-
PDF
- Split View
-
Views
-
Cite
Cite
Martin E.W. Hemels, Ans C.P. Wiesfeld, Dirk J. Van Veldhuisen, Maarten P. Van den Berg, Isabelle C. Van Gelder, Outcome of pharmacological rhythm control for new-onset persistent atrial fibrillation in patients with systolic heart failure: a comparison with patients with normal left ventricular function, EP Europace, Volume 9, Issue 4, April 2007, Pages 239–245, https://doi.org/10.1093/europace/eum011
Close - Share Icon Share
Abstract
Aims To compare outcome of a serial cardioversion strategy in atrial fibrillation (AF) patients with and without systolic heart failure (HF).
Methods and results In patients with new-onset persistent AF and systolic HF [left ventricular ejection fraction (LVEF) <0.40] outcome of a serial electrical cardioversion (ECV) and serial antiarrhythmic drug strategy was compared with a control group of patients without HF. Follow-up was 18 months. Sixty-four consecutive patients with systolic HF (mean age 64 ± 12 years, 50% coronary artery disease, LVEF 0.30 ± 0.07) were enrolled and compared with 48 consecutive patients without HF (mean age 66 ± 8 years, all LVEF >0.50, 40% lone AF). Success of ECV and occurrence of subacute and late recurrences in patients with and without HF were comparable. After the first relapse, AF was accepted in significantly more HF patients (23 vs. 4%, P < 0.01). Significantly less HF patients underwent serial ECV and antiarrhythmic drug approach (42 vs. 71%, respectively, P < 0.001). At the end of follow-up more HF patients were in permanent AF (45 vs. 29%, P = 0.03).
Conclusion Recurrence pattern after ECV is comparable between patients with and without systolic HF, but outcome of a serial cardioversion strategy is worse in HF patients, possibly related to a less stringent use of this approach.
Introduction
Atrial fibrillation (AF) and heart failure (HF) are very common and their incidence is steadily increasing.1,2 Both disorders profoundly increase cardiovascular morbidity and limit quality of life, and are often present in the same patient. In patients with chronic HF (CHF), there is an increased risk of AF development.2,3 With restoration and maintenance of sinus rhythm, left ventricular (LV) function, exercise capacity, and maximal oxygen consumption may improve.4,5 There is no consensus as to whether AF is an independent risk factor for morbidity and mortality in CHF,6,7 or just a marker of more advanced disease.8 Recent trials demonstrated that rate control may be adopted as first choice therapy in relatively asymptomatic patients.9–12 Whether this also holds for CHF patients is at present unknown.13 Drawbacks of pharmacological rhythm control, however, are the low-success rate and the risk of adverse effects including life-threatening proarrhythmia, especially in patients with impaired LV function.14–16 Outcome of a serial cardioversion approach is adverse in patients with CHF, but the exact cause is unknown. It may be due to more severe atrial structural remodelling and a lower efficacy and safety of antiarrhythmic drugs.2,15,17 In this study, we investigated the outcome of a serial cardioversion strategy for new-onset persistent AF in patients with and without systolic HF who were all treated according to our serial cardioversion strategy.11,18
Methods
Patient population
We included consecutive patients with and without systolic HF and a first episode of persistent AF referred for electrical cardioversion (ECV). All patients were prospectively followed at our AF outpatient clinic (from January 2002 to January 2004). Before ECV, all patients underwent echocardiography and exercise testing to evaluate underlying heart disease, and 24-h Holter monitoring to establish the persistence of AF.19 In order to prove LV systolic dysfunction in patients with HF, LV ejection fraction (LVEF) measurements were repeated 1 month after ECV, either in sinus rhythm or in (reappeared) AF after institution of adequate rate control drugs. Patients with HF were included if they had symptoms of HF [New York Heart Association (NYHA) class II-III] for >3 months and a LVEF <0.40. Left ventricular ejection fraction was assessed using radionuclide scanning or transthoracic echocardiography. We excluded patients with NYHA class IV HF, current or previous treatment with amiodarone, or a pacemaker. The outcome of ECV and the recurrence pattern after cardioversion were compared with a control group of consecutive patients with symptomatic persistent AF without systolic HF (LVEF >0.50 prior to ECV).
Study design
Our ECV protocol has been described before.11 In short, ECV was performed during light general anaesthesia by using 20 mg of etomidate intravenously. A calibrated mono- or bi-phasic defibrillator, which could store 360 or 200 J of energy, respectively, was used as cardioverter device. We started with 100 or 50 J of stored energy, respectively. Thereafter, energy load of successive shocks was doubled until sinus rhythm was restored or after two attempts at the highest level. All shocks were applied to the chest in an anterior-lateral paddle configuration. Immediate outcome of the shock (no sinus rhythm, immediate reinitiation of AF, sinus rhythm) was monitored by continuous 12-lead ECG for 5 min. Post-shock rhythm monitoring was secured by telemetry for 4 h. If after the first ECV procedure, sinus rhythm was restored during the in-hospital phase (for at least 4 h), patients did not receive an antiarrhythmic drug.
In case of shock failure, immediate reinitiation of AF (IRAF, defined as a relapse of AF <2 min after ECV), or recurrence of AF in the first 4 h after successful cardioversion a loading dose of amiodarone was given (600 mg daily for 4 weeks), thereafter ECV was repeated. The dose of amiodarone was then lowered to 200 mg daily. Time of recurrences during the first month were monitored by instructing the patients carefully to check their own pulse and/ or complaints on a daily basis and was confirmed by a 12-lead electrocardiogram.
In case of recurrence of AF within 6 months, ECV was repeated and patients received sotalol (160–320 mg daily, depending on body weight and renal function) afterwards, except when there was a contraindication for class III antiarrhythmic drugs (severe bradycardia, unmasked sick sinus syndrome, pre-existent long QT). If there was another recurrence within 6 months or contraindications to sotalol, amiodarone was instituted (aforementioned). Subsequently, in case of no chemical conversion, repeat ECV was performed and thereafter amiodarone was continued orally with a dose of 200 mg daily. In case of recurrences occurring >6 months after restoration of sinus rhythm, the drug eventually instituted was continued. In order to compare rhythm control in patients with and without HF, and since class IC drugs are contraindicated in HF patients, for the purpose of this study no class IC drugs were instituted in any patient included in the present study.
The primary endpoint was permanent AF, which was defined as a relapse of AF despite adequate amiodarone treatment (sum of plasma levels of amiodarone and desamiodarone >2.0 mg/L), if patients had a relapse of AF and antiarrhythmic drug-related adverse effects, contraindications to antiarrhythmic drugs, or if patients refused another ECV. Patients visited the outpatient department 1, 3, 6, 12, and 18 months after cardioversion. The follow-up was 18 months in all patients. At each visit, physical examination and a 12-lead electrocardiogram were performed.
From 4 weeks before until 4 weeks after ECV, all patients received acenocoumarol or fenprocoumon (International Normalized Ratio 2.5–3.5). Oral anticoagulation could be stopped or changed to aspirin 80–100 mg daily, if no new attempt at ECV was scheduled and patients had no risk factors for thromboembolic complications.
Statistical analysis
Baseline descriptive statistics are given as the mean ± SD or median (range) for continuous variables and counts with percentages for categorical variables. Differences between groups were evaluated by Students t-test or Mann–Whitney U test, depending on normality of the data, for continuous data and by Fisher's exact test or χ2 test for categorical data. For all time-to-event analyses, Kaplan–Meier estimates were used and were compared by the log-rank test. Univariate and multivariate Cox regression analyses were performed to determine predictors of permanent AF. The univariate analysed variables included gender, age, AF duration, hypertension, coronary artery disease, valvular heart disease, diabetes, NYHA class for HF, respiratory disease, body mass index, blood pressure, heart rate, echocardiographic atrial and ventricular dimensions, HF (impaired systolic LV function), and medication use. All univariate variables with P < 0.10 were added to the multivariate model. In multivariate models interaction was investigated. In all analyses, a value of P < 0.05 was considered statistically significant. Analyses were performed using the statistical package SPSS 11.0 (SPSS Inc, Chicago, IL, USA).
Results
Patient characteristics
A total of 112 consecutive patients, 64 with and 48 without HF were included. The baseline characteristics of these patient groups are listed in Table 1. Heart failure patients had a longer duration of persistent AF (median of 4 vs. 3 months, respectively, P = 0.02). Both atrial and ventricular diameters were larger in the HF group. Angiotensin converting enzyme inhibitors, angiotensin receptor blockers, digoxin, and diuretics were more often used in the HF patients, whereas non-HF patients were taking more often calcium channel blockers (verapamil or diltiazem) (all P < 0.05).
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Age, years | 64 ± 12 | 66 ± 8 | NS |
| Male sex, number (percentage of patients) | 46 (72) | 29 (60) | NS |
| Duration persistent AF (monthsa) | 4 (2–7) | 3 (1–5) | 0.02 |
| Heart rate pre-ECV | 92 ± 20 | 86 ± 23 | NS |
| Coronary artery disease, percentage of patients | 50 | 13 | <0.001 |
| Old myocardial infarction, percentage of patients | 33 | 8 | 0.002 |
| Coronary intervention (PTCA or CABG), percentage of patients | 20 | 6 | 0.04 |
| Valve disease, percentage of patients | |||
| Aortic | 4 | 3 | |
| Mitral | 30 | 15 | |
| Aortic and mitral | 3 | 4 | NS |
| History of hypertension, percentage of patients | 42 | 40 | NS |
| History of COPD, percentage of patients | 11 | 4 | NS |
| History of diabetes mellitus, percentage of patients | 14 | 8 | NS |
| No apparent heart disease, percentage of patients | — | 40 | <0.001 |
| History of thyreotoxicosis, percentage of patients | 3 | 2 | NS |
| NYHA for HF, percentage of patients | |||
| I | 0 | 100 | |
| II | 83 | 0 | |
| III | 17 | 0 | <0.001 |
| Body mass index | 26 ± 4 | 29 ± 6 | 0.002 |
| Blood pressure (mm Hg) | |||
| Systolic | 132 ± 23 | 145 ± 23 | 0.004 |
| Diastolic | 79 ± 12 | 88 ± 17 | 0.001 |
| Echocardiographic findings (mm) | |||
| Left atrium parasternal, long axis | 51 ± 7 | 47 ± 5 | <0.002 |
| Left atrium apical view, length | 72 ± 7 | 63 ± 8 | <0.001 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.003 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.03 |
| Left ventricular end-diastolic diameter | 60 ± 7 | 52 ± 5 | <0.001 |
| Left ventricular end-systolic diameter | 50 ± 8 | 36 ± 7 | <0.001 |
| Septal thickness | 10 ± 2 | 10 ± 2 | NS |
| Posterior-wall thickness | 10 ± 2 | 10 ± 2 | NS |
| Fractional shortening | 17 ± 7 | 31 ± 8 | <0.001 |
| LVEF | 0.30 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| Medication use (percentage of patients) | |||
| Acenocoumarol | 100 | 100 | NS |
| Betablockers | 39 | 53 | NS |
| Verapamil/Diltiazem | 23 | 48 | 0.007 |
| Digoxin | 73 | 52 | 0.02 |
| ACE-I/ARBs | 66 | 29 | 0.0001 |
| Diuretics | 63 | 35 | 0.005 |
| Statins | 19 | 10 | NS |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Age, years | 64 ± 12 | 66 ± 8 | NS |
| Male sex, number (percentage of patients) | 46 (72) | 29 (60) | NS |
| Duration persistent AF (monthsa) | 4 (2–7) | 3 (1–5) | 0.02 |
| Heart rate pre-ECV | 92 ± 20 | 86 ± 23 | NS |
| Coronary artery disease, percentage of patients | 50 | 13 | <0.001 |
| Old myocardial infarction, percentage of patients | 33 | 8 | 0.002 |
| Coronary intervention (PTCA or CABG), percentage of patients | 20 | 6 | 0.04 |
| Valve disease, percentage of patients | |||
| Aortic | 4 | 3 | |
| Mitral | 30 | 15 | |
| Aortic and mitral | 3 | 4 | NS |
| History of hypertension, percentage of patients | 42 | 40 | NS |
| History of COPD, percentage of patients | 11 | 4 | NS |
| History of diabetes mellitus, percentage of patients | 14 | 8 | NS |
| No apparent heart disease, percentage of patients | — | 40 | <0.001 |
| History of thyreotoxicosis, percentage of patients | 3 | 2 | NS |
| NYHA for HF, percentage of patients | |||
| I | 0 | 100 | |
| II | 83 | 0 | |
| III | 17 | 0 | <0.001 |
| Body mass index | 26 ± 4 | 29 ± 6 | 0.002 |
| Blood pressure (mm Hg) | |||
| Systolic | 132 ± 23 | 145 ± 23 | 0.004 |
| Diastolic | 79 ± 12 | 88 ± 17 | 0.001 |
| Echocardiographic findings (mm) | |||
| Left atrium parasternal, long axis | 51 ± 7 | 47 ± 5 | <0.002 |
| Left atrium apical view, length | 72 ± 7 | 63 ± 8 | <0.001 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.003 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.03 |
| Left ventricular end-diastolic diameter | 60 ± 7 | 52 ± 5 | <0.001 |
| Left ventricular end-systolic diameter | 50 ± 8 | 36 ± 7 | <0.001 |
| Septal thickness | 10 ± 2 | 10 ± 2 | NS |
| Posterior-wall thickness | 10 ± 2 | 10 ± 2 | NS |
| Fractional shortening | 17 ± 7 | 31 ± 8 | <0.001 |
| LVEF | 0.30 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| Medication use (percentage of patients) | |||
| Acenocoumarol | 100 | 100 | NS |
| Betablockers | 39 | 53 | NS |
| Verapamil/Diltiazem | 23 | 48 | 0.007 |
| Digoxin | 73 | 52 | 0.02 |
| ACE-I/ARBs | 66 | 29 | 0.0001 |
| Diuretics | 63 | 35 | 0.005 |
| Statins | 19 | 10 | NS |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive lung disease; PTCA, percutaneous transluminal coronary angioplasty.
aMedian (interquartile range).
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Age, years | 64 ± 12 | 66 ± 8 | NS |
| Male sex, number (percentage of patients) | 46 (72) | 29 (60) | NS |
| Duration persistent AF (monthsa) | 4 (2–7) | 3 (1–5) | 0.02 |
| Heart rate pre-ECV | 92 ± 20 | 86 ± 23 | NS |
| Coronary artery disease, percentage of patients | 50 | 13 | <0.001 |
| Old myocardial infarction, percentage of patients | 33 | 8 | 0.002 |
| Coronary intervention (PTCA or CABG), percentage of patients | 20 | 6 | 0.04 |
| Valve disease, percentage of patients | |||
| Aortic | 4 | 3 | |
| Mitral | 30 | 15 | |
| Aortic and mitral | 3 | 4 | NS |
| History of hypertension, percentage of patients | 42 | 40 | NS |
| History of COPD, percentage of patients | 11 | 4 | NS |
| History of diabetes mellitus, percentage of patients | 14 | 8 | NS |
| No apparent heart disease, percentage of patients | — | 40 | <0.001 |
| History of thyreotoxicosis, percentage of patients | 3 | 2 | NS |
| NYHA for HF, percentage of patients | |||
| I | 0 | 100 | |
| II | 83 | 0 | |
| III | 17 | 0 | <0.001 |
| Body mass index | 26 ± 4 | 29 ± 6 | 0.002 |
| Blood pressure (mm Hg) | |||
| Systolic | 132 ± 23 | 145 ± 23 | 0.004 |
| Diastolic | 79 ± 12 | 88 ± 17 | 0.001 |
| Echocardiographic findings (mm) | |||
| Left atrium parasternal, long axis | 51 ± 7 | 47 ± 5 | <0.002 |
| Left atrium apical view, length | 72 ± 7 | 63 ± 8 | <0.001 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.003 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.03 |
| Left ventricular end-diastolic diameter | 60 ± 7 | 52 ± 5 | <0.001 |
| Left ventricular end-systolic diameter | 50 ± 8 | 36 ± 7 | <0.001 |
| Septal thickness | 10 ± 2 | 10 ± 2 | NS |
| Posterior-wall thickness | 10 ± 2 | 10 ± 2 | NS |
| Fractional shortening | 17 ± 7 | 31 ± 8 | <0.001 |
| LVEF | 0.30 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| Medication use (percentage of patients) | |||
| Acenocoumarol | 100 | 100 | NS |
| Betablockers | 39 | 53 | NS |
| Verapamil/Diltiazem | 23 | 48 | 0.007 |
| Digoxin | 73 | 52 | 0.02 |
| ACE-I/ARBs | 66 | 29 | 0.0001 |
| Diuretics | 63 | 35 | 0.005 |
| Statins | 19 | 10 | NS |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Age, years | 64 ± 12 | 66 ± 8 | NS |
| Male sex, number (percentage of patients) | 46 (72) | 29 (60) | NS |
| Duration persistent AF (monthsa) | 4 (2–7) | 3 (1–5) | 0.02 |
| Heart rate pre-ECV | 92 ± 20 | 86 ± 23 | NS |
| Coronary artery disease, percentage of patients | 50 | 13 | <0.001 |
| Old myocardial infarction, percentage of patients | 33 | 8 | 0.002 |
| Coronary intervention (PTCA or CABG), percentage of patients | 20 | 6 | 0.04 |
| Valve disease, percentage of patients | |||
| Aortic | 4 | 3 | |
| Mitral | 30 | 15 | |
| Aortic and mitral | 3 | 4 | NS |
| History of hypertension, percentage of patients | 42 | 40 | NS |
| History of COPD, percentage of patients | 11 | 4 | NS |
| History of diabetes mellitus, percentage of patients | 14 | 8 | NS |
| No apparent heart disease, percentage of patients | — | 40 | <0.001 |
| History of thyreotoxicosis, percentage of patients | 3 | 2 | NS |
| NYHA for HF, percentage of patients | |||
| I | 0 | 100 | |
| II | 83 | 0 | |
| III | 17 | 0 | <0.001 |
| Body mass index | 26 ± 4 | 29 ± 6 | 0.002 |
| Blood pressure (mm Hg) | |||
| Systolic | 132 ± 23 | 145 ± 23 | 0.004 |
| Diastolic | 79 ± 12 | 88 ± 17 | 0.001 |
| Echocardiographic findings (mm) | |||
| Left atrium parasternal, long axis | 51 ± 7 | 47 ± 5 | <0.002 |
| Left atrium apical view, length | 72 ± 7 | 63 ± 8 | <0.001 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.003 |
| Right atrium apical view, length | 63 ± 7 | 60 ± 7 | 0.03 |
| Left ventricular end-diastolic diameter | 60 ± 7 | 52 ± 5 | <0.001 |
| Left ventricular end-systolic diameter | 50 ± 8 | 36 ± 7 | <0.001 |
| Septal thickness | 10 ± 2 | 10 ± 2 | NS |
| Posterior-wall thickness | 10 ± 2 | 10 ± 2 | NS |
| Fractional shortening | 17 ± 7 | 31 ± 8 | <0.001 |
| LVEF | 0.30 ± 0.07 | 0.54 ± 0.07 | <0.001 |
| Medication use (percentage of patients) | |||
| Acenocoumarol | 100 | 100 | NS |
| Betablockers | 39 | 53 | NS |
| Verapamil/Diltiazem | 23 | 48 | 0.007 |
| Digoxin | 73 | 52 | 0.02 |
| ACE-I/ARBs | 66 | 29 | 0.0001 |
| Diuretics | 63 | 35 | 0.005 |
| Statins | 19 | 10 | NS |
ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive lung disease; PTCA, percutaneous transluminal coronary angioplasty.
aMedian (interquartile range).
Cardioversion outcome, subacute, and late recurrences
Electrical cardioversion was successful in 86% (n = 55) and 87% (n = 42) of HF and non-HF patients, respectively (P = NS). Cardioversion was unsuccessful because of shock failure in 6 (9%) and because of IRAF in 3 (5%) of the HF patients vs. 3 (6%) of non-HF patients, respectively (P = NS). One month after ECV 44 (n = 28) vs. 46% (n = 22) of the HF vs. non-HF patients had a subacute recurrence (P = NS, Figure 1). Between 1 and 18 months of follow-up 28 (n = 18) vs. 21% (n = 10) of the HF vs. non-HF patients had their first (late) recurrence of AF (P = NS, Figure 1). In the HF group, late recurrence of AF coincided with an exacerbation of HF in two patients (3%). In the other patients, no relation with a trigger was observed. Fourteen percent of HF (n = 9) vs. 21% (n = 10) of non-HF patients had no recurrence of AF during 18 months of follow-up (P = NS). In the HF patients, LVEF remained significantly impaired 2 (1–3) months after ECV (0.34 ± 0.07), irrespective of the rhythm at that moment (0.33 ± 0.08 in sinus rhythm patients vs. 0.34 ± 0.09 in patients with relapsed AF).
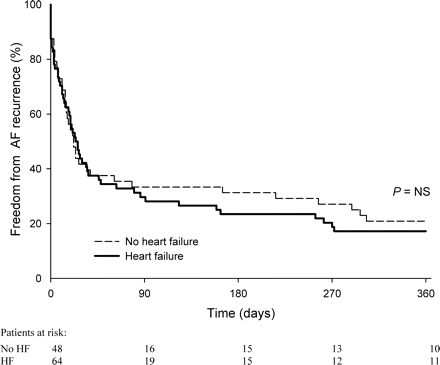
Freedom from any AF recurrences in HF vs. non-HF (no HF) patients.
Rhythm control and acceptance of AF
After 18 months of follow-up, AF was significantly more often accepted in the HF patients [45 (n = 29) vs. 29% (n = 14), respectively, P = 0.03, Figure 2]. In 15 (23%) vs. 2 (4%) of the HF vs. non-HF patients, AF was accepted after the first relapse of AF (P < 0.01, Table 2). Reasons for AF acceptance in the HF group were not severely symptomatic recurrence (47%, n = 7), patient refusal in (20%, n = 3), non-cardiac comorbidity (20%; cancer in two patients and dementia in one patient), and contraindication to antiarrhythmic drug treatment [13%; AV nodal conduction disturbances (n = 1) and sick sinus syndrome (n = 1)]. In the two patients of the non-HF group, AF was accepted after the first recurrence because of non-cardiac comorbidity. More ECVs were performed in the non-HF patients [median of 1 (0–4) vs. 0 (0–3), respectively, P = 0.02). Rhythm control with serial ECVs and serial institution of antiarrhythmic drugs was less often performed in HF patients [42 (n = 27) vs. 71% (n = 34), P < 0.001, respectively, Table 3]. Plasma levels of amiodarone and desethylamiodarone were comparable in the two groups, 1.3 ± 0.5 vs. 1.2 ± 0.5 mg/L for amiodarone and 0.9 ± 0.3 and 0.8 ± 0.3 mg/L for desethylamiodarone in the HF vs. non-HF patients respectively, P = NS. In the patients undergoing a ‘true’ serial ECV and antiarrhythmic, drug strategy outcome was comparable. Atrial fibrillation was accepted in 22 (6 of 27 patients) vs. 35% (12 of 34 patients) of the HF vs. non-HF patients (P = 0.45, Figure 3). Multivariate analysis did not show any parameter related to outcome, not even mitral valve disease which occurred more often in the HF group (Table 4).
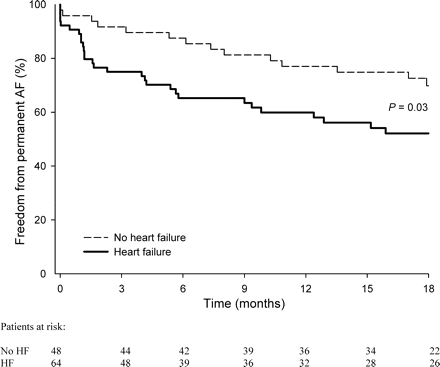
Freedom from permanent AF in patients with vs. patients without HF.
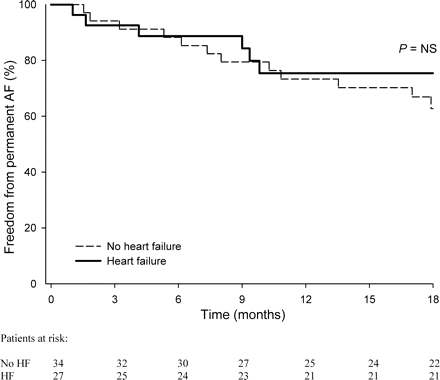
Freedom from permanent AF in patients with or without HF treated with serial ECV and antiarrhythmic drugs.
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| No recurrence, number (percentage of patients) | 9 (14) | 10 (21) | NS |
| Acceptance of AF after first relapse, number (percentage of patients) | 15 (23) | 2 (4) | 0.005 |
| Re-ECV, no AAD, number (percentage of patients) | 13 (20) | 2 (4) | 0.013 |
| Re-ECV and serial AAD, number (percentage of patients) | 27 (42) | 34 (71) | 0.003 |
| Total number of re-ECVs, median (range) | 0 (0–3) | 1 (0–4) | 0.033 |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| No recurrence, number (percentage of patients) | 9 (14) | 10 (21) | NS |
| Acceptance of AF after first relapse, number (percentage of patients) | 15 (23) | 2 (4) | 0.005 |
| Re-ECV, no AAD, number (percentage of patients) | 13 (20) | 2 (4) | 0.013 |
| Re-ECV and serial AAD, number (percentage of patients) | 27 (42) | 34 (71) | 0.003 |
| Total number of re-ECVs, median (range) | 0 (0–3) | 1 (0–4) | 0.033 |
AAD, antiarrhythmic drugs.
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| No recurrence, number (percentage of patients) | 9 (14) | 10 (21) | NS |
| Acceptance of AF after first relapse, number (percentage of patients) | 15 (23) | 2 (4) | 0.005 |
| Re-ECV, no AAD, number (percentage of patients) | 13 (20) | 2 (4) | 0.013 |
| Re-ECV and serial AAD, number (percentage of patients) | 27 (42) | 34 (71) | 0.003 |
| Total number of re-ECVs, median (range) | 0 (0–3) | 1 (0–4) | 0.033 |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| No recurrence, number (percentage of patients) | 9 (14) | 10 (21) | NS |
| Acceptance of AF after first relapse, number (percentage of patients) | 15 (23) | 2 (4) | 0.005 |
| Re-ECV, no AAD, number (percentage of patients) | 13 (20) | 2 (4) | 0.013 |
| Re-ECV and serial AAD, number (percentage of patients) | 27 (42) | 34 (71) | 0.003 |
| Total number of re-ECVs, median (range) | 0 (0–3) | 1 (0–4) | 0.033 |
AAD, antiarrhythmic drugs.
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Only sotalol treatment, number (percentage of patients) | 7 (11) | 12 (25) | 0.05 |
| Only amiodarone treatment, number (percentage of patients) | 18 (28) | 8 (17) | 0.16 |
| Amiodarone after sotalol treatment, number (percentage of patients) | 2 (3) | 14 (29) | <0.001 |
| Number of re-ECVs under AAD, median (range) | 1 (0–3) | 1 (0–3) | NS |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Only sotalol treatment, number (percentage of patients) | 7 (11) | 12 (25) | 0.05 |
| Only amiodarone treatment, number (percentage of patients) | 18 (28) | 8 (17) | 0.16 |
| Amiodarone after sotalol treatment, number (percentage of patients) | 2 (3) | 14 (29) | <0.001 |
| Number of re-ECVs under AAD, median (range) | 1 (0–3) | 1 (0–3) | NS |
AAD, antiarrhythmic drug.
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Only sotalol treatment, number (percentage of patients) | 7 (11) | 12 (25) | 0.05 |
| Only amiodarone treatment, number (percentage of patients) | 18 (28) | 8 (17) | 0.16 |
| Amiodarone after sotalol treatment, number (percentage of patients) | 2 (3) | 14 (29) | <0.001 |
| Number of re-ECVs under AAD, median (range) | 1 (0–3) | 1 (0–3) | NS |
| . | HF (n = 64) . | No HF (n = 48) . | P value . |
|---|---|---|---|
| Only sotalol treatment, number (percentage of patients) | 7 (11) | 12 (25) | 0.05 |
| Only amiodarone treatment, number (percentage of patients) | 18 (28) | 8 (17) | 0.16 |
| Amiodarone after sotalol treatment, number (percentage of patients) | 2 (3) | 14 (29) | <0.001 |
| Number of re-ECVs under AAD, median (range) | 1 (0–3) | 1 (0–3) | NS |
AAD, antiarrhythmic drug.
Predictors of occurrence of permanent AF in the total study population (n = 112)
| . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| HF | 2.0 | 1.0–3.7 | 0.038 | 1.9 | 1.0–3.7 | 0.067 |
| Diabetes | 2.0 | 0.9–4.3 | 0.081 | 1.8 | 0.8–3.9 | 0.15 |
| Diuretic use | 1.7 | 0.9–3.1 | 0.092 | 1.4 | 0.7–2.6 | 0.35 |
| Mitral valve disease | 0.9 | 0.5–1.8 | 0.79 | 0.8 | 0.4–1.6 | 0.49 |
| . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| HF | 2.0 | 1.0–3.7 | 0.038 | 1.9 | 1.0–3.7 | 0.067 |
| Diabetes | 2.0 | 0.9–4.3 | 0.081 | 1.8 | 0.8–3.9 | 0.15 |
| Diuretic use | 1.7 | 0.9–3.1 | 0.092 | 1.4 | 0.7–2.6 | 0.35 |
| Mitral valve disease | 0.9 | 0.5–1.8 | 0.79 | 0.8 | 0.4–1.6 | 0.49 |
CI, confidence interval; HR, hazard ratio. No interaction term was found statistically significant.
Predictors of occurrence of permanent AF in the total study population (n = 112)
| . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| HF | 2.0 | 1.0–3.7 | 0.038 | 1.9 | 1.0–3.7 | 0.067 |
| Diabetes | 2.0 | 0.9–4.3 | 0.081 | 1.8 | 0.8–3.9 | 0.15 |
| Diuretic use | 1.7 | 0.9–3.1 | 0.092 | 1.4 | 0.7–2.6 | 0.35 |
| Mitral valve disease | 0.9 | 0.5–1.8 | 0.79 | 0.8 | 0.4–1.6 | 0.49 |
| . | Univariate model . | Multivariate model . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P . | HR . | 95% CI . | P . |
| HF | 2.0 | 1.0–3.7 | 0.038 | 1.9 | 1.0–3.7 | 0.067 |
| Diabetes | 2.0 | 0.9–4.3 | 0.081 | 1.8 | 0.8–3.9 | 0.15 |
| Diuretic use | 1.7 | 0.9–3.1 | 0.092 | 1.4 | 0.7–2.6 | 0.35 |
| Mitral valve disease | 0.9 | 0.5–1.8 | 0.79 | 0.8 | 0.4–1.6 | 0.49 |
CI, confidence interval; HR, hazard ratio. No interaction term was found statistically significant.
Adverse effects
Adverse effects of sotalol (excessive QT duration prolongation) led to discontinuation in one patient in each group. Adverse effects of amiodarone led to discontinuation in six patients (9%) in the HF group [torsades de pointes (n = 1), skin rash (n = 1), thyrotoxicosis (n = 3), and elevated liver enzymes more than three times upper level of normal (n = 1)], and in six patients (13%) in the non-HF patients [skin rash (n = 2), prolongation of QT duration (n = 1), gastrointestinal problems (n = 1), thyrotoxicosis (n = 1), and blurred vision (n = 1)], P = NS. No stroke or major bleeding occurred during follow-up.
Discussion
Major findings
The present study shows that in patients with systolic HF and new-onset persistent AF outcome of a serial cardioversion strategy is inferior compared with patients without systolic HF. In part, this seems to be caused by a less stringent serial cardioversion approach. Less ECVs were performed and less antiarrhythmic drugs were instituted. More interestingly, the recurrence pattern of AF after ECV for new-onset persistent AF was similar in the two groups.
Outcome of serial cardioversion strategy in HF patients
Rate control is the recommended treatment strategy for the majority of patients with AF, but whether this also holds for patients with HF is at present still unknown.13 In patients with (severe) symptoms because of AF rhythm control remains first choice therapy.20 Rhythm control for patients with AF in the setting of HF, however, is even more unsuccessful than in patients without HF.17 Previous studies reported maintenance of sinus rhythm between 38 and 65% after a mean follow-up between 12 and 40 months.15,21–23 However, these studies were not specifically designed to study the effect of stringent rhythm control, and were not performed in patients with a first episode of persistent AF of ‘recent’ onset.
Our study also showed a poor success rate in the systolic HF patients, but less ECVs were performed and less antiarrhythmic drugs were instituted in these patients. Moreover, in a substantial part of the HF patients AF was accepted because of other reasons than refractory AF. In fact, patients who were treated according to the serial cardioversion strategy showed a similar outcome compared with the non-HF patients. On one hand, this suggests that the difference in the way of treatment and not the presence of HF was the main determinant of permanent AF in this study, and that a more stringent rhythm control approach in patients with HF may improve the success rate of rhythm control. On the other hand, there is no consensus whether this is worthwhile. It is unknown whether AF is an independent risk factor for morbidity and mortality in HF,6,7 or just a marker of more advanced disease.8 Furthermore, a substudy of Rate Control vs. Electrical cardioversion study (RACE) strongly suggested that even under optimal conditions of continuous anticoagulation, chronic sinus rhythm did not ameliorate the cardiovascular prognosis in this group of persistent AF patients.24 Remarkably, a substudy of the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study demonstrated that the presence of sinus rhythm was associated with a considerable reduction in the risk of death, whereas the presence of HF and antiarrhythmic drug use were associated with a higher risk of death.25 In line with this, Pappone et al. showed in a retrospective non-randomized analysis that restoration of sinus rhythm by pulmonary vein ablation reduced mortality and morbidity.26 Thus, at present, it remains uncertain whether restoring normal rhythm improves prognosis. In this respect, the results of the AF-CHF study are eagerly awaited.13
Recurrence pattern in HF vs. non-HF patients
Recurrence pattern of AF was not different between HF and non-HF patients. The electrophysiological substrate, though, for AF appears to be quite distinct in patients with and without HF.3,27–29 Nattel and colleagues showed that in a dog model with CHF, due to rapid ventricular pacing for 5 weeks, the action potential duration was not altered by HF at slow rates, but was, interestingly, increased at faster rates, contrary to action potential shortening in the atrial tachycardia models.27,30,31 Sanders et al. performed an electrophysiological and electroanatomical mapping study in 21 patients with symptomatic HF, without a history of AF, and 21 age-matched controls.3 In the HF patients they found significant anatomic and structural abnormalities (including atrial enlargement, regions of slow conduction and scarring), abnormalities in conduction, sinus node dysfunction, and increased refractoriness. These abnormalities were associated with an increased inducibility of AF.
The molecular mechanisms underlying atrial remodelling in HF patients are fairly complex. Activation of the renin angiotensin system, increasing atrial angiotensin II concentrations and atrial stretch may lead to apoptosis, fibrosis, and necrosis, setting the stage for reentry.32 Indeed, ACE inhibition33,34 and blockade of the angiotensin II type 1 receptor35 were able to prevent structural remodeling and AF promotion in dog models of ventricular tachycardiomyopathy. The clinical relevance has recently been illustrated.35–41
Limitations
The retrospective observational design and the small number of patients were major limitations of our study. A limited number of HF patients underwent re-ECVs and antiarrhythmic drug treatment according to the protocol. The presence and severity of diastolic HF in the control group of patients with preserved systolic LV function was not investigated. Although Figure 3 suggests a comparable outcome in HF vs. non-HF patients, it should be interpreted with caution because this subgroup of patients may also represent a positive selection of patients. Finally, differences in medication use at baseline in both groups may have influenced outcome.
Conclusion
This study shows that the recurrence pattern after ECV of new-onset AF is comparable between patients with and without HF. The outcome of a serial cardioversion strategy, however, was inferior in HF patients, at least in part due to less ECVs and less frequent institution of antiarrhythmic drugs. A more stringent serial cardioversion approach, in addition to optimal HF treatment, may improve rhythm control in HF patients. Whether restoring normal rhythm improves prognosis in patients with HF remains, however, uncertain.
References
- anti-arrhythmia agents
- atrial fibrillation
- heart failure, systolic
- ventricular function, left
- left ventricular ejection fraction
- coronary arteriosclerosis
- electric countershock
- heart failure
- follow-up
- pharmacology
- external cephalic version
- symptom onset
- permanent atrial fibrillation
- persistent atrial fibrillation
- rhythm



