-
PDF
- Split View
-
Views
-
Cite
Cite
Meelad I H Al-Jazairi, Bao-Oanh Nguyen, Ruben R De With, Marcelle D Smit, Bob Weijs, Anne H Hobbelt, Marco Alings, Jan G P Tijssen, Bastiaan Geelhoed, Hans L Hillege, Robert G Tieleman, Dirk J Van Veldhuisen, Harry J G M Crijns, Isabelle C Van Gelder, Yuri Blaauw, Michiel Rienstra, for the RACE 3 Investigators, Antiarrhythmic drugs in patients with early persistent atrial fibrillation and heart failure: results of the RACE 3 study, EP Europace, Volume 23, Issue 9, September 2021, Pages 1359–1368, https://doi.org/10.1093/europace/euab062
Close - Share Icon Share
Abstract
Maintaining sinus rhythm in patients with persistent atrial fibrillation (AF) is challenging. We explored the efficacy of class I and III antiarrhythmic drugs (AADs) in patients with persistent AF and mild to moderate heart failure (HF).
In the RACE 3 trial, patients with early persistent symptomatic AF and short history of mild to moderate HF with preserved or reduced left ventricular ejection fraction (LVEF) were randomized to targeted or conventional therapy. Both groups received AF and HF guideline-driven treatment. Additionally, the targeted-group received mineralocorticoid receptor antagonists, statins, angiotensin-converting enzyme inhibitors and/or receptor blockers, and cardiac rehabilitation. Class I and III AADs could be instituted in case of symptomatic recurrent AF. Eventually, pulmonary vein isolation could be performed. Primary endpoint was sinus rhythm on 7-day Holter after 1-year. Included were 245 patients, age 65 ± 9 years, 193 (79%) men, AF history was 3 (2–6) months, HF history 2 (1–4) months, 72 (29.4%) had HF with reduced LVEF. After baseline electrical cardioversion (ECV), 190 (77.6%) had AF recurrences; 108 (56.8%) received class I/III AADs; 19 (17.6%) flecainide, 36 (33.3%) sotalol, 3 (2.8%) dronedarone, 50 (46.3%) amiodarone. At 1-year 73 of 108 (68.0%) patients were in sinus rhythm, 44 (40.7%) without new AF recurrences. Maintenance of sinus rhythm was significantly better with amiodarone [n = 29/50 (58%)] compared with flecainide [n = 6/19 (32%)] and sotalol/dronedarone [n = 9/39 (23%)], P = 0.0064. Adverse events occurred in 27 (25.0%) patients, were all minor and reversible.
In stable HF patients with early persistent AF, AAD treatment was effective in nearly half of patients, with no serious adverse effects reported.
This article presents an analysis of the use of antiarrhythmic drugs in the Routine vs. Aggressive Upstream Rhythm Control for Prevention of Early Atrial Fibrillation in Heart Failure (RACE 3) trial, including per-individual timeline of all rhythm-related events during 1 year follow-up.
Almost a quarter of these patients with early persistent atrial fibrillation and early moderate heart failure maintained sinus rhythm for at least 1 year after a single electrical cardioversion.
Antiarrhythmic drugs (AADs) were effective in nearly half of patients, and mainly limited by reversible non-serious adverse effects.
Introduction
Atrial fibrillation (AF) is associated with increased risk of morbidity and mortality and the risk increases when AF occurs in combination with heart failure (HF).1 Studies have shown that sinus rhythm maintenance in certain subgroups of patients with persistent AF and HF may be associated with lower risk of morbidity and mortality.2 However, rhythm control strategies are limited by their efficacy and safety. Pharmacological rhythm control strategies are less effective than pulmonary vein isolation (PVI), and may be associated with adverse events.3 On the other hand, early management of underlying conditions of patients with persistent AF has been demonstrated to improve sinus rhythm maintenance4 and quality of life.5 In the Routine vs. Aggressive risk factor-driven upstream rhythm Control for prevention of Early AF in HF (RACE 3) trial, treating underlying conditions in patients with early AF and stable moderate HF was effective to improve blood pressure, lipid profile, weight, and HF. On top of that, this strategy was of added value to improve maintenance of sinus rhythm,4 even more if all targets for risk factor management were optimally achieved.6
We now hypothesize that AADs are still a viable and relatively safe option as a rhythm-control strategy in the management of patients with persistent AF and moderate HF.
Therefore, the aim of this analysis is to explore the outcome of rhythm control therapy in patients with early AF and short history of HF in the RACE 3 trial during 1-year follow-up, including the efficacy of class I and III antiarrhythmic drugs (AADs) in the whole study population, in both randomized arms, and in patients with HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HFrEF).
Methods
Study design and population
This post hoc analysis was performed in patients with early persistent AF and stable mild to moderate HF included in the RACE 3 trial.4,7 In brief, the RACE 3 study was a multicentre, prospective, randomized, open-label trial in patients with early persistent AF, and mild to moderate HF (ClinicalTrials.gov identifier NCT00877643). Moderate HF included HF with a preserved ejection fraction (HFpEF) or HF with a reduced ejection fraction (HFrEF). HFpEF was defined as left ventricular ejection fraction (LVEF) ≥45%, New York Heart Association (NYHA) functional Class II–III, and additional criteria consisting of echo parameters and/or elevated N-terminal pro-brain natriuretic peptide (NT-proBNP). HFrEF was defined as LVEF <45% and NYHA class I–III. All patients received treatment for AF and HF according to ESC guidelines,3,8 and were subsequently randomized to receive either targeted therapy of underlying conditions or conventional therapy. Patients in the targeted therapy group received four therapies on top of conventional therapy: mineralocorticoid receptor antagonists (MRAs), statins, angiotensin-converting enzyme inhibitors (ACE-Is) and/or angiotensin receptor blockers (ARBs), and cardiac rehabilitation including physical therapy, dietary restrictions, and counselling. Three weeks after inclusion, patients in both groups underwent electrical cardioversion (ECV). Follow-up duration was 1 year. Success was defined as sinus rhythm during ≥6/7th of assessable time on continuous 7-day Holter monitoring at 1-year. Predefined secondary endpoints comprised outcome of rhythm control therapy including sinus rhythm during ≥6/7th of assessable time on continuous 7-day Holter monitoring at 1-year without the need for class I or III ion-channel AADs and without the need for PVI, number of ECVs during follow-up, number of PVIs, and 100% sinus rhythm on the 7-day Holter at 1-year. All adverse events were prospectively documented and analysed for possible relation with study medication in the targeted group or AAD therapy, in addition to their possible effect on the discontinuation of AAD or study medication. Life-threatening adverse events were defined as adverse events requiring hospital admission (≥1 overnight stay), including drug-induced HF, conduction disturbances, and ventricular arrhythmias. To assess outcome of the present analysis we used the primary endpoint of the study (>6/7th sinus rhythm on 12-month Holter) and time to first AF recurrence (secondary endpoint).
Rhythm control therapy
In all patients included in the study, the treating cardiologist had already decided to pursue rhythm control strategy at inclusion, which was one of the inclusion criteria. The first (baseline) ECV was performed without institution of AADs but with a beta-blocker. After a symptomatic recurrence of persistent AF, class I or III AADs (flecainide, sotalol, or dronedarone in patients with HFpEF or amiodarone) could be instituted. The dose of sotalol was 160–320 mg daily, with regular ECG monitoring to detect any increase in QT-interval duration or any sign of pro-arrhythmic effects. In case of amiodarone administration, patients received 600 mg of amiodarone orally for 4 weeks, followed by an ECV (re-ECV).3 After 4 weeks, amiodarone was lowered to 200 mg daily. If the re-ECV was unsuccessful or a relapse of AF occurred, [desethyl]amiodarone plasma levels were evaluated. If the sum was more than 2 mg/L, i.e. within therapeutic range, amiodarone was deemed ineffective. In case of recurrences under adequate serum levels re-ECV was performed or PVI was offered to the patient. If the plasma levels were inadequate, higher dose of amiodarone was instituted for 2–4 weeks before re-ECV was performed. PVI was only considered if at least one class I or III AAD had failed.3
Statistical analysis
The primary objective of this substudy of the RACE 3 trial is to assess rhythm control therapy including the need for and efficacy of AADs in the RACE 3 study during 1-year follow-up. Baseline characteristics are given as mean±standard deviation for normally distributed variables, as median and inter-quartile range for non-normally distributed variables, and as number of patients with percentage for categorical variables. To compare baseline characteristics between groups χ2 test, Fisher’s exact test, T-test, Mann–Whitney test, Wilcoxon test, and Kruskal–Wallis test were used depending on the type of the variable. To assess sinus rhythm maintenance, cumulative rates for the time to AF recurrence was estimated using Kaplan–Meier method. The cumulative recurrence rates for different groups were then compared with each other using the Log-rank statistic. Analysis was done using R package (version 3.4.3). A two-sided P-value lower than 0.05 was considered statistically significant.
Results
Patients and baseline characteristics
All 245 patients included in RACE 3 were included in the present analysis (Table 1). Median time since first AF diagnosis was 3 (2–5) months and time since first diagnosis of HF was 2 (1–4) months. Seventy-two patients (29.4%) had HFrEF.
| . | Total population (n = 245)a . | Spontaneous conversion at baseline (n = 7) . | Successful baseline ECV with no AF recurrences (n = 57) . | Successful baseline ECV with AF recurrence(s) (n = 153) . | Failed baseline ECV (n = 27) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 60 ± 8 | 65 ± 10 | 65 ± 8 | 64 ± 11 | 0.523 |
| Women | 52 (21%) | 4 (57%) | 7 (12%) | 32 (21%) | 8 (30%) | 0.027 |
| Total duration AF (months) | 3 (2–5) | 3 (2–10) | 3 (1–5) | 3 (2–6) | 3 (2–4) | 0.203 |
| Total persistent AF (months) | 2 (1–4) | 2 (1–2) | 2 (1–4) | 2 (1–4) | 2 (2–4) | 0.484 |
| Duration heart failure (months) | 2 (1–4) | 1 (1–1) | 2 (1–3) | 2 (1–4) | 2 (2–4) | 0.022 |
| Hospital admission for HF | 36 (15%) | 0 (0%) | 8 (14%) | 20 (13%) | 7 (26%) | 0.279 |
| LVEF < 45% | 72 (29%) | 1 (14%) | 21 (37%) | 42 (27%) | 8 (30%) | 0.515 |
| Hypertension | 144 (59%) | 1 (14%) | 36 (63%) | 95 (62%) | 11 (41%) | 0.015 |
| Diabetes mellitus | 26 (11%) | 2 (29%) | 5 (9%) | 17 (11%) | 1 (4%) | 0.263 |
| Coronary artery disease | 33 (13%) | 1 (14%) | 8 (14%) | 21 (14%) | 3 (11%) | 1.000 |
| Ischaemic thromboembolic complication | 10 (4%) | 1 (14%) | 3 (5%) | 5 (3%) | 1 (4%) | 0.354 |
| Chronic obstructive pulmonary disease | 20 (8%) | 0 (0%) | 7 (12%) | 11 (7%) | 2 (7%) | 0.623 |
| CHA2-DS2-VASc scoreb | 2 (1–3) | 2 (0–2) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.757 |
| Symptoms | ||||||
| Palpitations | 101 (41%) | 5 (71%) | 16 (28%) | 64 (42%) | 16 (59%) | 0.021 |
| Dyspnoea | 193 (79%) | 6 (86%) | 43 (75%) | 121 (79%) | 22 (81%) | 0.989 |
| Fatigue | 146 (60%) | 3 (43%) | 31 (54%) | 95 (62%) | 16 (59%) | 0.663 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.196 |
| Body mass index (kg/m2) | 28 (26–31) | 29 (27–33) | 28 (25–31) | 28 (26–31) | 29 (26–31) | 0.682 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 15 | 121 ± 12 | 128 ± 16 | 129 ± 14 | 135 ± 19 | 0.174 |
| Diastolic | 82 ± 10 | 80 ± 8 | 82 ± 12 | 83 ± 9 | 81 ± 11 | 0.601 |
| Heart rate | 87 (78–97) | 98 (78–106) | 86 (77–95) | 88 (78–97) | 86 (76–94) | 0.705 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.934 |
| NT-ProBNP (pg/mL) | 1052 (698–1694) | 714 (630–1140) | 1107 (722–1735) | 1050 (692–1664) | 1152 (756–1696) | 0.553 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (40–47) | 42 (38–47) | 42 (39–47) | 44 (40–47) | 45 (37–48) | 0.666 |
| LA volume (mm/mL2) | 38 (31–48) | 38 (32–47) | 36 (32–48) | 38 (31–48) | 39 (30–44) | 0.986 |
| LVEF (%) | 50 (43–59) | 50 (48–56) | 50 (40–58) | 51 (43–59) | 53 (42–60) | 0.788 |
| Exercise test | ||||||
| Maximum load (W) | 130 (103–160) | 150 (106–169) | 140 (120–162) | 125 (100–160) | 129 (92–155) | 0.335 |
| . | Total population (n = 245)a . | Spontaneous conversion at baseline (n = 7) . | Successful baseline ECV with no AF recurrences (n = 57) . | Successful baseline ECV with AF recurrence(s) (n = 153) . | Failed baseline ECV (n = 27) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 60 ± 8 | 65 ± 10 | 65 ± 8 | 64 ± 11 | 0.523 |
| Women | 52 (21%) | 4 (57%) | 7 (12%) | 32 (21%) | 8 (30%) | 0.027 |
| Total duration AF (months) | 3 (2–5) | 3 (2–10) | 3 (1–5) | 3 (2–6) | 3 (2–4) | 0.203 |
| Total persistent AF (months) | 2 (1–4) | 2 (1–2) | 2 (1–4) | 2 (1–4) | 2 (2–4) | 0.484 |
| Duration heart failure (months) | 2 (1–4) | 1 (1–1) | 2 (1–3) | 2 (1–4) | 2 (2–4) | 0.022 |
| Hospital admission for HF | 36 (15%) | 0 (0%) | 8 (14%) | 20 (13%) | 7 (26%) | 0.279 |
| LVEF < 45% | 72 (29%) | 1 (14%) | 21 (37%) | 42 (27%) | 8 (30%) | 0.515 |
| Hypertension | 144 (59%) | 1 (14%) | 36 (63%) | 95 (62%) | 11 (41%) | 0.015 |
| Diabetes mellitus | 26 (11%) | 2 (29%) | 5 (9%) | 17 (11%) | 1 (4%) | 0.263 |
| Coronary artery disease | 33 (13%) | 1 (14%) | 8 (14%) | 21 (14%) | 3 (11%) | 1.000 |
| Ischaemic thromboembolic complication | 10 (4%) | 1 (14%) | 3 (5%) | 5 (3%) | 1 (4%) | 0.354 |
| Chronic obstructive pulmonary disease | 20 (8%) | 0 (0%) | 7 (12%) | 11 (7%) | 2 (7%) | 0.623 |
| CHA2-DS2-VASc scoreb | 2 (1–3) | 2 (0–2) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.757 |
| Symptoms | ||||||
| Palpitations | 101 (41%) | 5 (71%) | 16 (28%) | 64 (42%) | 16 (59%) | 0.021 |
| Dyspnoea | 193 (79%) | 6 (86%) | 43 (75%) | 121 (79%) | 22 (81%) | 0.989 |
| Fatigue | 146 (60%) | 3 (43%) | 31 (54%) | 95 (62%) | 16 (59%) | 0.663 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.196 |
| Body mass index (kg/m2) | 28 (26–31) | 29 (27–33) | 28 (25–31) | 28 (26–31) | 29 (26–31) | 0.682 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 15 | 121 ± 12 | 128 ± 16 | 129 ± 14 | 135 ± 19 | 0.174 |
| Diastolic | 82 ± 10 | 80 ± 8 | 82 ± 12 | 83 ± 9 | 81 ± 11 | 0.601 |
| Heart rate | 87 (78–97) | 98 (78–106) | 86 (77–95) | 88 (78–97) | 86 (76–94) | 0.705 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.934 |
| NT-ProBNP (pg/mL) | 1052 (698–1694) | 714 (630–1140) | 1107 (722–1735) | 1050 (692–1664) | 1152 (756–1696) | 0.553 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (40–47) | 42 (38–47) | 42 (39–47) | 44 (40–47) | 45 (37–48) | 0.666 |
| LA volume (mm/mL2) | 38 (31–48) | 38 (32–47) | 36 (32–48) | 38 (31–48) | 39 (30–44) | 0.986 |
| LVEF (%) | 50 (43–59) | 50 (48–56) | 50 (40–58) | 51 (43–59) | 53 (42–60) | 0.788 |
| Exercise test | ||||||
| Maximum load (W) | 130 (103–160) | 150 (106–169) | 140 (120–162) | 125 (100–160) | 129 (92–155) | 0.335 |
Data are presented as mean ± SD, number of patients (%), or median (IQR).
AF, atrial fibrillation; ECV, electrical cardioversion; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
One patient died before the baseline ECV.
The CHA2DS2-VASc score assesses thromboembolic risk. C, congestive heart failure/left ventricular dysfunction; H, hypertension; A2, age ≥75 years; D, diabetes mellitus; S2, stroke/transient ischaemic attack/systemic embolism; V, vascular disease; A, age 65–74 years; Sc, sex category (female sex).
| . | Total population (n = 245)a . | Spontaneous conversion at baseline (n = 7) . | Successful baseline ECV with no AF recurrences (n = 57) . | Successful baseline ECV with AF recurrence(s) (n = 153) . | Failed baseline ECV (n = 27) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 60 ± 8 | 65 ± 10 | 65 ± 8 | 64 ± 11 | 0.523 |
| Women | 52 (21%) | 4 (57%) | 7 (12%) | 32 (21%) | 8 (30%) | 0.027 |
| Total duration AF (months) | 3 (2–5) | 3 (2–10) | 3 (1–5) | 3 (2–6) | 3 (2–4) | 0.203 |
| Total persistent AF (months) | 2 (1–4) | 2 (1–2) | 2 (1–4) | 2 (1–4) | 2 (2–4) | 0.484 |
| Duration heart failure (months) | 2 (1–4) | 1 (1–1) | 2 (1–3) | 2 (1–4) | 2 (2–4) | 0.022 |
| Hospital admission for HF | 36 (15%) | 0 (0%) | 8 (14%) | 20 (13%) | 7 (26%) | 0.279 |
| LVEF < 45% | 72 (29%) | 1 (14%) | 21 (37%) | 42 (27%) | 8 (30%) | 0.515 |
| Hypertension | 144 (59%) | 1 (14%) | 36 (63%) | 95 (62%) | 11 (41%) | 0.015 |
| Diabetes mellitus | 26 (11%) | 2 (29%) | 5 (9%) | 17 (11%) | 1 (4%) | 0.263 |
| Coronary artery disease | 33 (13%) | 1 (14%) | 8 (14%) | 21 (14%) | 3 (11%) | 1.000 |
| Ischaemic thromboembolic complication | 10 (4%) | 1 (14%) | 3 (5%) | 5 (3%) | 1 (4%) | 0.354 |
| Chronic obstructive pulmonary disease | 20 (8%) | 0 (0%) | 7 (12%) | 11 (7%) | 2 (7%) | 0.623 |
| CHA2-DS2-VASc scoreb | 2 (1–3) | 2 (0–2) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.757 |
| Symptoms | ||||||
| Palpitations | 101 (41%) | 5 (71%) | 16 (28%) | 64 (42%) | 16 (59%) | 0.021 |
| Dyspnoea | 193 (79%) | 6 (86%) | 43 (75%) | 121 (79%) | 22 (81%) | 0.989 |
| Fatigue | 146 (60%) | 3 (43%) | 31 (54%) | 95 (62%) | 16 (59%) | 0.663 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.196 |
| Body mass index (kg/m2) | 28 (26–31) | 29 (27–33) | 28 (25–31) | 28 (26–31) | 29 (26–31) | 0.682 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 15 | 121 ± 12 | 128 ± 16 | 129 ± 14 | 135 ± 19 | 0.174 |
| Diastolic | 82 ± 10 | 80 ± 8 | 82 ± 12 | 83 ± 9 | 81 ± 11 | 0.601 |
| Heart rate | 87 (78–97) | 98 (78–106) | 86 (77–95) | 88 (78–97) | 86 (76–94) | 0.705 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.934 |
| NT-ProBNP (pg/mL) | 1052 (698–1694) | 714 (630–1140) | 1107 (722–1735) | 1050 (692–1664) | 1152 (756–1696) | 0.553 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (40–47) | 42 (38–47) | 42 (39–47) | 44 (40–47) | 45 (37–48) | 0.666 |
| LA volume (mm/mL2) | 38 (31–48) | 38 (32–47) | 36 (32–48) | 38 (31–48) | 39 (30–44) | 0.986 |
| LVEF (%) | 50 (43–59) | 50 (48–56) | 50 (40–58) | 51 (43–59) | 53 (42–60) | 0.788 |
| Exercise test | ||||||
| Maximum load (W) | 130 (103–160) | 150 (106–169) | 140 (120–162) | 125 (100–160) | 129 (92–155) | 0.335 |
| . | Total population (n = 245)a . | Spontaneous conversion at baseline (n = 7) . | Successful baseline ECV with no AF recurrences (n = 57) . | Successful baseline ECV with AF recurrence(s) (n = 153) . | Failed baseline ECV (n = 27) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 60 ± 8 | 65 ± 10 | 65 ± 8 | 64 ± 11 | 0.523 |
| Women | 52 (21%) | 4 (57%) | 7 (12%) | 32 (21%) | 8 (30%) | 0.027 |
| Total duration AF (months) | 3 (2–5) | 3 (2–10) | 3 (1–5) | 3 (2–6) | 3 (2–4) | 0.203 |
| Total persistent AF (months) | 2 (1–4) | 2 (1–2) | 2 (1–4) | 2 (1–4) | 2 (2–4) | 0.484 |
| Duration heart failure (months) | 2 (1–4) | 1 (1–1) | 2 (1–3) | 2 (1–4) | 2 (2–4) | 0.022 |
| Hospital admission for HF | 36 (15%) | 0 (0%) | 8 (14%) | 20 (13%) | 7 (26%) | 0.279 |
| LVEF < 45% | 72 (29%) | 1 (14%) | 21 (37%) | 42 (27%) | 8 (30%) | 0.515 |
| Hypertension | 144 (59%) | 1 (14%) | 36 (63%) | 95 (62%) | 11 (41%) | 0.015 |
| Diabetes mellitus | 26 (11%) | 2 (29%) | 5 (9%) | 17 (11%) | 1 (4%) | 0.263 |
| Coronary artery disease | 33 (13%) | 1 (14%) | 8 (14%) | 21 (14%) | 3 (11%) | 1.000 |
| Ischaemic thromboembolic complication | 10 (4%) | 1 (14%) | 3 (5%) | 5 (3%) | 1 (4%) | 0.354 |
| Chronic obstructive pulmonary disease | 20 (8%) | 0 (0%) | 7 (12%) | 11 (7%) | 2 (7%) | 0.623 |
| CHA2-DS2-VASc scoreb | 2 (1–3) | 2 (0–2) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.757 |
| Symptoms | ||||||
| Palpitations | 101 (41%) | 5 (71%) | 16 (28%) | 64 (42%) | 16 (59%) | 0.021 |
| Dyspnoea | 193 (79%) | 6 (86%) | 43 (75%) | 121 (79%) | 22 (81%) | 0.989 |
| Fatigue | 146 (60%) | 3 (43%) | 31 (54%) | 95 (62%) | 16 (59%) | 0.663 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.196 |
| Body mass index (kg/m2) | 28 (26–31) | 29 (27–33) | 28 (25–31) | 28 (26–31) | 29 (26–31) | 0.682 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 15 | 121 ± 12 | 128 ± 16 | 129 ± 14 | 135 ± 19 | 0.174 |
| Diastolic | 82 ± 10 | 80 ± 8 | 82 ± 12 | 83 ± 9 | 81 ± 11 | 0.601 |
| Heart rate | 87 (78–97) | 98 (78–106) | 86 (77–95) | 88 (78–97) | 86 (76–94) | 0.705 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.934 |
| NT-ProBNP (pg/mL) | 1052 (698–1694) | 714 (630–1140) | 1107 (722–1735) | 1050 (692–1664) | 1152 (756–1696) | 0.553 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (40–47) | 42 (38–47) | 42 (39–47) | 44 (40–47) | 45 (37–48) | 0.666 |
| LA volume (mm/mL2) | 38 (31–48) | 38 (32–47) | 36 (32–48) | 38 (31–48) | 39 (30–44) | 0.986 |
| LVEF (%) | 50 (43–59) | 50 (48–56) | 50 (40–58) | 51 (43–59) | 53 (42–60) | 0.788 |
| Exercise test | ||||||
| Maximum load (W) | 130 (103–160) | 150 (106–169) | 140 (120–162) | 125 (100–160) | 129 (92–155) | 0.335 |
Data are presented as mean ± SD, number of patients (%), or median (IQR).
AF, atrial fibrillation; ECV, electrical cardioversion; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
One patient died before the baseline ECV.
The CHA2DS2-VASc score assesses thromboembolic risk. C, congestive heart failure/left ventricular dysfunction; H, hypertension; A2, age ≥75 years; D, diabetes mellitus; S2, stroke/transient ischaemic attack/systemic embolism; V, vascular disease; A, age 65–74 years; Sc, sex category (female sex).
Rhythm control strategy
Baseline ECV was successful in 210 (88.6%) of 237 patients (Figure 1 and Table 1). Eight (3.3%) patients did not undergo baseline ECV, seven (2.9%) due to spontaneous conversion to sinus rhythm, and one (0.4%) due to death before ECV. Shock failure occurred in 13 (5.3%) and immediate re-initiation of AF occurred in 14 (5.7%) patients. During follow-up, additional ECVs were performed in 131 (53.5%) patients; 42 (17.1%) underwent ≥2 additional ECVs. Overall, AADs were instituted after recurrent symptomatic AF in 108 of 245 (44.1%) patients, 99 received only one AAD (17 received only flecainide, 29 sotalol, 3 dronedarone, and 50 amiodarone), and nine patients received more than one AAD during follow-up. Of the remaining 137 patients who did not use class I or III AAD, 127 (92.7%) received a beta-blocker. PVI was performed in five patients (2.0%), 281 (176–399) days after baseline. Table 2 shows baseline characteristics of all patients using AADs, based on success of AAD therapy at 1-year. Table 3 shows baseline characteristics of all patients using individual AADs. Figure 2 shows the timeline of rhythm control strategy for all individual patients who used AADs during follow-up.
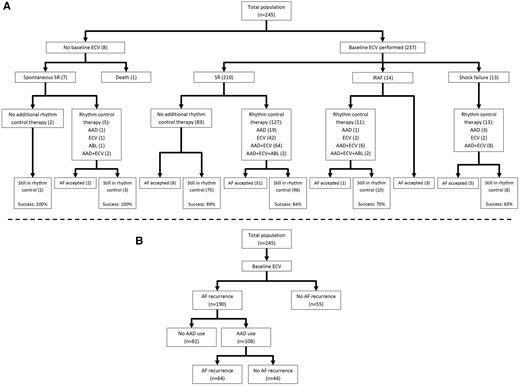
Course of rhythm control strategy followed in study population. (A) Primary endpoint: Sinus rhythm on 1-year Holter according to result of baseline electrical cardioversion and use of rhythm control therapies (Success: sinus rhythm >6/7 of the time on a 7-day Holter at 1 year). (B) Secondary endpoint: maintenance of sinus rhythm and use of antiarrhythmic drugs. AAD, antiarrhythmic drug; ABL, ablation; AF, atrial fibrillation; ECV, electrical cardioversion; IRAF, immediate reinitiation of atrial fibrillation; SR, sinus rhythm.
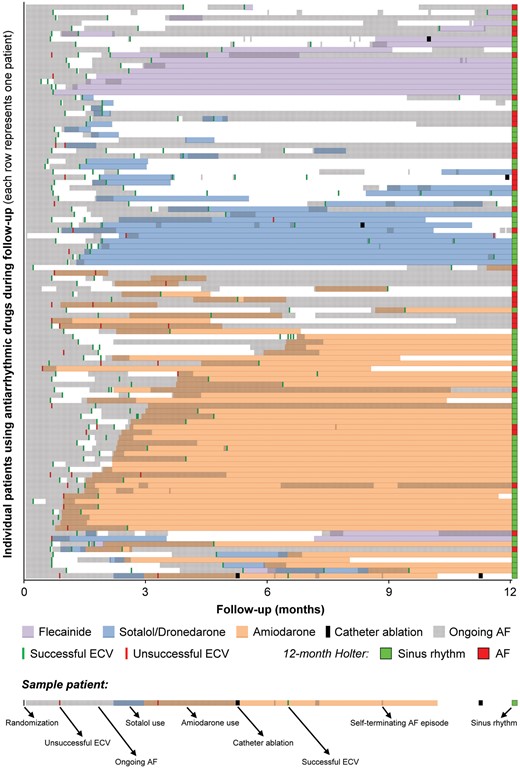
Timeline of events in patients using antiarrhythmic drugs (each row represents the timeline of one patient with all events related to rhythm control of that patient marked on the timeline). AF, atrial fibrillation; ECV, electrical cardioversion.
Baseline characteristics of patients using AADs during follow-up: based on success at 1-year
| . | All patients using AADs (N = 108) . | Success at 1-yeara (N = 35) . | Failure at 1-yeara (N = 73) . | P-value . |
|---|---|---|---|---|
| Age at index-moment (years) | 64 ± 9 | 65 ± 9 | 64 ± 8 | 0.434 |
| Women | 23 (21%) | 9 (26%) | 14 (19%) | 0.459 |
| Total duration AF (months) | 3 (2–5) | 3 (2–4) | 4 (2–5) | 0.291 |
| Total persistent AF (months) | 2 (1–4) | 3 (1–3) | 2 (1–4) | 0.551 |
| Duration heart failure (months) | 2 (1–3) | 2 (2–3) | 2 (1–4) | 0.741 |
| Previous hospital admission for HF | 13 (12%) | 7 (20%) | 6 (8%) | 0.113 |
| LVEF < 45% | 38 (35%) | 11 (31%) | 27 (37%) | 0.669 |
| Hypertension | 57 (53%) | 18 (51%) | 39 (53%) | 1.000 |
| Diabetes mellitus | 9 (8%) | 5 (14%) | 4 (5%) | 0.146 |
| Coronary artery disease | 15 (14%) | 4 (11%) | 11 (15%) | 0.770 |
| Ischaemic thromboembolic complication | 3 (3%) | 2 (6%) | 1 (1%) | 0.245 |
| Chronic obstructive pulmonary disease | 7 (6%) | 4 (11%) | 3 (4%) | 0.210 |
| CHA2-DS2-VASc score | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.412 |
| Symptoms | ||||
| Palpitations | 53 (49%) | 17 (49%) | 36 (49%) | 1.000 |
| Dyspnoea | 89 (82%) | 32 (91%) | 57 (78%) | 0.168 |
| Fatigue | 64 (59%) | 24 (69%) | 40 (55%) | 0.215 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.564 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (26–30) | 29 (26–31) | 0.410 |
| Blood pressure (mmHg) | ||||
| Systolic | 130 ± 16 | 130 ± 17 | 130 ± 16 | 0.957 |
| Diastolic | 83 ± 9 | 83 ± 9 | 82 ± 10 | 0.491 |
| Heart rate | 88 (80–97) | 88 (78–93) | 89 (80–98) | 0.452 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.435 |
| NT-ProBNP (pg/mL) | 1101 (695–1706) | 1052 (618–1366) | 1118 (716–1870) | 0.308 |
| Echocardiographic variables | ||||
| LA size, long axis (mm) | 44 (40–48) | 44 (42–48) | 44 (40–48) | 0.797 |
| LA volume (mm/mL2) | 40 (33–48) | 37 (32–44) | 43 (35–51) | 0.068 |
| LV ejection fraction (%) | 50 (40–56) | 47 (42–55) | 50 (40–58) | 0.688 |
| Exercise test | ||||
| Maximum load (W) | 135 (103–160) | 120 (102–160) | 140 (104–160) | 0.429 |
| . | All patients using AADs (N = 108) . | Success at 1-yeara (N = 35) . | Failure at 1-yeara (N = 73) . | P-value . |
|---|---|---|---|---|
| Age at index-moment (years) | 64 ± 9 | 65 ± 9 | 64 ± 8 | 0.434 |
| Women | 23 (21%) | 9 (26%) | 14 (19%) | 0.459 |
| Total duration AF (months) | 3 (2–5) | 3 (2–4) | 4 (2–5) | 0.291 |
| Total persistent AF (months) | 2 (1–4) | 3 (1–3) | 2 (1–4) | 0.551 |
| Duration heart failure (months) | 2 (1–3) | 2 (2–3) | 2 (1–4) | 0.741 |
| Previous hospital admission for HF | 13 (12%) | 7 (20%) | 6 (8%) | 0.113 |
| LVEF < 45% | 38 (35%) | 11 (31%) | 27 (37%) | 0.669 |
| Hypertension | 57 (53%) | 18 (51%) | 39 (53%) | 1.000 |
| Diabetes mellitus | 9 (8%) | 5 (14%) | 4 (5%) | 0.146 |
| Coronary artery disease | 15 (14%) | 4 (11%) | 11 (15%) | 0.770 |
| Ischaemic thromboembolic complication | 3 (3%) | 2 (6%) | 1 (1%) | 0.245 |
| Chronic obstructive pulmonary disease | 7 (6%) | 4 (11%) | 3 (4%) | 0.210 |
| CHA2-DS2-VASc score | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.412 |
| Symptoms | ||||
| Palpitations | 53 (49%) | 17 (49%) | 36 (49%) | 1.000 |
| Dyspnoea | 89 (82%) | 32 (91%) | 57 (78%) | 0.168 |
| Fatigue | 64 (59%) | 24 (69%) | 40 (55%) | 0.215 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.564 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (26–30) | 29 (26–31) | 0.410 |
| Blood pressure (mmHg) | ||||
| Systolic | 130 ± 16 | 130 ± 17 | 130 ± 16 | 0.957 |
| Diastolic | 83 ± 9 | 83 ± 9 | 82 ± 10 | 0.491 |
| Heart rate | 88 (80–97) | 88 (78–93) | 89 (80–98) | 0.452 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.435 |
| NT-ProBNP (pg/mL) | 1101 (695–1706) | 1052 (618–1366) | 1118 (716–1870) | 0.308 |
| Echocardiographic variables | ||||
| LA size, long axis (mm) | 44 (40–48) | 44 (42–48) | 44 (40–48) | 0.797 |
| LA volume (mm/mL2) | 40 (33–48) | 37 (32–44) | 43 (35–51) | 0.068 |
| LV ejection fraction (%) | 50 (40–56) | 47 (42–55) | 50 (40–58) | 0.688 |
| Exercise test | ||||
| Maximum load (W) | 135 (103–160) | 120 (102–160) | 140 (104–160) | 0.429 |
Data are presented as mean ± SD, number of patients (%), or median (IQR).
AAD, antiarrythmic drug; AF, atrial fibrillation; SR, sinus rhythm; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Based on primary Endpoint (>6/7 SR on 1-year Holter).
Baseline characteristics of patients using AADs during follow-up: based on success at 1-year
| . | All patients using AADs (N = 108) . | Success at 1-yeara (N = 35) . | Failure at 1-yeara (N = 73) . | P-value . |
|---|---|---|---|---|
| Age at index-moment (years) | 64 ± 9 | 65 ± 9 | 64 ± 8 | 0.434 |
| Women | 23 (21%) | 9 (26%) | 14 (19%) | 0.459 |
| Total duration AF (months) | 3 (2–5) | 3 (2–4) | 4 (2–5) | 0.291 |
| Total persistent AF (months) | 2 (1–4) | 3 (1–3) | 2 (1–4) | 0.551 |
| Duration heart failure (months) | 2 (1–3) | 2 (2–3) | 2 (1–4) | 0.741 |
| Previous hospital admission for HF | 13 (12%) | 7 (20%) | 6 (8%) | 0.113 |
| LVEF < 45% | 38 (35%) | 11 (31%) | 27 (37%) | 0.669 |
| Hypertension | 57 (53%) | 18 (51%) | 39 (53%) | 1.000 |
| Diabetes mellitus | 9 (8%) | 5 (14%) | 4 (5%) | 0.146 |
| Coronary artery disease | 15 (14%) | 4 (11%) | 11 (15%) | 0.770 |
| Ischaemic thromboembolic complication | 3 (3%) | 2 (6%) | 1 (1%) | 0.245 |
| Chronic obstructive pulmonary disease | 7 (6%) | 4 (11%) | 3 (4%) | 0.210 |
| CHA2-DS2-VASc score | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.412 |
| Symptoms | ||||
| Palpitations | 53 (49%) | 17 (49%) | 36 (49%) | 1.000 |
| Dyspnoea | 89 (82%) | 32 (91%) | 57 (78%) | 0.168 |
| Fatigue | 64 (59%) | 24 (69%) | 40 (55%) | 0.215 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.564 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (26–30) | 29 (26–31) | 0.410 |
| Blood pressure (mmHg) | ||||
| Systolic | 130 ± 16 | 130 ± 17 | 130 ± 16 | 0.957 |
| Diastolic | 83 ± 9 | 83 ± 9 | 82 ± 10 | 0.491 |
| Heart rate | 88 (80–97) | 88 (78–93) | 89 (80–98) | 0.452 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.435 |
| NT-ProBNP (pg/mL) | 1101 (695–1706) | 1052 (618–1366) | 1118 (716–1870) | 0.308 |
| Echocardiographic variables | ||||
| LA size, long axis (mm) | 44 (40–48) | 44 (42–48) | 44 (40–48) | 0.797 |
| LA volume (mm/mL2) | 40 (33–48) | 37 (32–44) | 43 (35–51) | 0.068 |
| LV ejection fraction (%) | 50 (40–56) | 47 (42–55) | 50 (40–58) | 0.688 |
| Exercise test | ||||
| Maximum load (W) | 135 (103–160) | 120 (102–160) | 140 (104–160) | 0.429 |
| . | All patients using AADs (N = 108) . | Success at 1-yeara (N = 35) . | Failure at 1-yeara (N = 73) . | P-value . |
|---|---|---|---|---|
| Age at index-moment (years) | 64 ± 9 | 65 ± 9 | 64 ± 8 | 0.434 |
| Women | 23 (21%) | 9 (26%) | 14 (19%) | 0.459 |
| Total duration AF (months) | 3 (2–5) | 3 (2–4) | 4 (2–5) | 0.291 |
| Total persistent AF (months) | 2 (1–4) | 3 (1–3) | 2 (1–4) | 0.551 |
| Duration heart failure (months) | 2 (1–3) | 2 (2–3) | 2 (1–4) | 0.741 |
| Previous hospital admission for HF | 13 (12%) | 7 (20%) | 6 (8%) | 0.113 |
| LVEF < 45% | 38 (35%) | 11 (31%) | 27 (37%) | 0.669 |
| Hypertension | 57 (53%) | 18 (51%) | 39 (53%) | 1.000 |
| Diabetes mellitus | 9 (8%) | 5 (14%) | 4 (5%) | 0.146 |
| Coronary artery disease | 15 (14%) | 4 (11%) | 11 (15%) | 0.770 |
| Ischaemic thromboembolic complication | 3 (3%) | 2 (6%) | 1 (1%) | 0.245 |
| Chronic obstructive pulmonary disease | 7 (6%) | 4 (11%) | 3 (4%) | 0.210 |
| CHA2-DS2-VASc score | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.412 |
| Symptoms | ||||
| Palpitations | 53 (49%) | 17 (49%) | 36 (49%) | 1.000 |
| Dyspnoea | 89 (82%) | 32 (91%) | 57 (78%) | 0.168 |
| Fatigue | 64 (59%) | 24 (69%) | 40 (55%) | 0.215 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.564 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (26–30) | 29 (26–31) | 0.410 |
| Blood pressure (mmHg) | ||||
| Systolic | 130 ± 16 | 130 ± 17 | 130 ± 16 | 0.957 |
| Diastolic | 83 ± 9 | 83 ± 9 | 82 ± 10 | 0.491 |
| Heart rate | 88 (80–97) | 88 (78–93) | 89 (80–98) | 0.452 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.435 |
| NT-ProBNP (pg/mL) | 1101 (695–1706) | 1052 (618–1366) | 1118 (716–1870) | 0.308 |
| Echocardiographic variables | ||||
| LA size, long axis (mm) | 44 (40–48) | 44 (42–48) | 44 (40–48) | 0.797 |
| LA volume (mm/mL2) | 40 (33–48) | 37 (32–44) | 43 (35–51) | 0.068 |
| LV ejection fraction (%) | 50 (40–56) | 47 (42–55) | 50 (40–58) | 0.688 |
| Exercise test | ||||
| Maximum load (W) | 135 (103–160) | 120 (102–160) | 140 (104–160) | 0.429 |
Data are presented as mean ± SD, number of patients (%), or median (IQR).
AAD, antiarrythmic drug; AF, atrial fibrillation; SR, sinus rhythm; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Based on primary Endpoint (>6/7 SR on 1-year Holter).
Baseline characteristics of patients using AADs during follow-up: based on individual antiarrhythmic drug groups
| . | No AAD (N = 137) . | Flecainide only (N = 17) . | Sotalol/dronedarone only (N = 32) . | Amiodarone only (N = 50) . | >1 AAD (N = 9) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 64 ± 10 | 66 ± 8 | 65 ± 8 | 56 ± 9 | 0.039 |
| Women | 29 (21%) | 3 (18%) | 11 (34%) | 8 (16%) | 1 (11%) | 0.360 |
| Total duration AF (months) | 3 (2–6) | 4 (3–6) | 3 (2–5) | 3 (2–5) | 4 (2–8) | 0.725 |
| Total persistent AF (months) | 2 (1–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.479 |
| Duration heart failure (months) | 2 (1–4) | 3 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.906 |
| Hospital admission for HF | 23 (17%) | 1 (6%) | 4 (12%) | 7 (14%) | 1 (11%) | 0.892 |
| LVEF < 45% | 34 (25%) | 3 (18%) | 9 (28%) | 25 (50%) | 1 (11%) | 0.009 |
| Hypertension | 87 (64%) | 6 (35%) | 24 (75%) | 22 (44%) | 5 (56%) | 0.010 |
| Diabetes mellitus | 17 (12%) | 2 (12%) | 5 (16%) | 2 (4%) | 0 (0%) | 0.314 |
| Coronary artery disease | 18 (13%) | 1 (6%) | 4 (12%) | 10 (20%) | 0 (0%) | 0.514 |
| Ischaemic thromboembolic complication | 7 (5%) | 0 (0%) | 1 (3%) | 2 (4%) | 0 (0%) | 1.000 |
| Chronic obstructive pulmonary disease | 13 (9%) | 3 (18%) | 1 (3%) | 3 (6%) | 0 (0%) | 0.416 |
| CHA2-DS2-VASc score | 2 (1–3) | 1 (1–3) | 2 (1–3) | 2 (1–3) | 1 (0–1) | 0.006 |
| Symptoms | ||||||
| Palpitations | 48 (35%) | 8 (47%) | 20 (62%) | 21 (42%) | 4 (44%) | 0.087 |
| Dyspnoea | 104 (76%) | 13 (76%) | 26 (81%) | 42 (84%) | 8 (89%) | 0.726 |
| Fatigue | 82 (60%) | 9 (53%) | 20 (62%) | 28 (56%) | 7 (78%) | 0.788 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.039 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.804 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (25–30) | 28 (26–30) | 29 (26–32) | 31 (26–32) | 0.625 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 14 | 134 ± 22 | 129 ± 13 | 129 ± 15 | 126 ± 20 | 0.900 |
| Diastolic | 82 ± 11 | 85 ± 12 | 82 ± 9 | 82 ± 8 | 84 ± 14 | 0.863 |
| ECG variables | ||||||
| Heart rate | 86 (76–99) | 87 (79–93) | 92 (85–100) | 88 (78–96) | 85 (83–92) | 0.561 |
| QRS (ms) | 96 (86–104) | 92 (88–96) | 91 (83–97) | 98 (86–106) | 93 (90–98) | 0.184 |
| QTc (ms) | 423 (399–442) | 429 (400–440) | 420 (393–441) | 424 (394–446) | 413 (402–426) | 0.990 |
| Laboratory results | ||||||
| NT-ProBNP (pg/mL) | 1036 (702–1608) | 1118 (743–1694) | 1136 (696–1908) | 1045 (692–1712) | 1101 (684–1410) | 0.968 |
| Potassium (mmol/L) | 4.3 (4.0–4.5) | 4.2 (3.9–4.6) | 4.3 (4.2–4.5) | 4.3 (4.0–4.6) | 4.3 (4.1–4.4) | 0.944 |
| eGFR (mL/min/1.73 m2) | 69 (59–79) | 72 (66–84) | 72 (66–83) | 75 (64–84) | 73 (62–81) | 0.227 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (39–47) | 46 (43–50) | 44 (41–46) | 44 (39–48) | 44 (40–45) | 0.337 |
| LA volume (mm/mL2) | 36 (29–47) | 44 (39–51) | 37 (33–50) | 39 (32–44) | 45 (34–51) | 0.159 |
| LV ejection fraction (%) | 53 (45–60) | 55 (47–56) | 50 (43–58) | 44 (39–55) | 53 (48–60) | 0.036 |
| Exercise test | ||||||
| Maximum load (W) | 128 (101–163) | 147 (125–162) | 106 (98–152) | 125 (105–150) | 160 (150–175) | 0.049 |
| . | No AAD (N = 137) . | Flecainide only (N = 17) . | Sotalol/dronedarone only (N = 32) . | Amiodarone only (N = 50) . | >1 AAD (N = 9) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 64 ± 10 | 66 ± 8 | 65 ± 8 | 56 ± 9 | 0.039 |
| Women | 29 (21%) | 3 (18%) | 11 (34%) | 8 (16%) | 1 (11%) | 0.360 |
| Total duration AF (months) | 3 (2–6) | 4 (3–6) | 3 (2–5) | 3 (2–5) | 4 (2–8) | 0.725 |
| Total persistent AF (months) | 2 (1–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.479 |
| Duration heart failure (months) | 2 (1–4) | 3 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.906 |
| Hospital admission for HF | 23 (17%) | 1 (6%) | 4 (12%) | 7 (14%) | 1 (11%) | 0.892 |
| LVEF < 45% | 34 (25%) | 3 (18%) | 9 (28%) | 25 (50%) | 1 (11%) | 0.009 |
| Hypertension | 87 (64%) | 6 (35%) | 24 (75%) | 22 (44%) | 5 (56%) | 0.010 |
| Diabetes mellitus | 17 (12%) | 2 (12%) | 5 (16%) | 2 (4%) | 0 (0%) | 0.314 |
| Coronary artery disease | 18 (13%) | 1 (6%) | 4 (12%) | 10 (20%) | 0 (0%) | 0.514 |
| Ischaemic thromboembolic complication | 7 (5%) | 0 (0%) | 1 (3%) | 2 (4%) | 0 (0%) | 1.000 |
| Chronic obstructive pulmonary disease | 13 (9%) | 3 (18%) | 1 (3%) | 3 (6%) | 0 (0%) | 0.416 |
| CHA2-DS2-VASc score | 2 (1–3) | 1 (1–3) | 2 (1–3) | 2 (1–3) | 1 (0–1) | 0.006 |
| Symptoms | ||||||
| Palpitations | 48 (35%) | 8 (47%) | 20 (62%) | 21 (42%) | 4 (44%) | 0.087 |
| Dyspnoea | 104 (76%) | 13 (76%) | 26 (81%) | 42 (84%) | 8 (89%) | 0.726 |
| Fatigue | 82 (60%) | 9 (53%) | 20 (62%) | 28 (56%) | 7 (78%) | 0.788 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.039 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.804 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (25–30) | 28 (26–30) | 29 (26–32) | 31 (26–32) | 0.625 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 14 | 134 ± 22 | 129 ± 13 | 129 ± 15 | 126 ± 20 | 0.900 |
| Diastolic | 82 ± 11 | 85 ± 12 | 82 ± 9 | 82 ± 8 | 84 ± 14 | 0.863 |
| ECG variables | ||||||
| Heart rate | 86 (76–99) | 87 (79–93) | 92 (85–100) | 88 (78–96) | 85 (83–92) | 0.561 |
| QRS (ms) | 96 (86–104) | 92 (88–96) | 91 (83–97) | 98 (86–106) | 93 (90–98) | 0.184 |
| QTc (ms) | 423 (399–442) | 429 (400–440) | 420 (393–441) | 424 (394–446) | 413 (402–426) | 0.990 |
| Laboratory results | ||||||
| NT-ProBNP (pg/mL) | 1036 (702–1608) | 1118 (743–1694) | 1136 (696–1908) | 1045 (692–1712) | 1101 (684–1410) | 0.968 |
| Potassium (mmol/L) | 4.3 (4.0–4.5) | 4.2 (3.9–4.6) | 4.3 (4.2–4.5) | 4.3 (4.0–4.6) | 4.3 (4.1–4.4) | 0.944 |
| eGFR (mL/min/1.73 m2) | 69 (59–79) | 72 (66–84) | 72 (66–83) | 75 (64–84) | 73 (62–81) | 0.227 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (39–47) | 46 (43–50) | 44 (41–46) | 44 (39–48) | 44 (40–45) | 0.337 |
| LA volume (mm/mL2) | 36 (29–47) | 44 (39–51) | 37 (33–50) | 39 (32–44) | 45 (34–51) | 0.159 |
| LV ejection fraction (%) | 53 (45–60) | 55 (47–56) | 50 (43–58) | 44 (39–55) | 53 (48–60) | 0.036 |
| Exercise test | ||||||
| Maximum load (W) | 128 (101–163) | 147 (125–162) | 106 (98–152) | 125 (105–150) | 160 (150–175) | 0.049 |
Data are presented as mean±SD, number of patients (%), or median (IQR).
AAD, antiarrythmic drug; AF, atrial fibrillation; SR, sinus rhythm; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Baseline characteristics of patients using AADs during follow-up: based on individual antiarrhythmic drug groups
| . | No AAD (N = 137) . | Flecainide only (N = 17) . | Sotalol/dronedarone only (N = 32) . | Amiodarone only (N = 50) . | >1 AAD (N = 9) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 64 ± 10 | 66 ± 8 | 65 ± 8 | 56 ± 9 | 0.039 |
| Women | 29 (21%) | 3 (18%) | 11 (34%) | 8 (16%) | 1 (11%) | 0.360 |
| Total duration AF (months) | 3 (2–6) | 4 (3–6) | 3 (2–5) | 3 (2–5) | 4 (2–8) | 0.725 |
| Total persistent AF (months) | 2 (1–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.479 |
| Duration heart failure (months) | 2 (1–4) | 3 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.906 |
| Hospital admission for HF | 23 (17%) | 1 (6%) | 4 (12%) | 7 (14%) | 1 (11%) | 0.892 |
| LVEF < 45% | 34 (25%) | 3 (18%) | 9 (28%) | 25 (50%) | 1 (11%) | 0.009 |
| Hypertension | 87 (64%) | 6 (35%) | 24 (75%) | 22 (44%) | 5 (56%) | 0.010 |
| Diabetes mellitus | 17 (12%) | 2 (12%) | 5 (16%) | 2 (4%) | 0 (0%) | 0.314 |
| Coronary artery disease | 18 (13%) | 1 (6%) | 4 (12%) | 10 (20%) | 0 (0%) | 0.514 |
| Ischaemic thromboembolic complication | 7 (5%) | 0 (0%) | 1 (3%) | 2 (4%) | 0 (0%) | 1.000 |
| Chronic obstructive pulmonary disease | 13 (9%) | 3 (18%) | 1 (3%) | 3 (6%) | 0 (0%) | 0.416 |
| CHA2-DS2-VASc score | 2 (1–3) | 1 (1–3) | 2 (1–3) | 2 (1–3) | 1 (0–1) | 0.006 |
| Symptoms | ||||||
| Palpitations | 48 (35%) | 8 (47%) | 20 (62%) | 21 (42%) | 4 (44%) | 0.087 |
| Dyspnoea | 104 (76%) | 13 (76%) | 26 (81%) | 42 (84%) | 8 (89%) | 0.726 |
| Fatigue | 82 (60%) | 9 (53%) | 20 (62%) | 28 (56%) | 7 (78%) | 0.788 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.039 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.804 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (25–30) | 28 (26–30) | 29 (26–32) | 31 (26–32) | 0.625 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 14 | 134 ± 22 | 129 ± 13 | 129 ± 15 | 126 ± 20 | 0.900 |
| Diastolic | 82 ± 11 | 85 ± 12 | 82 ± 9 | 82 ± 8 | 84 ± 14 | 0.863 |
| ECG variables | ||||||
| Heart rate | 86 (76–99) | 87 (79–93) | 92 (85–100) | 88 (78–96) | 85 (83–92) | 0.561 |
| QRS (ms) | 96 (86–104) | 92 (88–96) | 91 (83–97) | 98 (86–106) | 93 (90–98) | 0.184 |
| QTc (ms) | 423 (399–442) | 429 (400–440) | 420 (393–441) | 424 (394–446) | 413 (402–426) | 0.990 |
| Laboratory results | ||||||
| NT-ProBNP (pg/mL) | 1036 (702–1608) | 1118 (743–1694) | 1136 (696–1908) | 1045 (692–1712) | 1101 (684–1410) | 0.968 |
| Potassium (mmol/L) | 4.3 (4.0–4.5) | 4.2 (3.9–4.6) | 4.3 (4.2–4.5) | 4.3 (4.0–4.6) | 4.3 (4.1–4.4) | 0.944 |
| eGFR (mL/min/1.73 m2) | 69 (59–79) | 72 (66–84) | 72 (66–83) | 75 (64–84) | 73 (62–81) | 0.227 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (39–47) | 46 (43–50) | 44 (41–46) | 44 (39–48) | 44 (40–45) | 0.337 |
| LA volume (mm/mL2) | 36 (29–47) | 44 (39–51) | 37 (33–50) | 39 (32–44) | 45 (34–51) | 0.159 |
| LV ejection fraction (%) | 53 (45–60) | 55 (47–56) | 50 (43–58) | 44 (39–55) | 53 (48–60) | 0.036 |
| Exercise test | ||||||
| Maximum load (W) | 128 (101–163) | 147 (125–162) | 106 (98–152) | 125 (105–150) | 160 (150–175) | 0.049 |
| . | No AAD (N = 137) . | Flecainide only (N = 17) . | Sotalol/dronedarone only (N = 32) . | Amiodarone only (N = 50) . | >1 AAD (N = 9) . | P-value . |
|---|---|---|---|---|---|---|
| Age at index-moment (years) | 65 ± 9 | 64 ± 10 | 66 ± 8 | 65 ± 8 | 56 ± 9 | 0.039 |
| Women | 29 (21%) | 3 (18%) | 11 (34%) | 8 (16%) | 1 (11%) | 0.360 |
| Total duration AF (months) | 3 (2–6) | 4 (3–6) | 3 (2–5) | 3 (2–5) | 4 (2–8) | 0.725 |
| Total persistent AF (months) | 2 (1–4) | 3 (2–4) | 2 (1–3) | 2 (1–3) | 3 (1–3) | 0.479 |
| Duration heart failure (months) | 2 (1–4) | 3 (1–4) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.906 |
| Hospital admission for HF | 23 (17%) | 1 (6%) | 4 (12%) | 7 (14%) | 1 (11%) | 0.892 |
| LVEF < 45% | 34 (25%) | 3 (18%) | 9 (28%) | 25 (50%) | 1 (11%) | 0.009 |
| Hypertension | 87 (64%) | 6 (35%) | 24 (75%) | 22 (44%) | 5 (56%) | 0.010 |
| Diabetes mellitus | 17 (12%) | 2 (12%) | 5 (16%) | 2 (4%) | 0 (0%) | 0.314 |
| Coronary artery disease | 18 (13%) | 1 (6%) | 4 (12%) | 10 (20%) | 0 (0%) | 0.514 |
| Ischaemic thromboembolic complication | 7 (5%) | 0 (0%) | 1 (3%) | 2 (4%) | 0 (0%) | 1.000 |
| Chronic obstructive pulmonary disease | 13 (9%) | 3 (18%) | 1 (3%) | 3 (6%) | 0 (0%) | 0.416 |
| CHA2-DS2-VASc score | 2 (1–3) | 1 (1–3) | 2 (1–3) | 2 (1–3) | 1 (0–1) | 0.006 |
| Symptoms | ||||||
| Palpitations | 48 (35%) | 8 (47%) | 20 (62%) | 21 (42%) | 4 (44%) | 0.087 |
| Dyspnoea | 104 (76%) | 13 (76%) | 26 (81%) | 42 (84%) | 8 (89%) | 0.726 |
| Fatigue | 82 (60%) | 9 (53%) | 20 (62%) | 28 (56%) | 7 (78%) | 0.788 |
| EHRA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–3) | 2 (2–3) | 0.039 |
| NYHA class | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.804 |
| Body mass index (kg/m2) | 28 (26–31) | 28 (25–30) | 28 (26–30) | 29 (26–32) | 31 (26–32) | 0.625 |
| Blood pressure (mmHg) | ||||||
| Systolic | 129 ± 14 | 134 ± 22 | 129 ± 13 | 129 ± 15 | 126 ± 20 | 0.900 |
| Diastolic | 82 ± 11 | 85 ± 12 | 82 ± 9 | 82 ± 8 | 84 ± 14 | 0.863 |
| ECG variables | ||||||
| Heart rate | 86 (76–99) | 87 (79–93) | 92 (85–100) | 88 (78–96) | 85 (83–92) | 0.561 |
| QRS (ms) | 96 (86–104) | 92 (88–96) | 91 (83–97) | 98 (86–106) | 93 (90–98) | 0.184 |
| QTc (ms) | 423 (399–442) | 429 (400–440) | 420 (393–441) | 424 (394–446) | 413 (402–426) | 0.990 |
| Laboratory results | ||||||
| NT-ProBNP (pg/mL) | 1036 (702–1608) | 1118 (743–1694) | 1136 (696–1908) | 1045 (692–1712) | 1101 (684–1410) | 0.968 |
| Potassium (mmol/L) | 4.3 (4.0–4.5) | 4.2 (3.9–4.6) | 4.3 (4.2–4.5) | 4.3 (4.0–4.6) | 4.3 (4.1–4.4) | 0.944 |
| eGFR (mL/min/1.73 m2) | 69 (59–79) | 72 (66–84) | 72 (66–83) | 75 (64–84) | 73 (62–81) | 0.227 |
| Echocardiographic variables | ||||||
| LA size, long axis (mm) | 43 (39–47) | 46 (43–50) | 44 (41–46) | 44 (39–48) | 44 (40–45) | 0.337 |
| LA volume (mm/mL2) | 36 (29–47) | 44 (39–51) | 37 (33–50) | 39 (32–44) | 45 (34–51) | 0.159 |
| LV ejection fraction (%) | 53 (45–60) | 55 (47–56) | 50 (43–58) | 44 (39–55) | 53 (48–60) | 0.036 |
| Exercise test | ||||||
| Maximum load (W) | 128 (101–163) | 147 (125–162) | 106 (98–152) | 125 (105–150) | 160 (150–175) | 0.049 |
Data are presented as mean±SD, number of patients (%), or median (IQR).
AAD, antiarrythmic drug; AF, atrial fibrillation; SR, sinus rhythm; EHRA, European Heart Rhythm Association class for symptoms; HF, heart failure; LA, Left atrial; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-pro BNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association.
Outcome of rhythm control
At 1-year follow-up, 168 of 245 (68.6%) patients were in sinus rhythm for 6/7th of the time during 7-day Holter monitoring. Figure 1A shows the success percentages in different groups based on the result of baseline ECV. All seven patients who had spontaneous restoration of sinus rhythm at baseline were in sinus rhythm at 1-year. A total of 13 out of 27 (48%) patients with unsuccessful baseline ECV were in sinus rhythm at 1-year, compared with 148 out of 210 (70.5%) patients who had a successful ECV at baseline.
In total, 55 out of 245 patients (22.4%) maintained sinus rhythm throughout 1-year follow-up, i.e. had no documented AF episode during follow-up without any additional conventional rhythm control therapy. The other 190 (77.6%) patients had AF recurrences; 108 (56.8%) received class I or III AADs. Of those 108 patients 44 (40.7%) maintained sinus rhythm after starting the first AAD (Figure 1B). Among individuals who started with AADs, amiodarone was associated with significantly higher sinus rhythm maintenance than other AADs (58% for amiodarone vs. 32% for flecainide and 23% for sotalol, Figure 3A). There was no significant difference in rhythm control outcome between the targeted and conventional group (44% for targeted vs. 37% for conventional group), nor between patients with HFpEF and HFrEF (36% for HFpEF vs. 50% for HFrEF, Figure 3B and C). In patients treated with AADs, we could not detect parameters associated with sinus rhythm at 1-year of follow-up (Table 2).
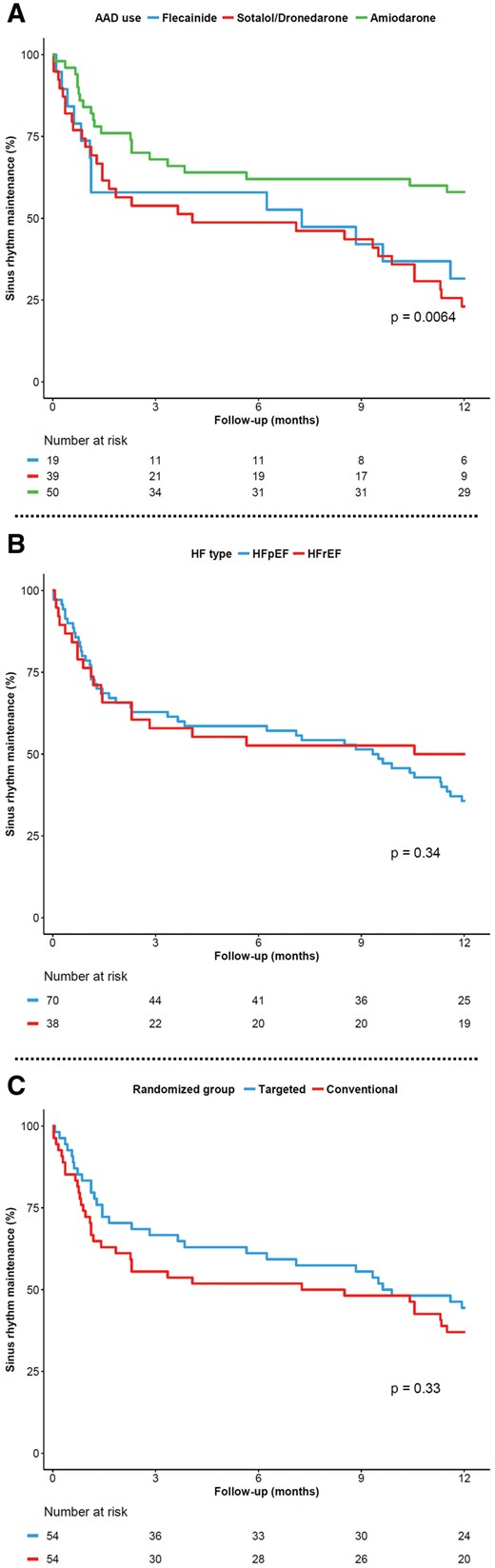
Kaplan–Meier curve showing AF-free survival in the study populationa. (A) For individual antiarrhythmic drugs used during follow-up, (B) based on type of heart failure, and (C) based on randomized group.
Adverse events and discontinuation of antiarrhythmic drugs
During follow-up, adverse events judged as possibly related to AAD treatment have been documented in 27 out of 108 (25.0%, Table 4). All were without any serious or life-threatening consequences and all were reversible after discontinuation of AAD. Overall, AADs were discontinued in 59 patients (54.6%). In 14 patients (13.0%) AADs were discontinued due to adverse events associated with the AAD (Table 4). In 36 patients (33.3%) AADs were discontinued due to AF recurrences, and in 9 patients (8.3%) because of long-term successful maintenance of sinus rhythm (after 109 ± 106 days, Table 4).
Adverse events associated with antiarrhythmic drugs and reasons for discontinuation
| . | Flecainide (n = 22) . | Sotalol >160 mg per day (n = 37) . | Amiodarone (n = 57) . | Dronedarone (n = 3) . |
|---|---|---|---|---|
| Max dose (mg/day) | 173 ± 46 | 258 ± 37 | 547 ± 144 | 800 ± 0 |
| Duration of use (days) | 146 (31–315) | 58 (24–259) | 273 (158–351) | 21 (17–42) |
| Total adverse events | 3 (13.6%)a | 9 (24.3%)a | 14 (24.6%)a | 1 (33.3%)a |
| Cardiovascular complications | 2 (1)b | 8 (7) | – | 1 (1) |
| Fatigue | 2 (1) | 3 (3) | – | – |
| Bradycardia | – | 2 (2) | – | 1 (1) |
| Hypotension + dizziness | – | 2 (2) | – | – |
| Cold extremities | – | 1 (0) | – | – |
| Dermatological complications | – | – | 5 (1) | – |
| Thyroid dysfunction | – | – | 5 (0) | – |
| Subclinical hypothyroidism | – | – | 3 (0) | |
| Hypothyroidism | – | – | 1 (0) | |
| Latent hyperthyroidism | – | – | 1 (0) | |
| Ophthalmic complications | – | – | 3 (2) | – |
| Blurred vision | – | – | 2 (2) | |
| Cornea deposit | – | – | 1 (0) | |
| Neurological problems | – | 1 (1) | – | – |
| Tinnitus | 1 (1) | |||
| Pulmonary complications | – | – | 1 (1) | – |
| Pneumonitis | 1 (1) | |||
| Gastrointestinal complications | 1 (0) | – | – | – |
| Diarrhoea | 1 (0) | |||
| AAD stopped due to AEs | 1 (4.5%) | 8 (21.6%) | 4 (7.0%) | 1 (33.3%) |
| AAD stopped due to AF recurrences | 7 (31.8%) | 16 (43.2%) | 11 (19.3%) | 2 (66.7%) |
| AAD stopped due to long-term sinus rhythm maintenance | 1 (4.5%) | 4 (10.8%) | 4 (7.0%) | 0 (0.0%) |
| AAD continued during follow-up | 13 (59.1%) | 9 (24.3%) | 38 (66.7%) | 0 (0.0%) |
| . | Flecainide (n = 22) . | Sotalol >160 mg per day (n = 37) . | Amiodarone (n = 57) . | Dronedarone (n = 3) . |
|---|---|---|---|---|
| Max dose (mg/day) | 173 ± 46 | 258 ± 37 | 547 ± 144 | 800 ± 0 |
| Duration of use (days) | 146 (31–315) | 58 (24–259) | 273 (158–351) | 21 (17–42) |
| Total adverse events | 3 (13.6%)a | 9 (24.3%)a | 14 (24.6%)a | 1 (33.3%)a |
| Cardiovascular complications | 2 (1)b | 8 (7) | – | 1 (1) |
| Fatigue | 2 (1) | 3 (3) | – | – |
| Bradycardia | – | 2 (2) | – | 1 (1) |
| Hypotension + dizziness | – | 2 (2) | – | – |
| Cold extremities | – | 1 (0) | – | – |
| Dermatological complications | – | – | 5 (1) | – |
| Thyroid dysfunction | – | – | 5 (0) | – |
| Subclinical hypothyroidism | – | – | 3 (0) | |
| Hypothyroidism | – | – | 1 (0) | |
| Latent hyperthyroidism | – | – | 1 (0) | |
| Ophthalmic complications | – | – | 3 (2) | – |
| Blurred vision | – | – | 2 (2) | |
| Cornea deposit | – | – | 1 (0) | |
| Neurological problems | – | 1 (1) | – | – |
| Tinnitus | 1 (1) | |||
| Pulmonary complications | – | – | 1 (1) | – |
| Pneumonitis | 1 (1) | |||
| Gastrointestinal complications | 1 (0) | – | – | – |
| Diarrhoea | 1 (0) | |||
| AAD stopped due to AEs | 1 (4.5%) | 8 (21.6%) | 4 (7.0%) | 1 (33.3%) |
| AAD stopped due to AF recurrences | 7 (31.8%) | 16 (43.2%) | 11 (19.3%) | 2 (66.7%) |
| AAD stopped due to long-term sinus rhythm maintenance | 1 (4.5%) | 4 (10.8%) | 4 (7.0%) | 0 (0.0%) |
| AAD continued during follow-up | 13 (59.1%) | 9 (24.3%) | 38 (66.7%) | 0 (0.0%) |
AAD, antiarrhythmic drug; AE, adverse event; AF, atrial fibrillation.
None was life-threatening or required emergency hospital admission.
Number between brackets represents number of cases that the adverse event led to discontinuation of AAD.
Adverse events associated with antiarrhythmic drugs and reasons for discontinuation
| . | Flecainide (n = 22) . | Sotalol >160 mg per day (n = 37) . | Amiodarone (n = 57) . | Dronedarone (n = 3) . |
|---|---|---|---|---|
| Max dose (mg/day) | 173 ± 46 | 258 ± 37 | 547 ± 144 | 800 ± 0 |
| Duration of use (days) | 146 (31–315) | 58 (24–259) | 273 (158–351) | 21 (17–42) |
| Total adverse events | 3 (13.6%)a | 9 (24.3%)a | 14 (24.6%)a | 1 (33.3%)a |
| Cardiovascular complications | 2 (1)b | 8 (7) | – | 1 (1) |
| Fatigue | 2 (1) | 3 (3) | – | – |
| Bradycardia | – | 2 (2) | – | 1 (1) |
| Hypotension + dizziness | – | 2 (2) | – | – |
| Cold extremities | – | 1 (0) | – | – |
| Dermatological complications | – | – | 5 (1) | – |
| Thyroid dysfunction | – | – | 5 (0) | – |
| Subclinical hypothyroidism | – | – | 3 (0) | |
| Hypothyroidism | – | – | 1 (0) | |
| Latent hyperthyroidism | – | – | 1 (0) | |
| Ophthalmic complications | – | – | 3 (2) | – |
| Blurred vision | – | – | 2 (2) | |
| Cornea deposit | – | – | 1 (0) | |
| Neurological problems | – | 1 (1) | – | – |
| Tinnitus | 1 (1) | |||
| Pulmonary complications | – | – | 1 (1) | – |
| Pneumonitis | 1 (1) | |||
| Gastrointestinal complications | 1 (0) | – | – | – |
| Diarrhoea | 1 (0) | |||
| AAD stopped due to AEs | 1 (4.5%) | 8 (21.6%) | 4 (7.0%) | 1 (33.3%) |
| AAD stopped due to AF recurrences | 7 (31.8%) | 16 (43.2%) | 11 (19.3%) | 2 (66.7%) |
| AAD stopped due to long-term sinus rhythm maintenance | 1 (4.5%) | 4 (10.8%) | 4 (7.0%) | 0 (0.0%) |
| AAD continued during follow-up | 13 (59.1%) | 9 (24.3%) | 38 (66.7%) | 0 (0.0%) |
| . | Flecainide (n = 22) . | Sotalol >160 mg per day (n = 37) . | Amiodarone (n = 57) . | Dronedarone (n = 3) . |
|---|---|---|---|---|
| Max dose (mg/day) | 173 ± 46 | 258 ± 37 | 547 ± 144 | 800 ± 0 |
| Duration of use (days) | 146 (31–315) | 58 (24–259) | 273 (158–351) | 21 (17–42) |
| Total adverse events | 3 (13.6%)a | 9 (24.3%)a | 14 (24.6%)a | 1 (33.3%)a |
| Cardiovascular complications | 2 (1)b | 8 (7) | – | 1 (1) |
| Fatigue | 2 (1) | 3 (3) | – | – |
| Bradycardia | – | 2 (2) | – | 1 (1) |
| Hypotension + dizziness | – | 2 (2) | – | – |
| Cold extremities | – | 1 (0) | – | – |
| Dermatological complications | – | – | 5 (1) | – |
| Thyroid dysfunction | – | – | 5 (0) | – |
| Subclinical hypothyroidism | – | – | 3 (0) | |
| Hypothyroidism | – | – | 1 (0) | |
| Latent hyperthyroidism | – | – | 1 (0) | |
| Ophthalmic complications | – | – | 3 (2) | – |
| Blurred vision | – | – | 2 (2) | |
| Cornea deposit | – | – | 1 (0) | |
| Neurological problems | – | 1 (1) | – | – |
| Tinnitus | 1 (1) | |||
| Pulmonary complications | – | – | 1 (1) | – |
| Pneumonitis | 1 (1) | |||
| Gastrointestinal complications | 1 (0) | – | – | – |
| Diarrhoea | 1 (0) | |||
| AAD stopped due to AEs | 1 (4.5%) | 8 (21.6%) | 4 (7.0%) | 1 (33.3%) |
| AAD stopped due to AF recurrences | 7 (31.8%) | 16 (43.2%) | 11 (19.3%) | 2 (66.7%) |
| AAD stopped due to long-term sinus rhythm maintenance | 1 (4.5%) | 4 (10.8%) | 4 (7.0%) | 0 (0.0%) |
| AAD continued during follow-up | 13 (59.1%) | 9 (24.3%) | 38 (66.7%) | 0 (0.0%) |
AAD, antiarrhythmic drug; AE, adverse event; AF, atrial fibrillation.
None was life-threatening or required emergency hospital admission.
Number between brackets represents number of cases that the adverse event led to discontinuation of AAD.
Discussion
We aimed to explore the need for and success of AAD use in a contemporary cohort of early persistent AF patients with short history of stable, mild to moderate HF included in the RACE 3 study. Our main findings were, first, that only one-quarter of the patients maintained sinus rhythm without additional ECVs and AADs during 1-year follow-up; secondly, AADs were instituted in ∼50% of the patients because of recurrent symptomatic AF; thirdly, in those patients with AF recurrences who were treated with AADs, 41% maintained sinus rhythm. Finally, adverse events occurred regularly, however, none of them was serious and all were reversible after discontinuation of AAD.
In our population of patients with early persistent AF and short-lasting moderate HF, AF recurrences occurred often, which is in agreement with previous studies.9 However, we observed a high recurrence rate despite the fact that our patients had a short history of persistent AF,10–12 and despite the fact that cardiovascular risk factors and comorbidities were treated in at least half of our patients (the targeted group).4 More than 50% underwent a repeat ECV, and AADs were instituted in over 40% of patients. Sinus rhythm was maintained in 40% of those who started AADs. As expected in patients with underlying risk factors and moderate HF, amiodarone was the most common AAD used, mainly due to its cardiovascular safety.13 Patients who received flecainide had a significantly lower CHA2DS2-VASc score and higher LV ejection fraction. In line with earlier studies, amiodarone showed a significantly higher success than the other AADs.10,14–16 We observed no parameters associated with successful AAD treatment. Additionally, we detected no significant difference in outcome of rhythm control between patients in the targeted and conventional group. The number of patients in these groups, however, was too small to draw conclusions. Similarly, there was no significant difference in the outcome of rhythm control between HFpEF and HFrEF patients. Again, number of patients, especially in the HFrEF group, was small and AAD use was not similar between groups, as most patients with HFrEF received amiodarone.
Adverse events related to AADs in our study were most often minor or moderate, but led to discontinuation in 13% of patients; 5% for flecainide, 22% for sotalol, 7% for amiodarone, and 33% for dronedarone. These results, particularly with respect to sotalol and amiodarone, seem to be in line with a previous review that reported 18% and 5% discontinuation rates due to adverse events for sotalol and amiodarone, respectively.12 Other individual studies, however, reported somewhat different results. In the AFFIRM study, discontinuation of AADs was reported in 21% of patients due to adverse events; 39% for flecainide, 16% for sotalol, and 13% for amiodarone.14 The CTAF trial reported 18% discontinuation rate due to adverse events for amiodarone, and 11% for sotalol or propafenone,15 while Bellandi et al.17 reported 9% and 10% discontinuation rate due to adverse events for propafenone and sotalol, respectively. We observed no ventricular proarrhythmia during follow-up. Proarrhythmia due to sotalol, nevertheless, may occur, even in the absence of structural heart disease. The new guidelines therefore recommend close monitoring of QT interval, serum potassium levels, renal function, and other proarrhythmia risk factors.3,18 Three patients suffered from bradycardia, two (5.4%) during sotalol and one during dronedarone. These results are difficult to compare with existing literature due to relatively small sample size and low percentages but are in line with most other studies.15,18
The majority of our patients continued the AAD to enhance long-term success. Ahmed et al.19 showed that continuous amiodarone therapy led to significantly lower AF recurrence rate and lower rate of all-cause mortality and cardiovascular hospitalizations compared with episodic, short-term, treatment with amiodarone. Additionally, Kirchhof et al.20 demonstrated that long-term flecainide treatment is more effective in preventing recurrence of persistent AF within one month after cardioversion, compared with short-term treatment.
Clinical implications
This post hoc analysis of RACE 3 shows that AADs still have a place in the treatment of persistent AF in patients with moderate HF.
Limitations and strengths
The number of patients in the study is relatively small, which affected the statistical power in some analyses and the ability to draw definitive conclusions. Furthermore, the follow-up duration was only 1 year. In addition, this is a post hoc analysis and AAD use was essentially left to discretion of treating cardiologist, despite general recommendations in study protocol about prescription of AADs. In some cases, this led to a judicious deviation from general treatment recommendations by the attending physician. We included both HFpEF and HFrEF patients which may lead to conclusion bias. Finally, the results of this trial only apply to patients with persistent AF and mild to moderate HF, and further conclusions cannot be drawn about a wider AF population. Strengths of the study include a well-defined group of patients with early AF.
Conclusion
In stable HF patients with early persistent AF, ECV and AAD treatment as needed (mainly amiodarone) is effective in less than half of patients, and mainly limited by reversible and non-serious adverse effects. Therefore, when carefully instituted, AADs are still a viable and relatively safe option as a rhythm-control strategy in the management of patients with persistent AF and moderate HF.
Funding
The study was supported by the Netherlands Heart Foundation (grant 2008B035). Unrestricted grants from AstraZeneca, Bayer, Biotronik, Boehringer-Ingelheim, Boston Scientific, Medtronic, Sanofi-Aventis, St Jude Medical paid to the Netherlands Heart Institute.
Conflict of interest: A.M.A. reports personal fees from Bayer, Boehringer-Ingelheim, Daiichi-Sankyo, Bristol-Myers-Squibb, Pfizer, and Sanofi. R.G.T. reports grants and personal fees from Bayer, Bristol-Myers-Squibb, Pfizer, and Daiichi-Sankyo. All other authors have no competing interests.
Data availability
All analyses done in this article were based on the data collected in the RACE 3 trial (registration number: Clinicaltrials.gov NCT00877643). The data underlying this article will be shared on reasonable request to the corresponding author.
References
AFFIRM First Antiarrhythmic Drug Substudy Investigators.



