-
PDF
- Split View
-
Views
-
Cite
Cite
Andreas Schuchert, Contributions of permanent cardiac pacing in the treatment of atrial fibrillation, EP Europace, Volume 5, Issue s1, 2003, Pages S36–S41, https://doi.org/10.1016/j.eupc.2004.06.003
Close - Share Icon Share
Abstract
Newer indications for permanent cardiac stimulation include the prevention of paroxysmal atrial fibrillation (AF) and cardiac resynchronisation in patients suffering from advanced heart failure. Direct comparisons between VVI and DDD or AAI pacing showed an advantage conferred by physiological pacing on the risk of developing AF during long-term follow-up in patients with sinus node dysfunction, AV block, or both. Furthermore, in patients with conventional pacing indications and paroxysmal atrial tachyarrhythmias, a high percentage of atrial pacing was associated with a lighter AF burden. This article reviews several important issues involved in the optimisation of cardiac pacing with a view to prevent paroxysmal AF by new, dedicated pacing algorithms. The AF Suppression™ algorithm significantly reduced the rates of symptomatic paroxysmal AF. This algorithm, which confers its benefit by maintaining the atrial pacing rate slightly above the spontaneous sinus rate, should be activated in patients with a history of atrial tachyarrhythmia. Implanting the lead in the low atrial septum seems to reduce further the frequency of tachyarrhythmic events. Future indications for this mode of pacing may be extended to patients at high risk of new-onset or recurrent AF, such as candidates for cardiac resynchronisation therapy or implantable cardioverter/defibrillator recipients.
Present-day permanent cardiac pacing is no longer limited to the prevention of bradycardia or asystole in patients with advanced atrioventricular (AV) block or sinus node dysfunction. Newer indications include (a) prevention of paroxysmal atrial fibrillation (AF), and (b) cardiac resynchronisation in patients suffering from advanced heart failure. This article reviews several important issues involved in the optimisation of cardiac pacing with a view to preventing paroxysmal AF ( Fig. 1 , Table 1 ).
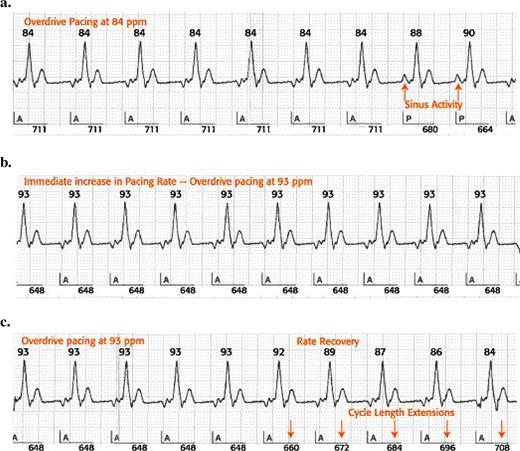
Operation of the AF Suppression™ Algorithm. When two P waves are sensed within 16 consecutive cardiac cycles (a), the device immediately paces the atrium at a higher rate (b) determined by the ongoing paced rate and the lower and upper rate overdrive settings. Overdrive pacing continues for a programmable number of cycles before slowing to a rate determined by rate recovery (c) and returning the device operation to the base rate , rest rate or sensor-indicated rate. Upon further sensing of spontaneous atrial events, the algorithm will return to overdrive pacing. See text for further explanations.
Atrial pacing and atrial fibrillation – results of randomised studies in patients with antibradycardia pacing indications
| Variable . | Comparison . | Follow-up . | AF decrease (%) . | Advantage . |
|---|---|---|---|---|
| Pacing mode | ||||
| Ref. [1] | AAI vs. VVI | 5.5 years | 46 | AAI ( P < 0.05) |
| Ref. [2] (MOST) | DDDR vs. VVIR | 33 months | 21 | AAIR ( P < 0.01) |
| Ref. [4] (CTOPP) | AAI/DDD vs. VVIR | 6.4 years | 20 | AAI/DDD ( P < 0.01) |
| Atrial OD pacing | ||||
| Ref. [8] (PAF-PACE) | RA OD vs. OAO | 3 × 4 weeks (CO) | 60 | RA OD ( P <0.01) |
| Ref. [9] (ADOPT) | RA OD vs. no OD | 6 months | 25 | RA OD ( P <0.01) |
| Ref. [10] | DDDR + RA OD vs. DDDR | 2 × 1 month (CO) | – | None |
| Ref. [11] (ASPECT) | DDDR + RA OD vs. DDDR | 2 × 3 months (CO) | – | None |
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Ref. [18] (OASES) | RA OD vs. DDDR | 2 × 3 months (CO) | 49 | RA OD ( P = 0.033) |
| Ref. [18] (OASES) | Low RA septal OD vs. DDDR | 2 × 3 months (CO) | 70 | Low RA septal OD ( P = 0.027) |
| Antitachycardia pacing | ||||
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Alternate pacing site | ||||
| Ref. [11] (ASPECT) | Septal vs. non-septal pacing | 2 × 3 months (CO) | 44 | Septal pacing ( P = 0.01) |
| Ref. [15] | RAA vs. BB pacing | ∼1 year | 28 | BB pacing ( P = 0.01) |
| Ref. [17] | RAA vs. low RA septal pacing | AF episodes/month | ∼80 | Low RA septal pacing |
| Dual site pacing | ||||
| Ref. [7] (DAPPAF) | Dual site vs. RAA vs. septum | 12 months | ∼35 | Dual site vs. septum ( P < 0.05) a |
| Variable . | Comparison . | Follow-up . | AF decrease (%) . | Advantage . |
|---|---|---|---|---|
| Pacing mode | ||||
| Ref. [1] | AAI vs. VVI | 5.5 years | 46 | AAI ( P < 0.05) |
| Ref. [2] (MOST) | DDDR vs. VVIR | 33 months | 21 | AAIR ( P < 0.01) |
| Ref. [4] (CTOPP) | AAI/DDD vs. VVIR | 6.4 years | 20 | AAI/DDD ( P < 0.01) |
| Atrial OD pacing | ||||
| Ref. [8] (PAF-PACE) | RA OD vs. OAO | 3 × 4 weeks (CO) | 60 | RA OD ( P <0.01) |
| Ref. [9] (ADOPT) | RA OD vs. no OD | 6 months | 25 | RA OD ( P <0.01) |
| Ref. [10] | DDDR + RA OD vs. DDDR | 2 × 1 month (CO) | – | None |
| Ref. [11] (ASPECT) | DDDR + RA OD vs. DDDR | 2 × 3 months (CO) | – | None |
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Ref. [18] (OASES) | RA OD vs. DDDR | 2 × 3 months (CO) | 49 | RA OD ( P = 0.033) |
| Ref. [18] (OASES) | Low RA septal OD vs. DDDR | 2 × 3 months (CO) | 70 | Low RA septal OD ( P = 0.027) |
| Antitachycardia pacing | ||||
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Alternate pacing site | ||||
| Ref. [11] (ASPECT) | Septal vs. non-septal pacing | 2 × 3 months (CO) | 44 | Septal pacing ( P = 0.01) |
| Ref. [15] | RAA vs. BB pacing | ∼1 year | 28 | BB pacing ( P = 0.01) |
| Ref. [17] | RAA vs. low RA septal pacing | AF episodes/month | ∼80 | Low RA septal pacing |
| Dual site pacing | ||||
| Ref. [7] (DAPPAF) | Dual site vs. RAA vs. septum | 12 months | ∼35 | Dual site vs. septum ( P < 0.05) a |
RA = right atrium; OD = overdrive; CO = crossover; RAA = right atrial appendage; BB = Bachmann's bundle.
a In patients treated with antiarrhythmic drugs.
Atrial pacing and atrial fibrillation – results of randomised studies in patients with antibradycardia pacing indications
| Variable . | Comparison . | Follow-up . | AF decrease (%) . | Advantage . |
|---|---|---|---|---|
| Pacing mode | ||||
| Ref. [1] | AAI vs. VVI | 5.5 years | 46 | AAI ( P < 0.05) |
| Ref. [2] (MOST) | DDDR vs. VVIR | 33 months | 21 | AAIR ( P < 0.01) |
| Ref. [4] (CTOPP) | AAI/DDD vs. VVIR | 6.4 years | 20 | AAI/DDD ( P < 0.01) |
| Atrial OD pacing | ||||
| Ref. [8] (PAF-PACE) | RA OD vs. OAO | 3 × 4 weeks (CO) | 60 | RA OD ( P <0.01) |
| Ref. [9] (ADOPT) | RA OD vs. no OD | 6 months | 25 | RA OD ( P <0.01) |
| Ref. [10] | DDDR + RA OD vs. DDDR | 2 × 1 month (CO) | – | None |
| Ref. [11] (ASPECT) | DDDR + RA OD vs. DDDR | 2 × 3 months (CO) | – | None |
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Ref. [18] (OASES) | RA OD vs. DDDR | 2 × 3 months (CO) | 49 | RA OD ( P = 0.033) |
| Ref. [18] (OASES) | Low RA septal OD vs. DDDR | 2 × 3 months (CO) | 70 | Low RA septal OD ( P = 0.027) |
| Antitachycardia pacing | ||||
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Alternate pacing site | ||||
| Ref. [11] (ASPECT) | Septal vs. non-septal pacing | 2 × 3 months (CO) | 44 | Septal pacing ( P = 0.01) |
| Ref. [15] | RAA vs. BB pacing | ∼1 year | 28 | BB pacing ( P = 0.01) |
| Ref. [17] | RAA vs. low RA septal pacing | AF episodes/month | ∼80 | Low RA septal pacing |
| Dual site pacing | ||||
| Ref. [7] (DAPPAF) | Dual site vs. RAA vs. septum | 12 months | ∼35 | Dual site vs. septum ( P < 0.05) a |
| Variable . | Comparison . | Follow-up . | AF decrease (%) . | Advantage . |
|---|---|---|---|---|
| Pacing mode | ||||
| Ref. [1] | AAI vs. VVI | 5.5 years | 46 | AAI ( P < 0.05) |
| Ref. [2] (MOST) | DDDR vs. VVIR | 33 months | 21 | AAIR ( P < 0.01) |
| Ref. [4] (CTOPP) | AAI/DDD vs. VVIR | 6.4 years | 20 | AAI/DDD ( P < 0.01) |
| Atrial OD pacing | ||||
| Ref. [8] (PAF-PACE) | RA OD vs. OAO | 3 × 4 weeks (CO) | 60 | RA OD ( P <0.01) |
| Ref. [9] (ADOPT) | RA OD vs. no OD | 6 months | 25 | RA OD ( P <0.01) |
| Ref. [10] | DDDR + RA OD vs. DDDR | 2 × 1 month (CO) | – | None |
| Ref. [11] (ASPECT) | DDDR + RA OD vs. DDDR | 2 × 3 months (CO) | – | None |
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Ref. [18] (OASES) | RA OD vs. DDDR | 2 × 3 months (CO) | 49 | RA OD ( P = 0.033) |
| Ref. [18] (OASES) | Low RA septal OD vs. DDDR | 2 × 3 months (CO) | 70 | Low RA septal OD ( P = 0.027) |
| Antitachycardia pacing | ||||
| Ref. [12] (ATTEST) | DDDR + RA OD + ATP vs. DDDR | 3 months | – | None |
| Alternate pacing site | ||||
| Ref. [11] (ASPECT) | Septal vs. non-septal pacing | 2 × 3 months (CO) | 44 | Septal pacing ( P = 0.01) |
| Ref. [15] | RAA vs. BB pacing | ∼1 year | 28 | BB pacing ( P = 0.01) |
| Ref. [17] | RAA vs. low RA septal pacing | AF episodes/month | ∼80 | Low RA septal pacing |
| Dual site pacing | ||||
| Ref. [7] (DAPPAF) | Dual site vs. RAA vs. septum | 12 months | ∼35 | Dual site vs. septum ( P < 0.05) a |
RA = right atrium; OD = overdrive; CO = crossover; RAA = right atrial appendage; BB = Bachmann's bundle.
a In patients treated with antiarrhythmic drugs.
Pacing system
The first issue addressed is the choice of “appropriate pacing devices”. Available options are either a VVI or a physiological (DDD or AAI) pacing system. Direct comparisons between the two systems revealed no advantage conferred by physiological pacing on overall survival, though it lowered the risk of developing AF during long-term follow-up [1–,4] . This benefit was observed in patients with sick sinus syndrome, AV block, or both [2,,4] .
Atrial pacing rate
A second important factor is the optimisation of the atrial pacing rate with respect to the spontaneous sinus rate. In patients with conventional pacing indications and paroxysmal atrial tachyarrhythmias, a high percentage of atrial pacing was associated with a lighter AF burden [5–,7] . Since the patients included in these studies were recipients of standard pacing systems, consistent atrial pacing was achieved by programming the lower atrial pacing rate above the mean sinus rate [8] . This effective approach has limitations, particularly in patients with sinus rates in a normal range, in whom the lower atrial paced rate has to be programmed between 75 and 90 bpm, often not tolerated over the long term [8] .
Most state-of-the-art dual-chamber pacemakers offer new functions, which continuously compare the lower atrial pacing rate with the actual sinus rate and allow the delivery of an atrial paced rate just above the spontaneous sinus rate. In St. Jude Medical pacing devices, the AF Suppression™ algorithm “controls” the sinus rate by increasing the paced atrial rate when two intrinsic beats are detected within any of 16 consecutive cardiac cycles ( Fig. 1 ). The rate of overdrive pacing and the duration of overdrive pacing are programmable. The lower rate overdrive (LRO) controls the degree of overdrive pacing between 45 and 59 bpm, and is set to pace at a rate 10 bpm faster than the spontaneous rate. The upper rate overdrive (URO) is effective between 150 and 180 bpm, and is set at 5 bpm above the intrinsic rate. The increase in overdrive rate between LRO and URO is based on linear regression. The maximum overdrive pacing rate is limited by the maximum sensor rate regardless of sensor activation. The rate dictated by the AF Suppression™ algorithm is consistently the same as or higher than the sensor-indicated rate. Once stable pacing is achieved, the system continues to pace at that rate for a number of overdrive pacing cycles programmable between 15 (nominal) and 40, before it decreases in search of the underlying rate. The dynamic rate recovery, which determines the decrease from overdrive pacing to base rate, is set at 8 ms per cycle for rates >100 bpm, and at 12 ms for rates between 45 and 100 bpm.
This easily implemented and programmable algorithm was prospectively studied in the single-blind randomised multicentre ADOPT trial, which included 288 patients with conventional pacing indications and a documented history of AF within the month prior to implantation of a DDDR pacing system [9] . The patients were randomly assigned to programming of the algorithm ON (treatment group, n = 130) or OFF (control group, n = 158). Over the following 6 months of follow-up, the patients were instructed to record all symptoms consistent with an episode of atrial tachyarrhythmia with a cardiac event monitor. The percentage of atrial pacing in the treatment group was significantly higher (93%) than in the control group (68%, P < 0.0001). Furthermore, the overall symptomatic AF burden, defined as the total number of AF days divided by the cumulative follow-up days, was 2.50% in the control group vs. 1.87% in the treatment group, a relative difference of 25% ( P = 0.005, Fig. 2 ). Limitations of this study were the evaluation of only symptomatic episodes verified by an external loop recorder, because extended diagnostic counters were not available in the implanted pacemakers. The total number of AF episodes as determined by the device memory revealed no significant reduction, but the mode switch algorithm in these devices was relatively insensitive compared with today's pacemakers.
![Reduction in AF burden by the AF Suppression™ algorithm over 6-month follow in the ADOPT trial. While a reduction was observed in both the control (open bars) and the treatment group (solid bars), AF burden decreased by significantly greater amounts in the actively treated group ( P = 0.005 at each follow-up visit). Reproduced with permission from the American College of Cardiology Foundation [9] .](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/europace/5/s1/10.1016_j.eupc.2004.06.003/3/m_S36_2.jpeg?Expires=1750191634&Signature=l0R7djbkJRHClPFuYTJWf4DS8fjnkiQuEtMY4JZysifOPedKT3kiQzQF4B9xXze6HAPFb9iIaJVJpqjBI55uxLTWws16yqXHpbenQzY7INZhkQeTsYOeFExcEOlAEd32QCmDQFef51BLyoLeHKPoPakbGYrJRA7D~QTcXruiONFvx4OAnNCMaGmgVOlbVFPMr8JnFtaGQ2f2tgqL9zLlX4nMJUlqnzBxQ3wl9N45wEgIqM~mB6-OVuPJPtvvjJP3vlrjf-RerKCB26k8noy~yPhyjthzQ-lyqXkLecNTI2u4dF9C-WmaxY6z6vj9wmDYdKHizFW~Z6VJoI2f5pAGFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Reduction in AF burden by the AF Suppression™ algorithm over 6-month follow in the ADOPT trial. While a reduction was observed in both the control (open bars) and the treatment group (solid bars), AF burden decreased by significantly greater amounts in the actively treated group ( P = 0.005 at each follow-up visit). Reproduced with permission from the American College of Cardiology Foundation [9] .
Nearly all pacemaker manufacturers have developed similar pacing functions. Ricci and co-workers reported the results of a randomised cross-over study of the consistent atrial pacing algorithm (Medtronic), designed to maintain a high percentage of atrial pacing [10] . Though it lowered the frequency of premature atrial complexes, this pacing function did not decrease the number of symptomatic episodes of AF, a result consistent with studies of other similar pacing algorithms.
Extended pacing functions, which initiate overdrive atrial pacing in response to specific events, for example after sensing premature atrial complexes or after spontaneous termination of an atrial tachyarrhythmia have also been tested. In ASPECT, a multicentre trial of three programmable pacing algorithms in 277 patients randomised between atrial septal vs. non-septal pacing, no objective benefit was conferred by the combined algorithms on the frequency of daily episodes of atrial tachyarrhythmias or on the overall atrial arrhythmic burden [11] .
Atrial antitachycardia pacing
Atrial antitachycardia pacing (ATP) can terminate atrial flutter or AF of recent onset in up to 50% of attempts. The ATTEST study tested the hypothesis that pace-termination of organised atrial tachyarrhythmias would prevent the development of AF [12] . In a parallel study design, 370 patients were randomised to DDDR pacing only, vs. DDDR pacing with atrial ATP and atrial pace prevention algorithms programmed ON. Neither the frequency of AF nor the AF burden were reduced by the delivery of preventive therapies and ATP [12] . This absence of therapeutic effect suggests that combining these algorithms may have both pro- and antiarrhythmic effects.
Alternate atrial pacing sites
The antiarrhythmic efficacy of pacing from one or two non-conventional atrial pacing sites has recently been evaluated ( Fig. 3 ). In studies from single institutions, the simultaneous implantation of two atrial leads was found effective in the prevention of recurrent AF [13,,14] , though, in a multicentre trial, it was less successful when tested as a sole mode of prevention [7] .
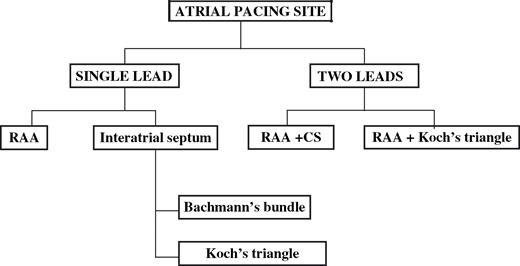
Different single and dual lead configurations applied among various studies of atrial pacing in the prevention of AF. RAA = right atrial appendage; CS = coronary sinus.
The most frequently used approach, currently, consists of implanting one lead in the upper or lower part of the atrial septum. Bailin and co-workers studied the implantation in the upper part of the atrial septum and demonstrated that patients with septal atrial leads had lower rates of permanent AF compared with patients with conventionally placed leads [15] . Padeletti introduced the lead implantation in the lower part of the atrial septum [16] . In a subsequent step the efficacy of pacing at this site combined with preventive pacing functions was examined. Though this strategy was not effective in two initial studies of preventive pacing functions [11,,17] , the Overdrive Atrial SEptum Stimulation trial (OASES), a prospective multicentre trial in 285 patients with paroxysmal AF and class I or II pacing indications, yielded different results. The symptomatic AF burden in patients with right atrial appendage leads was lowered by nearly 50%, from a mean of 76 min/day in the algorithm “OFF” group, to a mean of 38.9 min/day in the algorithm “ON” group ( P = 0.033). Among patients with atrial leads placed in the low atrial septum, the AF burden was lowered by over 70%, from a mean of 74 min/day in the algorithm “OFF” to a mean of 22 min/day in the algorithm “ON” group ( P = 0.027) [18] . Several studies completed thus far were open to methodological flaws and technical interference, such as inappropriate mode switch, atrial undersensing, or proarrhythmic effects of improper device programming. The ongoing PAFOS multicentre study was designed to eliminate these confounding factors. An interim analysis has confirmed that its design is appropriate to accurately measure the AF burden by allowing the individualisation of a broad range of AV delays [19] .
Candidates for preventive atrial pacing
Most studies discussed earlier included patients who had conventional pacing indications and a history of AF. In these patients the described approaches were successful. In the few studies performed in patients without symptomatic bradycardia atrial pacing had neutral effects on AF [20–,23] . At present, preventive atrial pacing may be appropriate in patients with AF only, e.g. prior to performing AV node ablation.
Conclusions
In patients with conventional pacing indications and a history of AF, DDD and AAI pacemakers reduce the risk of further AF during follow-up. In several randomised controlled trials, the AF Suppression™ algorithm significantly reduced the rates of paroxysmal AF. This algorithm, which confers its benefit by maintaining the atrial pacing rate slightly above the spontaneous sinus rate, should be activated in patients with a history of atrial tachyarrhythmia. Implanting the lead in the lower atrial septum seems to reduce further the frequency of tachyarrhythmic events measured by automatic mode switches. Future indications for this mode of pacing may be extended to patients at high risk of new-onset or recurrent AF, and candidates for cardiac resynchronisation therapy or implantable cardioverter/defibrillator patients.



