-
PDF
- Split View
-
Views
-
Cite
Cite
Birgitta Salmela, Jussi Jaakkola, Ksenia Kalatsova, Jaakko Inkovaara, Aapo L Aro, Konsta Teppo, Tero Penttilä, Olli Halminen, Jari Haukka, Jukka Putaala, Miika Linna, Pirjo Mustonen, Juha Hartikainen, K E Juhani Airaksinen, Mika Lehto, Sex- and age-specific differences in the use of antiarrhythmic therapies among atrial fibrillation patients: a nationwide cohort study, EP Europace, Volume 26, Issue 10, October 2024, euae264, https://doi.org/10.1093/europace/euae264
Close - Share Icon Share
Abstract
Atrial fibrillation (AF) patients frequently require active rhythm control therapy to maintain sinus rhythm and reduce symptom burden. Our study assessed whether antiarrhythmic therapies (AATs) are used disproportionately between men and women after new-onset AF.
The nationwide Finnish anticoagulation in AF registry-based linkage study covers all patients with new-onset AF in Finland during 2007–2018. Study outcomes included initiation of AATs in the form of antiarrhythmic drugs (AADs), cardioversion, or catheter ablation. The study population constituted of 229 565 patients (50% females). Women were older than men (76.6 ± 11.8 vs. 68.9 ± 13.4 years) and had higher prevalence of hypertension or hyperthyroidism, but lower prevalence of vascular disease, diabetes, renal disease, and cardiomyopathies than men. Overall, 17.6% of women and 25.1% of men were treated with any AAT. Women were treated with AADs more often than men in all age groups [adjusted subdistribution hazard ratio (aSHR) 1.223, 95% confidence interval (CI) 1.187–1.261]. Cardioversions were also performed less often on women than on men aged <65 years (aSHR 0.722, 95% CI 0.695–0.749), more often in patients ≥ 75 years (aSHR 1.166, 95% CI 1.108–1.227), while no difference between the sexes existed in patients aged 65–74 years. Ablations were performed less often in women aged <65 years (aSHR 0.908, 95% CI 0.826–0.998) and ≥75 years (aSHR 0.521, 95% CI 0.354–0.766), whereas there was no difference in patients aged 65–74 years.
Women used more AAD than men in all age groups but underwent fewer cardioversion and ablation procedures when aged <65 years.
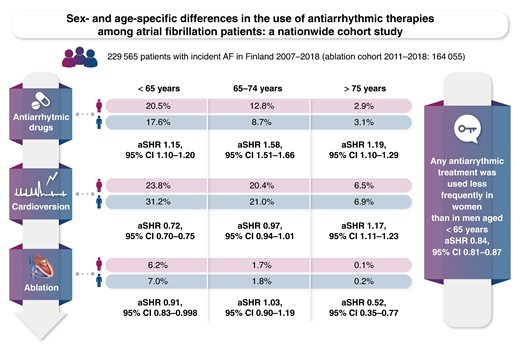
In this nationwide cohort including patients from all levels of care, the crude cumulative incidence of any antiarrhythmic therapies was lower in females than in men: 17.6% vs. 25.1% partly explained by their older age at initial atrial fibrillation diagnosis.
Among patients aged <65 years, the crude cumulative incidence of any antiarrhythmic therapies was lower in women than in men (36.1% vs. 40.3%) as women underwent less often cardioversion and ablation procedures despite having more frequent use of antiarrhythmic drugs (mainly flecainide).
Women had higher likelihood of receiving antiarrhythmic drugs also among patients aged 65–74 years whereas no difference was found in the utilization of cardioversion or ablation in comparison to men in this age group.
Among elderly patients (≥75 years), antiarrhythmic drugs and cardioversion were used more often in women than men, whereas ablations, mostly for typical atrial flutter, were performed more often on men than on women.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia in adults and is associated with severe complications such as stroke, heart failure, premature death, and reduced quality of life.1 Atrial fibrillation has a significant social and economic impact as its lifetime risk is 33% in Europeans aged 55 years.2,3 Ageing, obesity, and other comorbidities (hypertension, heart failure, coronary artery disease, valvular heart disease, etc.) contribute to the steadily increasing AF incidence.1,4,5 As a result, AF prevalence has increased by 33% during the last 20 years and currently constituting over 37 million cases worldwide.6 During the 21st century, AF prevalence in Finland has been reported to be as high as 5.2% of the adult population.7,8
Men tend to have their first AF at a younger age than women, and the age-adjusted incidence of AF is 1.5–2.0 times higher in men than in women, especially in North America and Europe.7,9–11 Asymptomatic AF is more common among men irrespective of age,12 while women often manifest higher mean heart rates during AF, experience more symptoms, and lower quality of life.9,13–19 Rhythm control with antiarrhythmic therapies (AATs) is indicated to control symptoms in selected AF patients, and early rhythm control therapy has been associated with a lower risk of adverse cardiovascular outcomes.20 However, lesser likelihood of AAT utilization has been reported among women, despite being more symptomatic and at a higher risk of developing heart failure.16,19,21,22
Our study explored whether the utilization of AAT or any of its modalities [antiarrhythmic drugs (AADs), cardioversion, and catheter ablation] differs between men and women among different age groups (<65, 65–74, and ≥75 years) in Finland.
Methods
Study cohort and data acquisition
This study was conducted within the Finnish anticoagulation in atrial fibrillation (FinACAF) study (ClinicalTrials identifier: NCT04645537; ENCePP identifier: EUPAS29845), a nationwide retrospective registry-based data linkage study of AF patients in Finland.7 Patients were identified from three national healthcare registers to cover all new patients with AF in Finland (hospitalizations and outpatient visits: HILMO; primary health care: AvoHILMO; and National Reimbursement Register upheld by Social Insurance Institute: KELA). Income data were obtained from the Finnish national Tax Register and data on the highest achieved educational level and mortality from Statistics Finland.
The inclusion criterion for the FinACAF register was an International Classification of Diseases, Tenth Revision (ICD-10) diagnosis code I48 (including AF and atrial flutter), recorded in any of the registers during 2004–2018. The exclusion criteria were age under 20 years at the time of first AF diagnosis (new-onset AF) and permanent migration abroad before 1 January 2019. This substudy focused on patients with new-onset AF, and therefore, patients with a recorded AF diagnosis or any oral anticoagulant purchase during 2004–2006 or within a year before the first AF diagnosis were excluded. Procedure-specific codes for ablation of AF and atrial (typical and atypical) flutter were introduced in Finland in 2010, and therefore, analyses of ablation of these arrhythmias were restricted to patients entering the cohort during 2011–2018 (ablation cohort). Figure 1 demonstrates patient selection and distribution.
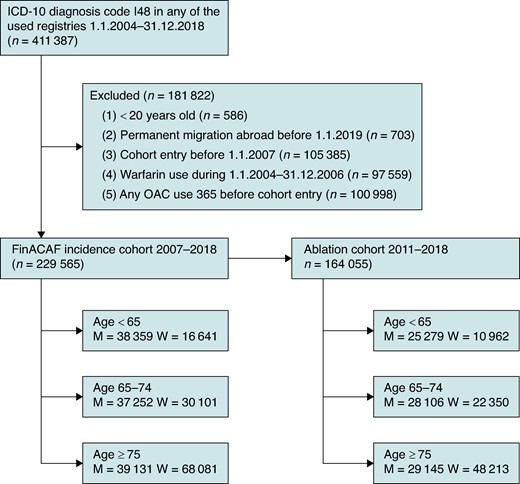
Flow chart of patient inclusion, exclusion, and distribution into different age groups.
Study protocol and outcomes
Follow-up began on the date of first recorded AF diagnosis during 2007–2018 and continued until death, 31 December 2018, or an outcome event, whichever occurred first. The outcomes of interest in the present study were the utilization of any AAT including all rhythm control modalities: AADs (Classes I and III), cardioversion, or AF catheter ablation (NSCP codes TFP44–46). Outcomes were considered to occur on the first date of fulfilled AAD prescription or recorded procedure code. Anatomical therapeutic chemical codes were used for identifying AAD prescriptions and Nordic classification of surgical procedures for catheter ablation and cardioversion procedures from different registries (see Supplementary material online, Tables S1–S3).
Study ethics
Personal identity codes are given to every resident in Finland, and they are used in all official registers, which allows reliable deterministic record linkage between different registers. The research group received individualized, but unidentifiable data including pseudonymized patient’s identification numbers. No patients were contacted at any phase of the study, and therefore, informed consent was waived. The study protocol was approved by the ethics committee of the Medical Faculty of Helsinki University, Helsinki, Finland (nr. 15/2017), and research permission was granted by the Helsinki University Hospital, Helsinki, Finland (HUS/46/2018). Permissions were obtained from the Finnish register holders (KELA 138/522/2018; THL 2101/5.05.00/2018; Population Register Centre VRK/1291/2019–3; Statistics Finland TK-53–1713–18/u1281; and Tax Register VH/874/07.01.03/2019). The study adheres to the principles of the Declaration of Helsinki as revised in 2013.
Statistical analyses
The normality assumption of continuous variables was assessed visually and with the Kolmogorov–Smirnov test of normality. Continuous data are presented as mean and standard deviation (SD) or median interquartile range (IQR) and categorical variables as absolute number and percentage.
Poisson regression analyses were conducted to determine the incidence rate ratios (IRRs) for the AAT groups. Additionally, Fine–Gray subdistribution hazards competing risks analyses were performed to estimate the effect of sex on the cumulative incidence of AAT initiation considering all-cause mortality as a competing event. The Poisson and Fine–Gray models were performed in the entire cohort and separately in three distinct age categories (<65 years, 65–74 years, and ≥75 years), and adjustments were made for the following baseline covariates: age, sex, hypertension, congestive heart failure, diabetes, prior stroke or TIA, coronary artery disease, dementia, abnormal renal function, abnormal liver function, hyperthyroidism, cancer, alcohol abuse, psychiatric disease, valvular disease, cardiomyopathy, conduction disease, pulmonary disease, sleep apnoea, education level, income level, and the year of cohort entry.
In addition to the age-stratified analyses, we also assessed the presence of interaction between sex and age in the use of AATs. In these analyses, age was treated as a categorical variable: under 40 years, in 5-year intervals from 40 to 90, and 90 or more (or up to 80 and then 80 or more in analyses on catheter ablation, as no procedures were performed in patients over 90 years of age). The interaction analyses were performed using Poisson regression, fitted with the abovementioned adjusting variables.
Statistical analyses were performed with the IBM SPSS Statistics software (version 27.0, SPSS, Inc., Chicago, Illinois) and R (version 4.0.5, https://www.R-project.org).
Results
Altogether, 229 565 patients with new-onset AF during 2007–2018 were identified. Patients’ mean age at the time of cohort entry was 72.7 (±13.2) years, and 50% (114 823) of them were women (Table 1). Women were significantly older than men (76.6 ± 11.8 vs. 68.9 ± 13.4 years, P < 0.001). Hypertension and hyperthyroidism were more common among women, whereas men presented more frequently with diabetes, coronary artery disease, history of myocardial infarction, vascular diseases, cardiomyopathies, abnormal renal function, and alcohol abuse (Table 1). The median CHA2DS2-VA and HAS-BLED scores did not differ between the sexes.
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Men . | Women . | Men . | Women . | Men . | Women . | Men . | Women . |
| . | n = 114 742 . | n = 114 823 . | n = 38 359 . | n = 16 641 . | n = 37 252 . | n = 30 101 . | n = 39 131 . | n = 68 081 . |
| Age, mean (SD) | 68.90 (13.44) | 76.57 (11.79) | 53.86 (9.38) | 55.73 (8.70) | 70.01 (3.11) | 70.54 (3.08) | 82.59 (4.93) | 84.33 (5.41) |
| Hypertension | 77 966 (67.9) | 92 288 (80.4) | 20 940 (54.6) | 10 773 (64.7) | 27 156 (72.9) | 23 689 (78.7) | 29 870 (76.3) | 57 826 (84.9) |
| Diabetes | 25 783 (22.5) | 23 764 (20.7) | 5842 (15.2) | 2275 (13.7) | 9948 (26.7) | 6322 (21.0) | 9993 (25.5) | 15 167 (22.3) |
| Coronary artery disease | 27 276 (23.8) | 24 406 (21.3) | 4178 (10.9) | 1100 (6.6) | 9100 (24.4) | 4286 (14.2) | 13 998 (35.8) | 19 020 (27.9) |
| Other vascular disease | 6417 (5.6) | 5244 (4.6) | 739 (1.9) | 201 (1.2) | 2281 (6.1) | 1002 (3.3) | 3397 (8.7) | 4041 (5.9) |
| Any vascular disease | 33 382 (29.1) | 30 971 (27.0) | 5462 (14.2) | 1634 (9.8) | 11 291 (30.3) | 5953 (19.8) | 16 629 (42.5) | 23 384 (34.3) |
| History of myocardial infarction | 11 053 (9.6) | 8964 (7.8) | 1998 (5.2) | 442 (2.7) | 3647 (9.8) | 1605 (5.3) | 5408 (13.8) | 6917 (10.2) |
| Cardiomyopathy | 2796 (2.4) | 1187 (1.0) | 1344 (3.5) | 331 (2.0) | 930 (2.5) | 414 (1.4) | 522 (1.3) | 442 (0.6) |
| Hyperlipidaemia | 53 331 (46.5) | 56 321 (49.1) | 12 080 (31.5) | 4720 (28.4) | 19 907 (53.4) | 15 894 (52.8) | 21 344 (54.5) | 35 707 (52.4) |
| Congestive heart failure | 17 718 (15.4) | 22 199 (19.3) | 3457 (9.0) | 1091 (6.6) | 5125 (13.8) | 3294 (10.9) | 9136 (23.3) | 17 814 (26.2) |
| Previous ischaemic stroke or TIA | 16 108 (14.0) | 19 267 (16.8) | 2446 (6.4) | 1198 (7.2) | 5378 (14.4) | 4175 (13.9) | 8284 (21.2) | 13 894 (20.4) |
| Abnormal renal function | 5029 (4.4) | 4102 (3.6) | 846 (2.2) | 297 (1.8) | 1487 (4.0) | 768 (2.6) | 2696 (6.9) | 3037 (4.5) |
| Abnormal liver function | 693 (0.6) | 467 (0.4) | 264 (0.7) | 107 (0.6) | 304 (0.8) | 176 (0.6) | 125 (0.3) | 184 (0.3) |
| Dementia | 4142 (3.6) | 7642 (6.7) | 106 (0.3) | 51 (0.3) | 529 (1.4) | 488 (1.6) | 3507 (9.0) | 7103 (10.4) |
| Alcohol abuse | 7641 (6.7) | 1917 (1.7) | 3857 (10.1) | 674 (4.1) | 2663 (7.1) | 741 (2.5) | 1121 (2.9) | 502 (0.7) |
| Bleeding history | 14 097 (12.3) | 10 442 (9.1) | 2658 (6.9) | 844 (5.1) | 4576 (12.3) | 2267 (7.5) | 6863 (17.5) | 7331 (10.8) |
| Hyperthyroidism | 475 (0.4) | 1693 (1.5) | 162 (0.4) | 326 (2.0) | 139 (0.4) | 444 (1.5) | 174 (0.4) | 923 (1.4) |
| Cancer | 21 893 (19.1) | 25 359 (22.1) | 2227 (5.8) | 1915 (11.5) | 6894 (18.5) | 6032 (20.0) | 12 772 (32.6) | 17 412 (25.6) |
| Psychiatric disease | 15 658 (13.6) | 15 345 (13.4) | 6655 (17.3) | 3133 (18.8) | 4919 (13.2) | 4045 (13.4) | 4084 (10.4) | 8167 (12.0) |
| Pulmonary disease | 20 858 (18.2) | 22 774 (19.8) | 5589 (14.6) | 3563 (21.4) | 7300 (19.6) | 6750 (22.4) | 7969 (20.4) | 12 461 (18.3) |
| Sleep apnoea | 6337 (5.5) | 2347 (2.0) | 2617 (6.8) | 629 (3.8) | 2619 (7.0) | 1021 (3.4) | 1101 (2.8) | 697 (1.0) |
| Valvular disease | 7409 (6.5) | 8470 (7.4) | 1577 (4.1) | 672 (4.0) | 2310 (6.2) | 1789 (5.9) | 3522 (9.0) | 6009 (8.8) |
| Conduction disease | 6135 (5.3) | 6402 (5.6) | 917 (2.4) | 580 (3.5) | 1753 (4.7) | 1248 (4.1) | 3465 (8.9) | 4574 (6.7) |
| Pacemaker | 3763 (3.3) | 3137 (2.7) | 555 (1.4) | 313 (1.9) | 1136 (3.0) | 597 (2.0) | 2072 (5.3) | 2227 (3.3) |
| CHA2DS2-VASc score, median [IQR] | 3 [1–4] | 4 [3–5] | 1 [0–2] | 2 [1–2] | 3 [2–4] | 3 [3–4] | 4 [3–5] | 5 [4–6] |
| CHA2DS2-VA score, median [IQR] | 3 [1–4] | 3 [2–4] | 1 [0–2] | 1 [1–1] | 3 [2–4] | 2 [2–3] | 4 [3–5] | 4 [3–5] |
| Modified HAS-BLED scorea | 2 [1–2] | 2 [2–3] | 1 [0–1] | 1 [0–1] | 2 [2–3] | 2 [2–2] | 2]2–3] | 2 [2–3] |
| Education level | ||||||||
| Primary school | 65 411 (57.0) | 78 659 (68.5) | 12 775 (33.3) | 4712 (28.3) | 21 052 (56.5) | 16 769 (55.7) | 31 584 (80.7) | 57 178 (84.0) |
| Upper secondary school | 27 018 (23.5) | 21 158 (18.4) | 15 223 (39.7) | 6559 (39.4) | 8308 (22.3) | 7797 (25.9) | 3487 (8.9) | 6802 (10.0) |
| Higher education | 22 313 (19.4) | 15 006 (13.1) | 10 361 (27.0) | 5370 (32.3) | 7892 (21.2) | 5535 (18.4) | 4060 (10.4) | 4104 (6.0) |
| Income, quintiles | ||||||||
| 1st | 14 711 (12.8) | 34 294 (29.9) | 2153 (5.6) | 1345 (8.1) | 4007 (10.8) | 6122 (20.3) | 8551 (21.9) | 26 827 (39.4) |
| 2nd | 15 680 (13.7) | 24 915 (21.7) | 2690 (7.0) | 1483 (8.97) | 4939 (13.3) | 6190 (20.6) | 8051 (20.6) | 17 242 (25.3) |
| 3rd | 23 351 (20.4) | 24 368 (21.2) | 4775 (12.4) | 2875 (17.3) | 8632 (23.2) | 7849 (26.1) | 9944 (25.4) | 13 644 (20.0) |
| 4th | 26 634 (23.2) | 19 726 (17.2) | 10 920 (28.5) | 6838 (41.1) | 8949 (24.0) | 6237 (20.7) | 6765 (17.3) | 6651 (9.8) |
| 5th | 34 366 (30.0) | 11 520 (10.0) | 17 821 (46.5) | 4100 (24.6) | 10 725 (28.8) | 3703 (12.3) | 5820 (14.9) | 3717 (5.5) |
| Beta-blockersb | 50 925 (44.4) | 62 006 (54.0) | 13 026 (34.0) | 6934 (41.7) | 17 861 (47.9) | 15 898 (52.8) | 20 038 (51.2) | 39 174 (57.5) |
| Non-dihydropyridine calcium channel blockersb | 1334 (1.2) | 2280 (2.0) | 264 (0.7) | 250 (1.5) | 444 (1.2) | 564 (1.9) | 626 (1.6) | 1466 (2.2) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Men . | Women . | Men . | Women . | Men . | Women . | Men . | Women . |
| . | n = 114 742 . | n = 114 823 . | n = 38 359 . | n = 16 641 . | n = 37 252 . | n = 30 101 . | n = 39 131 . | n = 68 081 . |
| Age, mean (SD) | 68.90 (13.44) | 76.57 (11.79) | 53.86 (9.38) | 55.73 (8.70) | 70.01 (3.11) | 70.54 (3.08) | 82.59 (4.93) | 84.33 (5.41) |
| Hypertension | 77 966 (67.9) | 92 288 (80.4) | 20 940 (54.6) | 10 773 (64.7) | 27 156 (72.9) | 23 689 (78.7) | 29 870 (76.3) | 57 826 (84.9) |
| Diabetes | 25 783 (22.5) | 23 764 (20.7) | 5842 (15.2) | 2275 (13.7) | 9948 (26.7) | 6322 (21.0) | 9993 (25.5) | 15 167 (22.3) |
| Coronary artery disease | 27 276 (23.8) | 24 406 (21.3) | 4178 (10.9) | 1100 (6.6) | 9100 (24.4) | 4286 (14.2) | 13 998 (35.8) | 19 020 (27.9) |
| Other vascular disease | 6417 (5.6) | 5244 (4.6) | 739 (1.9) | 201 (1.2) | 2281 (6.1) | 1002 (3.3) | 3397 (8.7) | 4041 (5.9) |
| Any vascular disease | 33 382 (29.1) | 30 971 (27.0) | 5462 (14.2) | 1634 (9.8) | 11 291 (30.3) | 5953 (19.8) | 16 629 (42.5) | 23 384 (34.3) |
| History of myocardial infarction | 11 053 (9.6) | 8964 (7.8) | 1998 (5.2) | 442 (2.7) | 3647 (9.8) | 1605 (5.3) | 5408 (13.8) | 6917 (10.2) |
| Cardiomyopathy | 2796 (2.4) | 1187 (1.0) | 1344 (3.5) | 331 (2.0) | 930 (2.5) | 414 (1.4) | 522 (1.3) | 442 (0.6) |
| Hyperlipidaemia | 53 331 (46.5) | 56 321 (49.1) | 12 080 (31.5) | 4720 (28.4) | 19 907 (53.4) | 15 894 (52.8) | 21 344 (54.5) | 35 707 (52.4) |
| Congestive heart failure | 17 718 (15.4) | 22 199 (19.3) | 3457 (9.0) | 1091 (6.6) | 5125 (13.8) | 3294 (10.9) | 9136 (23.3) | 17 814 (26.2) |
| Previous ischaemic stroke or TIA | 16 108 (14.0) | 19 267 (16.8) | 2446 (6.4) | 1198 (7.2) | 5378 (14.4) | 4175 (13.9) | 8284 (21.2) | 13 894 (20.4) |
| Abnormal renal function | 5029 (4.4) | 4102 (3.6) | 846 (2.2) | 297 (1.8) | 1487 (4.0) | 768 (2.6) | 2696 (6.9) | 3037 (4.5) |
| Abnormal liver function | 693 (0.6) | 467 (0.4) | 264 (0.7) | 107 (0.6) | 304 (0.8) | 176 (0.6) | 125 (0.3) | 184 (0.3) |
| Dementia | 4142 (3.6) | 7642 (6.7) | 106 (0.3) | 51 (0.3) | 529 (1.4) | 488 (1.6) | 3507 (9.0) | 7103 (10.4) |
| Alcohol abuse | 7641 (6.7) | 1917 (1.7) | 3857 (10.1) | 674 (4.1) | 2663 (7.1) | 741 (2.5) | 1121 (2.9) | 502 (0.7) |
| Bleeding history | 14 097 (12.3) | 10 442 (9.1) | 2658 (6.9) | 844 (5.1) | 4576 (12.3) | 2267 (7.5) | 6863 (17.5) | 7331 (10.8) |
| Hyperthyroidism | 475 (0.4) | 1693 (1.5) | 162 (0.4) | 326 (2.0) | 139 (0.4) | 444 (1.5) | 174 (0.4) | 923 (1.4) |
| Cancer | 21 893 (19.1) | 25 359 (22.1) | 2227 (5.8) | 1915 (11.5) | 6894 (18.5) | 6032 (20.0) | 12 772 (32.6) | 17 412 (25.6) |
| Psychiatric disease | 15 658 (13.6) | 15 345 (13.4) | 6655 (17.3) | 3133 (18.8) | 4919 (13.2) | 4045 (13.4) | 4084 (10.4) | 8167 (12.0) |
| Pulmonary disease | 20 858 (18.2) | 22 774 (19.8) | 5589 (14.6) | 3563 (21.4) | 7300 (19.6) | 6750 (22.4) | 7969 (20.4) | 12 461 (18.3) |
| Sleep apnoea | 6337 (5.5) | 2347 (2.0) | 2617 (6.8) | 629 (3.8) | 2619 (7.0) | 1021 (3.4) | 1101 (2.8) | 697 (1.0) |
| Valvular disease | 7409 (6.5) | 8470 (7.4) | 1577 (4.1) | 672 (4.0) | 2310 (6.2) | 1789 (5.9) | 3522 (9.0) | 6009 (8.8) |
| Conduction disease | 6135 (5.3) | 6402 (5.6) | 917 (2.4) | 580 (3.5) | 1753 (4.7) | 1248 (4.1) | 3465 (8.9) | 4574 (6.7) |
| Pacemaker | 3763 (3.3) | 3137 (2.7) | 555 (1.4) | 313 (1.9) | 1136 (3.0) | 597 (2.0) | 2072 (5.3) | 2227 (3.3) |
| CHA2DS2-VASc score, median [IQR] | 3 [1–4] | 4 [3–5] | 1 [0–2] | 2 [1–2] | 3 [2–4] | 3 [3–4] | 4 [3–5] | 5 [4–6] |
| CHA2DS2-VA score, median [IQR] | 3 [1–4] | 3 [2–4] | 1 [0–2] | 1 [1–1] | 3 [2–4] | 2 [2–3] | 4 [3–5] | 4 [3–5] |
| Modified HAS-BLED scorea | 2 [1–2] | 2 [2–3] | 1 [0–1] | 1 [0–1] | 2 [2–3] | 2 [2–2] | 2]2–3] | 2 [2–3] |
| Education level | ||||||||
| Primary school | 65 411 (57.0) | 78 659 (68.5) | 12 775 (33.3) | 4712 (28.3) | 21 052 (56.5) | 16 769 (55.7) | 31 584 (80.7) | 57 178 (84.0) |
| Upper secondary school | 27 018 (23.5) | 21 158 (18.4) | 15 223 (39.7) | 6559 (39.4) | 8308 (22.3) | 7797 (25.9) | 3487 (8.9) | 6802 (10.0) |
| Higher education | 22 313 (19.4) | 15 006 (13.1) | 10 361 (27.0) | 5370 (32.3) | 7892 (21.2) | 5535 (18.4) | 4060 (10.4) | 4104 (6.0) |
| Income, quintiles | ||||||||
| 1st | 14 711 (12.8) | 34 294 (29.9) | 2153 (5.6) | 1345 (8.1) | 4007 (10.8) | 6122 (20.3) | 8551 (21.9) | 26 827 (39.4) |
| 2nd | 15 680 (13.7) | 24 915 (21.7) | 2690 (7.0) | 1483 (8.97) | 4939 (13.3) | 6190 (20.6) | 8051 (20.6) | 17 242 (25.3) |
| 3rd | 23 351 (20.4) | 24 368 (21.2) | 4775 (12.4) | 2875 (17.3) | 8632 (23.2) | 7849 (26.1) | 9944 (25.4) | 13 644 (20.0) |
| 4th | 26 634 (23.2) | 19 726 (17.2) | 10 920 (28.5) | 6838 (41.1) | 8949 (24.0) | 6237 (20.7) | 6765 (17.3) | 6651 (9.8) |
| 5th | 34 366 (30.0) | 11 520 (10.0) | 17 821 (46.5) | 4100 (24.6) | 10 725 (28.8) | 3703 (12.3) | 5820 (14.9) | 3717 (5.5) |
| Beta-blockersb | 50 925 (44.4) | 62 006 (54.0) | 13 026 (34.0) | 6934 (41.7) | 17 861 (47.9) | 15 898 (52.8) | 20 038 (51.2) | 39 174 (57.5) |
| Non-dihydropyridine calcium channel blockersb | 1334 (1.2) | 2280 (2.0) | 264 (0.7) | 250 (1.5) | 444 (1.2) | 564 (1.9) | 626 (1.6) | 1466 (2.2) |
Values are presented as absolute number (%), mean (SD), or median [IQR].
CHA2DS2-VASc, congestive heart failure, hypertension, age at least 75 years, diabetes, history of ischaemic stroke or transient ischaemic attack, vascular disease, age 65–74 years, sex category (female); HAS-BLED, hypertension, abnormal renal or liver function, prior stroke, bleeding history, labile INR, elderly, alcohol abuse or drugs predisposing to bleeding; INR, international normalized ratio; IQR, interquartile range; SD, standard deviation; TIA, transient ischaemic attack.
aModified HAS-BLED score without labile INR.
bMedication use during preceding year from index date.
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Men . | Women . | Men . | Women . | Men . | Women . | Men . | Women . |
| . | n = 114 742 . | n = 114 823 . | n = 38 359 . | n = 16 641 . | n = 37 252 . | n = 30 101 . | n = 39 131 . | n = 68 081 . |
| Age, mean (SD) | 68.90 (13.44) | 76.57 (11.79) | 53.86 (9.38) | 55.73 (8.70) | 70.01 (3.11) | 70.54 (3.08) | 82.59 (4.93) | 84.33 (5.41) |
| Hypertension | 77 966 (67.9) | 92 288 (80.4) | 20 940 (54.6) | 10 773 (64.7) | 27 156 (72.9) | 23 689 (78.7) | 29 870 (76.3) | 57 826 (84.9) |
| Diabetes | 25 783 (22.5) | 23 764 (20.7) | 5842 (15.2) | 2275 (13.7) | 9948 (26.7) | 6322 (21.0) | 9993 (25.5) | 15 167 (22.3) |
| Coronary artery disease | 27 276 (23.8) | 24 406 (21.3) | 4178 (10.9) | 1100 (6.6) | 9100 (24.4) | 4286 (14.2) | 13 998 (35.8) | 19 020 (27.9) |
| Other vascular disease | 6417 (5.6) | 5244 (4.6) | 739 (1.9) | 201 (1.2) | 2281 (6.1) | 1002 (3.3) | 3397 (8.7) | 4041 (5.9) |
| Any vascular disease | 33 382 (29.1) | 30 971 (27.0) | 5462 (14.2) | 1634 (9.8) | 11 291 (30.3) | 5953 (19.8) | 16 629 (42.5) | 23 384 (34.3) |
| History of myocardial infarction | 11 053 (9.6) | 8964 (7.8) | 1998 (5.2) | 442 (2.7) | 3647 (9.8) | 1605 (5.3) | 5408 (13.8) | 6917 (10.2) |
| Cardiomyopathy | 2796 (2.4) | 1187 (1.0) | 1344 (3.5) | 331 (2.0) | 930 (2.5) | 414 (1.4) | 522 (1.3) | 442 (0.6) |
| Hyperlipidaemia | 53 331 (46.5) | 56 321 (49.1) | 12 080 (31.5) | 4720 (28.4) | 19 907 (53.4) | 15 894 (52.8) | 21 344 (54.5) | 35 707 (52.4) |
| Congestive heart failure | 17 718 (15.4) | 22 199 (19.3) | 3457 (9.0) | 1091 (6.6) | 5125 (13.8) | 3294 (10.9) | 9136 (23.3) | 17 814 (26.2) |
| Previous ischaemic stroke or TIA | 16 108 (14.0) | 19 267 (16.8) | 2446 (6.4) | 1198 (7.2) | 5378 (14.4) | 4175 (13.9) | 8284 (21.2) | 13 894 (20.4) |
| Abnormal renal function | 5029 (4.4) | 4102 (3.6) | 846 (2.2) | 297 (1.8) | 1487 (4.0) | 768 (2.6) | 2696 (6.9) | 3037 (4.5) |
| Abnormal liver function | 693 (0.6) | 467 (0.4) | 264 (0.7) | 107 (0.6) | 304 (0.8) | 176 (0.6) | 125 (0.3) | 184 (0.3) |
| Dementia | 4142 (3.6) | 7642 (6.7) | 106 (0.3) | 51 (0.3) | 529 (1.4) | 488 (1.6) | 3507 (9.0) | 7103 (10.4) |
| Alcohol abuse | 7641 (6.7) | 1917 (1.7) | 3857 (10.1) | 674 (4.1) | 2663 (7.1) | 741 (2.5) | 1121 (2.9) | 502 (0.7) |
| Bleeding history | 14 097 (12.3) | 10 442 (9.1) | 2658 (6.9) | 844 (5.1) | 4576 (12.3) | 2267 (7.5) | 6863 (17.5) | 7331 (10.8) |
| Hyperthyroidism | 475 (0.4) | 1693 (1.5) | 162 (0.4) | 326 (2.0) | 139 (0.4) | 444 (1.5) | 174 (0.4) | 923 (1.4) |
| Cancer | 21 893 (19.1) | 25 359 (22.1) | 2227 (5.8) | 1915 (11.5) | 6894 (18.5) | 6032 (20.0) | 12 772 (32.6) | 17 412 (25.6) |
| Psychiatric disease | 15 658 (13.6) | 15 345 (13.4) | 6655 (17.3) | 3133 (18.8) | 4919 (13.2) | 4045 (13.4) | 4084 (10.4) | 8167 (12.0) |
| Pulmonary disease | 20 858 (18.2) | 22 774 (19.8) | 5589 (14.6) | 3563 (21.4) | 7300 (19.6) | 6750 (22.4) | 7969 (20.4) | 12 461 (18.3) |
| Sleep apnoea | 6337 (5.5) | 2347 (2.0) | 2617 (6.8) | 629 (3.8) | 2619 (7.0) | 1021 (3.4) | 1101 (2.8) | 697 (1.0) |
| Valvular disease | 7409 (6.5) | 8470 (7.4) | 1577 (4.1) | 672 (4.0) | 2310 (6.2) | 1789 (5.9) | 3522 (9.0) | 6009 (8.8) |
| Conduction disease | 6135 (5.3) | 6402 (5.6) | 917 (2.4) | 580 (3.5) | 1753 (4.7) | 1248 (4.1) | 3465 (8.9) | 4574 (6.7) |
| Pacemaker | 3763 (3.3) | 3137 (2.7) | 555 (1.4) | 313 (1.9) | 1136 (3.0) | 597 (2.0) | 2072 (5.3) | 2227 (3.3) |
| CHA2DS2-VASc score, median [IQR] | 3 [1–4] | 4 [3–5] | 1 [0–2] | 2 [1–2] | 3 [2–4] | 3 [3–4] | 4 [3–5] | 5 [4–6] |
| CHA2DS2-VA score, median [IQR] | 3 [1–4] | 3 [2–4] | 1 [0–2] | 1 [1–1] | 3 [2–4] | 2 [2–3] | 4 [3–5] | 4 [3–5] |
| Modified HAS-BLED scorea | 2 [1–2] | 2 [2–3] | 1 [0–1] | 1 [0–1] | 2 [2–3] | 2 [2–2] | 2]2–3] | 2 [2–3] |
| Education level | ||||||||
| Primary school | 65 411 (57.0) | 78 659 (68.5) | 12 775 (33.3) | 4712 (28.3) | 21 052 (56.5) | 16 769 (55.7) | 31 584 (80.7) | 57 178 (84.0) |
| Upper secondary school | 27 018 (23.5) | 21 158 (18.4) | 15 223 (39.7) | 6559 (39.4) | 8308 (22.3) | 7797 (25.9) | 3487 (8.9) | 6802 (10.0) |
| Higher education | 22 313 (19.4) | 15 006 (13.1) | 10 361 (27.0) | 5370 (32.3) | 7892 (21.2) | 5535 (18.4) | 4060 (10.4) | 4104 (6.0) |
| Income, quintiles | ||||||||
| 1st | 14 711 (12.8) | 34 294 (29.9) | 2153 (5.6) | 1345 (8.1) | 4007 (10.8) | 6122 (20.3) | 8551 (21.9) | 26 827 (39.4) |
| 2nd | 15 680 (13.7) | 24 915 (21.7) | 2690 (7.0) | 1483 (8.97) | 4939 (13.3) | 6190 (20.6) | 8051 (20.6) | 17 242 (25.3) |
| 3rd | 23 351 (20.4) | 24 368 (21.2) | 4775 (12.4) | 2875 (17.3) | 8632 (23.2) | 7849 (26.1) | 9944 (25.4) | 13 644 (20.0) |
| 4th | 26 634 (23.2) | 19 726 (17.2) | 10 920 (28.5) | 6838 (41.1) | 8949 (24.0) | 6237 (20.7) | 6765 (17.3) | 6651 (9.8) |
| 5th | 34 366 (30.0) | 11 520 (10.0) | 17 821 (46.5) | 4100 (24.6) | 10 725 (28.8) | 3703 (12.3) | 5820 (14.9) | 3717 (5.5) |
| Beta-blockersb | 50 925 (44.4) | 62 006 (54.0) | 13 026 (34.0) | 6934 (41.7) | 17 861 (47.9) | 15 898 (52.8) | 20 038 (51.2) | 39 174 (57.5) |
| Non-dihydropyridine calcium channel blockersb | 1334 (1.2) | 2280 (2.0) | 264 (0.7) | 250 (1.5) | 444 (1.2) | 564 (1.9) | 626 (1.6) | 1466 (2.2) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Men . | Women . | Men . | Women . | Men . | Women . | Men . | Women . |
| . | n = 114 742 . | n = 114 823 . | n = 38 359 . | n = 16 641 . | n = 37 252 . | n = 30 101 . | n = 39 131 . | n = 68 081 . |
| Age, mean (SD) | 68.90 (13.44) | 76.57 (11.79) | 53.86 (9.38) | 55.73 (8.70) | 70.01 (3.11) | 70.54 (3.08) | 82.59 (4.93) | 84.33 (5.41) |
| Hypertension | 77 966 (67.9) | 92 288 (80.4) | 20 940 (54.6) | 10 773 (64.7) | 27 156 (72.9) | 23 689 (78.7) | 29 870 (76.3) | 57 826 (84.9) |
| Diabetes | 25 783 (22.5) | 23 764 (20.7) | 5842 (15.2) | 2275 (13.7) | 9948 (26.7) | 6322 (21.0) | 9993 (25.5) | 15 167 (22.3) |
| Coronary artery disease | 27 276 (23.8) | 24 406 (21.3) | 4178 (10.9) | 1100 (6.6) | 9100 (24.4) | 4286 (14.2) | 13 998 (35.8) | 19 020 (27.9) |
| Other vascular disease | 6417 (5.6) | 5244 (4.6) | 739 (1.9) | 201 (1.2) | 2281 (6.1) | 1002 (3.3) | 3397 (8.7) | 4041 (5.9) |
| Any vascular disease | 33 382 (29.1) | 30 971 (27.0) | 5462 (14.2) | 1634 (9.8) | 11 291 (30.3) | 5953 (19.8) | 16 629 (42.5) | 23 384 (34.3) |
| History of myocardial infarction | 11 053 (9.6) | 8964 (7.8) | 1998 (5.2) | 442 (2.7) | 3647 (9.8) | 1605 (5.3) | 5408 (13.8) | 6917 (10.2) |
| Cardiomyopathy | 2796 (2.4) | 1187 (1.0) | 1344 (3.5) | 331 (2.0) | 930 (2.5) | 414 (1.4) | 522 (1.3) | 442 (0.6) |
| Hyperlipidaemia | 53 331 (46.5) | 56 321 (49.1) | 12 080 (31.5) | 4720 (28.4) | 19 907 (53.4) | 15 894 (52.8) | 21 344 (54.5) | 35 707 (52.4) |
| Congestive heart failure | 17 718 (15.4) | 22 199 (19.3) | 3457 (9.0) | 1091 (6.6) | 5125 (13.8) | 3294 (10.9) | 9136 (23.3) | 17 814 (26.2) |
| Previous ischaemic stroke or TIA | 16 108 (14.0) | 19 267 (16.8) | 2446 (6.4) | 1198 (7.2) | 5378 (14.4) | 4175 (13.9) | 8284 (21.2) | 13 894 (20.4) |
| Abnormal renal function | 5029 (4.4) | 4102 (3.6) | 846 (2.2) | 297 (1.8) | 1487 (4.0) | 768 (2.6) | 2696 (6.9) | 3037 (4.5) |
| Abnormal liver function | 693 (0.6) | 467 (0.4) | 264 (0.7) | 107 (0.6) | 304 (0.8) | 176 (0.6) | 125 (0.3) | 184 (0.3) |
| Dementia | 4142 (3.6) | 7642 (6.7) | 106 (0.3) | 51 (0.3) | 529 (1.4) | 488 (1.6) | 3507 (9.0) | 7103 (10.4) |
| Alcohol abuse | 7641 (6.7) | 1917 (1.7) | 3857 (10.1) | 674 (4.1) | 2663 (7.1) | 741 (2.5) | 1121 (2.9) | 502 (0.7) |
| Bleeding history | 14 097 (12.3) | 10 442 (9.1) | 2658 (6.9) | 844 (5.1) | 4576 (12.3) | 2267 (7.5) | 6863 (17.5) | 7331 (10.8) |
| Hyperthyroidism | 475 (0.4) | 1693 (1.5) | 162 (0.4) | 326 (2.0) | 139 (0.4) | 444 (1.5) | 174 (0.4) | 923 (1.4) |
| Cancer | 21 893 (19.1) | 25 359 (22.1) | 2227 (5.8) | 1915 (11.5) | 6894 (18.5) | 6032 (20.0) | 12 772 (32.6) | 17 412 (25.6) |
| Psychiatric disease | 15 658 (13.6) | 15 345 (13.4) | 6655 (17.3) | 3133 (18.8) | 4919 (13.2) | 4045 (13.4) | 4084 (10.4) | 8167 (12.0) |
| Pulmonary disease | 20 858 (18.2) | 22 774 (19.8) | 5589 (14.6) | 3563 (21.4) | 7300 (19.6) | 6750 (22.4) | 7969 (20.4) | 12 461 (18.3) |
| Sleep apnoea | 6337 (5.5) | 2347 (2.0) | 2617 (6.8) | 629 (3.8) | 2619 (7.0) | 1021 (3.4) | 1101 (2.8) | 697 (1.0) |
| Valvular disease | 7409 (6.5) | 8470 (7.4) | 1577 (4.1) | 672 (4.0) | 2310 (6.2) | 1789 (5.9) | 3522 (9.0) | 6009 (8.8) |
| Conduction disease | 6135 (5.3) | 6402 (5.6) | 917 (2.4) | 580 (3.5) | 1753 (4.7) | 1248 (4.1) | 3465 (8.9) | 4574 (6.7) |
| Pacemaker | 3763 (3.3) | 3137 (2.7) | 555 (1.4) | 313 (1.9) | 1136 (3.0) | 597 (2.0) | 2072 (5.3) | 2227 (3.3) |
| CHA2DS2-VASc score, median [IQR] | 3 [1–4] | 4 [3–5] | 1 [0–2] | 2 [1–2] | 3 [2–4] | 3 [3–4] | 4 [3–5] | 5 [4–6] |
| CHA2DS2-VA score, median [IQR] | 3 [1–4] | 3 [2–4] | 1 [0–2] | 1 [1–1] | 3 [2–4] | 2 [2–3] | 4 [3–5] | 4 [3–5] |
| Modified HAS-BLED scorea | 2 [1–2] | 2 [2–3] | 1 [0–1] | 1 [0–1] | 2 [2–3] | 2 [2–2] | 2]2–3] | 2 [2–3] |
| Education level | ||||||||
| Primary school | 65 411 (57.0) | 78 659 (68.5) | 12 775 (33.3) | 4712 (28.3) | 21 052 (56.5) | 16 769 (55.7) | 31 584 (80.7) | 57 178 (84.0) |
| Upper secondary school | 27 018 (23.5) | 21 158 (18.4) | 15 223 (39.7) | 6559 (39.4) | 8308 (22.3) | 7797 (25.9) | 3487 (8.9) | 6802 (10.0) |
| Higher education | 22 313 (19.4) | 15 006 (13.1) | 10 361 (27.0) | 5370 (32.3) | 7892 (21.2) | 5535 (18.4) | 4060 (10.4) | 4104 (6.0) |
| Income, quintiles | ||||||||
| 1st | 14 711 (12.8) | 34 294 (29.9) | 2153 (5.6) | 1345 (8.1) | 4007 (10.8) | 6122 (20.3) | 8551 (21.9) | 26 827 (39.4) |
| 2nd | 15 680 (13.7) | 24 915 (21.7) | 2690 (7.0) | 1483 (8.97) | 4939 (13.3) | 6190 (20.6) | 8051 (20.6) | 17 242 (25.3) |
| 3rd | 23 351 (20.4) | 24 368 (21.2) | 4775 (12.4) | 2875 (17.3) | 8632 (23.2) | 7849 (26.1) | 9944 (25.4) | 13 644 (20.0) |
| 4th | 26 634 (23.2) | 19 726 (17.2) | 10 920 (28.5) | 6838 (41.1) | 8949 (24.0) | 6237 (20.7) | 6765 (17.3) | 6651 (9.8) |
| 5th | 34 366 (30.0) | 11 520 (10.0) | 17 821 (46.5) | 4100 (24.6) | 10 725 (28.8) | 3703 (12.3) | 5820 (14.9) | 3717 (5.5) |
| Beta-blockersb | 50 925 (44.4) | 62 006 (54.0) | 13 026 (34.0) | 6934 (41.7) | 17 861 (47.9) | 15 898 (52.8) | 20 038 (51.2) | 39 174 (57.5) |
| Non-dihydropyridine calcium channel blockersb | 1334 (1.2) | 2280 (2.0) | 264 (0.7) | 250 (1.5) | 444 (1.2) | 564 (1.9) | 626 (1.6) | 1466 (2.2) |
Values are presented as absolute number (%), mean (SD), or median [IQR].
CHA2DS2-VASc, congestive heart failure, hypertension, age at least 75 years, diabetes, history of ischaemic stroke or transient ischaemic attack, vascular disease, age 65–74 years, sex category (female); HAS-BLED, hypertension, abnormal renal or liver function, prior stroke, bleeding history, labile INR, elderly, alcohol abuse or drugs predisposing to bleeding; INR, international normalized ratio; IQR, interquartile range; SD, standard deviation; TIA, transient ischaemic attack.
aModified HAS-BLED score without labile INR.
bMedication use during preceding year from index date.
Use of rhythm control therapies in the whole cohort
Among the entire cohort, the crude cumulative incidences of any AAT (17.6% vs. 25.1%) including AADs (8.1% vs. 9.8%), cardioversion (12.7% vs. 19.6%), and ablation (1.4% vs. 2.8%) therapies were lower in women compared to men (Table 2 and Figure 2). After adjustment for confounding factors, female sex remained associated with lesser likelihood of any AAT use [adjusted subdistribution hazard ratio (aSHR) 0.934, 95% confidence interval (CI) 0.916–0.953], particularly cardioversion (aSHR 0.852, 95% CI 0.833–0.871), and AF ablation (aSHR 0.899, 95% CI 0.831–0.972), whereas women were more likely to be treated with AAD (mainly flecainide) (aSHR 1.223, 95% CI 1.187–1.261) (Tables 2 and Table 3). Among women, Class Ic drugs and among men Class III drugs were the most often used AADs (Table 4). Antiarrhythmic drug was initiated or catheter ablation performed within 1 year of the AF diagnosis for 7.6% of women and 9.5% of men (P < 0.001). The median time from AF diagnosis to ablation was longer in women compared to men (1.50, IQR 0.65–3.04, vs. 1.19, IQR 0.53–2.71, years, P < 0.001). Atrial fibrillation and typical atrial flutter ablations were the most common types of ablations performed both in women (71.9 and 38.1%) and men (64.1 and 48.2%) (Table 4).
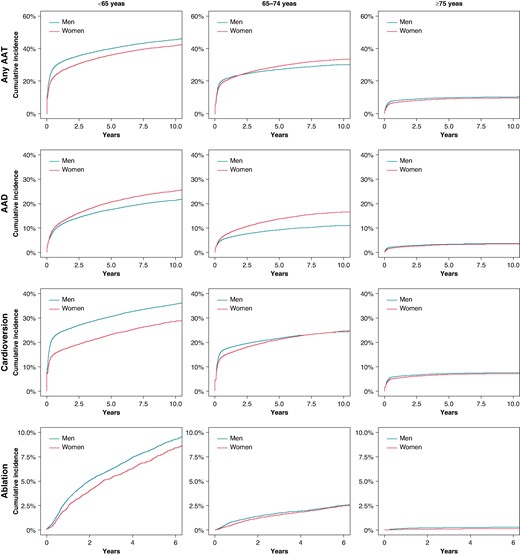
Antiarrhythmic therapies in three age groups: working age < 65 years, 65–74 years, and in the elderly ≥ 75 years. AAT, antiarrhythmic therapy; AAD, antiarrhythmic drugs; CV, cardioversion; Abl, ablation.
| Outcome . | Patient group . | Sex . | N . | Proportion of patients with events . | P-years (1000 years) . | Incidence (per 1000 P-years) . | Unadjusted IRR (95% CI) . | P value . | Adjusted IRR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| Any AAT | All | Male | 28 781 | 25.1% | 352.87 | 81.6 (80.6–82.5) | (Reference) | (Reference) | ||
| Female | 20 222 | 17.6% | 370.22 | 54.6 (53.9–55.4) | 0.670 (0.658–0.682) | <0.001 | 0.804 (0.801–0.807) | <0.001 | ||
| <65 years | Male | 15 444 | 40.3% | 134.91 | 114.5 (112.7–116.3) | (Reference) | (Reference) | |||
| Female | 6001 | 36.1% | 64.94 | 92.4 (90.1–94.8) | 0.807 (0.784–0.832) | <0.001 | 0.789 (0.765–0.815) | <0.001 | ||
| 65–74 years | Male | 9712 | 26.1% | 114.13 | 85.1 (83.4–86.8) | (Reference) | (Reference) | |||
| Female | 8402 | 27.9% | 103.04 | 81.5 (79.8–83.3) | 0.958 (0.931–0.987) | 0.004 | 1.026 (0.994–1.058) | 0.109 | ||
| ≥75 years | Male | 3625 | 9.3% | 103.82 | 34.9 (33.8–36.1) | (Reference) | (Reference) | |||
| Female | 5819 | 8.5% | 202.24 | 28.8 (28.0–29.5) | 0.824 (0.791–0.859) | <0.001 | 1.045 (1.000–1.093) | 0.050 | ||
| AAD | All | Male | 11 190 | 9.8% | 433.09 | 25.8 (25.3–26.3) | (Reference) | (Reference) | ||
| Female | 9249 | 8.1% | 418.48 | 22.1 (21.6–22.5) | 0.855 (0.832–0.879) | <0.001 | 1.195 (1.160–1.232) | <0.001 | ||
| <65 years | Male | 6749 | 17.6% | 178.15 | 37.9 (37.0–38.8) | (Reference) | (Reference) | |||
| Female | 3404 | 20.5% | 77.89 | 43.7 (42.2–45.2) | 1.154 (1.107–1.202) | <0.001 | 1.139 (1.089–1.190) | <0.001 | ||
| 65–74 years | Male | 3230 | 8.7% | 141.92 | 22.7 (22.0–23.5) | (Reference) | (Reference) | |||
| Female | 3846 | 12.8% | 123.00 | 31.2 (30.3–32.2) | 1.374 (1.311–1.440) | <0.001 | 1.532 (1.457–1.611) | <0.001 | ||
| ≥75 years | Male | 1211 | 3.1% | 113.01 | 10.7 (10.1–11.3) | (Reference) | (Reference) | |||
| Female | 1999 | 2.9% | 217.60 | 9.2 (8.8–9.6) | 0.857 (0.798–0.921) | <0.001 | 1.083 (1.003–1.169) | 0.042 | ||
| Cardioversion | All | Male | 22 482 | 19.6% | 385.07 | 58.3 (57.6–59.2) | (Reference) | (Reference) | ||
| Female | 14 531 | 12.7% | 398.95 | 36.4 (35.8–37.0) | 0.624 (0.611–0.637) | <0.001 | 0.798 (0.780–0.816) | <0.001 | ||
| <65 years | Male | 11 968 | 31.2% | 154.44 | 77.5 (76.1–78.9) | (Reference) | (Reference) | |||
| Female | 3968 | 23.8% | 76.37 | 52.0 (50.4–53.6) | 0.670 (0.647–0.695) | <0.001 | 0.671 (0.646–0.697) | <0.001 | ||
| 65–74 years | Male | 7827 | 21.0% | 123.24 | 63.5 (62.1–64.9) | (Reference) | (Reference) | |||
| Female | 6154 | 20.4% | 114.34 | 53.8 (52.5–55.2) | 0.847 (0.820–0.876) | <0.001 | 0.904 (0.872–0.937) | <0.001 | ||
| ≥75 years | Male | 2687 | 6.9% | 107.40 | 25.0 (24.1–26.0) | (Reference) | (Reference) | |||
| Female | 4409 | 6.5% | 208.24 | 21.2 (20.6–21.8) | 0.846 (0.807–0.888) | <0.001 | 1.070 (1.016–1.126) | 0.010 | ||
| Catheter ablationa | All | Male | 2341 | 2.8% | 254.71 | 9.2 (8.8–9.6) | (Reference) | (Reference) | ||
| Female | 1101 | 1.4% | 247.29 | 4.5 (4.2–4.7) | 0.484 (0.451–0.520) | <0.001 | 0.888 (0.822–0.960) | 0.003 | ||
| <65 years | Male | 1773 | 7.0% | 92.12 | 19.2 (18.4–20.2) | (Reference) | (Reference) | |||
| Female | 676 | 6.2% | 41.20 | 16.4 (15.2–17.73) | 0.853 (0.780–0.932) | <0.001 | 0.901 (0.819–0.991) | 0.031 | ||
| 65–74 years | Male | 500 | 1.8% | 89.31 | 5.6 (5.1–6.1) | (Reference) | (Reference) | |||
| Female | 377 | 1.7% | 76.24 | 4.9 (4.5–5.5) | 0.883 (0.773–1.010) | 0.069 | 1.007 (0.873–1.163) | 0.92394 | ||
| ≥75 years | Male | 68 | 0.2% | 73.28 | 0.9 (0.7–1.2) | (Reference) | (Reference) | |||
| Female | 48 | 0.1% | 129.85 | 0.4 (0.3–0.5) | 0.398 (0.275–0.576) | <0.001 | 0.494 (0.333–0.734) | <0.001 |
| Outcome . | Patient group . | Sex . | N . | Proportion of patients with events . | P-years (1000 years) . | Incidence (per 1000 P-years) . | Unadjusted IRR (95% CI) . | P value . | Adjusted IRR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| Any AAT | All | Male | 28 781 | 25.1% | 352.87 | 81.6 (80.6–82.5) | (Reference) | (Reference) | ||
| Female | 20 222 | 17.6% | 370.22 | 54.6 (53.9–55.4) | 0.670 (0.658–0.682) | <0.001 | 0.804 (0.801–0.807) | <0.001 | ||
| <65 years | Male | 15 444 | 40.3% | 134.91 | 114.5 (112.7–116.3) | (Reference) | (Reference) | |||
| Female | 6001 | 36.1% | 64.94 | 92.4 (90.1–94.8) | 0.807 (0.784–0.832) | <0.001 | 0.789 (0.765–0.815) | <0.001 | ||
| 65–74 years | Male | 9712 | 26.1% | 114.13 | 85.1 (83.4–86.8) | (Reference) | (Reference) | |||
| Female | 8402 | 27.9% | 103.04 | 81.5 (79.8–83.3) | 0.958 (0.931–0.987) | 0.004 | 1.026 (0.994–1.058) | 0.109 | ||
| ≥75 years | Male | 3625 | 9.3% | 103.82 | 34.9 (33.8–36.1) | (Reference) | (Reference) | |||
| Female | 5819 | 8.5% | 202.24 | 28.8 (28.0–29.5) | 0.824 (0.791–0.859) | <0.001 | 1.045 (1.000–1.093) | 0.050 | ||
| AAD | All | Male | 11 190 | 9.8% | 433.09 | 25.8 (25.3–26.3) | (Reference) | (Reference) | ||
| Female | 9249 | 8.1% | 418.48 | 22.1 (21.6–22.5) | 0.855 (0.832–0.879) | <0.001 | 1.195 (1.160–1.232) | <0.001 | ||
| <65 years | Male | 6749 | 17.6% | 178.15 | 37.9 (37.0–38.8) | (Reference) | (Reference) | |||
| Female | 3404 | 20.5% | 77.89 | 43.7 (42.2–45.2) | 1.154 (1.107–1.202) | <0.001 | 1.139 (1.089–1.190) | <0.001 | ||
| 65–74 years | Male | 3230 | 8.7% | 141.92 | 22.7 (22.0–23.5) | (Reference) | (Reference) | |||
| Female | 3846 | 12.8% | 123.00 | 31.2 (30.3–32.2) | 1.374 (1.311–1.440) | <0.001 | 1.532 (1.457–1.611) | <0.001 | ||
| ≥75 years | Male | 1211 | 3.1% | 113.01 | 10.7 (10.1–11.3) | (Reference) | (Reference) | |||
| Female | 1999 | 2.9% | 217.60 | 9.2 (8.8–9.6) | 0.857 (0.798–0.921) | <0.001 | 1.083 (1.003–1.169) | 0.042 | ||
| Cardioversion | All | Male | 22 482 | 19.6% | 385.07 | 58.3 (57.6–59.2) | (Reference) | (Reference) | ||
| Female | 14 531 | 12.7% | 398.95 | 36.4 (35.8–37.0) | 0.624 (0.611–0.637) | <0.001 | 0.798 (0.780–0.816) | <0.001 | ||
| <65 years | Male | 11 968 | 31.2% | 154.44 | 77.5 (76.1–78.9) | (Reference) | (Reference) | |||
| Female | 3968 | 23.8% | 76.37 | 52.0 (50.4–53.6) | 0.670 (0.647–0.695) | <0.001 | 0.671 (0.646–0.697) | <0.001 | ||
| 65–74 years | Male | 7827 | 21.0% | 123.24 | 63.5 (62.1–64.9) | (Reference) | (Reference) | |||
| Female | 6154 | 20.4% | 114.34 | 53.8 (52.5–55.2) | 0.847 (0.820–0.876) | <0.001 | 0.904 (0.872–0.937) | <0.001 | ||
| ≥75 years | Male | 2687 | 6.9% | 107.40 | 25.0 (24.1–26.0) | (Reference) | (Reference) | |||
| Female | 4409 | 6.5% | 208.24 | 21.2 (20.6–21.8) | 0.846 (0.807–0.888) | <0.001 | 1.070 (1.016–1.126) | 0.010 | ||
| Catheter ablationa | All | Male | 2341 | 2.8% | 254.71 | 9.2 (8.8–9.6) | (Reference) | (Reference) | ||
| Female | 1101 | 1.4% | 247.29 | 4.5 (4.2–4.7) | 0.484 (0.451–0.520) | <0.001 | 0.888 (0.822–0.960) | 0.003 | ||
| <65 years | Male | 1773 | 7.0% | 92.12 | 19.2 (18.4–20.2) | (Reference) | (Reference) | |||
| Female | 676 | 6.2% | 41.20 | 16.4 (15.2–17.73) | 0.853 (0.780–0.932) | <0.001 | 0.901 (0.819–0.991) | 0.031 | ||
| 65–74 years | Male | 500 | 1.8% | 89.31 | 5.6 (5.1–6.1) | (Reference) | (Reference) | |||
| Female | 377 | 1.7% | 76.24 | 4.9 (4.5–5.5) | 0.883 (0.773–1.010) | 0.069 | 1.007 (0.873–1.163) | 0.92394 | ||
| ≥75 years | Male | 68 | 0.2% | 73.28 | 0.9 (0.7–1.2) | (Reference) | (Reference) | |||
| Female | 48 | 0.1% | 129.85 | 0.4 (0.3–0.5) | 0.398 (0.275–0.576) | <0.001 | 0.494 (0.333–0.734) | <0.001 |
AAD, antiarrhythmic drugs; AAT, antiarrhythmic therapy; CI, confidence interval; IRR, incidence rate ratio.
aPatients entering the cohort from 2011 onwards.
| Outcome . | Patient group . | Sex . | N . | Proportion of patients with events . | P-years (1000 years) . | Incidence (per 1000 P-years) . | Unadjusted IRR (95% CI) . | P value . | Adjusted IRR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| Any AAT | All | Male | 28 781 | 25.1% | 352.87 | 81.6 (80.6–82.5) | (Reference) | (Reference) | ||
| Female | 20 222 | 17.6% | 370.22 | 54.6 (53.9–55.4) | 0.670 (0.658–0.682) | <0.001 | 0.804 (0.801–0.807) | <0.001 | ||
| <65 years | Male | 15 444 | 40.3% | 134.91 | 114.5 (112.7–116.3) | (Reference) | (Reference) | |||
| Female | 6001 | 36.1% | 64.94 | 92.4 (90.1–94.8) | 0.807 (0.784–0.832) | <0.001 | 0.789 (0.765–0.815) | <0.001 | ||
| 65–74 years | Male | 9712 | 26.1% | 114.13 | 85.1 (83.4–86.8) | (Reference) | (Reference) | |||
| Female | 8402 | 27.9% | 103.04 | 81.5 (79.8–83.3) | 0.958 (0.931–0.987) | 0.004 | 1.026 (0.994–1.058) | 0.109 | ||
| ≥75 years | Male | 3625 | 9.3% | 103.82 | 34.9 (33.8–36.1) | (Reference) | (Reference) | |||
| Female | 5819 | 8.5% | 202.24 | 28.8 (28.0–29.5) | 0.824 (0.791–0.859) | <0.001 | 1.045 (1.000–1.093) | 0.050 | ||
| AAD | All | Male | 11 190 | 9.8% | 433.09 | 25.8 (25.3–26.3) | (Reference) | (Reference) | ||
| Female | 9249 | 8.1% | 418.48 | 22.1 (21.6–22.5) | 0.855 (0.832–0.879) | <0.001 | 1.195 (1.160–1.232) | <0.001 | ||
| <65 years | Male | 6749 | 17.6% | 178.15 | 37.9 (37.0–38.8) | (Reference) | (Reference) | |||
| Female | 3404 | 20.5% | 77.89 | 43.7 (42.2–45.2) | 1.154 (1.107–1.202) | <0.001 | 1.139 (1.089–1.190) | <0.001 | ||
| 65–74 years | Male | 3230 | 8.7% | 141.92 | 22.7 (22.0–23.5) | (Reference) | (Reference) | |||
| Female | 3846 | 12.8% | 123.00 | 31.2 (30.3–32.2) | 1.374 (1.311–1.440) | <0.001 | 1.532 (1.457–1.611) | <0.001 | ||
| ≥75 years | Male | 1211 | 3.1% | 113.01 | 10.7 (10.1–11.3) | (Reference) | (Reference) | |||
| Female | 1999 | 2.9% | 217.60 | 9.2 (8.8–9.6) | 0.857 (0.798–0.921) | <0.001 | 1.083 (1.003–1.169) | 0.042 | ||
| Cardioversion | All | Male | 22 482 | 19.6% | 385.07 | 58.3 (57.6–59.2) | (Reference) | (Reference) | ||
| Female | 14 531 | 12.7% | 398.95 | 36.4 (35.8–37.0) | 0.624 (0.611–0.637) | <0.001 | 0.798 (0.780–0.816) | <0.001 | ||
| <65 years | Male | 11 968 | 31.2% | 154.44 | 77.5 (76.1–78.9) | (Reference) | (Reference) | |||
| Female | 3968 | 23.8% | 76.37 | 52.0 (50.4–53.6) | 0.670 (0.647–0.695) | <0.001 | 0.671 (0.646–0.697) | <0.001 | ||
| 65–74 years | Male | 7827 | 21.0% | 123.24 | 63.5 (62.1–64.9) | (Reference) | (Reference) | |||
| Female | 6154 | 20.4% | 114.34 | 53.8 (52.5–55.2) | 0.847 (0.820–0.876) | <0.001 | 0.904 (0.872–0.937) | <0.001 | ||
| ≥75 years | Male | 2687 | 6.9% | 107.40 | 25.0 (24.1–26.0) | (Reference) | (Reference) | |||
| Female | 4409 | 6.5% | 208.24 | 21.2 (20.6–21.8) | 0.846 (0.807–0.888) | <0.001 | 1.070 (1.016–1.126) | 0.010 | ||
| Catheter ablationa | All | Male | 2341 | 2.8% | 254.71 | 9.2 (8.8–9.6) | (Reference) | (Reference) | ||
| Female | 1101 | 1.4% | 247.29 | 4.5 (4.2–4.7) | 0.484 (0.451–0.520) | <0.001 | 0.888 (0.822–0.960) | 0.003 | ||
| <65 years | Male | 1773 | 7.0% | 92.12 | 19.2 (18.4–20.2) | (Reference) | (Reference) | |||
| Female | 676 | 6.2% | 41.20 | 16.4 (15.2–17.73) | 0.853 (0.780–0.932) | <0.001 | 0.901 (0.819–0.991) | 0.031 | ||
| 65–74 years | Male | 500 | 1.8% | 89.31 | 5.6 (5.1–6.1) | (Reference) | (Reference) | |||
| Female | 377 | 1.7% | 76.24 | 4.9 (4.5–5.5) | 0.883 (0.773–1.010) | 0.069 | 1.007 (0.873–1.163) | 0.92394 | ||
| ≥75 years | Male | 68 | 0.2% | 73.28 | 0.9 (0.7–1.2) | (Reference) | (Reference) | |||
| Female | 48 | 0.1% | 129.85 | 0.4 (0.3–0.5) | 0.398 (0.275–0.576) | <0.001 | 0.494 (0.333–0.734) | <0.001 |
| Outcome . | Patient group . | Sex . | N . | Proportion of patients with events . | P-years (1000 years) . | Incidence (per 1000 P-years) . | Unadjusted IRR (95% CI) . | P value . | Adjusted IRR (95% CI) . | P value . |
|---|---|---|---|---|---|---|---|---|---|---|
| Any AAT | All | Male | 28 781 | 25.1% | 352.87 | 81.6 (80.6–82.5) | (Reference) | (Reference) | ||
| Female | 20 222 | 17.6% | 370.22 | 54.6 (53.9–55.4) | 0.670 (0.658–0.682) | <0.001 | 0.804 (0.801–0.807) | <0.001 | ||
| <65 years | Male | 15 444 | 40.3% | 134.91 | 114.5 (112.7–116.3) | (Reference) | (Reference) | |||
| Female | 6001 | 36.1% | 64.94 | 92.4 (90.1–94.8) | 0.807 (0.784–0.832) | <0.001 | 0.789 (0.765–0.815) | <0.001 | ||
| 65–74 years | Male | 9712 | 26.1% | 114.13 | 85.1 (83.4–86.8) | (Reference) | (Reference) | |||
| Female | 8402 | 27.9% | 103.04 | 81.5 (79.8–83.3) | 0.958 (0.931–0.987) | 0.004 | 1.026 (0.994–1.058) | 0.109 | ||
| ≥75 years | Male | 3625 | 9.3% | 103.82 | 34.9 (33.8–36.1) | (Reference) | (Reference) | |||
| Female | 5819 | 8.5% | 202.24 | 28.8 (28.0–29.5) | 0.824 (0.791–0.859) | <0.001 | 1.045 (1.000–1.093) | 0.050 | ||
| AAD | All | Male | 11 190 | 9.8% | 433.09 | 25.8 (25.3–26.3) | (Reference) | (Reference) | ||
| Female | 9249 | 8.1% | 418.48 | 22.1 (21.6–22.5) | 0.855 (0.832–0.879) | <0.001 | 1.195 (1.160–1.232) | <0.001 | ||
| <65 years | Male | 6749 | 17.6% | 178.15 | 37.9 (37.0–38.8) | (Reference) | (Reference) | |||
| Female | 3404 | 20.5% | 77.89 | 43.7 (42.2–45.2) | 1.154 (1.107–1.202) | <0.001 | 1.139 (1.089–1.190) | <0.001 | ||
| 65–74 years | Male | 3230 | 8.7% | 141.92 | 22.7 (22.0–23.5) | (Reference) | (Reference) | |||
| Female | 3846 | 12.8% | 123.00 | 31.2 (30.3–32.2) | 1.374 (1.311–1.440) | <0.001 | 1.532 (1.457–1.611) | <0.001 | ||
| ≥75 years | Male | 1211 | 3.1% | 113.01 | 10.7 (10.1–11.3) | (Reference) | (Reference) | |||
| Female | 1999 | 2.9% | 217.60 | 9.2 (8.8–9.6) | 0.857 (0.798–0.921) | <0.001 | 1.083 (1.003–1.169) | 0.042 | ||
| Cardioversion | All | Male | 22 482 | 19.6% | 385.07 | 58.3 (57.6–59.2) | (Reference) | (Reference) | ||
| Female | 14 531 | 12.7% | 398.95 | 36.4 (35.8–37.0) | 0.624 (0.611–0.637) | <0.001 | 0.798 (0.780–0.816) | <0.001 | ||
| <65 years | Male | 11 968 | 31.2% | 154.44 | 77.5 (76.1–78.9) | (Reference) | (Reference) | |||
| Female | 3968 | 23.8% | 76.37 | 52.0 (50.4–53.6) | 0.670 (0.647–0.695) | <0.001 | 0.671 (0.646–0.697) | <0.001 | ||
| 65–74 years | Male | 7827 | 21.0% | 123.24 | 63.5 (62.1–64.9) | (Reference) | (Reference) | |||
| Female | 6154 | 20.4% | 114.34 | 53.8 (52.5–55.2) | 0.847 (0.820–0.876) | <0.001 | 0.904 (0.872–0.937) | <0.001 | ||
| ≥75 years | Male | 2687 | 6.9% | 107.40 | 25.0 (24.1–26.0) | (Reference) | (Reference) | |||
| Female | 4409 | 6.5% | 208.24 | 21.2 (20.6–21.8) | 0.846 (0.807–0.888) | <0.001 | 1.070 (1.016–1.126) | 0.010 | ||
| Catheter ablationa | All | Male | 2341 | 2.8% | 254.71 | 9.2 (8.8–9.6) | (Reference) | (Reference) | ||
| Female | 1101 | 1.4% | 247.29 | 4.5 (4.2–4.7) | 0.484 (0.451–0.520) | <0.001 | 0.888 (0.822–0.960) | 0.003 | ||
| <65 years | Male | 1773 | 7.0% | 92.12 | 19.2 (18.4–20.2) | (Reference) | (Reference) | |||
| Female | 676 | 6.2% | 41.20 | 16.4 (15.2–17.73) | 0.853 (0.780–0.932) | <0.001 | 0.901 (0.819–0.991) | 0.031 | ||
| 65–74 years | Male | 500 | 1.8% | 89.31 | 5.6 (5.1–6.1) | (Reference) | (Reference) | |||
| Female | 377 | 1.7% | 76.24 | 4.9 (4.5–5.5) | 0.883 (0.773–1.010) | 0.069 | 1.007 (0.873–1.163) | 0.92394 | ||
| ≥75 years | Male | 68 | 0.2% | 73.28 | 0.9 (0.7–1.2) | (Reference) | (Reference) | |||
| Female | 48 | 0.1% | 129.85 | 0.4 (0.3–0.5) | 0.398 (0.275–0.576) | <0.001 | 0.494 (0.333–0.734) | <0.001 |
AAD, antiarrhythmic drugs; AAT, antiarrhythmic therapy; CI, confidence interval; IRR, incidence rate ratio.
aPatients entering the cohort from 2011 onwards.
Risk estimates of AAT use according to female sex using the Fine–Gray subdistribution hazard model with all-cause death as a competing event
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . |
| Any AAT | 0.665 (0.653–0.677) | <0.001 | 0.934 (0.916–0.953) | <0.001 | 0.859 (0.834–0.885) | <0.001 | 0.839 (0.813–0.866) | <0.001 | 1.060 (1.030–1.090) | <0.001 | 1.084 (1.051–1.118) | <0.001 | 0.907 (0.871–0.946) | <0.001 | 1.141 (1.091–1.192) | <0.001 |
| CV | 0.618 (0.605–0.631) | <0.001 | 0.852 (0.833–0.871) | <0.001 | 0.727 (0.702–0.753) | <0.001 | 0.722 (0.695–0.749) | <0.001 | 0.955 (0.924–0.987) | 0.006 | 0.973 (0.940–1.008) | 0.130 | 0.931 (0.888–0.977) | 0.003 | 1.166 (1.108–1.227) | <0.001 |
| AAD | 0.813 (0.791–0.835) | <0.001 | 1.223 (1.187–1.261) | <0.001 | 1.180 (1.130–1.230) | <0.001 | 1.151 (1.102–1.203) | <0.001 | 1.480 (1.420–1.550) | <0.001 | 1.584 (1.508–1.663) | <0.001 | 0.933 (0.869–1.000) | 0.057 | 1.192 (1.104–1.287) | <0.001 |
| Ablationa | 0.470 (0.438–0.505) | <0.001 | 0.899 (0.831–0.972) | 0.008 | 0.873 (0.799–0.954) | 0.003 | 0.908 (0.826–0.998) | 0.046 | 0.937 (0.820–1.070) | 0.340 | 1.033 (0.896–1.192) | 0.650 | 0.420 (0.291–0.608) | <0.001 | 0.521 (0.354–0.766) | <0.001 |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . |
| Any AAT | 0.665 (0.653–0.677) | <0.001 | 0.934 (0.916–0.953) | <0.001 | 0.859 (0.834–0.885) | <0.001 | 0.839 (0.813–0.866) | <0.001 | 1.060 (1.030–1.090) | <0.001 | 1.084 (1.051–1.118) | <0.001 | 0.907 (0.871–0.946) | <0.001 | 1.141 (1.091–1.192) | <0.001 |
| CV | 0.618 (0.605–0.631) | <0.001 | 0.852 (0.833–0.871) | <0.001 | 0.727 (0.702–0.753) | <0.001 | 0.722 (0.695–0.749) | <0.001 | 0.955 (0.924–0.987) | 0.006 | 0.973 (0.940–1.008) | 0.130 | 0.931 (0.888–0.977) | 0.003 | 1.166 (1.108–1.227) | <0.001 |
| AAD | 0.813 (0.791–0.835) | <0.001 | 1.223 (1.187–1.261) | <0.001 | 1.180 (1.130–1.230) | <0.001 | 1.151 (1.102–1.203) | <0.001 | 1.480 (1.420–1.550) | <0.001 | 1.584 (1.508–1.663) | <0.001 | 0.933 (0.869–1.000) | 0.057 | 1.192 (1.104–1.287) | <0.001 |
| Ablationa | 0.470 (0.438–0.505) | <0.001 | 0.899 (0.831–0.972) | 0.008 | 0.873 (0.799–0.954) | 0.003 | 0.908 (0.826–0.998) | 0.046 | 0.937 (0.820–1.070) | 0.340 | 1.033 (0.896–1.192) | 0.650 | 0.420 (0.291–0.608) | <0.001 | 0.521 (0.354–0.766) | <0.001 |
Values are presented as SHR (95% CI).
AAD, antiarrhythmic drugs; AAT, antiarrhythmic therapy; CI, confidence interval; CV, cardioversion; SHR, subdistribution hazard ratio.
aPatients entering the cohort from 2011 onwards.
Risk estimates of AAT use according to female sex using the Fine–Gray subdistribution hazard model with all-cause death as a competing event
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . |
| Any AAT | 0.665 (0.653–0.677) | <0.001 | 0.934 (0.916–0.953) | <0.001 | 0.859 (0.834–0.885) | <0.001 | 0.839 (0.813–0.866) | <0.001 | 1.060 (1.030–1.090) | <0.001 | 1.084 (1.051–1.118) | <0.001 | 0.907 (0.871–0.946) | <0.001 | 1.141 (1.091–1.192) | <0.001 |
| CV | 0.618 (0.605–0.631) | <0.001 | 0.852 (0.833–0.871) | <0.001 | 0.727 (0.702–0.753) | <0.001 | 0.722 (0.695–0.749) | <0.001 | 0.955 (0.924–0.987) | 0.006 | 0.973 (0.940–1.008) | 0.130 | 0.931 (0.888–0.977) | 0.003 | 1.166 (1.108–1.227) | <0.001 |
| AAD | 0.813 (0.791–0.835) | <0.001 | 1.223 (1.187–1.261) | <0.001 | 1.180 (1.130–1.230) | <0.001 | 1.151 (1.102–1.203) | <0.001 | 1.480 (1.420–1.550) | <0.001 | 1.584 (1.508–1.663) | <0.001 | 0.933 (0.869–1.000) | 0.057 | 1.192 (1.104–1.287) | <0.001 |
| Ablationa | 0.470 (0.438–0.505) | <0.001 | 0.899 (0.831–0.972) | 0.008 | 0.873 (0.799–0.954) | 0.003 | 0.908 (0.826–0.998) | 0.046 | 0.937 (0.820–1.070) | 0.340 | 1.033 (0.896–1.192) | 0.650 | 0.420 (0.291–0.608) | <0.001 | 0.521 (0.354–0.766) | <0.001 |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . | Unadjusted SHR . | P value . | Adjusted SHR . | P value . |
| Any AAT | 0.665 (0.653–0.677) | <0.001 | 0.934 (0.916–0.953) | <0.001 | 0.859 (0.834–0.885) | <0.001 | 0.839 (0.813–0.866) | <0.001 | 1.060 (1.030–1.090) | <0.001 | 1.084 (1.051–1.118) | <0.001 | 0.907 (0.871–0.946) | <0.001 | 1.141 (1.091–1.192) | <0.001 |
| CV | 0.618 (0.605–0.631) | <0.001 | 0.852 (0.833–0.871) | <0.001 | 0.727 (0.702–0.753) | <0.001 | 0.722 (0.695–0.749) | <0.001 | 0.955 (0.924–0.987) | 0.006 | 0.973 (0.940–1.008) | 0.130 | 0.931 (0.888–0.977) | 0.003 | 1.166 (1.108–1.227) | <0.001 |
| AAD | 0.813 (0.791–0.835) | <0.001 | 1.223 (1.187–1.261) | <0.001 | 1.180 (1.130–1.230) | <0.001 | 1.151 (1.102–1.203) | <0.001 | 1.480 (1.420–1.550) | <0.001 | 1.584 (1.508–1.663) | <0.001 | 0.933 (0.869–1.000) | 0.057 | 1.192 (1.104–1.287) | <0.001 |
| Ablationa | 0.470 (0.438–0.505) | <0.001 | 0.899 (0.831–0.972) | 0.008 | 0.873 (0.799–0.954) | 0.003 | 0.908 (0.826–0.998) | 0.046 | 0.937 (0.820–1.070) | 0.340 | 1.033 (0.896–1.192) | 0.650 | 0.420 (0.291–0.608) | <0.001 | 0.521 (0.354–0.766) | <0.001 |
Values are presented as SHR (95% CI).
AAD, antiarrhythmic drugs; AAT, antiarrhythmic therapy; CI, confidence interval; CV, cardioversion; SHR, subdistribution hazard ratio.
aPatients entering the cohort from 2011 onwards.
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Any ablation | 2341 (2.8) | 1101 (1.4) | 1773 (7.0) | 676 (6.2) | 500 (1.8) | 377 (1.7) | 68 (0.2) | 48 (0.1) |
| Typical atrial flutter ablation (TFP44) | 1128 (1.4) | 419 (0.5) | 733 (2.9) | 219 (2.0) | 330 (1.2) | 157 (0.7) | 65 (0.2) | 43 (0.1) |
| Atypical atrial flutter ablation (TFP45) | 76 (0.1) | 58 (0.1) | 52 (0.2) | 30 (0.3) | 22 (0.1) | 25 (0.1) | 2 (0.0) | 3 (0.0) |
| Atrial fibrillation ablation (TFP46) | 1501 (1.8) | 792 (1.0) | 1292 (5.1) | 538 (4.9) | 206 (0.7) | 248 (1.1) | 3 (0.0) | 6 (0.0) |
| Class Ia | 108 (0.1) | 78 (0.1) | 46 (0.1) | 21 (0.1) | 36 (0.1) | 28 (0.1) | 26 (0.1) | 29 (0.0) |
| Quinidine | 49 (0.0) | 19 (0.0) | 13 (0.0) | 4 (0.0) | 18 (0.0) | 4 (0.0) | 18 (0.0) | 11 (0.0) |
| Disopyramide | 59 (0.1) | 59 (0.1) | 33 (0.1) | 17 (0.1) | 18 (0.0) | 24 (0.1) | 8 (0.0) | 18 (0.0) |
| Class Ic | 5872 (5.1) | 5735 (5.0) | 4258 (11.1) | 2648 (15.9) | 1375 (3.7) | 2443 (8.1) | 239 (0.6) | 644 (0.9) |
| Flecainide | 5825 (5.1) | 5702 (5.0) | 4241 (11.1) | 2640 (15.9) | 1357 (3.6) | 2434 (8.1) | 227 (0.6) | 628 (0.9) |
| Propafenone | 87 (0.1) | 72 (0.1) | 46 (0.1) | 27 (0.2) | 27 (0.1) | 26 (0.1) | 14 (0.0) | 19 (0.0) |
| Class III | 6273 (5.5) | 4419 (3.8) | 3248 (8.5) | 1207 (7.3) | 2050 (5.5) | 1782 (5.9) | 975 (2.5) | 1430 (2.1) |
| Amiodarone | 4504 (3.9) | 2971 (2.6) | 2352 (6.1) | 754 (4.5) | 1440 (3.9) | 1162 (3.9) | 712 (1.8) | 1055 (1.5) |
| Dronedarone | 813 (0.7) | 799 (0.7) | 504 (1.3) | 286 (1.7) | 259 (0.7) | 372 (1.2) | 50 (0.1) | 141 (0.2) |
| Sotalol | 1413 (1.2) | 1011 (0.9) | 695 (1.8) | 308 (1.9) | 500 (1.3) | 402 (1.3) | 236 (0.6) | 301 (0.4) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Any ablation | 2341 (2.8) | 1101 (1.4) | 1773 (7.0) | 676 (6.2) | 500 (1.8) | 377 (1.7) | 68 (0.2) | 48 (0.1) |
| Typical atrial flutter ablation (TFP44) | 1128 (1.4) | 419 (0.5) | 733 (2.9) | 219 (2.0) | 330 (1.2) | 157 (0.7) | 65 (0.2) | 43 (0.1) |
| Atypical atrial flutter ablation (TFP45) | 76 (0.1) | 58 (0.1) | 52 (0.2) | 30 (0.3) | 22 (0.1) | 25 (0.1) | 2 (0.0) | 3 (0.0) |
| Atrial fibrillation ablation (TFP46) | 1501 (1.8) | 792 (1.0) | 1292 (5.1) | 538 (4.9) | 206 (0.7) | 248 (1.1) | 3 (0.0) | 6 (0.0) |
| Class Ia | 108 (0.1) | 78 (0.1) | 46 (0.1) | 21 (0.1) | 36 (0.1) | 28 (0.1) | 26 (0.1) | 29 (0.0) |
| Quinidine | 49 (0.0) | 19 (0.0) | 13 (0.0) | 4 (0.0) | 18 (0.0) | 4 (0.0) | 18 (0.0) | 11 (0.0) |
| Disopyramide | 59 (0.1) | 59 (0.1) | 33 (0.1) | 17 (0.1) | 18 (0.0) | 24 (0.1) | 8 (0.0) | 18 (0.0) |
| Class Ic | 5872 (5.1) | 5735 (5.0) | 4258 (11.1) | 2648 (15.9) | 1375 (3.7) | 2443 (8.1) | 239 (0.6) | 644 (0.9) |
| Flecainide | 5825 (5.1) | 5702 (5.0) | 4241 (11.1) | 2640 (15.9) | 1357 (3.6) | 2434 (8.1) | 227 (0.6) | 628 (0.9) |
| Propafenone | 87 (0.1) | 72 (0.1) | 46 (0.1) | 27 (0.2) | 27 (0.1) | 26 (0.1) | 14 (0.0) | 19 (0.0) |
| Class III | 6273 (5.5) | 4419 (3.8) | 3248 (8.5) | 1207 (7.3) | 2050 (5.5) | 1782 (5.9) | 975 (2.5) | 1430 (2.1) |
| Amiodarone | 4504 (3.9) | 2971 (2.6) | 2352 (6.1) | 754 (4.5) | 1440 (3.9) | 1162 (3.9) | 712 (1.8) | 1055 (1.5) |
| Dronedarone | 813 (0.7) | 799 (0.7) | 504 (1.3) | 286 (1.7) | 259 (0.7) | 372 (1.2) | 50 (0.1) | 141 (0.2) |
| Sotalol | 1413 (1.2) | 1011 (0.9) | 695 (1.8) | 308 (1.9) | 500 (1.3) | 402 (1.3) | 236 (0.6) | 301 (0.4) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Any ablation | 2341 (2.8) | 1101 (1.4) | 1773 (7.0) | 676 (6.2) | 500 (1.8) | 377 (1.7) | 68 (0.2) | 48 (0.1) |
| Typical atrial flutter ablation (TFP44) | 1128 (1.4) | 419 (0.5) | 733 (2.9) | 219 (2.0) | 330 (1.2) | 157 (0.7) | 65 (0.2) | 43 (0.1) |
| Atypical atrial flutter ablation (TFP45) | 76 (0.1) | 58 (0.1) | 52 (0.2) | 30 (0.3) | 22 (0.1) | 25 (0.1) | 2 (0.0) | 3 (0.0) |
| Atrial fibrillation ablation (TFP46) | 1501 (1.8) | 792 (1.0) | 1292 (5.1) | 538 (4.9) | 206 (0.7) | 248 (1.1) | 3 (0.0) | 6 (0.0) |
| Class Ia | 108 (0.1) | 78 (0.1) | 46 (0.1) | 21 (0.1) | 36 (0.1) | 28 (0.1) | 26 (0.1) | 29 (0.0) |
| Quinidine | 49 (0.0) | 19 (0.0) | 13 (0.0) | 4 (0.0) | 18 (0.0) | 4 (0.0) | 18 (0.0) | 11 (0.0) |
| Disopyramide | 59 (0.1) | 59 (0.1) | 33 (0.1) | 17 (0.1) | 18 (0.0) | 24 (0.1) | 8 (0.0) | 18 (0.0) |
| Class Ic | 5872 (5.1) | 5735 (5.0) | 4258 (11.1) | 2648 (15.9) | 1375 (3.7) | 2443 (8.1) | 239 (0.6) | 644 (0.9) |
| Flecainide | 5825 (5.1) | 5702 (5.0) | 4241 (11.1) | 2640 (15.9) | 1357 (3.6) | 2434 (8.1) | 227 (0.6) | 628 (0.9) |
| Propafenone | 87 (0.1) | 72 (0.1) | 46 (0.1) | 27 (0.2) | 27 (0.1) | 26 (0.1) | 14 (0.0) | 19 (0.0) |
| Class III | 6273 (5.5) | 4419 (3.8) | 3248 (8.5) | 1207 (7.3) | 2050 (5.5) | 1782 (5.9) | 975 (2.5) | 1430 (2.1) |
| Amiodarone | 4504 (3.9) | 2971 (2.6) | 2352 (6.1) | 754 (4.5) | 1440 (3.9) | 1162 (3.9) | 712 (1.8) | 1055 (1.5) |
| Dronedarone | 813 (0.7) | 799 (0.7) | 504 (1.3) | 286 (1.7) | 259 (0.7) | 372 (1.2) | 50 (0.1) | 141 (0.2) |
| Sotalol | 1413 (1.2) | 1011 (0.9) | 695 (1.8) | 308 (1.9) | 500 (1.3) | 402 (1.3) | 236 (0.6) | 301 (0.4) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Any ablation | 2341 (2.8) | 1101 (1.4) | 1773 (7.0) | 676 (6.2) | 500 (1.8) | 377 (1.7) | 68 (0.2) | 48 (0.1) |
| Typical atrial flutter ablation (TFP44) | 1128 (1.4) | 419 (0.5) | 733 (2.9) | 219 (2.0) | 330 (1.2) | 157 (0.7) | 65 (0.2) | 43 (0.1) |
| Atypical atrial flutter ablation (TFP45) | 76 (0.1) | 58 (0.1) | 52 (0.2) | 30 (0.3) | 22 (0.1) | 25 (0.1) | 2 (0.0) | 3 (0.0) |
| Atrial fibrillation ablation (TFP46) | 1501 (1.8) | 792 (1.0) | 1292 (5.1) | 538 (4.9) | 206 (0.7) | 248 (1.1) | 3 (0.0) | 6 (0.0) |
| Class Ia | 108 (0.1) | 78 (0.1) | 46 (0.1) | 21 (0.1) | 36 (0.1) | 28 (0.1) | 26 (0.1) | 29 (0.0) |
| Quinidine | 49 (0.0) | 19 (0.0) | 13 (0.0) | 4 (0.0) | 18 (0.0) | 4 (0.0) | 18 (0.0) | 11 (0.0) |
| Disopyramide | 59 (0.1) | 59 (0.1) | 33 (0.1) | 17 (0.1) | 18 (0.0) | 24 (0.1) | 8 (0.0) | 18 (0.0) |
| Class Ic | 5872 (5.1) | 5735 (5.0) | 4258 (11.1) | 2648 (15.9) | 1375 (3.7) | 2443 (8.1) | 239 (0.6) | 644 (0.9) |
| Flecainide | 5825 (5.1) | 5702 (5.0) | 4241 (11.1) | 2640 (15.9) | 1357 (3.6) | 2434 (8.1) | 227 (0.6) | 628 (0.9) |
| Propafenone | 87 (0.1) | 72 (0.1) | 46 (0.1) | 27 (0.2) | 27 (0.1) | 26 (0.1) | 14 (0.0) | 19 (0.0) |
| Class III | 6273 (5.5) | 4419 (3.8) | 3248 (8.5) | 1207 (7.3) | 2050 (5.5) | 1782 (5.9) | 975 (2.5) | 1430 (2.1) |
| Amiodarone | 4504 (3.9) | 2971 (2.6) | 2352 (6.1) | 754 (4.5) | 1440 (3.9) | 1162 (3.9) | 712 (1.8) | 1055 (1.5) |
| Dronedarone | 813 (0.7) | 799 (0.7) | 504 (1.3) | 286 (1.7) | 259 (0.7) | 372 (1.2) | 50 (0.1) | 141 (0.2) |
| Sotalol | 1413 (1.2) | 1011 (0.9) | 695 (1.8) | 308 (1.9) | 500 (1.3) | 402 (1.3) | 236 (0.6) | 301 (0.4) |
Use of rhythm control therapies in different age groups
Patients aged <65 years
Among the working age patients (<65 years), the crude cumulative incidences of any AAT (36.1% vs. 40.3%), cardioversion (23.8% vs. 31.2%), and ablation (6.2% vs. 7.0%) were lower, and the cumulative incidence of AAD therapy (20.5% vs. 17.6%) was higher in women compared to men (Table 2 and Figure 2). Women received more often Class Ic drugs, i.e. flecainide (15.9% vs. 11.1%), but less often Class III drug amiodarone (4.5% vs. 6.1%). Female sex remained associated with lesser likelihood of use of any AAT (aSHR 0.839, 95% CI 0.813–0.866), cardioversion (aSHR 0.722, 95% CI 0.695–0.749), and ablation therapies (aSHR 0.908, 95% CI 0.826–0.998) and higher likelihood to receive AADs (aSHR 1.151, 95% CI 1.102–1.203) after adjustment for confounding factors in both the Poisson and the Fine–Gray models (Tables 2 and 3).
Patients aged 65–74 years
In patients aged 65–74 years, the crude cumulative incidences of any AAT (27.9% vs. 26.1%) or ablation (1.7% vs. 1.8%) did not differ between the sexes, whereas incidence of AAD therapy (12.8% vs. 8.7%) was higher in women compared to men and cardioversion (20.4% vs. 21.0%) lower (Table 2 and Figure 2). After adjusting for baseline characteristics, female sex remained associated with a higher likelihood of receiving any AAT in the Fine–Gray model (aSHR 1.084, 95% CI 1.051–1.118) and higher likelihood of receiving AADs in both the Poisson and the Fine–Gray models (aSHR 1.584, 95% CI 1.508–1.663), whereas no difference was found in the utilization of cardioversion or ablation (Tables 2 and 3) in comparison to men.
Patients aged ≥75 years
Among elderly patients (aged ≥75 years), the crude cumulative incidences of any AAT (8.5% vs. 9.3%) did not significantly differ between women and men. The crude cumulative incidences of AAD therapy (2.9% vs. 3.1%) and cardioversion (6.5% vs. 6.9%) were higher in women compared to men whereas ablation (0.1% vs. 0.2%) was lower (Table 2 and Figure 2). Ablation therapy was rare in this age group and mainly performed due to typical atrial flutter (Table 4). In multivariable analyses, female sex was associated with higher likelihood of receiving any AAT (aSHR 1.141, 95% CI 1.091–1.192), AAD (aSHR 1.192, 95% CI 1.104–1.287) and cardioversion (aSHR 1.166, 95% CI 1.108–1.227) and lesser likelihood of receiving ablation (aSHR 0.521, 95% CI 0.354–0.766) after adjusting for confounding factors (Tables 2 and 3).
Interaction analyses
A significant interaction between age and sex was observed in the use of all AAT modalities (P < 0.001 for all; Figure 3). The results of the interaction analyses were largely consistent with the abovementioned analyses with age stratified in three categories, though some additional granularity was noted. Any AAT was used less frequently in women compared to men under the age of 65 years and in patients over 90 years of age. Antiarrhythmic drugs were used more often in women than in men in the age range from 55 to 80, whereas among the youngest and oldest patients, women were less likely to be treated with AADs. For cardioversions, female sex was associated with a lower incidence of procedures in patients under the age of 70, as well as in those over 90 years of age. Finally, female sex was associated with a lower incidence of catheter ablations in the youngest patients under the age of 40 and in the oldest patients over 75 years of age, but not in other age categories.
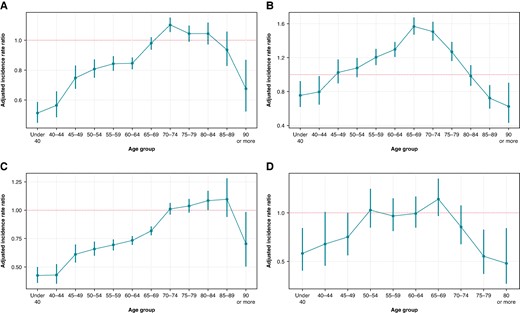
Adjusted incidence ratios and 95% confidence intervals of any AAT (A), AAD (B), cardioversion (C), and ablation (D) comparing women with men (broken line).
Use of rate control therapies
Women were treated more often with rate control drugs (beta-blockers, non-dihydropyridine calcium channel blockers, and digitalis) (P < 0.001) than men (Table 5). Pacemaker implantations and atrioventricular nodal ablations were more frequent among women in comparison to men (P < 0.001).
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Beta-blockers | 91 006 (79.3) | 95 309 (83.0) | 31 514 (82.2) | 14 197 (85.3) | 31 260 (83.9) | 27 016 (89.8) | 28 232 (72.1) | 54 096 (79.5) |
| NDHP calcium channel blockers | 3238 (2.8) | 4799 (4.2) | 1394 (3.6) | 922 (5.5) | 1078 (2.9) | 1585 (5.3) | 766 (2.0) | 2292 (3.4) |
| Digitalis | 15 194 (13.2) | 23 588 (20.5) | 4840 (12.6) | 2029 (12.2) | 5335 (14.3) | 6009 (20.0) | 5019 (12.8) | 15 550 (22.8) |
| AV node ablation (TFP47) | 290 (0.3) | 470 (0.4) | 136 (0.4) | 86 (0.5) | 107 (0.3) | 189 (0.6) | 47 (0.1) | 195 (0.3) |
| Pacemaker implantation | 5688 (5.0) | 6946 (6.0) | 1017 (2.7) | 687 (4.1) | 2074 (5.6) | 2004 (6.7) | 2598 (6.6) | 4263 (6.3) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Beta-blockers | 91 006 (79.3) | 95 309 (83.0) | 31 514 (82.2) | 14 197 (85.3) | 31 260 (83.9) | 27 016 (89.8) | 28 232 (72.1) | 54 096 (79.5) |
| NDHP calcium channel blockers | 3238 (2.8) | 4799 (4.2) | 1394 (3.6) | 922 (5.5) | 1078 (2.9) | 1585 (5.3) | 766 (2.0) | 2292 (3.4) |
| Digitalis | 15 194 (13.2) | 23 588 (20.5) | 4840 (12.6) | 2029 (12.2) | 5335 (14.3) | 6009 (20.0) | 5019 (12.8) | 15 550 (22.8) |
| AV node ablation (TFP47) | 290 (0.3) | 470 (0.4) | 136 (0.4) | 86 (0.5) | 107 (0.3) | 189 (0.6) | 47 (0.1) | 195 (0.3) |
| Pacemaker implantation | 5688 (5.0) | 6946 (6.0) | 1017 (2.7) | 687 (4.1) | 2074 (5.6) | 2004 (6.7) | 2598 (6.6) | 4263 (6.3) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Beta-blockers | 91 006 (79.3) | 95 309 (83.0) | 31 514 (82.2) | 14 197 (85.3) | 31 260 (83.9) | 27 016 (89.8) | 28 232 (72.1) | 54 096 (79.5) |
| NDHP calcium channel blockers | 3238 (2.8) | 4799 (4.2) | 1394 (3.6) | 922 (5.5) | 1078 (2.9) | 1585 (5.3) | 766 (2.0) | 2292 (3.4) |
| Digitalis | 15 194 (13.2) | 23 588 (20.5) | 4840 (12.6) | 2029 (12.2) | 5335 (14.3) | 6009 (20.0) | 5019 (12.8) | 15 550 (22.8) |
| AV node ablation (TFP47) | 290 (0.3) | 470 (0.4) | 136 (0.4) | 86 (0.5) | 107 (0.3) | 189 (0.6) | 47 (0.1) | 195 (0.3) |
| Pacemaker implantation | 5688 (5.0) | 6946 (6.0) | 1017 (2.7) | 687 (4.1) | 2074 (5.6) | 2004 (6.7) | 2598 (6.6) | 4263 (6.3) |
| . | All patients . | <65 years . | 65–74 years . | ≥75 years . | ||||
|---|---|---|---|---|---|---|---|---|
| . | Male . | Female . | Male . | Female . | Male . | Female . | Male . | Female . |
| Beta-blockers | 91 006 (79.3) | 95 309 (83.0) | 31 514 (82.2) | 14 197 (85.3) | 31 260 (83.9) | 27 016 (89.8) | 28 232 (72.1) | 54 096 (79.5) |
| NDHP calcium channel blockers | 3238 (2.8) | 4799 (4.2) | 1394 (3.6) | 922 (5.5) | 1078 (2.9) | 1585 (5.3) | 766 (2.0) | 2292 (3.4) |
| Digitalis | 15 194 (13.2) | 23 588 (20.5) | 4840 (12.6) | 2029 (12.2) | 5335 (14.3) | 6009 (20.0) | 5019 (12.8) | 15 550 (22.8) |
| AV node ablation (TFP47) | 290 (0.3) | 470 (0.4) | 136 (0.4) | 86 (0.5) | 107 (0.3) | 189 (0.6) | 47 (0.1) | 195 (0.3) |
| Pacemaker implantation | 5688 (5.0) | 6946 (6.0) | 1017 (2.7) | 687 (4.1) | 2074 (5.6) | 2004 (6.7) | 2598 (6.6) | 4263 (6.3) |
Discussion
Our nationwide cohort study revealed that rhythm control appears to be less intensive among women compared to men in AF patients under 65 years of age. In this age group, overall AATs, as well as ablation and cardioversion, were less frequently utilized in women, whereas they were treated more frequently with AADs (mainly flecainide).
The age-stratified analyses of the current study provided important new information on possible sex disparities in the real-life management of AF. Our findings signal clinically meaningful sex inequality in symptom control—a relevant part of ESC 2020 AF guideline recommendation of Atrial fibrillation Better Care (ABC) holistic treatment (‘A’ anticoagulation/stroke avoidance, ‘B’ symptom control, and ‘C’ cardiovascular and comorbidity optimization) of AF.1 Notably, it appeared that the rhythm control strategy was overall less often chosen in women, particularly among those under 65 years of age. Moreover, when rhythm control strategy was pursued, women were more often treated with AADs and less often with catheter ablation, which is unfortunate as catheter ablation has been shown to be more effective than AADs in reducing overall AF burden, as well as AF-related symptoms and hospitalizations.16,23 In contrast with the current finding of lower AAT use among women, a prior study within the FinACAF cohort did not find meaningful sex disparities in the use of anticoagulation.24
The higher utilization of especially Class Ic drugs and lower use of amiodarone in women are most likely due to the lower prevalence of ischaemic and structural heart diseases in women compared to men.2 However, women are known to have a higher risk of side effects and even life-threatening ventricular arrhythmias with AADs.15,25 Unfortunately, we do not have data on adverse events related to AATs. Previously reported higher rate of complications after AF ablation in women can be mostly explained by the older age and comorbidity burden at the time of ablation rather than sex itself.17,26–28 Furthermore, the incidence of major procedural complications is very low in both men and women.
In total, ablation (for AF or atrial flutter) was performed only in 2.1% of the patients in the ablation cohort, but the ablations were performed relatively early in both men and women as approximately 65% of the ablations were performed within 2 years after the initial AF diagnosis. Rhythm control therapy with AADs or ablation was initiated less often within 1 year of the AF diagnosis in women than in men. This may have adverse prognostic implications, as early initiation of rhythm control therapy for AF in the recent EAST-AFNET 4 study was associated with lower risk of cardiovascular death, stroke, and cardiac hospitalizations.20 In the subgroup analysis of the study, the benefit was at least comparable for women and those in the oldest tertile and especially pronounced in those with a high CHA2DS2-VASc score. Furthermore, AXAFA-AFNET 5 study showed similar short-term beneficial trends regarding efficacy, safety, and quality of life after AF ablation for both women and men.29 Women have been reported to experience more often AF recurrence in long-term follow-up, but they have also been older with more comorbidities than men.28,30 Further studies are needed to analyse whether reported sex differences in AF recurrence are partly explained by the longer delays seen in women before initiation of rhythm control therapies.
In the present study, 50% of the patients were women, but they were significantly older than men, and only 30% of patients under 65 years were women. Evident sex difference in the age-adjusted AF incidence and prevalence reflects to women’s under-representation in randomized clinical AF trials.31,32 In addition to smaller body size and fewer traditional AF risk factors compared to middle-aged men, oestrogen plays a protective role against AF before menopause in women.4,33 Indeed, AF incidence increases rapidly in men after 50 years, whereas in women, this occurs approximately 10 years later.2 Additionally, adipokine levels which may contribute to AF perpetuation have been reported to be higher in women (at least in postmenopausal women).34
The recommendations between rhythm vs. rate control strategies were based on patient’s clinical features, symptoms, and patient preferences during our study period.1,35 In general, women tend to be more symptomatic during AF, but they report also more atypical symptoms such as fatigue and dyspnoea, whereas men report more often palpitations and effort intolerance.36,37 In permanent AF, the lower quality of life in women may be due to higher age and comorbidity burden in comparison to men.18 Atypical symptoms in women, however, may predispose to failure in recognizing AF as the cause of symptoms and lead to rate control instead of rhythm control strategy. On the other hand, even with typical AF symptoms, women have been reported to be treated less often with rhythm control therapies compared to men.26,27,38 Evaluation of sex differences is clinically relevant mostly in patients younger than 75 years, since in older patients, rate control is often the most appropriate treatment strategy for both women and men.
Strengths and limitations
The most important strengths of our study include the large cohort size, comparison of three age groups, and the use of comprehensive nationwide healthcare registers covering uniquely all levels of care, as well as information of medication, socio-economic background, and income.
Our main study limitations are inherent to real-world retrospective register studies, such as lack of some clinical data, i.e. severity of AF symptoms and recurrency, body mass index, left ventricular ejection fraction, and left atrial dimensions, which all are weighed when treatment options (rhythm vs. rate control therapy) are chosen according to clinical practice guidelines. Additionally, data on adverse effects of AATs as well as the recurrence of AF were not included in the FinACAF register. Our relatively long study period (11 years) enabled some minor changes in patient selection and treatment options that may have been reflected in results despite relatively unchanged rhythm management guidelines. We treated death as a competing risk in our analyses, but we acknowledge that there may be other competing risks, such as incidence of heart failure, myocardial infarction, and bleeding events, which may limit the use of certain AATs, that were not accounted for.
Further prospective studies are needed to analyse whether the differences found in the utilization of AATs correlate with patient characteristics such as comorbidities, body mass index, and AF recurrence rate and AF symptoms. Otherwise, the sex differences found in this study, especially in patients aged under 65 years, are not justified as these differences may entail reduced quality of life and a higher risk of major outcomes in women than in men. Importantly, both the 2020 published ABC holistic pathway recommendation and accumulating knowledge of prognostic effect of early initiation of rhythm control therapies after our study period will possibly lead to diminished sex differences in use of AATs even if atypical AF symptoms in women are not fully recognized.
Conclusions
Our nationwide cohort study demonstrated that women aged under 65 years were less likely to receive AAT than men, whereas in all age groups, the utilization of AADs was higher in women than in men.
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the Aarne Koskelo Foundation, Finnish Foundation for Cardiovascular Research, and Helsinki and Uusimaa Hospital District research fund (TYH2019309).
Data availability
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Finnish national register holders (KELA, Finnish Institute for Health and Welfare, Population Register Center, and Tax Register) through Findata (https://findata.fi/en/).
References
Author notes
Conflict of interest: B.S.: speaker—BMS-Pfizer Alliance and Boehringer Ingelheim; member of advisory board—Pfizer; educational grants—Medtronic and Abbott. K.K.: Finnish Foundation for Cardiovascular Research. J.I.: speaker—Boehringer Ingelheim, BMS-Pfizer Alliance, and Boston Scientific; educational grants—Boston Scientific, Johnson & Johnson, and Medtronic. A.L.A.: research grants—Finnish Foundation for Cardiovascular Research and Sigrid Juselius Foundation; speaker—Abbott, Johnson & Johnson, Sanofi, Bayer, and Boehringer Ingelheim. K.T.: research grants—the Finnish Foundation for Cardiovascular Research, Aarne and Aili Turunen Foundation, and Finnish State Research funding. T.P.: BMS-Pfizer Alliance and Boehringer Ingelheim. J.P.: speaker—Bayer, Boehringer Ingelheim, BMS-Pfizer Alliance, and Abbott; advisory board—Portola, Novo Nordisk, and Herantis Pharma; visiting editor—Terve Media; stock ownership—VitalSignum. M.Li.: speaker—BMS-Pfizer Alliance, Bayer, and Boehringer Ingelheim. P.M.: consultant—Roche, BMS-Pfizer Alliance, Novartis Finland, Boehringer Ingelheim, and MSD Finland. J.H.: research grants—the Finnish Foundation for Cardiovascular Research; advisory board member—BMS-Pfizer Alliance, Novo Nordisk, and Amgen; speaker—Cardiome and Bayer. K.E.J.A.: research grants—the Finnish Foundation for Cardiovascular Research; speaker—Bayer, Pfizer, and Boehringer Ingelheim; advisory board member—Bayer, Pfizer, and AstraZeneca. M.Le.: consultant—BMS-Pfizer Alliance, Bayer, Boehringer Ingelheim, and MSD; speaker—BMS-Pfizer Alliance, Bayer, Boehringer Ingelheim, MSD, Terve Media, and Orion Pharma; research grants—Aarne Koskelo Foundation, the Finnish Foundation for Cardiovascular Research, and Helsinki and Uusimaa Hospital District research fund. All remaining authors have declared no conflicts of interest.



