-
PDF
- Split View
-
Views
-
Cite
Cite
Ki-Woon Kang, Hui-Nam Pak, Junbeom Park, Jin Gyu Park, Jae Sun Uhm, Boyoung Joung, Moon-Hyoung Lee, Chun Hwang, Additional linear ablation from the superior vena cava to right atrial septum after pulmonary vein isolation improves the clinical outcome in patients with paroxysmal atrial fibrillation: prospective randomized study, EP Europace, Volume 16, Issue 12, December 2014, Pages 1738–1745, https://doi.org/10.1093/europace/euu226
Close - Share Icon Share
Abstract
Although circumferential pulmonary vein isolation (CPVI) has been considered as the cornerstone for paroxysmal atrial fibrillation (PAF) ablation, there has been a substantial recurrence rate. We conducted a prospectively randomized study to evaluate whether additional linear ablation from the superior vena cava (SVC) to the right atrial (RA) septum (SVC-L) improves the clinical outcome.
This study enroled 200 patients with PAF (male 74.5%, 56.8 ± 11.7 years old) randomly assigned to either the CPVI (n = 100) or CPVI + SVC-L (n = 100) groups. An RA isthmus ablation was performed in all patients. The CPVI + SVC-L group required a longer ablation procedure time (82.7 ± 17.9 min) than the CPVI group (63.6 ± 16.8 min, P < 0.001). The complication rates were 5% in CPVI + SVC-L group and 2% in CPVI group, respectively (P = 0.445). Two CPVI + SVC-L group patients had post-procedural sinus node dysfunction, which recovered within 24 h. During 12.2 ± 5.3 months of follow-up, the recurrence rate was significantly lower in the CPVI + SVC-L group (6%) than the CPVI group (27%, P < 0.001). The post-procedural 3-month follow-up heart rate variability in the CPVI + SVC-L group showed a significantly greater reduction in the rMSSD (25.2 ± 13.7 vs. 13.7 ± 8.5 ms, P < 0.001), HF (10.2 ± 7.1 vs. 5.5 ± 5.8 ms2, P < 0.001), and LF/HF (1.6 ± 0.5 vs. 0.9 ± 0.3, P < 0.001) than in the CPVI group.
In spite of a longer procedure time and risk of transient sinus node dysfunction, an SVC-L in addition to CPVI improved the clinical outcome of catheter ablation, and was associated with post-procedural autonomic neural remodelling in patients with PAF.
Patients with paroxysmal atrial fibrillation were randomly assigned to circumferential pulmonary vein isolation (CPVI) group vs. CPVI with additional linear ablation from superior vena cava to right atrial septum (SVC-L) group.
In spite of a longer procedure time and risk of transient sinus node dysfunction, an SVC-L in addition to CPVI improved the clinical outcome of catheter ablation at over 1-year follow-up, and was associated with post-procedural autonomic neural remodelling.
Introduction
Radiofrequency catheter ablation (RFCA) became the standard therapy for rhythm control in symptomatic atrial fibrillation (AF) patients who are refractory to antiarrhythmic drugs or as a first-line rhythm control strategy.1 Circumferential pulmonary vein isolation (CPVI) has been considered as the cornerstone of the catheter ablation of AF, especially in patients with paroxysmal AF (PAF).2 Although CPVI reduces the AF burden significantly, there is a substantial recurrence rate during the long-term follow-up. To improve the procedural success rate, additional left atrial (LA) linear ablation or complex fractionated atrial electrogram-guided ablation has been suggested to modify the substrate of AF with conflicting results.3,4 For the patients with PAF, although 90% of AF triggers originate from the pulmonary veins (PVs), the remainder of the triggers arise from non-PV foci, and the superior vena cava (SVC) is one of the most common non-PV foci.1,5,6 Autonomic modulation, especially vagal suppression, improves the clinical outcome of the RFCA of AF.7 Cardiac autonomic nerves and ganglionate plexi are located along the epicardial area of the CPVI.8 The third fat pad, the gate keeper ganglia of the cardiac parasympathetic nerve, is located at the junction of the SVC, right atrial (RA) septum, and ascending aorta.8 Because the RA posterior wall and septum have non-uniform anisotropy and disorganized electrical conduction,9 the interatrial septum is the major locus for unstable reentry, and a septal linear ablation from the fossa ovalis to Bachmann's bundle or the inferior vena cava has been shown to reduce AF in experimental studies.10 Therefore, we hypothesized that the septal linear ablation from the SVC septal aspect to the fossa ovalis (SVC-L) in addition to CPVI would improve the clinical outcome of catheter ablation in patients with PAF. We compared the clinical efficacy and safety between the two different ablation strategies (CPVI + SVC-L vs. CPVI alone) as a prospectively randomized study.
Methods
Study population
The study protocol adhered to the Declaration of Helsinki and was approved by the institutional review board of Severance Hospital. Proper written informed consent was obtained from all patients. This study was a single-centre prospective randomized study conducted in Severance Hospital, Yonsei University. The study prospectively enroled 200 patients who underwent RFCA for symptomatic drug-refractory PAF. Exclusion criteria were: (i) permanent or persistent AF; (ii) associated structural heart disease other than left ventricular (LV) hypertrophy; (iii) intracardiac thrombi; (iv) an LV ejection fraction <50%; (v) AF with rheumatic valvular disease; (vi) previous AF ablation; and (vii) a history of cardiac surgery. The patients were prospectively and randomly assigned to two groups according to the method of the RFCA: CPVI alone (n = 100); and CPVI + SVC-L (n = 100). All antiarrhythmic drugs were discontinued for more than five half-lives before the procedure. Anticoagulation therapy was maintained at a target prothrombin time international normalized ratio of 2.0–3.0 for at least 4 weeks before the procedure and continued until the day of the procedure. Both transthoracic echocardiography and transoesophageal echocardiography were performed before the procedure to determine whether the patients had any combined structural heart disease or LA thrombi. We imaged all patients with three-dimensional (3D) spiral CT (64 Channel, Light Speed Volume CT, Philips, Brilliance 63) to visually define the anatomy of the LA and PVs.
Electrophysiological mapping procedure
An electrophysiology study was performed using a decapolar catheter (WOVEN diagnostic catheter, Brard Electrophysiology) in the high RA, duo-decapolar catheter (St Jude Medical Inc.) in the low RA and inside the coronary sinus, and quardripolar catheter at the His bundle recording region. Bipolar intracardiac electrograms were recorded using the PruckaCardioLab Electrophysiology system (General Electric Medical Systems), and RFCA was performed in all patients using 3D electroanatomical mapping (NavX; St Jude Medical) or CARTO (Biosense Webster) merged with the 3D spiral computed tomography (CT). After a double transseptal punctures, a circumferential PV mapping catheter (Lasso; Biosense-Webster) was introduced into the LA, and systemic anticoagulation was achieved with intravenous heparin to maintain an activated clotting time of 350–500 s during the procedure. For electroanatomical mapping, the 3D geometry of both the LA and PV was generated using the NavX or CARTO mapping system and then merged with the 3D spiral CT images.
Radiofrequency catheter ablation
For the CPVI ablation, contiguous circumferential lesions were created at the level of the LA antrum encircling the right and left PVs using an open irrigation 3.5 mm tip deflectable irrigation tip catheter (25–35 W; 47°C 30 s in each point). The endpoint of the CPVI was the electrical isolation of all PV potentials, which was confirmed by Lasso catheter mapping during sinus rhythm, RA pacing, and/or an isoproterenol infusion after 30 min. All patients underwent a cavotricuspid isthmus ablation with an endpoint of bidirectional conduction block. The SVC-L ablation was started from the highest septal aspect of the SVC while observing the SVC potentials (30 W, 30 s ablation in each point). The tip of the catheter was directed towards the septal side guided by the left anterior oblique (LAO) and right anterior oblique (RAO) 30° views (Figure 1A–C) and the 3D electroanatomical map (Figure 1D and E). At the SVC–RA junction, we increased the RF power to 35 W and continued the linear ablation lesion to the upper posterior limbus of the fossa ovalis (Figure 1C). We stayed 30 s for each site of ablation watching the reduction of voltage amplitude and elimination of sharp potential along the SVC-L (Figure 2). We confirmed the PVI one more time under an isoproterenol infusion before finishing the procedure.

An SVC-L ablation shown in the LAO 35° and RAO 35° fluoroscopic views, and a 3D electroanatomical map. (A) The SVC-L ablation was started at the highest level of the septal aspect of the SVC while observing the SVC potentials. (B) At the SVC–RA junction, the RF power was increased to 35 W. (C) The end of the SVC-L ablation was at the upper posterior limbus of the foramen ovale. (D, E) Anterior posterior and posterior anterior views of the 3D electroanatomical map showing the SVC-L ablation sites.
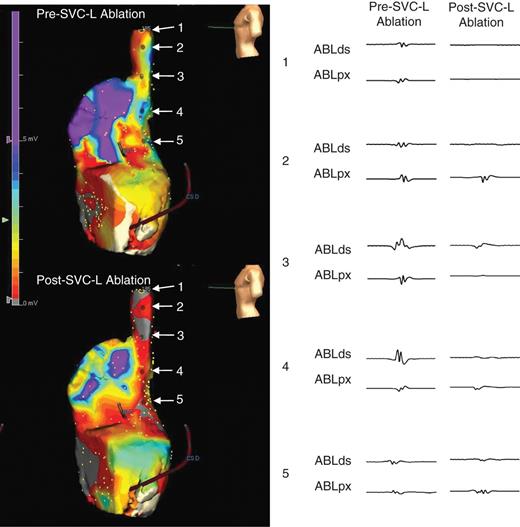
Right atrial voltage maps before and after SVC-L ablation (left lateral views) and electrogram changes along the SVC-L.
Post-ablation management and follow-up
After the RFCA, the patients were discharged without any antiarrhythmic drugs and were followed up at the outpatient clinic at 1, 3, 6, and 12 months and then every 6 months thereafter. Electrocardiography (ECG) was performed at each visit. Holter monitor (24 or 48 h) and/or event recorder recordings were evaluated at 3 and 6 months and every 6 months thereafter according to the 2012 HRS/EHRA/ECAS Expert Consensus Statement guidelines.1 Whenever a patient reported any symptoms of palpitations suggestive of arrhythmia recurrence, Holter monitoriong or event monitor recordings were obtained. We defined a recurrence of AF as any episode of AF or atrial tachycardia (AT) of at least 30 s in duration. Any ECG-documented AF/AT recurrence within 3 months of the blanking period after the RFCA was defined as an early recurrence, and an ECG-documented AF recurrence after 3 months was diagnosed as a recurrence.1 If there was a recurrence of AF or AT beyond the first 3 months after the RFCA, then a re-do procedure could be performed if the patient wanted to proceed.
Analysis of heart rate variability by Holter monitoring
We analysed the heart rate variability (HRV) from the 24 h Holter monitoring taken both pre- and 3 months post-RFCA in 59 patients with a GE Marquette MARS 8000 Holter analyzer (GE Medical System). We excluded the patients whose HRV was not analysable because of sinus node dysfunction or a high number of AF episodes (>1 h or >20 times per day) or any other arrhythmia episodes. After identifying each QRS complex, the numerical series of the RR intervals was calculated. Only high-quality recordings were considered for analysis. All recordings were digitized and reviewed by an experienced operator. Premature ventricular beats, premature atrial beats, and all electrical artefact were also excluded from the HRV analysis. The HRV parameters were used as indicators of the autonomic nerve activity according to the previously published guidelines.11 The mean heart rate (b.p.m.) and the following time-domain parameter, the root-mean square of the differences between successive NN intervals (rMSSD), were analysed: The following frequency-domain parameters were calculated: low-frequency component (LF; 0.04–0.15 Hz), high-frequency component (HF; 0.15–0.40 Hz), and LF/HF ratio. Because we used a total recording of the rhythm for a duration of 24 h, we used the absolute power of the frequency-domain measurements, and not the normalized value.12 The HF and rMSSD were indicators of the parasympathetic nervous activity, and the LF and LF/HF ratio reflected the sympathetic nervous activity.
Data analysis
We chose the sample size on the basis of a statistical analysis to prove the superiority of the CPVI with SVC-L ablation, which was described in a previous study comparing ablation strategies in patients with PAF.8 Continuous variables are expressed as the mean ± SD and were compared by an independent two sample t-test analysis. Categorical variables are presented as frequencies (%) and were compared by an χ2 analysis or Fisher's exact test for small numbers, as appropriate. To identify the confounding factors associated with AF recurrence, the collected clinical variables were analysed using a univariate Cox proportional hazards regression. A multivariate Cox proportional hazards regression was performed to determine the independent association between the two groups. A model discrimination was assessed with the use of a Harrell's C statistic. A Kaplan–Meier analysis was used to determine the probability of freedom from arrhythmia recurrence after the RFCA. A P < 0.05 was considered significant.
Results
Patient characteristics
The patients were prospectively and randomly assigned to undergo CPVI (n = 100) or CPVI + SVC-L (n = 100). The mean age was 56.8 ± 11.7 years, 149 patients (74.5%) were male. The mean CHADS2 score was 0.8 ± 1.0. Table 1 summarizes the patient characteristics between the two groups. The mean CHADS2 score (P = 0.030) and the proportion of the patients older than 75 years (P = 0.007) were higher in the CPVI + SVC-L group than in the CPVI group. Otherwise, there was no significant difference in the clinical and echocardiographic parameters between the two groups.
Clinical characteristics and comparison of study patients between CPVI and CPVI + SVC-L
| . | All patients . | HRV-analysed patients . | ||||
|---|---|---|---|---|---|---|
| CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . | CPVI (n = 28) . | CPVI + SVC-L (n = 31) . | P-value . | |
| Age (years) | 55.65 ± 11.87 | 57.89 ± 11.46 | 0.176 | 55.36 ± 9.80 | 56.35 ± 15.36 | 0.765 |
| Male (%) | 74.00 | 75.00 | 0.871 | 71.43 | 70.97 | 0.969 |
| LA size (mm) | 39.53 ± 6.22 | 40.09 ± 5.69 | 0.505 | 39.00 ± 5.82 | 40.10 ± 5.20 | 0.448 |
| LA volume index (mL/m2) | 30.43 ± 10.06 | 31.55 ± 11.16 | 0.473 | 28.97 ± 6.77 | 29.98 ± 9.40 | 0.641 |
| Ejection fraction (%) | 63.37 ± 9.89 | 64.60 ± 9.38 | 0.369 | 63.71 ± 10.63 | 67.06 ± 7.33 | 0.170 |
| E/E′ | 9.35 ± 3.29 | 10.69 ± 6.82 | 0.090 | 10.10 ± 3.65 | 9.84 ± 4.11 | 0.801 |
| CHADS2 score | 0.67 ± 0.92 | 0.98 ± 1.08 | 0.030* | 0.57 ± 0.69 | 1.13 ± 1.06 | 0.019* |
| Heart failure (%) | 3.00 | 3.00 | >0.999 | 3.57 | 3.23 | >0.999 |
| Hypertension (%) | 41.00 | 53.00 | 0.089 | 42.86 | 61.29 | 0.157 |
| Age >75 years (%) | 0 | 8.00 | 0.007* | 0 | 9.68 | 0.239 |
| Diabetes mellitus (%) | 11.00 | 17.00 | 0.221 | 10.71 | 25.81 | 0.137 |
| Stroke (%) | 5.00 | 7.00 | 0.552 | 0.00 | 3.23 | >0.999 |
| TIA (%) | 1.00 | 2.00 | >0.999 | 0.00 | 3.23 | >0.999 |
| . | All patients . | HRV-analysed patients . | ||||
|---|---|---|---|---|---|---|
| CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . | CPVI (n = 28) . | CPVI + SVC-L (n = 31) . | P-value . | |
| Age (years) | 55.65 ± 11.87 | 57.89 ± 11.46 | 0.176 | 55.36 ± 9.80 | 56.35 ± 15.36 | 0.765 |
| Male (%) | 74.00 | 75.00 | 0.871 | 71.43 | 70.97 | 0.969 |
| LA size (mm) | 39.53 ± 6.22 | 40.09 ± 5.69 | 0.505 | 39.00 ± 5.82 | 40.10 ± 5.20 | 0.448 |
| LA volume index (mL/m2) | 30.43 ± 10.06 | 31.55 ± 11.16 | 0.473 | 28.97 ± 6.77 | 29.98 ± 9.40 | 0.641 |
| Ejection fraction (%) | 63.37 ± 9.89 | 64.60 ± 9.38 | 0.369 | 63.71 ± 10.63 | 67.06 ± 7.33 | 0.170 |
| E/E′ | 9.35 ± 3.29 | 10.69 ± 6.82 | 0.090 | 10.10 ± 3.65 | 9.84 ± 4.11 | 0.801 |
| CHADS2 score | 0.67 ± 0.92 | 0.98 ± 1.08 | 0.030* | 0.57 ± 0.69 | 1.13 ± 1.06 | 0.019* |
| Heart failure (%) | 3.00 | 3.00 | >0.999 | 3.57 | 3.23 | >0.999 |
| Hypertension (%) | 41.00 | 53.00 | 0.089 | 42.86 | 61.29 | 0.157 |
| Age >75 years (%) | 0 | 8.00 | 0.007* | 0 | 9.68 | 0.239 |
| Diabetes mellitus (%) | 11.00 | 17.00 | 0.221 | 10.71 | 25.81 | 0.137 |
| Stroke (%) | 5.00 | 7.00 | 0.552 | 0.00 | 3.23 | >0.999 |
| TIA (%) | 1.00 | 2.00 | >0.999 | 0.00 | 3.23 | >0.999 |
Values are given as mean ± SD for continuous variables and percentages for categorical variables.
*Statistically significant.
LA, left atrium; TIA, transient ischaemic attack.
Clinical characteristics and comparison of study patients between CPVI and CPVI + SVC-L
| . | All patients . | HRV-analysed patients . | ||||
|---|---|---|---|---|---|---|
| CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . | CPVI (n = 28) . | CPVI + SVC-L (n = 31) . | P-value . | |
| Age (years) | 55.65 ± 11.87 | 57.89 ± 11.46 | 0.176 | 55.36 ± 9.80 | 56.35 ± 15.36 | 0.765 |
| Male (%) | 74.00 | 75.00 | 0.871 | 71.43 | 70.97 | 0.969 |
| LA size (mm) | 39.53 ± 6.22 | 40.09 ± 5.69 | 0.505 | 39.00 ± 5.82 | 40.10 ± 5.20 | 0.448 |
| LA volume index (mL/m2) | 30.43 ± 10.06 | 31.55 ± 11.16 | 0.473 | 28.97 ± 6.77 | 29.98 ± 9.40 | 0.641 |
| Ejection fraction (%) | 63.37 ± 9.89 | 64.60 ± 9.38 | 0.369 | 63.71 ± 10.63 | 67.06 ± 7.33 | 0.170 |
| E/E′ | 9.35 ± 3.29 | 10.69 ± 6.82 | 0.090 | 10.10 ± 3.65 | 9.84 ± 4.11 | 0.801 |
| CHADS2 score | 0.67 ± 0.92 | 0.98 ± 1.08 | 0.030* | 0.57 ± 0.69 | 1.13 ± 1.06 | 0.019* |
| Heart failure (%) | 3.00 | 3.00 | >0.999 | 3.57 | 3.23 | >0.999 |
| Hypertension (%) | 41.00 | 53.00 | 0.089 | 42.86 | 61.29 | 0.157 |
| Age >75 years (%) | 0 | 8.00 | 0.007* | 0 | 9.68 | 0.239 |
| Diabetes mellitus (%) | 11.00 | 17.00 | 0.221 | 10.71 | 25.81 | 0.137 |
| Stroke (%) | 5.00 | 7.00 | 0.552 | 0.00 | 3.23 | >0.999 |
| TIA (%) | 1.00 | 2.00 | >0.999 | 0.00 | 3.23 | >0.999 |
| . | All patients . | HRV-analysed patients . | ||||
|---|---|---|---|---|---|---|
| CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . | CPVI (n = 28) . | CPVI + SVC-L (n = 31) . | P-value . | |
| Age (years) | 55.65 ± 11.87 | 57.89 ± 11.46 | 0.176 | 55.36 ± 9.80 | 56.35 ± 15.36 | 0.765 |
| Male (%) | 74.00 | 75.00 | 0.871 | 71.43 | 70.97 | 0.969 |
| LA size (mm) | 39.53 ± 6.22 | 40.09 ± 5.69 | 0.505 | 39.00 ± 5.82 | 40.10 ± 5.20 | 0.448 |
| LA volume index (mL/m2) | 30.43 ± 10.06 | 31.55 ± 11.16 | 0.473 | 28.97 ± 6.77 | 29.98 ± 9.40 | 0.641 |
| Ejection fraction (%) | 63.37 ± 9.89 | 64.60 ± 9.38 | 0.369 | 63.71 ± 10.63 | 67.06 ± 7.33 | 0.170 |
| E/E′ | 9.35 ± 3.29 | 10.69 ± 6.82 | 0.090 | 10.10 ± 3.65 | 9.84 ± 4.11 | 0.801 |
| CHADS2 score | 0.67 ± 0.92 | 0.98 ± 1.08 | 0.030* | 0.57 ± 0.69 | 1.13 ± 1.06 | 0.019* |
| Heart failure (%) | 3.00 | 3.00 | >0.999 | 3.57 | 3.23 | >0.999 |
| Hypertension (%) | 41.00 | 53.00 | 0.089 | 42.86 | 61.29 | 0.157 |
| Age >75 years (%) | 0 | 8.00 | 0.007* | 0 | 9.68 | 0.239 |
| Diabetes mellitus (%) | 11.00 | 17.00 | 0.221 | 10.71 | 25.81 | 0.137 |
| Stroke (%) | 5.00 | 7.00 | 0.552 | 0.00 | 3.23 | >0.999 |
| TIA (%) | 1.00 | 2.00 | >0.999 | 0.00 | 3.23 | >0.999 |
Values are given as mean ± SD for continuous variables and percentages for categorical variables.
*Statistically significant.
LA, left atrium; TIA, transient ischaemic attack.
Efficacy and safety of the SVC-L ablation
Table 2 compares the procedural efficacy and safety between the two groups. Despite the total procedure time (P < 0.001) and ablation time (P < 0.001) being significantly longer in the CPVI + SVC-L group than in the CPVI group, the complication rates did not significantly differ between the two groups (5% in CPVI + SVC-L group vs. 2% in CPVI group, P = 0.445). In the CPVI + SVC-L group, there were two cases of sinus node dysfunction, but both recovered within 24 h. Right phrenic nerve paralysis occurred in one patient after the CPVI + SVC-L. That patient had an SVC trigger non-PV focus where the phrenic nerve was captured by high output pacing. Right phrenic nerve damage may have occurred during the focal ablation at the lateral aspect of the SVC, and not during the SVC-L ablation itself, and recovered after 3 weeks. During 12.2 ± 5.3 months of follow-up, the recurrence rates were significantly lower in the CPV + SVC-L group than the CPVI group (6 vs. 27%, P < 0.001). In the Cox regression analysis, the CPVI + SVC-L was considered as being independently associated with the AF-free clinical status after the RFCA [hazard ratio (HR): 0.093, 95% confidence interval (CI): 0.014–0.646, P = 0.016, Table 3]. The Kaplan–Meier analysis for the AF-free survival showed a significantly lower AF recurrence in the CPVI + SVC-L group than in the CPVI group (log-rank P < 0.001, Figure 3). In terms of the type of recurrent atrial tachyarrhythmia, AT recurrence was more common in the CPVI + SVC-L group (66.7%, 4 of 6) than in the CPVI group (11.1%, 3 of 27, P = 0.011, Table 2).
| . | CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . |
|---|---|---|---|
| Total ablation duration (min) | 63.6 ± 16.8 | 82.7 ± 17.9 | <0.001 |
| Total procedural time (min) | 157 ± 27 | 171 ± 23 | <0.001 |
| Fluoroscopic time (min) | 40 ± 10 | 37 ± 8 | 0.019 |
| Complications (%) | 2.00 | 5.00 | 0.445 |
| Haemopericardium (%) | 2.00 | 2.00 | |
| Transient sinus node dysfunction (%) | 0.00 | 2.00 | |
| Transient phrenic nerve palsy (%) | 0.00 | 1.00 | |
| Early recurrence (%) | 23.00 | 21.00 | 0.733 |
| Recurrence after third month (%) | 27.00 | 6.00 | <0.001 |
| AT : AF recurrence | 3 : 24 | 4 : 2 | 0.011 |
| . | CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . |
|---|---|---|---|
| Total ablation duration (min) | 63.6 ± 16.8 | 82.7 ± 17.9 | <0.001 |
| Total procedural time (min) | 157 ± 27 | 171 ± 23 | <0.001 |
| Fluoroscopic time (min) | 40 ± 10 | 37 ± 8 | 0.019 |
| Complications (%) | 2.00 | 5.00 | 0.445 |
| Haemopericardium (%) | 2.00 | 2.00 | |
| Transient sinus node dysfunction (%) | 0.00 | 2.00 | |
| Transient phrenic nerve palsy (%) | 0.00 | 1.00 | |
| Early recurrence (%) | 23.00 | 21.00 | 0.733 |
| Recurrence after third month (%) | 27.00 | 6.00 | <0.001 |
| AT : AF recurrence | 3 : 24 | 4 : 2 | 0.011 |
Values are given as mean ± SD for continuous variables and percentages for categorical variables.
| . | CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . |
|---|---|---|---|
| Total ablation duration (min) | 63.6 ± 16.8 | 82.7 ± 17.9 | <0.001 |
| Total procedural time (min) | 157 ± 27 | 171 ± 23 | <0.001 |
| Fluoroscopic time (min) | 40 ± 10 | 37 ± 8 | 0.019 |
| Complications (%) | 2.00 | 5.00 | 0.445 |
| Haemopericardium (%) | 2.00 | 2.00 | |
| Transient sinus node dysfunction (%) | 0.00 | 2.00 | |
| Transient phrenic nerve palsy (%) | 0.00 | 1.00 | |
| Early recurrence (%) | 23.00 | 21.00 | 0.733 |
| Recurrence after third month (%) | 27.00 | 6.00 | <0.001 |
| AT : AF recurrence | 3 : 24 | 4 : 2 | 0.011 |
| . | CPVI (n = 100) . | CPVI + SVC-L (n = 100) . | P-value . |
|---|---|---|---|
| Total ablation duration (min) | 63.6 ± 16.8 | 82.7 ± 17.9 | <0.001 |
| Total procedural time (min) | 157 ± 27 | 171 ± 23 | <0.001 |
| Fluoroscopic time (min) | 40 ± 10 | 37 ± 8 | 0.019 |
| Complications (%) | 2.00 | 5.00 | 0.445 |
| Haemopericardium (%) | 2.00 | 2.00 | |
| Transient sinus node dysfunction (%) | 0.00 | 2.00 | |
| Transient phrenic nerve palsy (%) | 0.00 | 1.00 | |
| Early recurrence (%) | 23.00 | 21.00 | 0.733 |
| Recurrence after third month (%) | 27.00 | 6.00 | <0.001 |
| AT : AF recurrence | 3 : 24 | 4 : 2 | 0.011 |
Values are given as mean ± SD for continuous variables and percentages for categorical variables.
| Parameter . | Univariate . | Multivariate [0.834 (0.090)a] . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Clinical parameters | ||||
| Age | 1.012 (0.966–1.060) | 0.624 | ||
| Age >75 years | 1.770 (0.226–13.835) | 0.586 | ||
| Male gender | 4.255 (0.544–33.247) | 0.168 | 2.812 (0.353–22.388) | 0.329 |
| Hypertension | 1.101 (0.336–3.608) | 0.874 | ||
| Diabetes | 0.433 (0.055–3.383) | 0.425 | ||
| LA size | 1.040 (0.935–1.157) | 0.473 | ||
| LA volume index | 1.005 (0.938–1.078) | 0.883 | ||
| LVEF | 0.974 (0.930–1.020) | 0.262 | ||
| E/Em | 0.973 (0.825–1.148) | 0.749 | ||
| CHADS2 score | 0.938 (0.491–1.792) | 0.846 | ||
| Group parameter | ||||
| CPVI + SVC-L | 0.178 (0.038–0.824) | 0.027 | 0.093 (0.014–0.646) | 0.016 |
| Parameter . | Univariate . | Multivariate [0.834 (0.090)a] . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Clinical parameters | ||||
| Age | 1.012 (0.966–1.060) | 0.624 | ||
| Age >75 years | 1.770 (0.226–13.835) | 0.586 | ||
| Male gender | 4.255 (0.544–33.247) | 0.168 | 2.812 (0.353–22.388) | 0.329 |
| Hypertension | 1.101 (0.336–3.608) | 0.874 | ||
| Diabetes | 0.433 (0.055–3.383) | 0.425 | ||
| LA size | 1.040 (0.935–1.157) | 0.473 | ||
| LA volume index | 1.005 (0.938–1.078) | 0.883 | ||
| LVEF | 0.974 (0.930–1.020) | 0.262 | ||
| E/Em | 0.973 (0.825–1.148) | 0.749 | ||
| CHADS2 score | 0.938 (0.491–1.792) | 0.846 | ||
| Group parameter | ||||
| CPVI + SVC-L | 0.178 (0.038–0.824) | 0.027 | 0.093 (0.014–0.646) | 0.016 |
aC-statistic (SD).
LA, left atrium; LVEF, left ventricular ejection fraction.
| Parameter . | Univariate . | Multivariate [0.834 (0.090)a] . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Clinical parameters | ||||
| Age | 1.012 (0.966–1.060) | 0.624 | ||
| Age >75 years | 1.770 (0.226–13.835) | 0.586 | ||
| Male gender | 4.255 (0.544–33.247) | 0.168 | 2.812 (0.353–22.388) | 0.329 |
| Hypertension | 1.101 (0.336–3.608) | 0.874 | ||
| Diabetes | 0.433 (0.055–3.383) | 0.425 | ||
| LA size | 1.040 (0.935–1.157) | 0.473 | ||
| LA volume index | 1.005 (0.938–1.078) | 0.883 | ||
| LVEF | 0.974 (0.930–1.020) | 0.262 | ||
| E/Em | 0.973 (0.825–1.148) | 0.749 | ||
| CHADS2 score | 0.938 (0.491–1.792) | 0.846 | ||
| Group parameter | ||||
| CPVI + SVC-L | 0.178 (0.038–0.824) | 0.027 | 0.093 (0.014–0.646) | 0.016 |
| Parameter . | Univariate . | Multivariate [0.834 (0.090)a] . | ||
|---|---|---|---|---|
| HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | |
| Clinical parameters | ||||
| Age | 1.012 (0.966–1.060) | 0.624 | ||
| Age >75 years | 1.770 (0.226–13.835) | 0.586 | ||
| Male gender | 4.255 (0.544–33.247) | 0.168 | 2.812 (0.353–22.388) | 0.329 |
| Hypertension | 1.101 (0.336–3.608) | 0.874 | ||
| Diabetes | 0.433 (0.055–3.383) | 0.425 | ||
| LA size | 1.040 (0.935–1.157) | 0.473 | ||
| LA volume index | 1.005 (0.938–1.078) | 0.883 | ||
| LVEF | 0.974 (0.930–1.020) | 0.262 | ||
| E/Em | 0.973 (0.825–1.148) | 0.749 | ||
| CHADS2 score | 0.938 (0.491–1.792) | 0.846 | ||
| Group parameter | ||||
| CPVI + SVC-L | 0.178 (0.038–0.824) | 0.027 | 0.093 (0.014–0.646) | 0.016 |
aC-statistic (SD).
LA, left atrium; LVEF, left ventricular ejection fraction.
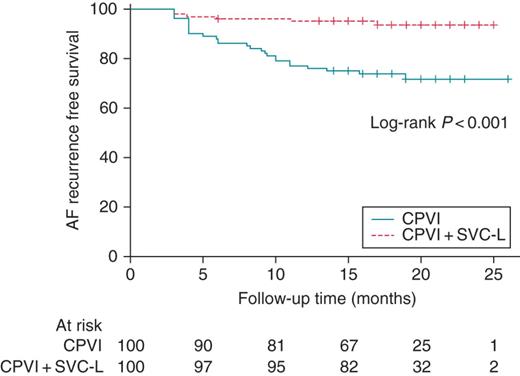
Kaplan–Meier curve comparing the clinical outcomes of the CPVI and CPVI + SVC-L.
The change in the heart rate variability parameters after the atrial fibrillation catheter ablation
Among 200 patients included, 59 had both pre-RFCA and 3-month post-RFCA Holter recordings that were analysable for the HRV after excluding Holter recordings with sinus node dysfunction or a high number of AF episodes (see Supplementary material online, Supplemental Table). Therefore, 31 patients in the CPVI + SVC-L group and 28 in the CPVI group were finally compared for the HRV analyses (Table 1). Although the pre-RFCA mean heart rate, rMSSD, HF domain, and LF/HF ratio did not differ between the two groups, the mean heart rate 3 months post-RFCA was significantly higher (82 ± 12 vs. 69 ± 9 b.p.m., P < 0.001), and rMSSD (13.7 ± 8.5 vs. 21.4 ± 12.9 ms, P = 0.009), HF domain (5.6 ± 5.9 vs. 9.0 ± 7.1 ms2, P = 0.050), and LF/HF ratio (0.93 ± 0.36 vs. 1.36 ± 0.51, P < 0.001) significantly lower in the CPVI + SVC-L group than in the CPVI alone group (Figure 4).
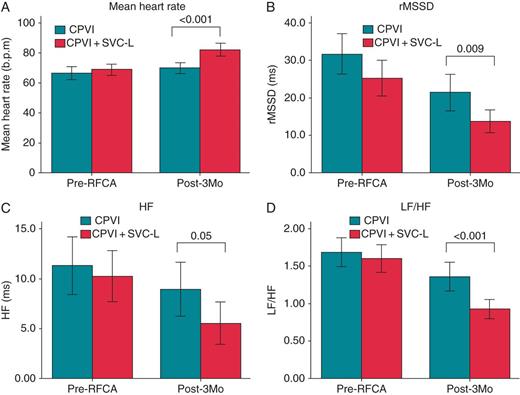
A comparison of the pre-RFCA and post-RFCA 3-month mean heart rate (A) and HRV (B–D) depending on the ablation strategy.
Discussion
In this prospectively randomized comparison study, we demonstrated that additional SVC-L ablation added to the CPVI improved the clinical outcome after the PAF catheter ablation, in spite of a longer procedure time. A significantly greater reduction in the parasympathetic activity was observed after the additional SVC-L in the 3-month post-RFCA HRV analysis. The risk of sinus node dysfunction should be considered during SVC-L ablation, although all those were recovered within a day.
Rationale for additional SVC-L ablation in paroxymal atrial fibrillation patients
The SVC has been considered as a common non-PV trigger site.5 However, we conducted linear ablation from the septal aspect of the SVC to the RA septum in this study instead of performing a circumferential SVC isolation. The purpose of the SVC-L ablation was not to target the SVC trigger, but was the autonomic remodelling and arrhythmogenic septum, which mimicked the cavo-caval line in the maze surgery.13 Therefore, we can summarize the rationale for the additional SVC-L ablation in the PAF patients as follows. First, the SVC-L ablation had a partial denervation effect on the third fat pad, which is the gate keeper of the ganglia of the cardiac autonomic nerve at the junction of the SVC–RA,14 and the abundant autonomic nerve ganglia in Waterston's groove.15 In the current study, the autonomic denervation effect by the SVC-L ablation was proven by the follow-up HRV study. Secondly, the RA posterior septal wall has shown to have increased non-uniform anisotropy, disorganized electrical activity, and conduction deterioration. The SVC and sinus venarum also have been known to be common foci for ectopic AT, and contain HCN4-positive cells with automaticity.16 Thirdly, considering the interatrial septum and Bachmann's bundle as common sites of unstable reentrant circuits, the SVC-L ablation might have an antiarrhythmic effect by diminishing the probability of multiple reentrant circuits including the septum. The interatrial septum has two layers, and the SVC-L ablation line matches well with the ablation line on the anterior aspect of the right-side PV antrum. Therefore, a biatrial septal linear ablation may generate a transmural lesion formation, blocking any transseptal reentry, especially with highly efficient open irrigation tip catheters.17 In this study, we performed bidirectional block of the RA isthmus in all patients. The rationale for the RA isthmus ablation in the patients with PAF was to gain beneficial effects of vagal denervation at the cardiac crux and a partial critical mass reduction as described previously.18 We also expected that the SVC-L ablation would block any septal reentry, and the RA isthmus block prevented the reentry around the tricuspid annulus. However, it is unclear whether or not the RA isthmus ablation potentiated the SVC-L ablation effects.
Potential complications of the SVC-L ablation
For the SVC-L ablation during the AF catheter ablation, the operator has to keep in mind the potential of collateral damage. We experienced two cases of sinus node dysfunction in the SVC-L group. Although both patients recovered within a day, that might have been due to damage to the sinus nodal artery.19 However, the long-term outcome should be monitored in these patients, and sinus nodal artery damage may induce permanent sinus node dysfunction in patients with advanced coronary artery disease.20 Because we ablated the septal aspect of the SVC, sinus node dysfunction was less likely due to direct damage to the sinus node. The risk of right phrenic nerve injury should be monitored, especially, when you rotate the catheter to the posterior lateral side of the SVC. The SVC-L ablation site is also close to the proximal right coronary artery, ascending aorta, and right pulmonary artery. The atrioventricular node exists just anterior to the foramen ovale. Superior vena cava stenosis is recognized as a rare complication after an SVC ablation.21 We conducted immediate post-SVC-L ablation SVC venograms in the first five cases, and there was no significant SVC stenosis.
Limitations
This study included a highly selected group of patients with PAF, referred for catheter ablation, and the number of patients was also limited. There was no definite electrophysiological ablation endpoint for the SVC-L lesion, because it was an anatomically oriented ablation and we never experienced acute autonomic response during SVC-L ablation. Although we defined the change in the follow-up HRV, we did not prove the location of the third fat pad by nerve stimulation. The causal relationship between the SVC-L ablation site and potential mechanisms of the automaticity or septal reentry were not proven directly. We did not compare the long-term clinical outcome or delayed complications. Although fluoroscopic time was slightly shorter in the SVC-L group than in the CPVI alone group, it may be associated with small number of included patients. Generally, we conducted SVC-L ablation by 3D mapping guidance with minimal fluoroscopy time.
Conclusion
Additional SVC-L ablation added to the CPVI with RA isthmus block, required a longer procedure time, but significantly improved the clinical outcome of the PAF ablation. The third month post-ablation HRV suggested significant cardiac autonomic neural remodelling after the SVC-L ablation. However, the risk of collateral damage, including sinus node dysfunction, should be considered. Although all SVC-L ablation-related sinus node dysfunction recovered within 24 h, further study with higher number of patients and longer follow-up duration is warranted.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by a grant (A085136) from the Korea Health 21 R&D Project, Ministry of Health and Welfare, and a grant (7-2013-0362) from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (MSIP).
Conflict of interest: none declared.
References
Author notes
Co-correspondence.



