing rate in patients with permanent AF and VVI(R) pacing can be established from any one of the following three parameters: the MHR, the RCP, or the pacing rate that determines a P% in the 60–80% range.
Our study evaluated the effects of different pacing rates only in the patients at rest. Of course, an optimal control of rate irregularity would require an automatic algorithm incorporated into the implanted device, in order to guarantee a continuous and effective adjustment of the pacing rate to the evolving conditions of each patient. An automatic algorithm would only be able to evaluate the RCP or the current P%, since evaluation of the MHR would imply switching off stimulation. However, the study investigated neither the intra-patient variability of the CP with repeated tests, nor its dependence on the pacing rate, nor its variability during exercise, and the analysis of MHR and P% was made on a sample of only 1 min ECG recording. Nevertheless, the results are in accordance with those of Greenhut [7] showing that the pacing rate required to reduce ventricular rate variability was closely approximated by the mean ventricular rate during intrinsic AF, even if these two studies started from different assumptions and used different methods.
It has been hypothesised that the compensatory pause in AF is the consequence of concealed retrograde conduction. However, investigating the electrophysiological origin of this phenomenon was not the purpose of our study [8,,9].
The standard pacing technique is based on right ventricular apical pacing, which may impair left ventricular function with regard to the spontaneous rhythm [16]. Our results are therefore applicable to every ventricular pacing technique or site, as our study only addressed the choice of the best pacing rate for rhythm stabilisation. Application of our method to a biventricular pacemaker [17] or to a pacemaker stimulating His bundle [18,,19] or RV outflow tract [20] may obviate the possible adverse haemodynamic effect of RV apical pacing, while offering the advantage of a stable ventricular rate. Paced patients with ischaemic heart disease could also benefit from rate regularisation by decreasing the incidence of short cardiac cycles [21], since coronary blood flow occurs during ventricular diastole and in short cardiac intervals the time available for coronary blood flow is limited [22,,23]. On the other hand, the effect of pacing site on the mechanical efficiency of the myocardium and the potentially negative influence of a slightly higher HR should be considered and evaluated.
Conclusions
The RCP enables us to estimate, during pacing, what the spontaneous MHR would be without pacing. This provides a reference parameter for use in stabilising the rhythm without increasing the heart rate. The value of the optimal pacing rate can be determined by the MHR of the patient with the pacemaker OFF, by the RCP or by the corresponding P%.
References
Author notes
*This work was performed at the Cardiovascular Department, Cecina Hospital.



